Abstract
There is an accumulating body of evidence indicating strong association between inflammation and the pathogenesis of atrial fibrillation (AF). IL-10 is a multifunctional anti-inflammatory cytokine that down-regulates cell-mediated immune responses and cytotoxic inflammatory responses. The aim of the present study is to investigate the association of IL-10 gene -592A/C polymorphism with AF in Han Chinese. 117 AF patients and 100 healthy volunteers were eligible for this study. The PCR-based restriction fragment length polymorphism (PCR-RFLP) technique was used to assess the genotypes frequencies. The distribution of the IL-10 -592A/C genotypes (AA, AC, and CC) was 55.00%, 35.00%, and 10.00% in the controls, and 71.79%, 23.08%, and 5.13% in AF subjects, respectively (p = 0.0335). The frequency of the A allele in the AF group was significantly higher than that in the control group (83.33% vs 72.50%, p = 0.0063). Compared with the CC genotype, the AA genotype had increased risk of AF in both unadjusted and adjusted analyses. The average serum IL-10 levels in AA genotype were statistically lower than in AC + CC genotype (p = 0.0000). These findings suggest that IL-10 -592A/C polymorphism is associated with AF and the A allele has increased risk for AF in Han Chinese.
Keywords: Atrial fibrillation, interleukin-10, genetic polymorphism, Chinese
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia seen in clinical practice, affecting 1-2% of the general population [1-3]. The prevalence of AF doubles with each advancing decade from the age of 50 years, affecting approximately 0.5% of individuals aged at 40-50 years and 5-15% of persons at 80 years [1]. In China, morbidity related to AF is 0.77% in the adult population [4]. Compared with people in sinus rhythm, those in AF have a 6-fold increased risk of stroke and twofold increased risk of death. For those with rheumatic heart disease, the risk of stroke is increased up to 18-fold [2,5-7]. Despite the extensive studies, the pathophysiological mechanisms in AF, however, remain unclear.
Interleukin (IL)-10 is a multifunctional cytokine involved in both innate and adaptive immune response [8,9]. Interleukin-10 inhibits the production of pro-inflammatory cytokines by inhibition of T-helper 1 (Th1) lymphocytes and stimulation of B lymphocytes and Th2 lymphocytes and thus downregulates the inflammatory response [10]. As an inflammatory cytokine, IL-10 participates in the development of various diseases, such as chronic infection, kidney disease, cancer, and cardiovascular disease [11,12]. The gene encoding IL-10 is located on chromosome 1 (1q31-1q32). Three functional promoter single nucleotide polymorphisms (SNPs) in the IL-10 locus at -1082 (A to G, rs1800896), -819 (C to T, rs1800871), and -592 (A to C, rs1800872) from the transcriptional start site have been confirmed, and -819C/T is in tight linkage disequilibrium with -592A/C [13,14]. However, the -1082G allele is extremely rare in Chinese Han population [15].
Based on these findings, we carried out a case-control study of IL-10 gene -592A/C polymorphism to evaluate its putative association with AF in Han Chinese.
Subjects and methods
Study subject
A total of 117 AF patients were eligible for this study. AF was defined according to the European Society of Cardiology (ESC) Guidelines for the management of AF [1] as replacement of sinus P waves by rapid oscillations or fibrillatory waves that varied in size, shape, and timing, which were associated with an irregular ventricular response when atrioventricular conduction was intact. The presence of AF was determined from history, followed by serial electrocardiogram or ambulatory electrocardiographic monitoring. 100 healthy volunteers in the corresponding period served as controls. The control subjects were judged to be free of AF by history, clinical examination, electrocardiography, and dynamic electrocardiography. All study participants were enrolled at the Affiliated Hospital of Nantong University and unrelated Han nationality. Details of medical history, family history, and clinical symptoms were obtained from all participants using a standardized questionnaire, together with information of drug intake and cigarette smoking. Study participants with acute coronary syndrome, hypertrophic cardiomyopathy, significant valvular disease, left ventricular dysfunction (ejection fraction < 50%), and neoplastic, renal, liver, or thyroid diseases were excluded. The study has been approved by the Medical Ethics Committee of Soochow University, and written informed consent was obtained from all participants.
Biochemical analysis
Venous blood samples were obtained after at least a 10-hour overnight fast and then centrifuged at 2500 rpm for 30 minutes at 4°C and immediately stored at -80°C until analysis. Measurement of total cholesterol (TC), high density lipoprotein-cholesterol (HDL-C), low density lipoprotein-cholesterol (LDL-C), and triglycerides (TG) was performed as described previously [16-18]. The serum IL-10 levels were analyzed using a standard enzyme-linked immunosorbent assay (ELISA) using a commercially available kit (R&D Systems, Minneapolis, Minnesota, USA) according to manufacturer’s instructions.
