Abstract
Exercise in cold environments can cause significant metabolic regulation and antioxidant behavior. For discussing enzymatic responses towards cold adaptation, we investigated enzyme activities of adenylate cyclase (AC) and phosphodiesterase (PDE) in liver, skeletal muscle, and brown adipose tissue (BAT), as well as Na+·K+ ATPase and Na+/K+ ratio in blood. Malondialdehyde (MDA) and superoxide dismutase (SOD) activity in blood were also studied to address the effect of cold adaptation on oxidative damage and antioxidant system. Experimental results indicated that enzyme activities in liver, skeletal muscle and BAT maintained relatively constant for the control group. For the cold adaptation group, enzyme activities in liver and skeletal muscle were in high levels at the beginning, and then gradually decreased to similar values with the control group. However, enzyme activities in BAT performed an increasing trend and significantly higher than the control at the end. In addition, decreased oxidative damage and activated antioxidant system was observed along with the cold adaptation process.
Keywords: Cold adaptation, adenylate cyclase, phosphodiesterase, Na+·K+ ATPase, malondialdehyde, superoxide dismutase
Introduction
Cold exposure is a common issue in several sporting events (winter swimming, etc.) and special work in both indoor and outdoor conditions (fishing, forestry, dairy industries, etc.) [1]. In recent years, exercise in cold environments has attracted much attention on its potential benefits for athletes [2,3]. Exercise in cold environments means that human body has to produce more heat for maintaining normal body core temperature, involving a series of physiological response within human body to change the utilization patterns of energy substances [4-6]. These metabolic processes lead to the enhancement of blood pressure, heart rate, and oxygen consumption [7]. It is well known that the increase of oxygen consumption (or energy consumption) can be mainly due to the thermogenesis effect and elimination of lactic acid [8]. Although some studies indicated that the local hypothermia can delay the release of lactic acid, the metabolic block of lactic acid from reduced blood supply resulted in lactic acid accumulations to some extent and obviously increased the respiratory quotient [9,10].
Exercise in cold environments results in significant variation to body metabolisms. Sink et al. carried out the training project under different temperatures with the training strength of 50% VO2max and 60% VO2max, and the concentrations of plasma fatty acids and glycerol (as the lipid metabolic markers) significantly increased at 0°C environmental condition [11]. Fink et al. made the excise project with the training strength of 70%~80% VO2max, and they found that the concentrations of triglyceride in muscle decreased for 23% under cold environments, and only 11% under warm condition. Parkin et al. found that after the exhausting excise of 70% VO2max, muscle glycogen concentration in vastus lateralis was 30% higher under cold environments (3°C) than that under normal temperature (20°C) [12]. Febbraio et al. attributed the decrease of glycogen decomposition rate to the decrease of local muscle temperature and the sympathetic nervous system excitability [13]. They concluded that high lipid utilization and low glycogen utilization occurred under cold environments. As a special physiological response towards cold exposure, the phenomenon can be explained as a protection mechanism for maintaining body core temperature [14]. Since the demand of glycogen is significantly increased under cold environments, skeletal muscle has to obtain more energy from lipid. In this way, more energy can be saved for making sure the organ function to sustain life [15].
The variation of body metabolisms under cold environments can be fundamentally due to metabolic regulations by relative important enzymes and compounds. For further understanding the stress/response metabolic mechanisms towards exercise in cold environments, in this study, we investigated enzyme activities of adenylate cyclase (AC) and phosphodiesterase (PDE) in liver, skeletal muscle, and brown adipose tissue (BAT) of rats, as well as Na+·K+ ATPase, K+ concentration, and Na+ concentration in blood. In addition, malondialdehyde (MDA) concentration and superoxide dismutase (SOD) activity were measured to discuss oxidative damage and free radical metabolism during cold adaptation process.
Materials and methods
Samples
One hundred Sprague-Dawley (SD) rats (50 males and 50 females) were chosen as the samples in this study, which was approved by the Ethics Committee in our institution. The rats were fed in the same conditions and all performed perfect physical status. While the rats were about 8 weeks old and had a weight range from 156 g to 203 g, they were prepared for the following experiment.
