Abstract
Objective: Mean platelet volume (MPV), which is determined by a routine complete blood count, is a parameter that is usually overlooked by clinicians. The present study was designed to investigate the association between MPV and different disease states in patients with hepatitis B virus (HBV) infection. Methods: A total of 120 patients, including 17 with acute hepatitis B (AHB), 62 with chronic hepatitis B (CHB), and 41 with chronic severe hepatitis B (CSHB), as well as 58 healthy controls (HCs) were enrolled in the study. At study entry, blood samples were collected from all subjects to examine liver function and renal function, determine the international normalized ratio and perform routine hematological tests. Results: We demonstrated that MPV was significantly increased in CSHB and CHB patients compared with HCs and AHB patients (all P<0.05). Among the patient groups, the CSHB patients had the highest MPV. Increased MPV was clinically associated with severe liver disease. Conclusions: MPV is significantly increased in chronic HBV-infected patients and is associated with disease severity; thus, it may serve as an important biomarker.
Keywords: Mean platelet volume, hepatitis B virus infection, biomarker
Introduction
Mean platelet volume (MPV), a parameter routinely determined by complete blood count analyzers, indicates the average size of platelets and reflects the platelet production rate and stimulation [1]. Some studies have reported that MPV is a predictor of cardiovascular risk, stroke risk, and overall vascular mortality [2-4]. MPV is also associated with worse clinical prognosis in acute myocardial infarction and acute stroke [5,6]. Recently, the involvement of MPV in several hepatic diseases, such as steatosis, cirrhosis and hepatitis, has also been investigated [7-9]. Hepatic steatosis and fatty liver disease are directly associated with atherosclerosis and cardiovascular disease risk factors, and several previous studies have observed increased MPVs in patients with these conditions [7,9]. However, the role of MPV in other liver diseases without direct links to atherosclerosis, such as hepatitis B virus (HBV) infection, has not been fully clarified to date. HBV is a major public health problem in China, and it is estimated that this virus infects 2 billion people worldwide, with 350 million chronic cases and 60,000 deaths each year [10,11]. Minimal research has been conducted to investigate the relationship between MPV and the different disease states of HBV infection, such as acute hepatitis B (AHB), chronic hepatitis B (CHB) and chronic severe hepatitis B (CSHB). Therefore, we conducted this study to assess MPV in HBV-infected patients.
Materials and methods
Subjects
Adult HBV-infected patients (≥18 years of age) who were admitted to the First Affiliated Hospital of Zhejiang University College of Medicine and diagnosed with AHB, CHB or CSHB were consecutively recruited between August 1, 2012 and August 1, 2013. All patients were given standard medical treatments, including energy supplements and intravenous infusions of albumin and plasma, and all patients received preventive treatment for complications. The exclusion criteria were as follows: (a) atherosclerotic heart disease, heart failure, peripheral vascular disease, hematological disorders, chronic obstructive pulmonary disease or renal insufficiency; (b) malignancy, such as hepatocellular carcinoma; (c) pregnancy; (d) ongoing steroid, PEGylated interferon, or nucleoside analog therapy; and (e) autoimmune liver disease or concurrent HCV, hepatitis D virus, hepatitis G virus, or HIV infection. During the same time period, 58 age- and sex-matched healthy controls (HCs) were recruited from community-dwelling individuals who presented for their yearly physical examinations and had no specific complaints or illnesses requiring treatment. None of the patients or controls used medications such as aspirin, oral contraceptives, oral anticoagulants or corticosteroids. This study was performed in accordance with the Declaration of Helsinki, and the procedures have been approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University College of Medicine. Written informed consent was obtained from all study participants prior to their enrollment.