Genetic analysis
Genomic DNA was extracted from peripheral blood leukocytes by the salting-out method with minimal modifications. Determination of IL-10 gene -592A/C genotypes was performed by polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP) as described previously [19]. A 465 bp PCR amplification product was generated from genomic DNA using the following primers: 5’-AACTTCTTCCACCCCATCTTT-3’ (sense) and 5’-ATCCTCAAAGTTCCCAAGCAG-3’ (antisense). PCR reactions were carried out with 200 ng of genomic DNA in a total volume of 25 μL, containing 1.5 mmol/L Mg2+, 0.02 µmol of each of the four dNTPs, 40 pmol of each of the primers, and 1.5 U of DNA polymerase. Thermal processing started with 94°C for 5 min, and 38 cycles at 94°C for 45 s, 52°C for 38 s and 72°C for 90 s, this was followed by a final extension at 72°C for 10 min. All PCR reactions were carried out in a Perkin-Elmer 9600 thermal cycler (Foster City, CA). 10 μL of the PCR product was digested using the 2 U of the RsaⅠrestriction endonuclease (New England Biolabs, Beverly, MA, USA) in 25 μL volumes at 37°C for 1 h. The digestion products were then separated by electrophoresis on 1.5% agarose gel, stained with ethidium bromide.
Statistical analysis
All continuous variables are expressed as the mean and standard deviation (SD). Student’s t-test was used to compare continuous variables from two groups. Genotypes and allele frequencies were obtained by direct count. Differences in the distribution of alleles and genotypes between the groups, and deviations from the Hardy-Weinberg equilibrium were assessed by χ2 test. All significant tests were two-tailed and were considered statistically significant at p < 0.05. SPSS for Windows version 11.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Results
Characteristics of the study subjects
The clinical characteristics of all participants enrolled in the study are shown in Table 1. No significant differences were seen between the two groups with regard to gender, prevalence of hypertension and diabetes, smoking status, leukocyte count, LDL-C, HDL-C, and TG. However, compared with the controls, AF patients had older ages, larger left atrial dimension, and higher serum IL-10 and TC levels.
Table 1.
Clinical characteristics of AF and control subjects
| Characteristics | AF (n = 117) | Controls (n = 100) | p value |
|---|---|---|---|
| Age (years) | 64.37 ± 13.11 | 59.10 ± 4.04 | 0.0001 |
| Gender (% male) | 57.26 | 60.00 | 0.6835 |
| Hypertension (n, %) | 65 (55.55) | 43 (43.00) | 0.0652 |
| Diabetes mellitus (n, %) | 16 (13.68) | 8 (8.00) | 0.1840 |
| Smoking (n, %) | 38 (32.48) | 33 (33.00) | 0.9350 |
| Leukocyte count (109/L) | 5.60 ± 1.44 | 5.46 ± 1.21 | 0.4435 |
| Left atrial dimension (mm) | 44.56 ± 6.83 | 33.06 ± 1.71 | 0.0000 |
| TC (mmol/L) | 4.43 ± 1.14 | 3.63 ± 0.56 | 0.0000 |
| LDL-C (mmol/L) | 2.41 ± 0.96 | 2.45 ± 0.49 | 0.7067 |
| HDL-C (mmol/L) | 1.23 ± 0.32 | 1.19 ± 0.32 | 0.3597 |
| TG (mmol/L) | 1.30 ± 0.85 | 1.31 ± 0.71 | 0.9259 |
| IL-10 (ng/L) | 12.78 ± 6.05 | 18.65 ± 5.52 | 0.0000 |
AF, atrial fibrillation; TC, total cholesterol; LDL-C, low density lipoprotein-cholesterol; HDL-C, high density lipoprotein-cholesterol; TG, triglycerides; IL-10, interleukin-10.
Distributions of IL-10 -592A/C genotypes and allele frequencies
PCR product digested with RsaⅠyields 405 and 60 bp fragments when A is at position -592. According to electrophoresis, the fragments were 405 bp + 60 bp (AA genotype), 465 bp (CC genotype) and 465 bp + 405 bp + 60 bp (AC genotype), respectively, as shown in Figure 1. 10% of the PCR samples were directly sequenced to ensure the reliability of the digestion. The sequencing results were consistent with the results by PCR-RFLP, as shown in Figure 2.
Figure 1.
