Abstract
The safety of antibiotics has been becoming an important worldwide concern. As an inevitable and widespread existing impurity of β-lactam antibiotics, pivaloylacylation-7ADCA may has potential impact on drug safety. However, due to the restriction on traditional drug production technique, purified pivaloylacylation-7ADCA cannot be acquired and thus the toxicity of pivaloylacylation-7ADCA remains completely unknown. In this study, we firstly assessed the genotoxicity of newly purified pivaloylacylation-7ADCA. A series of well-designed experiments, including bacterial reverse mutation assay (Ames assay), mouse lymphoma assay (TK gene mutation test), chromosomal aberration assay, in vivo mouse micronucleus test and single cell gel electrophoresis assay (comet assay), were performed in genotoxicity assessment of pivaloylacylation-7ADCA at three different genetic endpoints, i.e. gene mutation, chromosome aberration or breakage, and DNA strand breaks. No genotoxicity were observed at all tested genetic endpoints, suggesting that pivaloylacylation-7ADCA has no mutagenic effect. To our knowledge, this is the first systematic assessment on the toxicity of newly synthesized pivaloylacylation-7ADCA, which should be an important part of the drug safety evaluation of β-lactam antibiotics. Moreover, our study is expected to serve as a reference for the genotoxicity assessment of other antibiotic impurities, by using purified impurity as test sample and by combining a group of well-designed genotoxic assays with different species, major genetic endpoints and in vivo/vitro tests.
Keywords: Genotoxicity, risk assessment, β-lactam antibiotics, pivaloylacylation-7ADCA
Introduction
Since their discovery in 1920s,antibiotics have undergone an ever-accelerating development. So far, about 120 categories of antibiotics have been produced and consumed in medical applications worldwide, among which penicillin, cephalothin, tetracycline, aminoside and macrolide are the most commonly used [1]. Most of antibiotics in current markets are produced via microbial fermentation processes or synthetic technologies combined with chemical processes. Compared to simple synthesis processes, the fermentation processes involve more complex chemical reactions and thus have much more difficulties in quality control. Therefore, the emergence of impurities is inevitable in antibiotic-production related to fermentation process. Researches and clinical trials have demonstrated that many adverse drug reactions, such as anaphylaxis, are not caused by antibiotics themselves, but by the impurities [2]. How to assess and control the impurities in antibiotics products now becomes a crucial issue in drug quality control. Close attention has been paid by global drug regulatory agencies, and, as a result of enduring co-efforts, lots of new technologies and instruments have been invented and applied into the area. Guideline on setting specifications for related impurities in antibiotics, launched in 2010 by European Medicines Agency (EMA), proposes the European Union’s opinions on how to control the impurities in antibiotics production, which also clearly points out the limitations on identification, confirmation and reporting of impurities in all antibiotics produced via fermentation processes or semi-synthetic processes [3]. Meanwhile, It is highly required that the impurities whose amount exceeds the quality control threshold should undergo toxicological evaluation. Genotoxic Impurity Limits Guiding Principles, also issued by the EMA in 2011, aims to manage impurities with genotoxicity and is accepted by the US Food & Drug Administration (FDA) [4]. In China, requirements on medicine impurities control and toxicological safety evaluation are at present keeping in line with international standards, yet requiring more international principles to be introduced into as reference in Chinese drug-approval process.Genotoxicity is one of such main factors determining drug safety for it offers a reliable reference in evaluation of the carcinogenicity of drugs and impurities. It is, therefore, an important test index accepted by many nations [5].