Experimental procedures
The rats were randomly divided into the control group and the cold adaption group with 50 individuals in each group. All the experiments were carried out in a temperature-controlled swimming chamber (Western Environmental Chamber, Napa, CA). For each day during the experimental process, the rats in the control group swam in the chamber at 20°C for 30 min, and the rats in the cold adaption group experienced a cold swimming excise at 4°C for 30 min. At the end of the 1, 2, 3, 4, and 6 weeks, ten rats in each group were randomly chosen and killed respectively by decapitation. Blood sample, left liver lobule, intestinal muscle of right calf, and BAT in scapular region were taken for analyzing enzyme activities and other items.
Analytical methods
Enzyme activities of AC were measured by the unlabeled substrate method described in a previous study [16]. Enzyme activities of PDE were investigated by using cytochemical procedures described by Okruhlicova et al. [17]. K+ concentration and Na+ concentration in blood were examined by a Beckman CX-3 ion selective electrode. Na+·K+ ATPase activities, MDA concentration, and SOD activities were analyzed by using kits from Nanjing Jiancheng Bioengineering Institute, including the Na+·K+ ATPase assay kit, the MDA assay kit (TBA method), and the SOD assay kit (hydroxylamine method), respectively.
Statistical analysis
The experimental results were shown as mean ± standard deviati Kolmogorov-Smirnov test was used to ensure normality. The analysis of variance (ANOVA) was conducted to locate the differences with the level of signon. Differences in experimental data between groups were evaluated by statistical analysis, which was performed with the use of the commercially available SPSS 16.0 software. The independent samples t tests were used to characterize the anthropometric and baseline data between groups. Theificance set at P < 0.05.
Results
Effects of cold adaptation on AC enzyme activities
Table 1 represents time-course profiles of AC enzyme activities in liver, skeletal muscle and BAT. AC enzyme activities in the control group maintained relatively constant during the whole period. In the cold adaptation group, AC enzyme activities performed a decrease trend in liver and skeletal muscle, and gradually increased in BAT. After six weeks of cold adaption, AC enzyme activities were almost the same in liver (control: 2.49 umol/mg-prot·h; cold adaption: 2.66 umol/mg-prot·h), and skeletal muscle (control: 2.19 umol/mg-prot·h; cold adaption: 2.32 umol/mg-prot·h) for the two groups. However, AC enzyme activities in BAT were significantly higher (7.35 umol/mg-prot·h) in the cold adaption group than those in the control (2.26 umol/mg-prot·h).
Table 1.
Time-course profiles of AC enzyme activities in liver, skeletal muscle, and BAT
| Time | Liver (umol/mg-prot·h) | Skeletal muscle (umol/mg·h) | BAT (umol/mg-prot·h) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Control | Cold adaption | Control | Cold adaption | Control | Cold adaption | |
| 1 week | 2.22 ± 0.23 | 4.26 ± 0.40* | 2.07 ± 0.33 | 4.23 ± 0.52* | 2.16 ± 0.42 | 5.10 ± 0.58* |
| 2 weeks | 2.31 ± 0.26 | 3.70 ± 0.32* | 2.14 ± 0.35 | 3.98 ± 0.47* | 2.53 ± 0.33 | 5.94 ± 0.52* |
| 3 weeks | 2.42 ± 0.27 | 3.24 ± 0.27* | 1.98 ± 0.26 | 3.45 ± 0.38* | 2.25 ± 0.35 | 6.21 ± 0.60* |
| 4 weeks | 2.58 ± 0.29 | 2.86 ± 0.22 | 2.22 ± 0.23 | 2.53 ± 0.32 | 2.37 ± 0.22 | 6.81 ± 0.72* |
| 6 weeks | 2.49 ± 0.25 | 2.66 ± 0.30 | 2.19 ± 0.27 | 2.32 ± 0.22 | 2.26 ± 0.29 | 7.35 ± 0.66* |
Represents the difference between two groups is significant (P < 0.05).