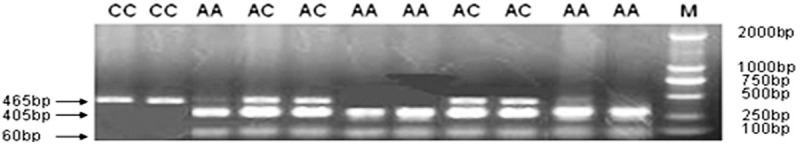
Electrophoresis results of IL-10 -592A/C genotypes.
Figure 2.
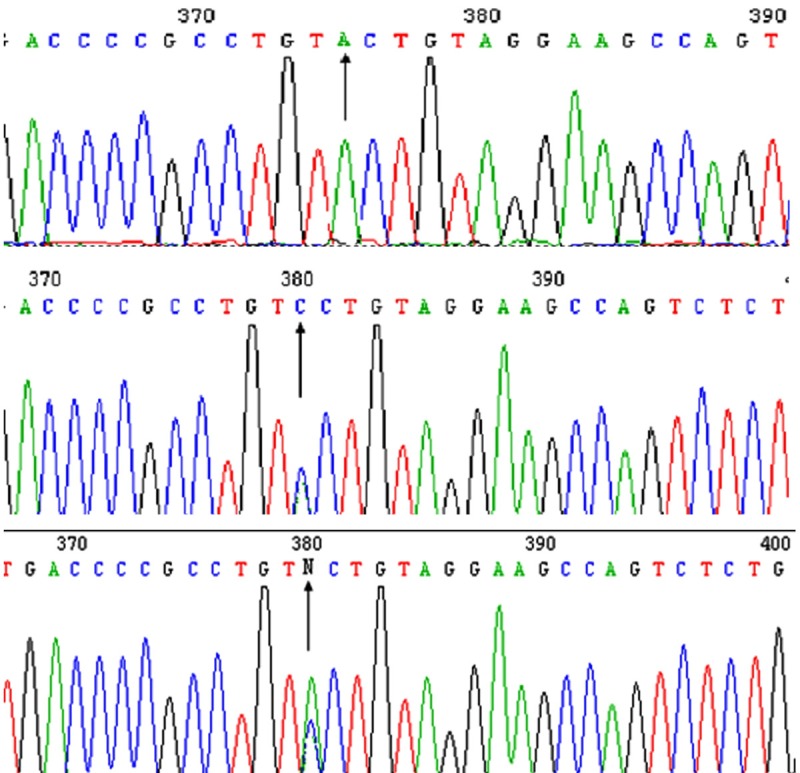
Sequencing results of IL-10 -592A/C genotypes. The sequencing results of the -592A/C genotypes showed that there was only a single A peak in AA genotype, a single C peak in CC genotype and the overlaping A and C peaks in AC genotypes (black arrow).
Table 2 summarizes the distributions of IL-10 -592A/C genotypes and allele frequencies for two groups. The genotype distribution among the subjects was in Hardy-Weinberg equilibrium in both the control group (χ2 =1.44947, p = 0.2215) and the AF group (χ2 = 3.3508, p = 0.0672). The distribution of the IL-10 -592A/C genotypes (AA, AC, and CC) was 55.00%, 35.00%, and 10.00% in the controls, and 71.79%, 23.08%, and 5.13% in AF subjects, respectively (p = 0.0335). The frequency of the A allele in the AF group was significantly higher than that in the control group (83.33% vs 72.50%, p = 0.0063). Compared with the CC genotype, the AA genotype had a 2.0826-fold increased risk of AF (crude odds ratio [OR] = 2.0826, 95% confidence interval [CI] = 1.1856-3.6583, P = 0.0101). After being adjusted for age, gender, prevalence of hypertension and diabetes, serum levels of lipids and IL-10, and left atrial dimension, the association persisted (adjusted OR = 1.8408, 95% CI = 1.0525-3.2196, p = 0.0316) (Table 3).
Table 2.
Distribution of the IL-10 -592A/C genotypes and alleles in AF and control subjects
| Groups | n | Genotypes frequencies (n, %) | Alleles frequencies (n, %) | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| AA | AC | CC | A | C | ||
| controls | 100 | 55 (55.00) | 35 (35.00) | 10 (10.00) | 145 (72.50) | 55 (27.50) |
| AF | 117 | 84 (71.79) | 27 (23.08) | 6 (5.13) | 195 (83.33) | 39 (16.67) |
| p value | 0.0335 | 0.0063 | ||||
Table 3.