Clinical diagnosis
The criteria used for diagnosing AHB, CHB and CSHB were in accordance with the 2000 Xi’an Viral Hepatitis Management Guidelines recommended by the Chinese Society of Infectious Diseases and Parasitology and the Society of Hepatology of the Chinese Medical Association [12]. Briefly, AHB patients are defined as individuals who display hepatitis B surface Ag (HBsAg)-negative conversion within 6 months after the initial onset of HBV symptoms. CHB patients are defined as individuals who show evidence of hepatitis B infection for more than 6 months. CSHB is defined by a history of CHB or liver cirrhosis, with serum HBsAg positivity for more than 6 months and a serum total bilirubin level that is more than 10 times higher than the normal level (i.e., 171 μmol/L), with at least one of the following five signs of liver failure: prothrombin activity of less than 40%, hepatic encephalopathy, ascites, progressive reduction in liver size, and hepatorenal syndrome. In addition, liver cirrhosis was diagnosed based on clinical (e.g., physical stigmata of cirrhosis), biochemical (e.g., decreased serum albumin and increased serum globulin levels), and ultrasonographic or computed tomography (CT) findings (e.g., nodular liver surface, coarsened echogenicity of liver parenchyma, enlarged spleen, and/or ascites) [13]. Splenomegaly was diagnosed using an abdominal CT examination, and patients with a spleen size >14 cm according to CT and other examinations were considered to have splenomegaly [14].
Laboratory analysis
Blood samples were collected from all patients within 24 h of admission and after they had fasted overnight, and samples were obtained from the 58 HCs at the time of recruitment. As part of a complete blood cell count, the platelet count, MPV and hemoglobin level were evaluated using an XE-2100 automated hematology analyzer (Sysmex Corp., Kobe, Japan). Normal MPVs in our laboratory range between 7.4 and 12.0 fl, and a preselected cutoff value of 10.0 fl was chosen. Serum creatinine, serum albumin, total protein, total bilirubin and alanine transaminase (ALT) levels were measured using a Hitachi 704 Analyzer (Boehringer Mannheim Diagnostics), and the international normalized ratio (INR) was generated using a Sysmex CA1500 fully automatic analyzer (Sysmex Corp., Hyogo, Japan). At baseline, demographic and clinical characteristics were collected, including the model for end-stage liver disease (MELD) score (with higher scores indicating more severe illness).
MELD score
Liver disease severity was evaluated via the MELD score, which uses the patient’s serum bilirubin and creatinine levels and the INR for prothrombin time to predict survival. The MELD score was calculated using an online calculator (http://www.mayoclinic.org/gi-rst/mayomodel7.html).
Statistical analysis
All continuous variables were expressed as the mean ± standard deviation (SD) or medians (range), and categorical data were expressed as percentages. The differences in the variables were analyzed using analysis of variance (ANOVA) or the Kruskal-Wallis tests. The chi-square test was used for categorical data, as appropriate. Correlations between the variables were examined using Spearman’s correlations test. Statistical analyses were performed using the statistical package SPSS version 12.0 (SPSS Inc, Chicago, IL), and the level of statistical significance was set at P<0.05.
Results
Increased MPV levels in HBV-infected patients
A total of 120 HBV-infected patients, including 17 with AHB, 62 with CHB, and 41 with CSHB, in addition to 58 HCs were recruited during the study period (Table 1). Our study showed that the MPV in the CSHB patients was significantly higher compared with that in the HCs (P<0.001), CHB patients (P=0.033), and AHB patients (P<0.001). The CHB patients had higher MPVs than the AHB patients and HCs (P=0.010 and P<0.001, respectively). However, comparisons of MPV between the AHB patients and HCs revealed no significant differences (P=0.510) (Figure 1). There was a positive correlation between the MELD scores and MPVs (r=0.362, P<0.001), while a significant negative correlation was observed between the platelet counts and MPVs (r=-0.359, P<0.001) (Figure 2).
Table 1.