β-lactam antibiotics, such as penicillin and cephalosporin, is the most common antibiotics in terms of annual bulk production (more than 3×107 kg/year), annual sales (over $ 15 billion), and market share (more than 70% of the total antibiotic market) [6]. At present, most of β-lactam antibiotics refer to a large family of semi-synthetic antibiotics which is produced via fermentation process by complex chemical reactions. However, in case of poor quality control and/or incomplete refinement, the final products are usually contaminated by part of the raw materials and intermediate material such as 7-aminodesacetoxycephalosporanic acid (7-ADCA), which inevitably lead to the generation of pivaloylacylation-7ADCA, an widely existing impurity in β-lactam antibiotic production [7]. The impurity pivaloylacylation-7ADCA may has a potential impact both on efficacy reducing and activity weakening, even a genotoxicity to threat the safety of cefadroxil. Therefore, the evaluation for the genotoxicity of pivaloylacylation-7ADCA is in urgent need. Unfortunately, previous drug safety studies, due to the restriction on drug production technique, mostly based on the evaluation of combined antibiotics and the impurities as a whole [8], which means only the safety of the mixture could be evaluated while the detailed safety information of specific impurities remain completely unknown. Most recently, Sichuan Industrial Institute of Antibiotics, National Pharmaceutical Group, has achieved a breakthrough in its effort to identify and synthesize purified pivaloylacylation-7ADCA. So, in the present study, the genotoxicity of the purified pivaloylacylation-7ADCA was firstly studied in specific, by conducting a series of well-designed assays combined with different species, three major genetic endpoints and in vivo/vitro tests. To our knowledge, this is the first report in specific on the toxicity of the new synthesized pivaloylacylation-7ADCA, which should be an important part of the drug safety evaluation of β-lactam antibiotics. Moreover, we systematically designed a group of well-designed genotoxic assays with different species, major genetic endpoints and in vivo/vitro tests, which may improve the reliability as well as preciseness of the results. Therefore, our study is highly expected to serve as a reference for the genotoxicity assessments of other antibiotic impurities.
Materials and methods
The main reagents
Pivaloylacylation-7ADCA in this study, purity ≥99.9%, was kindly provided by Sichuan Industrial Institute of Antibiotics, National Pharmaceutical Group. Pivaloylacylation-7ADCA is dissolved in dimethyl sulfoxide (DMSO, Sigma Co., Missouri, USA).
The rat liver microsomal enzyme (S9) was mad by our laboratory, as described previously [9], and stored at -80°C until use. The final metabolizing system (S9 mixture) contained glucose-6-phosphate (G6P, 4.4 mM), β-Nicotinamide adenine dinucleotide phosphate (NADP, 0.84 mM), potassium chloride (KCl, 30 mM), NaHCO3 (0.032%) and S9 fraction (10%). Cultures treated in the absence of S9 mixture received an equivalent volume of KCl (150 mM).
THMG media for TK gene mutation test was prepared with 3 μg/ml thymine, 5 μg/ml hypoxanthine, 0.1 μg/ml methopterin and 7.5 μg/ml glycin.
Cell culture and Salmonella typhimurium strains
Cell lines
The Chinese hamster lung cell line and mouse lymphoma L5178Y (TK+/-) cell line were purchased from Shanghai Cell Bank, Chinese Academy of Sciences and maintained at 37°C in a humidified atmosphere of 5% (ν/ν) CO2 in air.
The CHL cells were routinely maintained in high glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% (ν/ν) fetal bovine serum (Minhai Biotechnology Co., Beijing, China), 100 unit/ml of penicillin and 100 μg/ml of streptomycin.
Mouse lymphoma L5178Y (TK+/-) cells were cultured in RPMI 1640 medium (Minhai Biotechnology Co., Beijing, China) containing 10% (ν/ν) heat-inactivated horse serum (Minhai Biotechnology Co., Beijing, China), 200 μg/ml sodium pyruvate, 100 unit/ml penicillin and 100 μg/ml streptomycin in routine culture.
Salmonella typhimurium strains
The histidine-dependent auxotrophic mutants of Salmonella typhimurium (strains TA97, TA98, TA100 and TA102) used in this study were stored in the liquid nitrogen container. The eligibility of the strains has been verified by genetic properties before the experiments.