Relative risk of AF according to IL-10 -592A/C genotypes
| Genotypes | OR (95% CI) | p value | ORa (95% CI) | p value |
|---|---|---|---|---|
| CC | 1.00 | 1.00 | ||
| AC | 0.5571 (0.3074-1.0099) | 0.0525 | 0.6130 (0.3425-1.0973) | 0.0985 |
| AA | 2.0826 (1.1856-3.6583) | 0.0101 | 1.8408 (1.0525-3.2196) | 0.0316 |
| AA+AC | 0.4865 (0.1703 -1.3897) | 0.1715 | 0.5149 (0.1917-1.3829) | 0.1821 |
Adjusted for age, gender, prevalence of hypertension and diabetes, serum levels of lipids and IL-10, and left atrial dimension.
OR, odds ratio; CI, confidence interval.
The distributions of IL-10 -592A/C genotypes and allele frequencies in AF patients according to the presentation and duration of the arrhythmia were also investigated. No statistical differences were observed between the paroxysmal AF and the non-paroxysmal AF group (Table 4).
Table 4.
Distribution of the IL-10 -592A/C genotypes and alleles in paroxysmal AF and non-paroxysmal AF group
| Groups | n | Genotypes frequencies (n, %) | Alleles frequencies (n, %) | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| AA | AC | CC | A | C | ||
| Paroxysmal AF | 33 | 24 (72.73) | 6 (18.18) | 3 (9.09) | 54 (81.82) | 12 (18.18) |
| non-paroxysmal AF | 84 | 60 (71.43) | 21 (25.00) | 3 (3.57) | 141 (83.93) | 27 (16.07) |
| p value | 0.3886 | 0.6967 | ||||
Effects of the different genotypes on serum IL-10 levels
The effects of the different genotypes on serum IL-10 levels are depicted in Figure 3. Since the numbers of individuals with the CC genotype were small, the carriers of the C allele (AC + CC) were pooled into one group. The average serum IL-10 levels (ng/L) in AA genotype (11.78 ± 5.40) were statistically lower than in AC + CC genotype (18.64 ± 9.73) (p = 0.0000).
Figure 3.
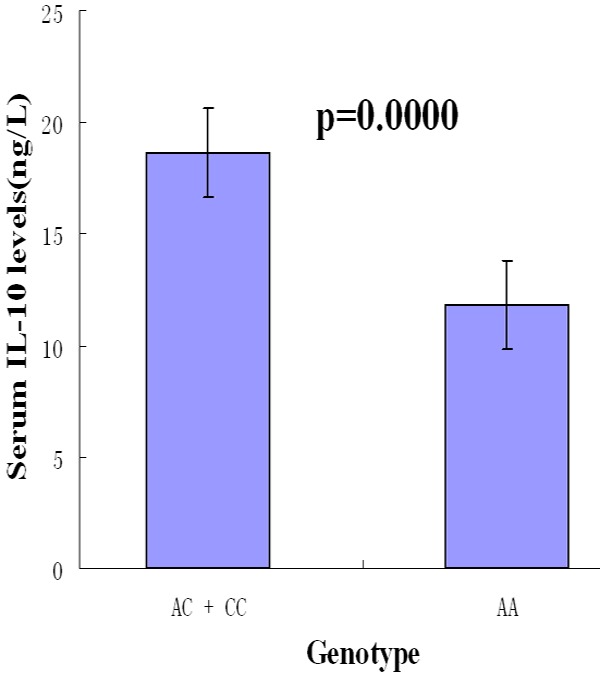
Effects of the different genotypes on serum IL-10 levels.
Discussion
The major finding of the present study is that there is a strong association between the IL-10 -592A/C polymorphim and risk of developing AF. Compared with the CC genotype, the AA genotype had a 2.0826-fold increased risk of AF. Subjects with AA genotype also had lower serum IL-10 levels than those with AC + CC genotype. These findings support the hypothesis that inflammation plays a role in the underlying mechanisms of AF.
In the past few years, much attention has been devoted to assess the role of low-grade inflammation in AF. Inflammatory stimuli may lead to structural remodeling of atria that promotes progression and persistence of AF. Histological evidence to support the association between inflammation and AF has been derived from several sources [20]. In addition, some studies have shown that circulating levels of inflammatory mediators or markers, such as interleukin IL-6 and high-sensitivity C-reactive protein (hs-CRP), were increased in patients with AF and were associated with unsuccessful cardioversion [5]. Furthermore, treatment with anti-inflammatory agents, such as statins, in AF patients was associated with a significant decrease in the risk of arrhythmia recurrence after successful cardioversion [2].