Clinical characteristics of the study participants
| AHB (n=17) | CHB (n=62) | CSHB (n=41) | Healthy controls (n=58) | P | |
|---|---|---|---|---|---|
| Age (year)* | 40.0±10.8 | 46.7±12.4 | 49.0±13.4 | 44.8±9.0 | 0.109 |
| Gender (male/female)** | 12/5 | 42/20 | 32/9 | 42/16 | 0.724 |
| Total bilirubin (μmol/L)*** | 32 (24-65) | 41 (27-73) | 308 (226-399) | 12 (9-15) | <0.001 |
| ALT (U/L)*** | 597 (372-1139) | 48 (37-109) | 104 (63-189) | 15 (11-22) | 0.026 |
| INR* | 1.29±0.41 | 1.21±0.26 | 2.05±0.56 | 0.89±0.23 | 0.015 |
| HBsAg positive (n) | 17 | 62 | 41 | 0 | - |
| HBeAg positive (n, %) | 8 (47) | 62 | 26 (63) | 0 | - |
| HBcAb IgM positive (n) | 17 | 0 | 0 | 0 | - |
| HBV-DNA positive (n, %) | 13 (76) | 62 | 33 (80) | 0 | - |
| MPV (fl)* | 10.8±1.5 | 11.7±1.2 | 12.3±0.8 | 10.5±0.9 | <0.001 |
Data are expressed as the number of patients (%), mean ± SD or medians (interquartile range). Abbreviations: ALT, alanine aminotransferase; INR, international normalized ratio; AHB, acute hepatitis B; CHB, chronic hepatitis B; CSHB, chronic severe hepatitis B; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B e antigen; HBcAb, hepatitis B core antibody; HBV, hepatitis B virus.
One way ANOVA Test;
Chi-Square Test;
Kruskal-Wallis Test as compared among the four groups.
Figure 1.
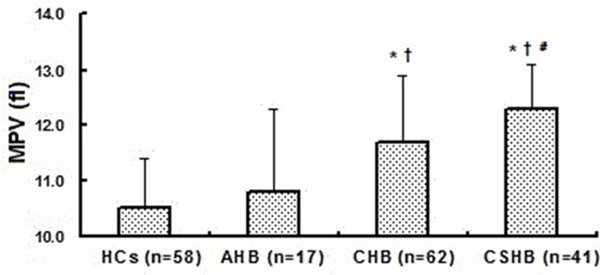
The association between MPV and various stages of HBV infection. Data are shown as the mean ± standard deviation. P values were calculated using the One-Way ANOVA and posthoc Tukey HSD. (*) P<0.001 when compared to HCs. (†) P<0.05 when compared to AHB group. (#) P<0.05 when compared to CHB group.
Figure 2.
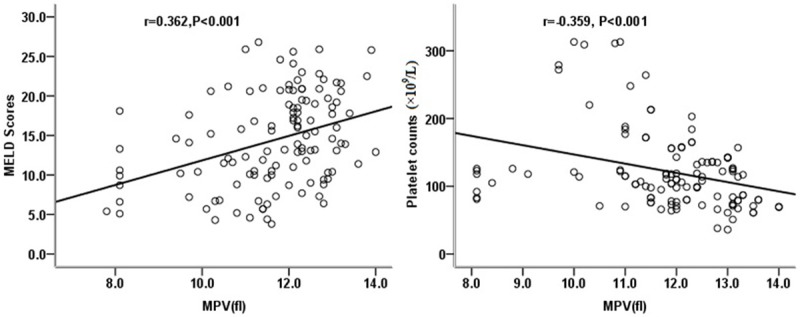
Scatter diagrams showing the correlation between MELD scores and MPV and the correlation between platelet counts and MPV in patients with HBV infections. Coefficients (r) and P-values were calculated using Spearman correlation analysis.
Baseline characteristics and baseline factors compared with MPV
The patients were divided into three groups based on their MPVs: group A (MPV≤10.0 fl), group B (>10.0 fl but <12.0 fl) and group C (≥12.0 fl). The clinical and laboratory results associated with the MPV are listed in Table 2. Patients with higher MPVs were more likely to have severe liver disease, lower total protein and albumin levels, and higher INR and total bilirubin levels. Moreover, highly elevated MPV levels were associated with a higher rate of clinical complications, such as cirrhosis, splenomegaly and thrombocytopenia. Creatinine level, sex, age, and hemoglobin level were not significantly different among the three groups.
Table 2.