Effects of cold adaptation on PDE enzyme activities
Enzyme activities of PDE in liver, skeletal muscle and BAT are listed in Table 2. For the control group, the enzyme activities of PDE were relatively stable, with the values ranged from 1.95-2.14 umol/mg-prot·h (liver), 2.07-2.55 umol/mg-prot·h (skeletal muscle), and 2.72-2.92 umol/mg-prot·h (BAT), respectively. For the cold adaption group, PDE enzyme activities performed a decrease trend in liver and skeletal muscle, and finally kept at 2.19 umol/mg-prot·h and 2.37 umol/mg-prot·h, respectively. Impressively, PDE enzyme activities in BAT dramatically increased for the cold adaption group and finally reached 8.07 umol/mg-prot·h.
Table 2.
Time-course profiles of PDE enzyme activities in liver, skeletal muscle, and BAT
| Time | Liver (umol/mg-prot·h) | Skeletal muscle (umol/mg·h) | BAT (umol/mg-prot·h) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Control | Cold adaption | Control | Cold adaption | Control | Cold adaption | |
| 1 week | 2.18 ± 0.31 | 3.18 ± 0.31* | 2.39 ± 0.19 | 4.69 ± 0.52* | 2.72 ± 0.35 | 4.52 ± 0.61* |
| 2 weeks | 2.24 ± 0.27 | 3.06 ± 0.28* | 2.55 ± 0.26 | 4.20 ± 0.47* | 2.83 ± 0.36 | 5.76 ± 0.72* |
| 3 weeks | 1.95 ± 0.22 | 2.68 ± 0.32* | 2.33 ± 0.29 | 3.66 ± 0.43* | 2.92 ± 0.42 | 6.23 ± 0.69* |
| 4 weeks | 2.08 ± 0.26 | 2.25 ± 0.19 | 2.19 ± 0.32 | 2.65 ± 0.31 | 2.86 ± 0.47 | 7.62 ± 0.74* |
| 6 weeks | 2.12 ± 0.20 | 2.19 ± 0.21 | 2.07 ± 0.31 | 2.37 ± 0.32 | 2.79 ± 0.38 | 8.07 ± 0.79* |
Represents the difference between two groups is significant (P < 0.05).
Effects of cold adaptation on enzyme activities of Na+·K+ ATPase
Figure 1 showed the variation trend of enzyme activities of Na+·K+ ATPase for the two groups. For the control, the enzyme activities of Na+·K+ ATPase was almost the same all the time with little fluctuation (0.21-0.25 umol/mg-prot·h). For the cold adaption group, the enzyme activities of Na+·K+ ATPase experienced a continuous drop from 0.46 ± 0.11 umol/mg-prot·h to 0.30 ± 0.08 umol/mg-prot·h. At the end of the experiment, the cold adaptation group showed relatively higher Na+·K+ ATPase activity than the control group.
Figure 1.
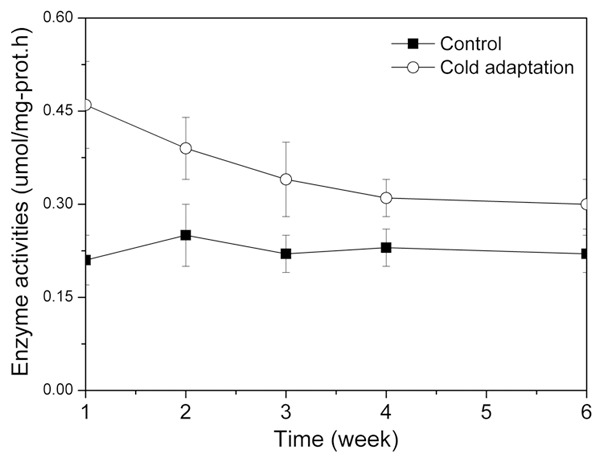
Enzyme activities of Na+·K+ ATPase at different time for the control group and the cold adaptation group.
