Abstract
Despite recent developments reported in studies of (-)-epigallocatechin-3-gallate (EGCG), its early preventive effect of mitigating bone loss is not well understood. We investigated the effect of EGCG in preventing bone loss in ovariectomized (OVX) female rats, and explored the possible underlying mechanisms. Twelve-week-old female Sprague-Dawley rats, were divided into 3 groups: group A received intraperitoneal EGCG for 12 consecutive weeks, begun 3 days after ovariectomy; group B received ovariectomy alone; group C, received a sham operation. At the end of the experiment, tibias and femurs were harvested for: (1) micro-CT scanning and measurement of bone mineral density (BMD) and bone morphological parameters; (2) a 3-point bending test; (3) HE staining and an immunohistological study investigating Sema4D expression. Results: The BMD and BV/TV of group A were significantly higher than for the OVX group. The trabecular separation (Tb.Sp) of group A was significantly lower than for group B. Results from the 3-point bending test showed no statistical significance among all the groups. Bone histological studies indicated that trabecular bone was denser in group C, while group B had less dense trabecular bone, and the bone morphological status of group A was intermediate between groups A and C. The immunohistological study demonstrated that Sema4D was more highly expressed as a percentage of the brown-stained area in group B than in the other 2 groups. Conclusion: EGCG had a positive effect on mitigating bone loss in ovariectomized rats, and it inhibited Sema4D expression in bone tissue. Early stage supplementation of EGCG at a dose of 10 mg/kg/day after the onset of ovariectomy did not entirely eliminate bone loss.
Keywords: EGCG, osteoporosis, bone loss, microarchitecture, BMD, Sema4D
Introduction
Osteoporosis has become a global public health issue as the aging of the population increases. It is a degenerative disease characterized by low bone mass, an increased rate of bone fragility, and susceptibility to fractures, especially of the hip, spine and wrist [1]. Postmenopausal women have a high risk of osteoporosis due to lack of estrogen [2]. Although various drugs for the treatment and prevention of osteopenia and osteoporosis are available, the development of new, effective and economical medicines that cause fewer complications is still urgent.
Green tea, produced from the dried leaves of Camellia sinensis, is a popular beverage worldwide [3]. Antioxidant effects are a widely recognized peculiarity of tea polyphenols [4,5], which might also regulate estrogen deficiency-induced bone loss in postmenopausal women [4,5]. Catechins comprise more than 80% of green tea polyphenols, mainly consisting of (-)-epicatechin (EC), (-)-epicatechin gallate (ECG), (-)-epigallocatechin (EGC) and (-)-epigallocatechin-3-gallate (EGCG), which accounted for more than 50% of the total catechins [6]. Supported by in vitro studies [3,7], an in vivo study that focused on the therapeutic effects of EGCG on the bone microarchitecture of osteoporosis in a rodent model has been carried out, and it demonstrated that EGCG could mitigate bone loss in ovariectomized (OVX) rats, but total recovery of the bone structure was not observed [8]. Whether early preventive supplementation of EGCG could achieve an ideal recovery of bone structure in rodent models has not been demonstrated.
Semaphorins were reported to be involved in the cell-to-cell communication between osteoclasts and osteoblasts [9,10]. Evidence suggested that osteoclasts express semaphorin4D (Sema4D or CD100), which potently inhibits bone formation [9]. Consequently, Sema4D provides a new therapeutic target for anti-osteoporosis treatment. However, whether EGCG regulates bone metabolism via this signal pathway is unknown, and related in vivo studies are rare. Therefore, we conducted this study in female ovariectomized (OVX) rats and intervened with EGCG in the early stages after ovariectomy to assess the preventive effects on bone microstructure and their possible mechanisms. In addition, we also studied the expression of Sema4D in bone tissue.