Clinical and laboratory characteristics of the patients with various mean MPVs (fl) upon admission
| Group A (MPV≤10.0 fl, n=15) | Group B (10.0<MPV<12.0 fl, n=40) | Group C (MPV≥12.0 fl, n=65) | P | |
|---|---|---|---|---|
| AHB/CHB/CSHB (n) | 8/6/1 | 5/26/9 | 4/30/31 | <0.001 |
| MPV (fl)* | 8.88±0.87 | 11.15±0.52 | 12.64±0.53 | <0.001 |
| Age (year)* | 40.0±10.64 | 48.12±13.91 | 46.51±13.15 | 0.129 |
| Gender (male/female)** | 11/4 | 34/6 | 45/20 | 0.191 |
| Hemoglobin (g/dL)* | 133.14±28.68 | 118.73±22.08 | 112.31±24.13 | 0.238 |
| Total protein (g/L)* | 66.96±7.65 | 62.39±6.53 | 61.2±7.75 | 0.035 |
| Albumin (g/L)* | 40.60±6.22 | 35.73±5.17 | 33.95±5.61 | 0.001 |
| INR* | 1.28±0.38 | 1.48±0.48 | 1.68±0.58 | 0.015 |
| Creatinine (mmol/L)* | 62.57±12.96 | 66.22±34.76 | 70.43±32.78 | 0.686 |
| Total bilirubin (μmol/L)*** | 48 (28-122) | 92(36-177) | 132 (48-301) | 0.034 |
| MELD score*** | 10.3(7.1-14.2) | 11.2 (6.7-16.0) | 16.6 (13.0-20.8) | <0.001 |
| Hepatic cirrhosis (yes/no)** | 5/10 | 15/25 | 41/24 | 0.014 |
| Splenomegaly (yes/no)** | 2/13 | 10/30 | 31/34 | 0.009 |
| Platelet counts (×109/L)*** | 121.0 (98.5-199.0) | 115.0 (92.0-178.8) | 99.0 (73.3-134.3) | 0.022 |
Data are expressed as the number of patients (%), mean ± SD or medians (interquartile range). Abbreviations: MPV, mean platelet volume; INR, international normalized ratio; ALT, alanine aminotransferase; MELD score, model for end-stage liver disease score.
One way ANOVA Test;
Chi-Square Test;
Kruskal-Wallis Test as compared among the three groups.
The MELD scores in groups A, B, and C were 10.1 (7.1-11.3), 11.6 (7.2-16.4) and 17.1 (13.0-20.8), respectively. There was a stepwise increase in MELD scores with increased MPVs (P=0.33 between groups A and B, and P<0.05 between groups B and C) (Figure 3).
Figure 3.
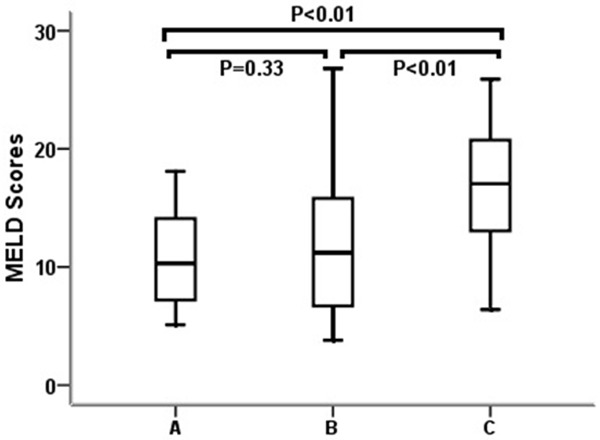
Comparisons of the MELD scores among the patients with various MPVs. Patients were divided into three groups based on MPV levels: groups A (≤10 fl), B (>10 fl, but <12 fl) and C (≥12 fl). Data are expressed as box plots, in which the horizontal lines illustrate the 25th, 50th, and 75th percentiles of the MPV levels. The vertical lines represent the 5th and 95th percentiles. The P values for multiple comparisons were calculated using the nonparametric Kruskal-Wallis test.