Experimental animals and protocol
The healthy adult Kunming mice (8 weeks of age, weighing 25-30 g) in specific pathogen free (SPF) level were purchased from Shanghai Slac Laboratory Company (Shanghai, China), and maintained in a climate-controlled environment with a 12 h light/dark cycle. Five mice were housed per cage and acclimatized for two weeks before the experiment starting. The mice had free access to food and water which were provided through the food chamber on the top of the cage throughout the experiment period.
All animal experiments were approved by Care and Use of Animals Center of Sichuan University. All animal protocols in this study were all approved by the Ethics Committee of Sichuan University and conducted in strict accordance with the principles outlined in the NIH Guide for the Care and Use of Laboratory Animal (AAALAC International accredited in 1998). Every effort was made to minimize animal pain, suffering and distress and to reduce the number of animal used.
Bacterial reverse mutation assay (ames assay)
The mutagenicity of pivaloylacylation-7ADCA was assessed by a bacterial reverse mutation assay according to the OECD Guideline, No. 471 [10]. The assay was conducted by the plate incorporation method both in the presence and absence of S9 mixture. Salmonella typhimurium strains TA97, TA98, TA100 and TA102 were used as test strains. Pivaloylacylation-7ADCA was designed with concentrations of 0 (sterile purified water), 8, 40, 200, 1000, 5000 μg/plate. DMSO was utilized as the solvent control. The positive controls contained 2-aminofluorene (2-AF, 10 μg/plate vs. TA97, TA98, TA100 with S9), 1,8-dihydroxyanthraquinone (50 μg/plate vs. TA102 with S9), 2, 4, 7-trinitrofluoren-9-one (2,4,7-tNFO, 0.2 μg/plate vs. TA97, TA98 without S9), sodium azide (SA, 1.5 μg/plate vs. TA100 without S9), mitomycin C (MMC, 0.5 μg/plate vs.TA102 without S9). In brief, 100 μl compound solutions and 100 μl bacterial culture, with and without 500 μl S9 mixture, were added to each tube containing 2 ml top agar. The mixture was quickly poured across the surface of a basal (minimal glucose) agar plate. After incubation at 37°C for 48 h, the number of revertant colonies was scored according to the description by Gatehouse D [11]. The result was considered as positive for mutagenicity if there was 1) a two fold increase in the number of the negative control or 2) a dose-dependent increase in the number of revertant colonies in one or more strains [12].
Mouse lymphoma assay (TK gene mutation test)
The TK gene mutation test was performed according to the International Conference on Harmonisation (ICH) guidance [13]. L5178Y mouse lymphoma cells were used as test cell lines. Dosages ranging from 625 to 5000 μg/ml were selected as the final concentrations of pivaloylacylation-7ADCA, meanwhile 5 μg/ml methyl methane sulphonate (MMS, Sigma Co., Missouri, USA) and DMSO were used as positive control and solvent control, respectively. The cells were cultured in THMG medium for 24 h and then transferred into THG medium (without methopterin) for 48 h to remove the pre-existing mutants. Then cells were treated with test substances for 10 h, and the cell density was subsequently recorded for 2 d to calculate relative suspension growth (RSG). Meanwhile, the cells were seeded into 96-well plates at a density of 1.6 cells per well for calculation of plating efficiency (PE). All plates were incubated for 12 d to score the mutant colonies and calculate relative viability (RV). The indexes used in this assay were relative viability (RV), relative suspension growth (RSG), relative total growth (RTG) and mutant frequency (MF), among which, RTG (%) was calculated as RSG×RV (%) [14-17].