Materials and methods
Experimental characteristics and group design
Soochow University permits and approvals this work and study. Virgin female OVX Sprague-Dawley (SD) rats were used because (a) this study was focused on simulating the condition of postmenopausal women, for whom pregnancy and lactation might influence bone structure [11] and (b) OVX rats that simulate estrogen deficiency are currently a widely used animal model for postmenopausal osteoporosis [12]. Rats were purchased from Shanghai Laboratory Center, China. This animal study was approved by the Institutional Animal Care and Use Committee. EGCG (99% purity) was purchased from Hangzhou Ebeikar Tea Development CO., LTD, Hangzhou, China. All rats were raised in a temperature-controlled room at approximately 23±1°C and maintained on a regular rodent chow diet with distilled water given ad libitum throughout the experimental period. After a 1-week acclimation phase, 35 rats were weighed and assigned into 3 groups with no statistically significant differences among the pre-operative weights: Group A (n=15) was ovariectomized and EGCG was given intraperitoneally at 10 mg/kg/day, beginning 3 days after ovariectomy and continuing for 12 consecutive weeks; this group was labeled as OVX+ EGCG/IP; Group B consisted of ovariectomized controls (n=10), labeled as OVX, and Group C consisted of sham-operated controls (n=10), labeled as SHAM.
Surgical procedures
Ovariectomy was performed under intraperitoneal anesthesia. A ventral middle incision approximately 1.5-2.5 centimeters was made in the lower abdomen. After separation of muscles and exposure of the retroperitoneal fat tissues, a Y-shaped uterus appeared. Two ovaries were present, resembling two irregular-shaped masses distal to the fallopian tubes bilaterally. The fallopian tubes were ligated at the junction with the ovary and the ovaries were excised bilaterally. For sham-operated rats, similar sized retroperitoneal fat tissues were removed instead. Finally, the incision was closed layer by layer, and 0.1 ml penicillin (4000 units per rat) was injected to prevent inflammation.
Sample preparations
At the end of the experiment, the rats were anesthetized and euthanized, and the final body weight was recorded. The distal femur and proximal tibiae were harvested and adhering soft tissues were removed. The right femur and tibia samples were preserved in 4% paraformaldehyde for histology and immunohistochemistry testing. The left femur samples were kept in a 10% cold phosphate buffer solution (PBS) for micro-CT examination and the 3 point-bending test.
Micro-CT analysis
The bone microarchitecture of the distal left femur samples was evaluated by μCT (Skyscan 1176; Skyscan, Antwerp, Belgium) and related software (CT-analyser, Version: 1.10.11.0). All scans were performed in a fixed matrix resulting in an isotropic voxel resolution of 18 μm. Data were collected every 0.5° rotation step through 180°. The volume of interest (VOI) of the trabecular bone of the femur included the secondary spongiosa in 100 cross-sectional slices of the distal femur beginning 50 slices up from the growth plate region, and the VOI of the cortical bone of the femur was composed of 100 cross-sectional slices of the distal femur beginning 350 slices up from the growth plate region [13]. Trabecular parameters for the femur included bone mineral density (BMD), trabecular bone volume (BV), total bone volume (TV), the fraction (BV/TV, %), trabecular number (Tb.N, n/mm), trabecular thickness (Tb.Th, μm) and trabecular separation (Tb.Sp, μm) calculated by Skyscan software CTscan. Cortical thicknesses were also calculated by measuring the average values of 4 quartile points at a single cross-section 340 slices up from the femoral growth plate and repeating the measurement twice, at intervals of 100 slices.
Histological presentations
The distal right femur and proximal right tibiae were harvested and assessed for the effect of EGCG on trabecular bone tissues. Decalcified bone tissue histological sections embedded in paraplast were made and observed. Each section was 5 μm thick and was stained with hematoxylin and eosin.
Immunohistochemistry study
Right distal femur and proximal tibiae were fixed overnight in 4% paraformaldehyde in PBS and decalcified for 5 weeks in 0.5 M EDTA, pH 7.4. Sections of right distal femur and proximal tibiae samples 5-μm thick were made. The sections were deparaffinized in xylene and washed twice with a graded series of ethanol solutions. Blocking solutions were applied. Sections were incubated in 0.01 M sodium citrate buffer for 10 minutes at 100°C for antigen retrieval. Purified mouse anti-CD100 (BD Bioscience) was used. After incubation with anti-CD100 overnight at 4°C in a humid chamber, a phosphate buffer was used for washing, and a secondary antibody (Gene Tech) was applied before incubation with horseradish peroxidase-conjugated streptavidin. HRP activity was measured using diaminobenzidine (DAB) as a substrate. Stained samples were observed under an Axio imager M1 microscope (Zeiss, Germany) equipped with a camera and software. The relative density of immunostaining (density/area) and the positive area percentage was measured using Image-Pro plus 5, Media Cybernetics, Silver Spring, Maryland, USA.