Discussion
In this study, we found that CHB and CSHB patients had significantly higher MPVs compared with AHB patients and HCs. Additionally, the CSHB patients had the highest MPVs among all of the patients. It is well known that AHB patients are less commonly observed in the clinical setting because 90-95% of adult patients have spontaneously self-limited acute hepatitis without obvious manifestations. Additionally, before visiting physicians, these patients often enter a convalescence period after a short-term acute phase. Thus, there was no significant difference in MPV between the AHB patients and HCs. We speculate that these differences are an important factor influencing disease progression and may serve as an important marker for patients with HBV infection. Moreover, trials are currently underway to investigate the relationship between MPV and liver diseases. For example, Turhan et al. reported that MPV was significantly higher in an inactive HBsAg carrier group compared with a control group [15]. Furthermore, Shin et al. reported a significant correlation between MPV and non-alcoholic fatty liver disease in an obese patient population [16]. More recently, Qi et al. demonstrated that MPV is a simple and non-invasive routine laboratory parameter and that elevated MPV levels might be an independent predictor of cirrhosis in patients with chronic HBV infections [17]. In contrast, Ceylan et al. observed that the MPV was lower among patients with severe fibrosis compared with patients with milder fibrosis [18]. These differences may largely reflect differences in the patients’ stage of liver disease in the differing studies.
The mechanism underlying the increased MPV in HBV-infected patients is not fully understood. Some published studies have reported an increase in the entry of young platelets into circulation from the bone marrow because of the increased levels of inflammatory cytokines in patients with inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease, thereby leading to increased MPV [19]. Similar to the above-mentioned diseases, chronic hepatitis is also characterized by inflammation; thus, the MPV is suggested to increase. Furthermore, the increased levels of interleukin-6 caused by inflammation have also been reported to trigger an increase in the platelet production in patients infected with CHB [20]. On the other hand, thrombocytopenia is one of the most frequent hematological disorders in patients with HBV-related liver diseases. According to our previous research, peripheral platelet destruction or sequestration is the major mechanism underlying thrombocytopenia, and hypersplenism is an important cause of HBV-related hepatitis [21]. In the current study, MPV was negatively correlated with platelet counts, and we also observed that highly elevated MPVs were associated with a higher rate of clinical complications, such as thrombocytopenia. Based on our findings, it is reasonable to suggest that MPV is an indicator of platelet activation, systemic inflammation and infection.
We note that AHB, CHB and CSHB patients were diagnosed in accordance with the 2000 Xi’an Viral Hepatitis Management Guidelines recommended by the Chinese Society of Infectious Diseases and Parasitology and the Society of Hepatology of the Chinese Medical Association [12]. In China, ‘CSHB’ usually refers to the fatal form of chronic hepatitis, which resembles ‘liver failure caused by chronic hepatitis B’ in Western countries. Although this term is not used uniformly on a global scale, CSHB often encompasses serious clinical courses with fatal consequences. Acute attacks may occur in some CHB patients. Moreover, the disease may develop into liver failure (i.e., CSHB) due to various factors, such as HBV mutations, co-infection with other hepatotropic viruses, long-term corticosteroid treatment, and bacterial infections over the course of a disease. Although the application of newly developed drugs with an artificial liver support system is effective in some cases, the mortality rate of CSHB may still reach 80%-100%. For these patients, orthotopic liver transplantation might be the last option. Therefore, the identification of novel predictive biomarkers is critical in the therapeutic management of CSHB. In this study, we demonstrated an increased frequency of liver-related complications, such as cirrhosis and splenomegaly, in patients with higher MPVs. Furthermore, we also observed that MPV was positively correlated with the MELD score. Over the past decade, MELD scores have served as the most widely used method of determining organ allocation in liver transplantation. This model, which includes variables related to both liver and renal function, was implemented in the U.S. in 2002 and is currently being used in many countries to classify patients with cirrhosis who are awaiting transplantation according to the severity of their liver disease [22]. Our previous study reported that the model for MELD scores was associated with the prognosis of patients with (HBV)-related acute-on-chronic liver failure [23]. MPV is significantly increased in patients with HBV; thus, together with disease severity, MPV may serve as an important biomarker.