Effects of cold adaptation on K+ concentration, Na+ concentration and Na+/K+ ratio
Table 3 illustrates the variation of K+ concentration, Na+ concentration and Na+/K+ ratio at different times for the two groups. K+ concentration, Na+ concentration and Na+/K+ ratio for the control group were relative steady all the time. However, for the cold adaption group, K+ concentration indicated an increasing trend and Na+ concentration decreased along with the adaption time, leading to a remarkable decrease of Na+/K+ ratio (from 0.38 ± 0.08 to 0.18 ± 0.05). At the end of the cold adaptation process, we can not find significant differences of K+ concentration, Na+ concentration and Na+/K+ ratio for the two groups.
Table 3.
Time-course profiles of K+, Na+ concentration and Na+/K+ ratio in liver, skeletal muscle, and BAT
| Time | K+ (umol/mg-prot) | Na+ (umol/mg-prot) | Na+/K+ (umol/mg-prot) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Control | Cold adaption | Control | Cold adaption | Control | Cold adaption | |
| 1 week | 9.23 ± 0.68 | 8.44 ± 0.51* | 1.66 ± 0.17 | 3.21 ± 0.38* | 0.18 ± 0.06 | 0.38 ± 0.08* |
| 2 weeks | 9.52 ± 0.77 | 8.24 ± 0.57* | 1.81 ± 0.23 | 2.39 ± 0.32* | 0.19 ± 0.07 | 0.29 ± 0.07* |
| 3 weeks | 9.14 ± 0.70 | 8.68 ± 0.49 | 1.74 ± 0.26 | 2.08 ± 0.26 | 0.19 ± 0.06 | 0.24 ± 0.07 |
| 4 weeks | 9.35 ± 0.76 | 9.02 ± 0.71 | 1.68 ± 0.20 | 1.98 ± 0.28 | 0.18 ± 0.05 | 0.22 ± 0.05 |
| 6 weeks | 9.42 ± 0.74 | 9.11 ± 0.69 | 1.60 ± 0.22 | 1.64 ± 0.23 | 0.17 ± 0.04 | 0.18 ± 0.05 |
Represents the difference between two groups is significant (P < 0.05).
Effects of cold adaptation on MDA concentration and SOD activity
The change of MDA concentrations for the two groups is shown in Figure 2. For all the time, MDA concentration for the control group was almost the constant around 1.6 nmol/mg-prot. For the cold adaptation group, the MDA concentration was extremely high at the beginning (3.68 nmol/mg-prot.), followed by a rapid decrease to 1.62 nmol/mg-prot. At the end, MDA concentrations were almost the same for the two groups. Figure 3 illustrated the variation trend of SOD activities for the two groups. For the control group, SOD activities performed less fluctuation ranged from 221.08 U/mg-prot to 256.78 U/mg-prot. For the cold adaptation group, SOD activities slowly increased from 157.81 U/mg-prot to 267.11 U/mg-prot.
Figure 2.
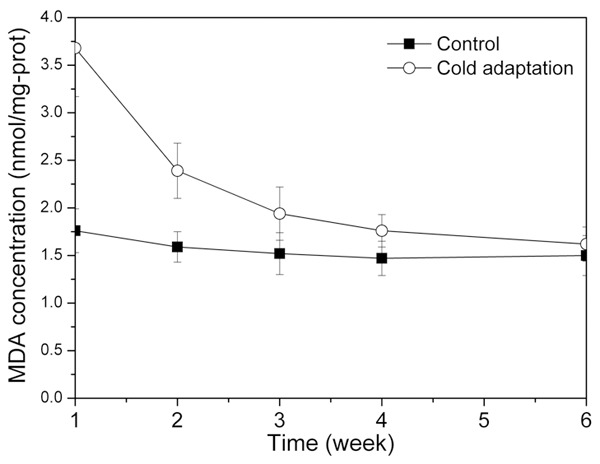
MDA concentrations at different time for the control group and the cold adaptation group.
Figure 3.