Biomechanical test
A three-point bending test was applied to test the mechanical strength of the intact femur. The bones were wrapped in gauze soaked in isotonic saline and placed in a moisture box at room temperature 4 hours before mechanical testing [14]. A biomechanical test machine (Instron E10000, USA) and associated software (Bluehill 2, USA) were employed. The femurs were placed between two metal supports with a distance of 18 mm between them, while the loading pin was compressed at the anterior surface in the middle of the femoral shaft with a blunt metal edge. A load was applied at a rate of 5 mm/min and directed to the mid-diaphyseal region. Compression of the loading pin continued until a fracture occurred. The biomechanical parameters bending displacement (mm), ultimate bending stress (N) and elastic modulus (MPa) to break bones, and modulus of elasticity were recorded and evaluated.
Statistical analysis
Data are presented as the mean ± standard deviation and were analyzed with SPSS software, version 19.0 (SPSS Inc., Chicago, IL). The Kolmogorov-Smirnov normality test for each continuous variable was performed. A 95% confidence interval was applied, and a p value less than 0.05 determined statistical significance. A one-way analysis of variance (ANOVA) was applied to compare different groups for BMD, bone morphological parameters (trabecular BV, TV, BV/TV, Tb.N, Tb.Th, Tb.Sp and cortical thickness), biomechanical test parameters (bending displacement, ultimate bending stress and elastic modulus), and immunostaining parameters in the immunohistochemistry test (density/area and the positive area percentage). Post hoc multiple comparisons were made if statistical significance was indicated by the one-way ANOVA (P<0.05).
Results
Body weight
Before ovariectomy, there was no statistical significance among any of the treatment groups. At the end of the experiment, the body weight was significantly higher in the OVX group compared with the SHAM group. EGCG supplementation did not reduce body weight as the body weight in the OVX+ EGCG/IP group at the end of the experiment did not show any significant differences with the OVX group (Table 1).
Table 1.
Changes of body weight before and after treatment
| Group | OVX+ EGCG/IP (Group A) | OVX (Group B) | SHAM (Group C) |
|---|---|---|---|
| Before treatment, g | 241.83±10.43 | 237.20±9.51 | 239.20±9.74 |
| After treatment, g | 352.92±14.69## | 361.67±16.39## | 311.10±11.49** |
| Difference value, g | 111.08±9.89**,## | 124.44±8.71## | 71.90±9.48** |
Abbreviations: OVX for ovariectomy; SHAM for sham operation. All data are presented as the mean ± standard deviation.
P<0.01 vs. SHAM group;
P<0.01 vs. OVX group.
Bone morphological parameters
Measured from the trabecular bone of the distal femur of the female SD rats, the BMD of the SHAM group was significantly higher than in the other 2 groups (P<0.01), while the BMD of OVX+ EGCG/IP group was significantly higher than in the OVX group (P<0.05) (Table 2). Regarding the measurements of the trabecular bone morphological parameters of the distal femur in female SD rats, the SHAM group had a higher BV/TV, TbTh, and a lower TbSp than the OVX group (P<0.01), while the OVX+ EGCG/IP group had a higher BV/TV and a lower TbSp than the OVX group (P<0.05). There was no significant difference in TbN among the 3 groups. Regarding the measurement of the cortical bone morphological parameters of the distal femur in female SD rats (Table 3), the SHAM group had a higher BV/TV than the OVX+ EGCG/IP group (P<0.01) and the OVX group (P<0.05), while there was no significant difference between the OVX+ EGCG/IP group and the OVX group (P>0.05) (Figure 1).
Table 2.
Bone morphological parameters in the trabecular bone of the distal femur in female SDrats
| Group | OVX+ EGCG/IP (Group A) | OVX (Group B) | SHAM (Group C) |
|---|---|---|---|
| BMD | 0.081±0.030*,## | 0.046±0.026## | 0.192±0.034** |
| BV/TV, % | 40.11±10.01*,## | 28.93±7.60## | 81.53±9.17** |
| TbTh, mm | 0.19±0.02## | 0.17±0.01## | 0.27±0.05** |
| TbN, mm-1 | 2.14±0.39 | 1.70±0.41 | 2.08±1.63 |
| TbSp, mm | 0.41±0.12*,## | 0.57±0.18## | 0.13±0.02** |
Abbreviations: OVX for ovariectomy; SHAM for sham operation; BV for bone volume; TV for total volume; TbTh for trabecular thickness; TbN for trabecular number; TbSp for trabecular separation. All data are presented as the mean ± standard deviation.