A few limitations of this study warrant consideration. First, the study was non-randomized and was conducted at a single center; therefore, it was subject to selection bias. Investigations involving a larger sample size and prospective analyses are necessary. Second, MPVs were not monitored during the course of disease progression; therefore, it remains unclear whether the MPV is elevated in a stepwise manner when a patient’s condition progressively deteriorates. Knowledge regarding cytokine levels might have aided in establishing a mechanism for the observed results. Therefore, the mechanistic pathways that increase the MPV in patients with HBV infection were only partially investigated in the present study.
In conclusion, the MPV is significantly increased in patients infected with HBV and is associated with the severity of the disease. Because the MPV is a parameter that is easily obtained at no additional cost from routine complete blood cell counts and is highly reproducible, it may serve as an important biomarker. However, further studies with larger patient cohorts are required to verify our findings.
Disclosure of conflict of interest
None.
Abbreviations
- MPV
mean platelet volume
- HBV
hepatitis B virus
- HCs
healthy controls
- AHB
acute hepatitis B
- CHB
chronic hepatitis B
- CSHB
chronic severe hepatitis B
- MELD score
Model for End-stage Liver Disease score
- INR
international normalized ratio
- ALT
alanine aminotransferase
References
- 1.Ekiz F, Yuksel O, Kocak E, Yilmaz B, Altinbas A, Coban S, Yuksel I, Uskudar O, Koklu S. Mean platelet volume as a fibrosis marker in patients with chronic hepatitis B. J Clin Lab Anal. 2011;25:162–165. doi: 10.1002/jcla.20450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bath P, Algert C, Chapman N, Neal B. Association of mean platelet volume with risk of stroke among 3134 individuals with history of cerebrovascular disease. Stroke. 2004;35:622–626. doi: 10.1161/01.STR.0000116105.26237.EC. [DOI] [PubMed] [Google Scholar]
- 3.Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, Mohler ER, Reilly MP, Berger JS. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:148–156. doi: 10.1111/j.1538-7836.2009.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slavka G, Perkmann T, Haslacher H, Greisenegger S, Marsik C, Wagner OF, Endler G. Mean platelet volume may represent a predictive parameter for overall vascular mortality and ischemic heart disease. Arterioscler Thromb Vasc Biol. 2011;31:1215–1218. doi: 10.1161/ATVBAHA.110.221788. [DOI] [PubMed] [Google Scholar]
- 5.Greisenegger S, Endler G, Hsieh K, Tentschert S, Mannhalter C, Lalouschek W. Is elevated mean platelet volume associated with a worse outcome in patients with acute ischemic cerebrovascular events? Stroke. 2004;35:1688–1691. doi: 10.1161/01.STR.0000130512.81212.a2. [DOI] [PubMed] [Google Scholar]
- 6.Tekbas E, Kara AF, Ariturk Z, Cil H, Islamoglu Y, Elbey MA, Soydinc S, Ulgen MS. Mean platelet volume in predicting short- and long-term morbidity and mortality in patients with or without ST-segment elevation myocardial infarction. Scand J Clin Lab Invest. 2011;71:613–619. doi: 10.3109/00365513.2011.599416. [DOI] [PubMed] [Google Scholar]
- 7.Lee WS, Kim TY. Alcoholic fatty liver disease and alcoholic liver cirrhosis may be differentiated with mean platelet volume and platelet distribution width. Platelets. 2010;21:584–585. doi: 10.3109/09537104.2010.500423. [DOI] [PubMed] [Google Scholar]
- 8.Turhan O, Coban E, Inan D, Yalcin AN. Increased mean platelet volume in chronic hepatitis B patients with inactive disease. Med Sci Monit. 2010;16:CR202–205. [PubMed] [Google Scholar]