In vitro mammalian chromosomal aberration assay
To further detect whether or not pivaloylacylation-7ADCA induces chromosome aberration, the in vitro chromosome aberration assay was conducted according to the OECD Guideline No. 473 [18]. The assay was also conducted in the presence and absence of S9 mixture. The Chinese hamster lung (CHL) cells were seeded into six-well plate at a density of 1×105 cells per well. After adherence, cells were exposed to a range dose of pivaloylacylation-7ADCA (1250, 2500 and 5000 μg/ml). Cells treated with DMSO alone were employed as the solvent control. In the absence of S9 mixture, cells treated by 0.1 μg/ml of mitomycin C (MMC) were used as positive control; while in the presence of S9, cells treated by 20 μg/ml of cyclophosphamide (CP) was employed as the positive control. After treatment for a short-term exposure (24 h) and a continuous exposure (48 h), cells were harvested after 0.5 μg/ml colchicine treatment for 2.5 h. 100 cells at metaphases per slide were analyzed under light microscope (200× magnification, Leica, Germany) with 10% (ν/ν) Giemsa stained. Then the frequency of chromosome aberrations was calculated. Results were evaluated according to the following criteria for structural or numerical chromosomal aberration frequencies: negative <4.9%; uncertain 5-10%; positive ≥10% [19].
In vivo mouse micronucleus test
The in vivo micronucleus test was another important assay to detect chromosome aberration in interphase cells [20]. The Kunming mice in specific pathogen free (SPF) level, a widely used model for drug safety assessment, were used in our study. The animal protocols in this study were all approved by the Ethics Committee of Sichuan University and conducted in strictly accordance with the principles outlined in the NIH Guide for the Care and Use of Laboratory Animal (AAALAC International accredited in 1998).
An acute oral toxicity test was firstly performed to determine the suitable concentration of pivaloylacylation-7ADCA that did not induce toxic effect on mice. Health male and female mice were assigned to groups of five rats of each sex. An oral gavage administration of pivaloylacylation-7ADCA ranging from 0-1000 mg/kg /day was conducted once a day for 2 days. Then all animals were observed, and the mortality, clinical signs, body weight changes, and gross findings were recorded for 14 days. The preliminary result showed that oral administration of pivaloylacylation-7ADCA at a dose of at 500 mg/kg/day did not induce any toxic effect on Kunming mice. According to the OECD guidelines, this dose of pivaloylacylation-7ADCA was selected as the maximum dose. Test next was the genotoxicity of pivaloylacylation-7ADCA on chromosome level. Briefly, fifty SPF mice at 8 weeks of age (weighing 25-30 g) were set into 5 groups (5 animals per sex in each group). Mice in three treated groups were administered by oral gavage 30 h and 6 h prior to mice sacrifice, at doses of 125, 250 and 500 mg/kg body weight of pivaloylacylation-7ADCA. The positive control group was given an intraperitoneal injection (i.p.) of cyclophosphamide (CP) dissolved in physiological saline at a dose of 40 mg/kg body weight; while the solvent control group received sodium carboxyl methyl cellulose (CMC-Na) by oral gavage at 0.1 mg/kg body weight. After treatment, animals were subsequently sacrificed by carbon dioxide asphyxiation. Bone marrow smears were prepared from each treatment group after mice sacrifice, according to the OECD Guideline No. 474 [21]. After fixed by methanol containing 1% (ν/ν) acetic acid for 15 min, the smears were air dried at room temperature and stained with 10% (ν/ν) of Giemsa for 10 min and examined under light microscope (400× magnification, Leica, Germany). Counting of micronuclei was done according to the method of Kimura A et al [22].
Polychromatic erythrocytes (PCEs) and normochromatic erythrocytes (NCEs) were identified according to the methods of Hayashi et al [23]. The ratio of PCE/NCE was determined by examination of 200 erythrocytes from each animal. The frequency of micronucleated cells was analyzed by examination of 1000 erythrocytes. The results were considered as positive if 1) the test substance caused a significant dose-dependent increase in the frequency of micronucleated cells compared with negative group, or 2) the frequency of micronucleated cells in any pivaloylacylation-7ADCA treated group counted three times larger than that of negative group.