P<0.01 vs. SHAM group;
P<0.05 vs. OVX group;
P<0.01 vs. OVX group.
Table 3.
Bone morphological parameters in the cortical bone of the distal femur in female SD rats
| Group | OVX+ EGCG/IP (Group A) | OVX (Group B) | SHAM (Group C) |
|---|---|---|---|
| BV/TV, % | 55.39±3.50## | 57.51±1.89# | 62.86±3.46* |
| Cortical thickness, mm | 0.629±0.023 | 0.626±0.034 | 0.634±0.010 |
Abbreviations: OVX for ovariectomy; SHAM for sham operation; BV for bone volume; TV for total volume. All data are presented as the mean ± standard deviation.
P<0.05 vs. SHAM group;
P<0.01 vs. SHAM group;
P<0.05, vs. OVX group.
Figure 1.
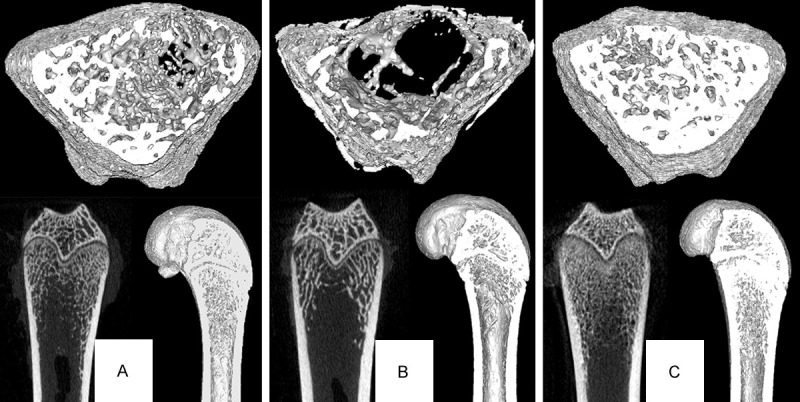
Micro-CT analysis of the distal femur of female SD rats. Each group contains 3 images. The top is the cross-sectional image of trabecular bone, the left bottom is the original coronal image of CT scanning, and the right bottom is the 3D reconstruction image. The images clearly show that group A and group C have a more dense distribution of trabecular bone than group B.
Three-point bending test
Although the SHAM group had higher average values of bending displacement, ultimate bending stress, and elastic modulus than the other 2 groups, there was no significant difference among the 3 groups (Table 4).
Table 4.
Biomechanical test parameters of the femur shaft in female SD rats
| Group | OVX+ EGCG/IP (Group A) | OVX (Group B) | SHAM (Group C) |
|---|---|---|---|
| Bending displacement, mm | 1.03±0.31 | 1.01±0.27 | 1.03±0.36 |
| Ultimate bending stress, N | 158.35±17.94 | 125.84±6.10 | 145.73±18.70 |
| Elastic modulus, MPa | 4079.91±1004.41 | 3248.77±391.94 | 3846.10±600.47 |
Abbreviations: OVX for ovariectomy; SHAM for sham operation. All data are presented as the mean ± standard deviation.
Histological presentations
In the histological study as indicated by to the HE staining, the trabecular bone distribution was denser and the trabecular number was higher in the SHAM group and the OVX+ EGCG/IP group than in the OVX group (Figure 2).
Figure 2.
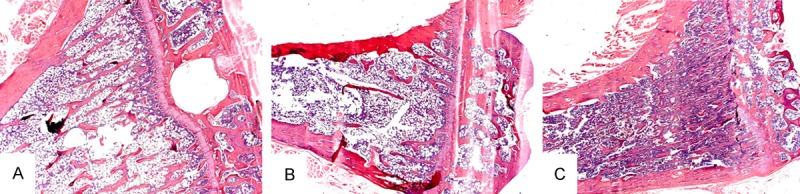
The HE staining of the proximal tibia of female SD rats indicates that group (B) had a sparser trabecular distribution, while group (A) and (C) had a more dense distribution (×25).
Immunohistochemistry study
The results of immunohistochemistry study are illustrated in Table 5. The brown-stained area is the positive area, and it was expressed mainly along the bone surface. The Brown/Total area ratio was significantly higher in the OVX group than in the other 2 groups (P<0.01), but there was no statistical difference of the Brown/Total area ratio between the OVX+ EGCG/IP group and the SHAM group. Similar results were observed for the mean IOD (Figure 3).