IL-10 is a multifunctional anti-inflammatory cytokine that downregulates cell-mediated immune responses and cytotoxic inflammatory responses [8,12]. Its effects are directed mainly against functions of mononuclear cells, T lymphocytes and polymorphonuclear leukocytes. Furthermore, IL-10 plays a role in inhibition of cell adhesion molecules, monocyte chemotactic protein-1, tissue factor, fibrinogen, matrix metalloproteinase-9, T-lymphocyte granulocyte-macrophage colony-stimulation factor, inducible nitric oxide synthase and smooth muscle cell proliferation [21,22]. Moreover, a potent ability of IL-10 to suppress TNF-α, IL-1α, IL-1β, IL-6, IL-12, and interferon-γ production makes it one of the most important immunoregulator as well as a mediator of inflammatory process [8,23]. Published data also have shown that TNF-α and IL-6 are associated with AF [24].
It has been reported that 50-75% of the variation in IL-10 production is genetically controlled [25,26]. Among the most studied three SNPs in the IL-10 promotor region, -819C/T is in tight linkage disequilibrium with -592A/C [13,14]. However, the distribution of various genotypes for the IL-10 promoter polymorphic sites differ significantly between ethnic populations [12]. Our previous study found that IL-10 -1082 G allele is extremely rare in Chinese Han population [15], the result is similar to other reports in eastern Asian populations apart from a slight variation [27,28]. Of 98 Japanese subjects, only 1 GG homozygous was detected, while in Korean population, the frequency of GG genotype was only 0.81%. In contrast, the genotypes distributions of IL-10 -1082 A/G in several Caucasian groups were similar, although the A allele frequency in European Caucasians increased with higher latitudes, with the highest found in a Finnish population [29-31]. The prevalence of IL-10 -592A/C polymorphism also varies from population to population. In our study of Chinese healthy subjects, the IL-10 -592 A allele frequency was 72.50%, which is very similar to this frequency in southern Chinese (67.00%) [32], Korean (62.00%) [33] and Japanese (67.20%) [34], but markedly different from Caucasians (21.00%) [35]. The ethnic differences suggest that IL-10 promotor polymorphisms could be a useful anthropologic genetic marker. The frequency of the IL-10 -592 A allele was significantly increased in our AF patients, suggesting that it might represent a candidate genetic marker to predict the development of AF for an individual.
The present study also found lower serum IL-10 levels in AF patients than in controls, and subjects with AA genotype had lower serum IL-10 levels than those with AC + CC genotype. The position -592 is in an area containing putative binding sites for IL-6 and STAT-1 [36], the two important signaling pathway molecules initiating atrial fibrosis and AF [24]. Thus, the higher AA genotype frequency and lower circulating IL-10 levels in AF patients may facilitate the pro-inflammatory cytokines initiating and maintaining atrial fibrosis and AF.
As AF is often associated with other cardiac and systemic disorders, it is not generally appreciated that AF may be inherited. However, accumulating studies have provided evidence of a genetic contribution to AF. A few studies have identified Mendelian variants in selected families, which increase susceptibility to AF [37]. In addition, the future risk for offspring AF in parental compared with no parental AF was increased by 1.85 times in the Framingham Heart Study [38] and 1.77 times in unselected families in Iceland [39]. Furthermore, most patients with AF have one or more identifiable risk factors, but many or even most individuals with these same risk factors do not develop AF, indicating that there are probably genetic factors that predispose some of them to the AF. Several investigations have been trying to unravel some of these genetic backgrounds with the use of association studies. In some case-control studies, genetic polymorphisms of cardiac sodium channel (SCN5A), tissue inhibitors of matrix metalloproteinases-2 (TIMP-2), renin-angiotensin system (RAS), IL-6, and cholesteryl ester transfer protein (CETP) were identified as risk factors for AF [17]. A genome-scan analysis performed in three populations of European descent and a Chinese population found a strong association between two sequence variants at chromosome 4q25 and AF [40].
Several limitations need to be addressed. Firstly, we could not exclude the presence of previous asymptomatic AF in the control group because these conclusions were based solely on the medical history of the interviews with the participants. The relatively limited cohort size restricts the generalizability of our results. Secondly, except IL-10, other inflammatory cytokines, might be measured to clarify possible causative mediators. Finally, although all the study subjects were Han Chinese population and thus the possibility of ethnicity as a confounding factor could be excluded, the association of the IL-10 -592A/C polymorphism and AF in other populations remains unknown and needs further study.
In conclusion, our data support that IL-10 -592A/C polymorphism is associated with AF and the A allele has increased risk for AF in Han Chinese. Given the inherent limitations of case-control studies and the complex nature of genetic susceptibility for chronic degenerative diseases, the prospective and interventional clinical studies with larger sample size are required to be conducted in individual ethnic groups to confirm our observations.