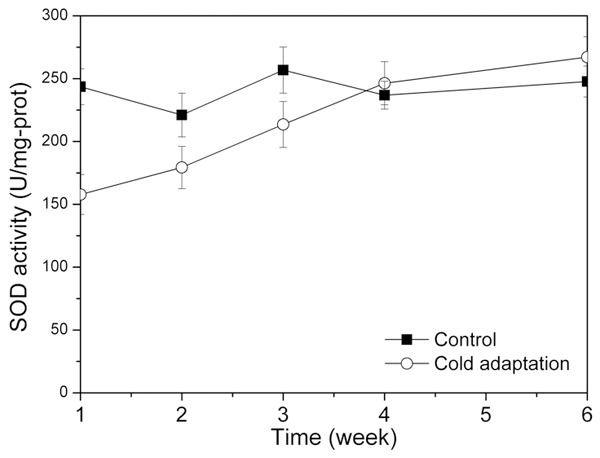
SOD activities at different time for the control group and the cold adaptation group.
Discussion
Recently, exercise in cold environments became a hot topic for enhancing exercise efforts of athletes. Under the cold conditions, human body can alter the neuroendocrine regulation and metabolic levels to maintain core temperature and normal life activities [19]. After a long period of cold stimulation, a series of biochemical and physiological regulations are induced to increase cold resistance capability, which finally leads to the status of cold adaptation. Generally, the cold adaptation process can be divided into two phase: cold stimulation and cold adaptation, characterized by the variations of important enzymes and compounds
In this study, for the beginning three weeks, AC and PDE activities in liver and skeletal muscle of the cold adaptation group were significantly larger than those of the control group (P < 0.05). For the following three weeks, AC and PDE activities in BAT significantly increased and played an important role in heat production. It can be concluded that the beginning three weeks was cold stimulation period, which was characterized by main heat production by liver and skeletal muscle. In this stage, enhanced catecholamine hormones had regulatory effects on tissue cells, resulting in the reinforcement of AC and PDE activities. By catalyzing cytoplasm ATP into cAMP, Fat kinase was activated to strengthen lipolysis. More free fatty acids were produced to supply abundant substrates for oxidative phosphorylation of tissues, leading to the enhancement of tissue heat production [20].
For the following three weeks, it can be recognized as the cold adaptation period. In this stage, heat production in liver and skeletal muscle decreased and enzyme activities in BAT performed higher values. In previous studies, it has been proved that cold adaptation was mainly achieved by increased heat production from BAT and regulated by sympathetic nervous system [21]. During the process, express of some important components in BAT (uncoupling proteins, thyroxine- 5’ - iodine enzyme, lipoprotein lipase, etc.) was increased to accelerate the decomposition of triglyceride in plasma lipoprotein, resulting in strengthened heat production and decrease of body fat [22]. Since lower body fat concentration might be more susceptible towards sympathetic stimulation, heat production process can be better operated to maintain core temperature, and further strengthen athletes’ physique [23].
In addition, cold adaptation process led to the increase of Na+·K+ ATPase activity and Na+/K+ ratio, which act vital efforts on maintain normal physiological function of cells. It has been assumed that exposure in cold environments induced the production of more catecholamine hormones, which act on sodium ion channel of cell membrane and increase the sodium ion concentration. Sodium pump was activated to uptake sodium and release potassium. At the same time, ATP was largely decomposed to release much energy for meeting metabolic requirements of body [24].
Exercises in cold environments were accompanied by enhanced metabolic processes and energy consumption. Moreover, more oxygen free radicals were simultaneously produced to make damage towards lipids, proteins and DNA. It was reported that cold stimulation led to the decrease of SOD activity in rat hypothalamus and adrenal medulla [25]. Kaushik et al. found that cold exposure led to the change of oxidative stress and antioxidant system of rat tissues [26]. In this study, we investigate the effect of cold adaptation on oxidative damage and antioxidant system. MDA and SOD were chosen as the important indexes to evaluate the contents of oxidative stress injury and antioxidant repair. We concluded that cold adaptation process can strengthen the oxidative stress level and decrease the antioxidant ability at the beginning. However, systemic adaptation was successfully established after a long period, including the activation of antioxidant system, intervention of repair/elimination system, and change of redox sensitive transcription. Based on effective removal of oxygen free radicals, virtuous circle can be made to strengthen the adaptation capability of body antioxidant defense system.
Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (No. HEUCF141602). The authors acknowledge the work of animal feeding by Animal experimental center of Harbin Medical University, and the relevant equipments provided by Department of Radiology, Harbin Medical University Cancer Hospital.
Disclosure of conflict of interest
None.
References
- 1.Sormunen E, Rissanen S, Oksa J, Pienimaki T, Remes J, Rintamaki H. Muscular activity and thermal responses in men and women during repetitive work in cold environments. Ergonomics. 2009;52:964–976. doi: 10.1080/00140130902767413. [DOI] [PubMed] [Google Scholar]
- 2.Lucia A, Hoyos J, Perez M, Santalla A, Chicharro JL. Inverse relationship between VO2max and economy/efficiency in worldclass cyclists. Med Sci Sports Exerc. 2002;34:2079–2084. doi: 10.1249/01.MSS.0000039306.92778.DF. [DOI] [PubMed] [Google Scholar]
- 3.Hong JH, Kim KJ, Suzuki K, Lee IS. Effect of cold acclimation on antioxidant status in cold acclimated skaters. J Physiol Anthropol. 2008;27:255–262. doi: 10.2114/jpa2.27.255. [DOI] [PubMed] [Google Scholar]
- 4.Moseley L, Jeukendrup AE. The reliability of cycling efficiency. Med Sci Sports Exerc. 2001;33:621–627. doi: 10.1097/00005768-200104000-00017. [DOI] [PubMed] [Google Scholar]
- 5.Chappell MA, Hammond KA. Maximal aerobic performance of deer mice in combined cold and exercise challenges. J Comp Physiol B. 2004;174:41–48. doi: 10.1007/s00360-003-0387-z. [DOI] [PubMed] [Google Scholar]
- 6.Muller MD, Ryan EJ, Bellar DM, Kim CH, Blankfield RP, Muller SM, Glickman EL. The influence of interval versus continuous exercise on thermoregulation, torso hemodynamics, and finger dexterity in the cold. Eur J Appl Physiol. 2010;109:857–867. doi: 10.1007/s00421-010-1416-8. [DOI] [PubMed] [Google Scholar]
- 7.Spriet LL. Regulation of skeletal muscle fat oxidation during exercise in humans. Med Sci Sports Exerc. 2002;34:1477–1484. doi: 10.1097/00005768-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Layden JD, Patterson MP, Nimmo MA. Effect of reduced ambient temperature on fat utilization during submaximal exercise. Med Sci Sports Exerc. 2002;34:774–779. doi: 10.1097/00005768-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Febbraio MA, Chiu A, Angus DJ, Arkinstall MJ, Hawley JA. Effects of carbohydrate ingestion before and during exercise on glucose kinetics and performance. J Appl Physiol. 2000;89:2220–2226. doi: 10.1152/jappl.2000.89.6.2220. [DOI] [PubMed] [Google Scholar]
- 10.Watt MJ, Heigenhauser GJ, Dyck DJ, Spriet LL. Intramuscular triacylglycerol, glycogen and acetyl group metabolism during 4 h of moderate exercise in man. J Physiol. 2002;541:969–978. doi: 10.1113/jphysiol.2002.018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sink KR, Thomas TR, Araujo J, Hill SF. Fat energy use and plasma lipid changes associated with exercise intensity and temperature. Eur J Appl Physiol Occup Physiol. 1989;58:508–513. doi: 10.1007/BF02330705. [DOI] [PubMed] [Google Scholar]
- 12.Fink WJ, Costill DL, van Handel PJ. Leg muscle metabolism during exercise in the cold. Eur J Appl Physiol Occup Physiol. 1975;34:183–190. doi: 10.1007/BF00999931. [DOI] [PubMed] [Google Scholar]