Table 5.
Results of immunohistochemistry study of Sema4D
| Group | OVX+ EGCG/IP (Group A) | OVX (Group B) | SHAM (Group C) |
|---|---|---|---|
| Brown/Total area, % | 15.08±12.51** | 45.29±4.63## | 21.32±14.80** |
| Mean IOD | 0.026±0.019** | 0.065±0.010## | 0.029±0.010** |
Abbreviations: OVX for ovariectomy; SHAM for sham operation; IOD for integrated option density. All data are presented as the mean ± standard deviation.
P<0.01 statistical significance achieved in comparison with the SHAM group;
P<0.01 statistical significance achieved in comparison with the OVX group.
Figure 3.
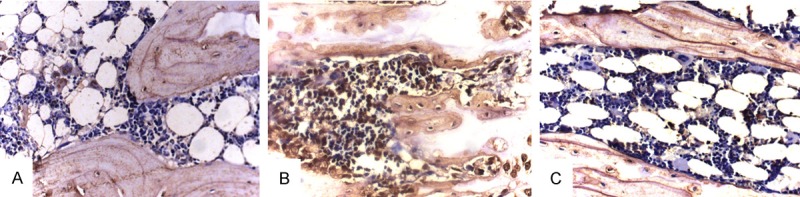
The Immunohistochemistry study of Sema4D expression in bone tissue in female SD rats demonstrated that group (B) has a higher expression shown by an increase in the size of the brown stained area, while groups (A) and (C) have a relatively lower positive area (×400).
Discussion
In our study, a model of OVX estrogen deficiency-induced female Sprague-Dawley rats was successfully applied to assess the effect of EGCG supplementation on bone microarchitecture and to investigate possible mechanisms. The conventional model of estrogen deficiency-induced female rats in researching osteoporosis or osteopenia was established in 12-week-old female rats [8,15], but some OVX models used rats less than 12 weeks old [16,17] and other models used rats as old as 8 months old [18]. It should be taken into consideration that the rats were still in the growing phase [17] in these models. In our study, we used the OVX group and the SHAM group as 2 control groups for comparisons.
A study by Shen et al. [19] found that, although GTP supplementation for 16 weeks markedly improved both femoral BMD and the microarchitecture of trabecular and cortical bone in the tibia and femur in middle-aged 14-month-old female rats, GTP did not completely prevent bone loss due to aging plus estrogen deficiency in OVX rats. Similar results were observed in a study by Chen et al. [8] who studied a 3-month-old female OVX rat model. EGCG supplementation was begun at 12 weeks post-operatively and maintained for 12 weeks. Chen et al. that found that EGCG supplementation could mitigate bone loss in OVX rats, but the OVX rats still had a relative low BMD, BV/TV, TbTh, and TbN compared to sham-operated rats.
A study reported that major changes in the bone structure of estrogen deficiency-induced rats were detected within the first 3 months after ovariectomy [20] demonstrated that early intervention is important. In our study, we decided to intervene in 12-week-old female OVX rats with EGCG 3 days after ovariectomy to investigate the magnitude of any preventive effects of EGCG on the bone microarchitecture as a simulation of the early stages of post-menopause without the influence of senility [17,21].
As illustrated by our results, decreases of BMD, BV/TV, TbTh, and an increase of TbSp in the distal femur of trabecular bone were detected in the OVX group (Table 2). The results in the EGCG-intervened estrogen deficiency-induced group suggested an attenuation of bone loss in female OVX rats. However, in comparison with the SHAM group, the OVX+ EGCG/IP group had relatively lower parameters for BMD, BV/TV, and higher parameters of TbSp. Although the average value of parameters of TbTh and TbN were higher in the OVX+ EGCG/IP group than in the OVX group, no statistical significance was observed. The results were similar to those from an experiment conducted by Shen et al. [19] who used GTP for early intervention 2 weeks after ovariectomy in middle-aged female rats and did not observe complete prevention of bone loss due to aging plus estrogen deficiency.