Acknowledgements
This work was supported by the Clinical Medicine Special Foundation of Nantong City (HS2013069), and “Summit of the Six Top Talents” Program of Jiangsu Province (2009046), P. R. China.
Disclosure of conflict of interest
None.
References
- 1.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le HJY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 2.Pan M, Zhu JH, Jiang WP, Liu ZH, Li HM, Yu XH, Yang XJ. Inflammation: a possible pathogenic link to atrial fibrillation. Med Hypotheses. 2006;67:1305–1307. doi: 10.1016/j.mehy.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 3.Han ZH, Ren XJ, Wang Y. Anticoagulation management of patients with long-term warfarin therapy after valve replacement during the perioperative period of pacemaker implantation. Int J Clin Exp Med. 2013;6:594–598. [PMC free article] [PubMed] [Google Scholar]
- 4.Hu D, Sun Y. Epidemiology, risk factors for stroke, and management of atrial fibrillation in China. J Am Coll Cardiol. 2008;52:865–868. doi: 10.1016/j.jacc.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 5.Lip GY, Patel JV, Hughes E, Hart RG. High-sensitivity C-reactive protein and soluble CD40 ligand as indices of inflammation and platelet activation in 880 patients with nonvalvular atrial fibrillation: relationship to stroke risk factors, stroke risk stratification schema, and prognosis. Stroke. 2007;38:1229–1237. doi: 10.1161/01.STR.0000260090.90508.3e. [DOI] [PubMed] [Google Scholar]
- 6.Gao SP, Deng XT, Ge LJ, Luan H, Zheng JG, Chen C, Jiang MH, Pan M. Is inflammation linked to thrombogenesis in atrial fibrillation. Int J Cardiol. 2011;149:260–261. doi: 10.1016/j.ijcard.2011.02.046. [DOI] [PubMed] [Google Scholar]
- 7.Deng XT, Jiang MH, Zhu JH, Ge LJ, Guo J, Gao SP, Zheng JG, Luan H, Shi GL, Wang RX, Shi HF, Pan M. The Association of Interleukin 6-634C/G polymorphism with left atrial thrombus and severe spontaneous echocontrast in patients with atrial fibrillation. Clin Appl Thromb Hemost. 2013;19:673–678. doi: 10.1177/1076029612457706. [DOI] [PubMed] [Google Scholar]
- 8.Li C, Tong W, Liu B, Zhang A, Li F. The -1082A>G polymorphism in promoter region of interleukin-10 and risk of digestive cancer: a meta-analysis. Sci Rep. 2014;4:5335. doi: 10.1038/srep05335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwacha MG, Zhang Q, Rani M, Craig T, Oppeltz RF. Burn enhances toll-like receptor induced responses by circulating leukocytes. Int J Clin Exp Med. 2012;5:136–144. [PMC free article] [PubMed] [Google Scholar]
- 10.Xue H, Lin B, An J, Zhu Y, Huang G. Interleukin-10-819 promoter polymorphism in association with gastric cancer risk. BMC Cancer. 2012;12:102. doi: 10.1186/1471-2407-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sancakdar E, Guven AS, Uysal EB, Kaya A, Deveci K, Karapinar H, Akkar I. Evaluation of cytokines as Th1/Th2 markers in pathogenesis of children with Crimean-Congo hemorrhagic fever. Int J Clin Exp Med. 2014;7:751–757. [PMC free article] [PubMed] [Google Scholar]
- 12.Hsueh KC, Lin YJ, Chang JS, Wan L, Tsai YH, Tsai CH, Chen CP, Tsai FJ. Association of interleukin-10 A-592C polymorphism in Taiwanese children with Kawasaki disease. J Korean Med Sci. 2009;24:438–442. doi: 10.3346/jkms.2009.24.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan XF, Yang SJ, Loh M, Xie Y, Wen YY, Tian Z, Huang H, Lan H, Chen F, Soong R, Yang CX. Interleukin-10 gene promoter polymorphisms and risk of gastric cancer in a Chinese population: single nucleotide and haplotype analyses. Asian Pac J Cancer Prev. 2013;14:2577–2582. doi: 10.7314/apjcp.2013.14.4.2577. [DOI] [PubMed] [Google Scholar]
- 14.Koss K, Fanning GC, Welsh KI, Jewell DP. Interleukin-10 gene promoter polymorphism in English and Polish healthy controls. Polymerase chain reaction haplotyping using 3’ mismatches in forward and reverse primers. Genes Immun. 2000;1:321–324. doi: 10.1038/sj.gene.6363669. [DOI] [PubMed] [Google Scholar]