Single cell gel electrophoresis assay (comet assay)
Alkaline comet assay was used to detect DNA damage including single-strand and double-strand breaks, as well as alkali-labile sites [24]. Briefly, the L5178Y mouse lymphoma cells were seeded at a density of 1×105 cells/well in 24-well plates and cultured overnight. Then cells were treated by pivaloylacylation-7ADCA treatment with a concentration of 625, 1250, 2500 and 5000 μg/ml for 24 h, along with the solvent control (DMSO) and positive control methyl methane sulphonate (MMS, 5.0 μg/ml). The comet assay was performed as described previously [25,26]. In brief, 20 μl single-cell suspensions were mixed with 80 μl low-melting point agarose (LMPA, 0.65%) and spread on the microscope slide pre-coated with 100 μl of normal-melting point agarose (NMPA, 0.8%). After solidified for 10 min at 4°C, slides were soaked in freshly prepared lysis solution at 4°C in the dark for 1 h. Slides were then immersed in electrophoresis buffer for 30 min at room temperature to allow DNA unwinding, and then subjected to electrophoresis at 25 V/cm for 30 min. Then slides were gently washed three times with distilled water, stained with 20 μg/ml ethidium bromide solution and analyzed under a fluorescence microscope (DMLB2, Leica, Wetzlar, Germany) at 200× magnification. Tail intensity (% of tail DNA) and olive tail moment (OTM) were used as the qualitative and quantitative measurement of DNA damage, and were obtained using come assay score software (CASP) [25,26]. 50 randomly selected comet cells per slide were analyzed for Tail intensity and OTMs.
Statistical methods
All the experiments were performed at least three times. Statistical analysis was performed by SPSS 13.0 software. The results of Ames assay and comet assay were expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used to evaluate significant difference among various experimental groups and least-significant difference (LSD) was employed for multi-group comparison. Poisson distribution was used to analyze the data of TK gene mutation assay, chromosomal aberration assay and mouse micronucleus assay. P<0.05 was considered as statistically significant.
Results
Pivaloylacylation-7ADCA had no mutagenic effects in Salmonella typhimurium strains TA97, TA98, TA100 and TA102
The mutagenicity of pivaloylacylation-7ADCA was firstly assessed by Ames assay. The results show that all positive drugs including 2-AF, 1,8-dihydroxyanthraquinone, 2,4,7-tNFO, SA and MMC, did induce more than 5-fold increases in the number of revertant colonies in all test strains, which confirmed the sensitivity and liability of the test system. However, for all test strains, the revertant colony numbers induced by pivaloylacylation-7ADCA (ranging from 8 μg/plate-5000 μg/plate) were similar to that of the negative control (Table 1). Moreover, no dose-dependent increase in the revertant colony number was observed with the increasing dosages of pivaloylacylation-7ADCA (Table 1), suggesting that pivaloylacylation-7ADCA can no exert mutagenic effect on Salmonella typhimurium strains TA97, TA98, TA100 and TA102 either in the absence or presence of S9 mixture.
Table 1.
Effect of pivaloylacylation-7ADCA on revertant colonies in Ames assay
| Dose (μg/plate) | The revertant number/plate (X̅±S) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| TA97 | TA98 | TA100 | TA102 | |||||
|
|
|
|
|
|||||
| -S9 | +S9 | -S9 | +S9 | -S9 | +S9 | -S9 | +S9 | |
| 0 | 120.7±15.1 | 118.9±13.9 | 36.7±5.8 | 36.8±6.0 | 142.6±18.1 | 142.1±15.4 | 254.2±13.8 | 255.2±14.1 |