Cellular studies support the beneficial effect of EGCG in preventing bone loss via its effects on osteoblasts and osteoclasts. EGCG could stimulate osteoblastogenesis via: 1) enhancing the mRNA expression of osteogenic genes, such as alkaline phosphatase activity, osteocalcin, and core binding factor a1, eventually stimulating mineralization [3,22]; 2) increasing proliferation and differentiation of osteoblasts via the Wnt signaling pathway [23]; 3) improving the survival of osteoblasts through the inhibition of TNF-α and interleukin-6 production [24]; 4) suppressing endothelin-1-induced interleukin-6 synthesis in osteoblasts through inhibition of p44/p42 mitogen-activated protein kinase [25]; 5) enhancing prostaglandin F2α-induced vascular endothelial growth factor via the up-regulation of SAPK/JNK activation in osteoblastic-like MC3T3-E1 cells [26]. Meanwhile, EGCG could influence osteoclasts via: 1) inhibiting the survival of differentiated osteoclasts and increasing apoptosis in osteoclasts [27,28]; 2) stimulating osteoclastic cell death via the Fenton reaction and caspase activation [28,29]; 3) suppressing the formation of osteoclastic cells via inhibiting matrix and metalloproteinase-9 expression [27].
As previously reported by Hirschberg et al., Sema4D positively regulates neuron migration during cortical development [30]. As indicated by Negishi-Koga et al., Sema4D, is an axon guidance molecule and is expressed in osteoclasts, and could potently inhibit bone formation. Through the binding of Sema4D to its receptor Plexin-B1 in osteoblasts, the activation of the small GTPase RhoA resulted, and bone formation was inhibited by suppression of IGF-1 signaling and by modulation of osteoblast motility [9]. Negishi-Koga et al. found that bone volume, trabecular thickness, and bone strength was significantly greater in Sema4D-/- mice and Plxnb1-/- mice compared to wild-type mice, suggesting an osteosclerotic phenotype in Sema4D-/- mice and Plxnb1-/- mice. Meanwhile, blocking the Sema4D-Plexin-B1 interaction could be a new and potentially effective strategy for increasing bone formation.
According to our immunohistochemistry study, the OVX group had relatively higher positive area percentage of Sema4D expression and mean IOD than the other 2 groups, and EGCG supplementation decreased the expression of Sema4D mainly along the osteoclast and osteoblast surface. These results indicated that in female estrogen-deficient rats, the possible mechanism of bone loss is likely to be an increase in the expression of Sema4D by osteoclasts, which act on osteoblasts to further suppress osteogenesis; moreover, the intervention of EGCG can reduce the expression of Sema4D to a certain degree. Although Plexin-B1 is responsible for recognizing Sema4D, some recognition is mediated by other receptors, such as Plexin-B2 [9]. Thus the expression of Plexin-B1 has a limited role in explaining the observed effects.
EGCG has positive effect on mitigation of bone loss in ovariectomized rats, and inhibits the expression of Sema4D in bone tissue. Early supplementation of EGCG at a dose of 10 mg/kg/day after ovariectomy does not entirely prevent bone loss. Further studies exploring the underlying mechanisms, different dose supplementations of EGCG, and a middle-aged rat model should be conducted.
Acknowledgements
This study was sponsored by the following foundations: 1, National Natural Science Foundation of China (81071451, 81171689, 81301559), 2, Jiangsu Natural Science Foundation (BK2011264), 3, Suzhou Science Education And Health Project (KJXW2012004), 4, Jiangsu Graduate Student Innovation Project (CXZZ12-0840), 5, Suzhou Basic Application Study Project (SYS201211).
Disclosure of conflict of interest
None.