- 15.Guo J, He YH, Chen F, Jiang MH, Gao SP, Su YM, Shi GL, Deng XT, Zhu JH, Pan M. The A to G polymorphism at -1082 of the interleukin-10 gene is rare in the Han Chinese population. Mol Med Rep. 2012;6:894–896. doi: 10.3892/mmr.2012.995. [DOI] [PubMed] [Google Scholar]
- 16.Pan M, Jiang MH, Wei MF, Liu ZH, Jiang WP, Geng HH, Cui ZC, Zhang DL, Zhu JH. Association of angiotensin-converting enzyme gene 2350G>A polymorphism with myocardial infarction in a Chinese population. Clin Appl Thromb Hemost. 2009;15:435–442. doi: 10.1177/1076029608316013. [DOI] [PubMed] [Google Scholar]
- 17.Li J, Song J, Jiang MH, Zheng JG, Gao SP, Zhu JH, Pan M. Interleukin-6 promoter polymorphisms and susceptibility to atrial fibrillation in elderly han chinese patients with essential hypertension. J Interferon Cytokine Res. 2012;32:542–547. doi: 10.1089/jir.2012.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang MH, Su YM, Tang JZ, Shen YB, Deng XT, Yuan DS, Wu J, Pan M, Huang ZW. Angiotensin-converting enzyme gene 2350 G/A polymorphism and susceptibility to atrial fibrillation in Han Chinese patients with essential hypertension. Clinics (Sao Paulo) 2013;68:1428–1432. doi: 10.6061/clinics/2013(11)08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards-Smith CJ, Jonsson JR, Purdie DM, Bansal A, Shorthouse C, Powell EE. Interleukin-10 promoter polymorphism predicts initial response of chronic hepatitis C to interferon alfa. Hepatology. 1999;30:526–530. doi: 10.1002/hep.510300207. [DOI] [PubMed] [Google Scholar]
- 20.Verheule S, Wilson E, Everett T 4th, Shanbhag S, Golden C, Olgin J. Alterations in atrial electrophysiology and tissue structure in a canine model of chronic atrial dilatation due to mitral regurgitation. Circulation. 2003;107:2615–2622. doi: 10.1161/01.CIR.0000066915.15187.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plourde M, Vohl MC, Bellis C, Carless M, Dyer T, Dolley G, Marette A, Despres JP, Bouchard C, Blangero J, Perusse L. A variant in the LRRFIP1 gene is associated with adiposity and inflammation. Obesity (Silver Spring) 2013;21:185–192. doi: 10.1002/oby.20242. [DOI] [PubMed] [Google Scholar]
- 22.Suarez A, Castro P, Alonso R, Mozo L, Gutierrez C. Interindividual variations in constitutive interleukin-10 messenger RNA andprotein levels and their association with genetic polymorphisms. Transplantation. 2003;75:711–717. doi: 10.1097/01.TP.0000055216.19866.9A. [DOI] [PubMed] [Google Scholar]
- 23.Abanmi A, Al HF, Zouman A, Kudwah A, Jamal MA, Arfin M, Tariq M. Association of Interleukin-10 gene promoter polymorphisms in Saudi patients with vitiligo. Dis Markers. 2008;24:51–57. doi: 10.1155/2008/210609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedrichs K, Klinke A, Baldus S. Inflammatory pathways underlying atrial fibrillation. Trends Mol Med. 2011;17:556–563. doi: 10.1016/j.molmed.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Reuss E, Fimmers R, Kruger A, Becker C, Rittner C, Hohler T. Differential regulation of interleukin-10 production by genetic and environmentalfactors--a twin study. Genes Immun. 2002;3:407–413. doi: 10.1038/sj.gene.6363920. [DOI] [PubMed] [Google Scholar]
- 26.Westendorp RG, Langermans JA, Huizinga TW, Elouali AH, Verweij CL, Boomsma DI, Vandenbroucke JP. Genetic influence on cytokine production and fatal meningococcal disease. Lancet. 1997;349:170–173. doi: 10.1016/s0140-6736(96)06413-6. [DOI] [PubMed] [Google Scholar]
- 27.Ito M, Takahashi H, Fuse K, Hirono S, Washizuka T, Kato K, Yamazaki F, Inano K, Furukawa T, Komada M, Aizawa Y. Polymorphisms of tumor necrosis factor-alpha and interleukin-10 genes in Japanesepatients with idiopathic dilated cardiomyopathy. Jpn Heart J. 2000;41:183–191. doi: 10.1536/jhj.41.183. [DOI] [PubMed] [Google Scholar]