- 9.Ozhan H, Aydin M, Yazici M, Yazgan O, Basar C, Gungor A, Onder E. Mean platelet volume in patients with non-alcoholic fatty liver disease. Platelets. 2010;21:29–32. doi: 10.3109/09537100903391023. [DOI] [PubMed] [Google Scholar]
- 10.Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet. 2003;362:2089–2094. doi: 10.1016/S0140-6736(03)15108-2. [DOI] [PubMed] [Google Scholar]
- 11.Sun Z, Ming L, Zhu X, Lu J. Prevention and control of hepatitis B in China. J Med Virol. 2002;67:447–450. doi: 10.1002/jmv.10094. [DOI] [PubMed] [Google Scholar]
- 12.Management scheme of diagnostic and therapy criteria of viral hepatitis. Chinese J Hepatol. 2000;6:324–329. [Google Scholar]
- 13.Shakil AO, Kramer D, Mazariegos GV, Fung JJ, Rakela J. Acute liver failure: clinical features, outcome analysis, and applicability of prognostic criteria. Liver Transpl. 2000;6:163–169. doi: 10.1002/lt.500060218. [DOI] [PubMed] [Google Scholar]
- 14.Dardenne AN. The spleen. In: Meire HB, Dewbury KCD, editors. Clinical Ultrasound. Edinburgh: Churchill Livingstone; 1993. pp. 353–365. [Google Scholar]
- 15.Purnak T, Olmez S, Torun S, Efe C, Sayilir A, Ozaslan E, Tenlik I, Kalkan IH, Beyazit Y, Yuksel O. Mean platelet volume is increased in chronic hepatitis C patients with advanced fibrosis. Clin Res Hepatol Gastroenterol. 2013;37:41–46. doi: 10.1016/j.clinre.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 16.Shin WY, Jung DH, Shim JY, Lee HR. The association between non-alcoholic hepatic steatosis and mean platelet volume in an obese Korean population. Platelets. 2011;22:442–446. doi: 10.3109/09537104.2010.540049. [DOI] [PubMed] [Google Scholar]
- 17.Qi XT, Wan F, Lou Y, Ye B, Wu D. The mean platelet volume is a potential biomarker for cirrhosis in chronic hepatitis B virus infected patients. Hepatogastroenterology. 2014;61:456–459. [PubMed] [Google Scholar]
- 18.Ceylan B, Mete B, Fincanci M, Aslan T, Akkoyunlu Y, Ozgunes N, Colak O, Gunduz A, Senates E, Ozaras R, Inci A, Tabak F. A new model using platelet indices to predict liver fibrosis in patients with chronic hepatitis B infection. Wien Klin Wochenschr. 2013;125:453–460. doi: 10.1007/s00508-013-0394-3. [DOI] [PubMed] [Google Scholar]
- 19.Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011;17:47–58. doi: 10.2174/138161211795049804. [DOI] [PubMed] [Google Scholar]
- 20.Kaser A, Brandacher G, Steurer W, Kaser S, Offner FA, Zoller H, Theurl I, Widder W, Molnar C, Ludwiczek O, Atkins MB, Mier JW, Tilg H. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98:2720–2725. doi: 10.1182/blood.v98.9.2720. [DOI] [PubMed] [Google Scholar]
- 21.Dou J, Lou Y, Wu J, Lu Y, Jin Y. Thrombocytopenia in patients with hepatitis B virus-related chronic hepatitis: Evaluation of the immature platelet fraction. Platelets. 2014;25:399–404. doi: 10.3109/09537104.2013.832742. [DOI] [PubMed] [Google Scholar]
- 22.Freeman RB Jr, Wiesner RH, Harper A, McDiarmid SV, Lake J, Edwards E, Merion R, Wolfe R, Turcotte J, Teperman L. The new liver allocation system: moving toward evidence-based transplantation policy. Liver Transpl. 2002;8:851–858. doi: 10.1053/jlts.2002.35927. [DOI] [PubMed] [Google Scholar]
- 23.Mao W, Ye B, Lin S, Fu Y, Chen Y, Chen Y. Prediction value of model for end-stage liver disease scoring system on prognosis in the acute on chronic liver failure patients with plasma exchange treatment. ASAIO J. 2010;56:475–478. doi: 10.1097/MAT.0b013e3181e6bf13. [DOI] [PubMed] [Google Scholar]