References
- 1.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795. [Google Scholar]
- 2.Saika M, Inoue D, Kido S, Matsumoto T. 17beta-estradiol stimulates expression of osteoprotegerin by a mouse stromal cell line, ST-2, via estrogen receptor-alpha. Endocrinology. 2001;142:2205–2212. doi: 10.1210/endo.142.6.8220. [DOI] [PubMed] [Google Scholar]
- 3.Chen CH, Ho ML, Chang JK, Hung SH, Wang GJ. Green tea catechin enhances osteogenesis in a bone marrow mesenchymal stem cell line. Osteoporos Int. 2005;16:2039–2045. doi: 10.1007/s00198-005-1995-0. [DOI] [PubMed] [Google Scholar]
- 4.Hegarty VM, May HM, Khaw KT. Tea drinking and bone mineral density in older women. Am J Clin Nutr. 2000;71:1003–1007. doi: 10.1093/ajcn/71.4.1003. [DOI] [PubMed] [Google Scholar]
- 5.Shen CL, Wang P, Guerrieri J, Yeh JK, Wang JS. Protective effect of green tea polyphenols on bone loss in middle-aged female rats. Osteoporos Int. 2008;19:979–990. doi: 10.1007/s00198-007-0527-5. [DOI] [PubMed] [Google Scholar]
- 6.Nagle DG, Ferreira D, Zhou YD. Epigallocatechin-3-gallate (EGCG): chemical and biomedical perspectives. Phytochemistry. 2006;67:1849–1855. doi: 10.1016/j.phytochem.2006.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin RW, Chen CH, Wang YH, Ho ML, Hung SH, Chen IS, Wang GJ. (-)-Epigallocatechin gallate inhibition of osteoclastic differentiation via NF-kappaB. Biochem Biophys Res Commun. 2009;379:1033–1037. doi: 10.1016/j.bbrc.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Chen CH, Kang L, Lin RW, Fu YC, Lin YS, Chang JK, Chen HT, Lin SY, Wang GJ, Ho ML. (-)-Epigallocatechin-3-gallate improves bone microarchitecture in ovariectomized rats. Menopause. 2013;20:687–694. doi: 10.1097/GME.0b013e31828244f0. [DOI] [PubMed] [Google Scholar]
- 9.Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH, Takayanagi H. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat Med. 2011;17:1473–1480. doi: 10.1038/nm.2489. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi M, Nakashima T, Taniguchi M, Kodama T, Kumanogoh A, Takayanagi H. Osteoprotection by semaphorin 3A. Nature. 2012;485:69–74. doi: 10.1038/nature11000. [DOI] [PubMed] [Google Scholar]
- 11.Bowman BM, Siska CC, Miller SC. Greatly increased cancellous bone formation with rapid improvements in bone structure in the rat maternal skeleton after lactation. J Bone Miner Res. 2002;17:1954–1960. doi: 10.1359/jbmr.2002.17.11.1954. [DOI] [PubMed] [Google Scholar]
- 12.Kalu DN, Liu CC, Hardin RR, Hollis BW. The aged rat model of ovarian hormone deficiency bone loss. Endocrinology. 1989;124:7–16. doi: 10.1210/endo-124-1-7. [DOI] [PubMed] [Google Scholar]
- 13.Shen CL, Yeh JK, Samathanam C, Cao JJ, Stoecker BJ, Dagda RY, Chyu MC, Wang JS. Protective actions of green tea polyphenols and alfacalcidol on bone microstructure in female rats with chronic inflammation. J Nutr Biochem. 2011;22:673–680. doi: 10.1016/j.jnutbio.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Sha M, Guo Z, Fu J, Li J, Yuan CF, Shi L, Li SJ. The effects of nail rigidity on fracture healing in rats with osteoporosis. Acta Orthop. 2009;80:135–138. doi: 10.1080/17453670902807490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang ZG, Bai D, Liu MJ, Li Y, Pan JH, Liu H, Wang WL, Xiang LH, Xiao GG, Ju DH. Therapeutic effect of aqueous extract from Ecliptae herba on bone metabolism of ovariectomized rats. Menopause. 2013;20:232–240. doi: 10.1097/gme.0b013e318265e7dd. [DOI] [PubMed] [Google Scholar]
- 16.Tantikanlayaporn D, Wichit P, Weerachayaphorn J, Chairoungdua A, Chuncharunee A, Suksamrarn A, Piyachaturawat P. Bone sparing effect of a novel phytoestrogen diarylheptanoid from Curcuma comosa Roxb. in ovariectomized rats. PLoS One. 2013;8:e78739. doi: 10.1371/journal.pone.0078739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perilli E, Le V, Ma B, Salmon P, Reynolds K, Fazzalari NL. Detecting early bone changes using in vivo micro-CT in ovariectomized, zoledronic acid-treated, and sham-operated rats. Osteoporos Int. 2010;21:1371–1382. doi: 10.1007/s00198-009-1082-z. [DOI] [PubMed] [Google Scholar]