- 28.Garbuzova VY, Gurianova VL, Stroy DA, Dosenko VE, Parkhomenko AN, Ataman AV. Association of matrix Gla protein gene allelic polymorphisms (G(-7)-->A, T(-138)-->C and Thr(83)-->Ala) with acute coronary syndrome in the Ukrainian population. Exp Clin Cardiol. 2012;17:30–33. [PMC free article] [PubMed] [Google Scholar]
- 29.Arosio B, Mastronardi L, Vergani C, Annoni G. Intereleukin-10 promoter polymorphism in mild cognitive impairment and in itsclinical evolution. Int J Alzheimers Dis. 2010;2010 doi: 10.4061/2010/854527. LID - 854527 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galley HF, Lowe PR, Carmichael RL, Webster NR. Genotype and interleukin-10 responses after cardiopulmonary bypass. Br J Anaesth. 2003;91:424–426. doi: 10.1093/bja/aeg174. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Maderuelo D, Arnalich F, Serantes R, Gonzalez A, Codoceo R, Madero R, Vazquez JJ, Montiel C. Interferon-gamma and interleukin-10 gene polymorphisms in pulmonary tuberculosis. Am J Respir Crit Care Med. 2003;167:970–975. doi: 10.1164/rccm.200205-438BC. [DOI] [PubMed] [Google Scholar]
- 32.Mok CC, Lanchbury JS, Chan DW, Lau CS. Interleukin-10 promoter polymorphisms in Southern Chinese patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1090–1095. doi: 10.1002/1529-0131(199806)41:6<1090::AID-ART16>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 33.Chin HJ, Na KY, Kim SJ, Oh KH, Kim YS, Lim CS, Kim S, Chae DW. Interleukin-10 promoter polymorphism is associated with the predisposition to the development of IgA nephropathy and focal segmental glomerulosclerosis in Korea. J Korean Med Sci. 2005;20:989–993. doi: 10.3346/jkms.2005.20.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tegoshi H, Hasegawa G, Obayashi H, Nakano K, Kitagawa Y, Fukui M, Matsuo S, Deguchi M, Ohta M, Nishimura M, Nakamura N, Yoshikawa T. Polymorphisms of interferon-gamma gene CA-repeat and interleukin-10 promoter region (-592A/C) in Japanese type I diabetes. Hum Immunol. 2002;63:121–128. doi: 10.1016/s0198-8859(01)00363-9. [DOI] [PubMed] [Google Scholar]
- 35.Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet. 1997;24:1–8. doi: 10.1111/j.1365-2370.1997.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 36.Kube D, Platzer C, von KA, Straub H, Bohlen H, Hafner M, Tesch H. Isolation of the human interleukin 10 promoter. Characterization of the promoter activity in Burkitt’s lymphoma cell lines. Cytokine. 1995;7:1–7. doi: 10.1006/cyto.1995.1001. [DOI] [PubMed] [Google Scholar]
- 37.Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, Xu WY, Jin HW, Sun H, Su XY, Zhuang QN, Yang YQ, Li YB, Liu Y, Xu HJ, Li XF, Ma N, Mou CP, Chen Z, Barhanin J, Huang W. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 38.Fox CS, Parise H, Sr DRB, Lloyd-Jones DM, Vasan RS, Wang TJ, Levy D, Wolf PA, Benjamin EJ. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291:2851–2855. doi: 10.1001/jama.291.23.2851. [DOI] [PubMed] [Google Scholar]
- 39.Arnar DO, Thorvaldsson S, Manolio TA, Thorgeirsson G, Kristjansson K, Hakonarson H, Stefansson K. Familial aggregation of atrial fibrillation in Iceland. Eur Heart J. 2006;27:708–712. doi: 10.1093/eurheartj/ehi727. [DOI] [PubMed] [Google Scholar]
- 40.Gudbjartsson DF, Arnar DO, Helgadottir A, Gretarsdottir S, Holm H, Sigurdsson A, Jonasdottir A, Baker A, Thorleifsson G, Kristjansson K, Palsson A, Blondal T, Sulem P, Backman VM, Hardarson GA, Palsdottir E, Helgason A, Sigurjonsdottir R, Sverrisson JT, Kostulas K, Ng MC, Baum L, So WY, Wong KS, Chan JC, Furie KL, Greenberg SM, Sale M, Kelly P, MacRae CA, Smith EE, Rosand J, Hillert J, Ma RC, Ellinor PT, Thorgeirsson G, Gulcher JR, Kong A, Thorsteinsdottir U, Stefansson K. Variants conferring risk of atrial fibrillation on chromosome 4q25. Nature. 2007;448:353–357. doi: 10.1038/nature06007. [DOI] [PubMed] [Google Scholar]