- 13.Febbraio MA, Snow RJ, Stathis CG, Carey MF. Blunting the rise in body temperature reduces muscle glycogenolysis during exercise in humans. Exp Physiol. 1996;81:685–693. doi: 10.1113/expphysiol.1996.sp003969. [DOI] [PubMed] [Google Scholar]
- 14.Layden JD, Pattenson MJ, Nimmo MA. Effects of reduced ambient temperature on fat utilization during submaximal exercise. Med Sci Sports Exerc. 2002;34:774–779. doi: 10.1097/00005768-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Muller MD, Kim CH, Bellar DM, Ryan EJ, Seo YS, Muller SM, Glickman EL. Effect of cold acclimatization on exercise economy in the cold. Eur J Appl Physiol. 2012;112:795–800. doi: 10.1007/s00421-011-2038-5. [DOI] [PubMed] [Google Scholar]
- 16.Ross EM, Howlett AC, Ferguson KM, Gilman AG. Reconstitution of hormone-sensitive adenylate cyclase activity with resolved components of the enzyme. J Biol Chem. 1978;253:6401–6412. [PubMed] [Google Scholar]
- 17.Okruhlicova L, Klenerova V, Hynie S, Sida P. In situ detection of cyclic AMP-phosphodiesterase activity in the heart of Lewis and Sprague-Dawley rats: the effect of restraint stress or amphetamine application. Histol Histopathol. 2004;19:719–726. doi: 10.14670/HH-19.719. [DOI] [PubMed] [Google Scholar]
- 18.Jankovi A, Buzadz B, Kora A, Petrovi V, Vasilijevi A, Kora B. Antioxidative defense organization in retroperitoneal white adipose tissue during acclimation to cold-The involvement of L-arginine/NO pathway. J Therm Biol. 2009;34:358–365. [Google Scholar]
- 19.Shephard RJ. Fat metabolism, exercise, and the cold. Can J Sport Sci. 1992;17:83–90. [PubMed] [Google Scholar]
- 20.Vybiral S, Lesna I, Jansky L, Zeman V. Thermoregulation in winter swimmers and physiological significance of human catecholamine thermogenesis. Exp Physiol. 2000;85:321–326. [PubMed] [Google Scholar]
- 21.Sulloa A, Brizzia G, Maffulli N. Deiodinating activity in the brown adipose tissue of rats following short cold exposure after strenuous exercise. Physiol Behav. 2003;80:399–403. doi: 10.1016/j.physbeh.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Simonsen L, Stallknecht B, Bülow J. Contribution of skeletal muscle and adipose tissue to adrenaline-induced thermogenesis in man. Int J Obes Relat Metab Disord. 1993;17:S47–51. [PubMed] [Google Scholar]
- 23.Schaefer CD, Staples JF. Mitochondrial metabolism in mammalian cold-acclimation: Magnitude and mechanisms of fatty acid uncoupling. J Therm Biol. 2006;31:355–361. [Google Scholar]
- 24.Teulier L, Rouanet JL, Letexier D, Romestaing C, Belouze M, Rey B, Duchamp C, Roussel D. Cold acclimation induced non shivering thermogenesis in birds is associated with upregulation of avian UCP but not with innate uncoupling or altered ATP efficiency. J Exp Biol. 2010;213:2476–2482. doi: 10.1242/jeb.043489. [DOI] [PubMed] [Google Scholar]
- 25.Yüksel Ş, Asma D. Effects of extended cold exposure on antioxidant defense system of rat hypothalamic-pituitary-adrenal axis. J Therm Biol. 2006;31:313–317. [Google Scholar]
- 26.Kaushik S, Kaur J. Chronic cold exposure affects the antioxidant defense system in various rat tissues. Clin Chim Acta. 2003;333:69–77. doi: 10.1016/s0009-8981(03)00171-2. [DOI] [PubMed] [Google Scholar]