- 18.Ammann P, Bourrin S, Bonjour JP, Brunner F, Meyer JM, Rizzoli R. The new selective estrogen receptor modulator MDL 103,323 increases bone mineral density and bone strength in adult ovariectomized rats. Osteoporos Int. 1999;10:369–376. doi: 10.1007/s001980050242. [DOI] [PubMed] [Google Scholar]
- 19.Shen CL, Yeh JK, Stoecker BJ, Chyu MC, Wang JS. Green tea polyphenols mitigate deterioration of bone microarchitecture in middle-aged female rats. Bone. 2009;44:684–690. doi: 10.1016/j.bone.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Boyd SK, Davison P, Muller R, Gasser JA. Monitoring individual morphological changes over time in ovariectomized rats by in vivo micro-computed tomography. Bone. 2006;39:854–862. doi: 10.1016/j.bone.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Lane NE, Haupt D, Kimmel DB, Modin G, Kinney JH. Early estrogen replacement therapy reverses the rapid loss of trabecular bone volume and prevents further deterioration of connectivity in the rat. J Bone Miner Res. 1999;14:206–214. doi: 10.1359/jbmr.1999.14.2.206. [DOI] [PubMed] [Google Scholar]
- 22.Vali B, Rao LG, El-Sohemy A. Epigallocatechin-3-gallate increases the formation of mineralized bone nodules by human osteoblast-like cells. J Nutr Biochem. 2007;18:341–347. doi: 10.1016/j.jnutbio.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Mount JG, Muzylak M, Allen S, Althnaian T, McGonnell IM, Price JS. Evidence that the canonical Wnt signalling pathway regulates deer antler regeneration. Dev Dyn. 2006;235:1390–1399. doi: 10.1002/dvdy.20742. [DOI] [PubMed] [Google Scholar]
- 24.Nelson-Dooley C, Della-Fera MA, Hamrick M, Baile CA. Novel treatments for obesity and osteoporosis: targeting apoptotic pathways in adipocytes. Curr Med Chem. 2005;12:2215–2225. doi: 10.2174/0929867054864886. [DOI] [PubMed] [Google Scholar]
- 25.Tokuda H, Takai S, Hanai Y, Matsushima-Nishiwaki R, Hosoi T, Harada A, Ohta T, Kozawa O. (-)-Epigallocatechin gallate suppresses endothelin-1-induced interleukin-6 synthesis in osteoblasts: inhibition of p44/p42 MAP kinase activation. FEBS Lett. 2007;581:1311–1316. doi: 10.1016/j.febslet.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 26.Tokuda H, Takai S, Matsushima-Nishiwaki R, Akamatsu S, Hanai Y, Hosoi T, Harada A, Ohta T, Kozawa O. (--)-epigallocatechin gallate enhances prostaglandin F2alpha-induced VEGF synthesis via upregulating SAPK/JNK activation in osteoblasts. J Cell Biochem. 2007;100:1146–1153. doi: 10.1002/jcb.21104. [DOI] [PubMed] [Google Scholar]
- 27.Yun JH, Pang EK, Kim CS, Yoo YJ, Cho KS, Chai JK, Kim CK, Choi SH. Inhibitory effects of green tea polyphenol (-)-epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J Periodontal Res. 2004;39:300–307. doi: 10.1111/j.1600-0765.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa H, Wachi M, Woo JT, Kato M, Kasai S, Takahashi F, Lee IS, Nagai K. Fenton reaction is primarily involved in a mechanism of (-)-epigallocatechin-3-gallate to induce osteoclastic cell death. Biochem Biophys Res Commun. 2002;292:94–101. doi: 10.1006/bbrc.2002.6622. [DOI] [PubMed] [Google Scholar]
- 29.Islam S, Islam N, Kermode T, Johnstone B, Mukhtar H, Moskowitz RW, Goldberg VM, Malemud CJ, Haqqi TM. Involvement of caspase-3 in epigallocatechin-3-gallate-mediated apoptosis of human chondrosarcoma cells. Biochem Biophys Res Commun. 2000;270:793–797. doi: 10.1006/bbrc.2000.2536. [DOI] [PubMed] [Google Scholar]
- 30.Hirschberg A, Deng S, Korostylev A, Paldy E, Costa MR, Worzfeld T, Vodrazka P, Wizenmann A, Gotz M, Offermanns S, Kuner R. Gene deletion mutants reveal a role for semaphorin receptors of the plexin-B family in mechanisms underlying corticogenesis. Mol Cell Biol. 2010;30:764–780. doi: 10.1128/MCB.01458-09. [DOI] [PMC free article] [PubMed] [Google Scholar]