| 8 | 112.5±13.9 | 113.4±11.8 | 33.1±5.9 | 34.2±5.2 | 136.8±16.4 | 140.6±14.1 | 252.5±18.6 | 248.4±14.9 |
| 40 | 120.0±18.4 | 115.5±15.4 | 30.9±6.3 | 35.1±7.0 | 136.7±10.5 | 137.4±11.0 | 244.2±18.1 | 250.0±14.3 |
| 200 | 114.7±16.2 | 107.0±11.4 | 30.8±10.8 | 31.4±10.3 | 134.6±18.7 | 133.4±13.4 | 263.6±13.4 | 250.2±12.3 |
| 1000 | 115.9±17.9 | 115.6±15.8 | 35.5±9.0 | 33.1±11.6 | 133.5±12.8 | 140.4±11.6 | 247.5±17.6 | 253.4±12.7 |
| 5000 | 118.2±15.1 | 114.3±14.8 | 35.0±10.0 | 36.2±10.2 | 136.0±14.9 | 128.8±13.9 | 256.4±17.0 | 251.7±12.1 |
| Solvent control | 115.3±14.3 | 114.6±12.2 | 34.1±9.0 | 34.1±10.3 | 144.2±15.1 | 144.3±12.9 | 251.2±11.2 | 252.6±11.5 |
| Positive control | 1122.8±148.1a | 1090.5±121.7a | 875.9±109.8a | 852.8±112.3a | 1135.6±116.5a | 1085.1±107.2a | 1260.6±97.4a | 1105.6±98.9a |
The mutagenicity of pivaloylacylation-7ADCA was firstly assessed by Ames assay. Four test strains (TA97, TA98, TA100 and TA102) were exposed to pivaloylacylation-7ADCA, along with solvent control 1‰ (ν/ν) DMSO and positive controls and incubated for 48 h. The positive controls contained 2-aminofluorene (2-AF, 10 μg/plate vs. TA97, TA98, TA100 with S9), 1,8-dihydroxyanthraquinone (50 μg/plate vs. TA102 with S9), 2, 4, 7-trinitrofluoren-9-one (2,4,7-tNFO, 0.2 μg/plate vs. TA97, TA98 without S9), sodium azide (SA, 1.5 μg/plate vs. TA100 without S9), mitomycin C (MMC, 0.5 μg/plate vs.TA102 without S9). Data were obtained from three independent experiments and were expressed as mean ± standard deviation (S.D.).
denotes more than 2-fold increase in the number of revertant colonies of the negative control.
Statistical significance was set as P<0.05
Pivaloylacylation-7ADCA would not induce gene mutation in L5178Y mouse lymphoma cells
TK gene mutation test were performed to further detect whether or not pivaloylacylation-7ADCA would cause gene mutation. The results were shown in Table 2. The mutation frequency (MF) of the positive control group was 271.90×106, over 3-fold higher than that of the DMSO control group (P<0.05), which confirmed the sensitivity and liability of the test system. With the increasing dosages of pivaloylacylation-7ADCA, the values of RS, RV, RSG and RTG all decreased in a dose-dependent manner, suggesting that within the experimental dosage range, pivaloylacylation-7ADCA can induce cytotoxicity in L5178Y mouse lymphoma cells. However, no significant differences were observed in the MF value among each pivaloylacylation-7ADCA groups [(69.7~133.33) ×10-6] and the DMSO control group (77.90×10-6) (P>0.05). On the contrary, the MF value in pivaloylacylation-7ADCA group with the highest dose (133.33×10-6) was remarkably lower than that of the positive control group (271.90×10-6) (P<0.05), suggesting that even at the dosages with cytotoxicity, pivaloylacylation-7ADCA did not induce gene mutation in L5178Y cells.
Table 2.
The effects of pivaloylacylation-7ADCA on TK gene mutation
| Dose (μg/ml) | RV (%) | RSG (%) | RTG (%) | MF (×10-6) |
|---|---|---|---|---|
| 625 | 92.48 | 94.50 | 87.40 | 69.70 |
| 1250 | 82.95 | 89.13 | 73.93 | 80.37 |
| 2500 | 64.14 | 70.27 | 45.07 | 102.40 |
| 5000 | 57.57 | 56.63 | 32.60 | 133.33 |
| DMSO control | 100.00 | 100.00 | 100.00 | 77.90 |
| Positive control | 91.19 | 71.03 | 64.77 | 271.90a |
TK gene mutation test were performed to further detect whether or not pivaloylacylation-7ADCA caused gene mutation. Four dose of pivaloylacylation-7ADCA, along with positive control methyl methane sulphonate (MMS, 5 μg/ml) and solvent control 1‰ (ν/ν) DMSO were tested in this assay. The mutation frequency (MF) was expressed as the number of mutants per 106 viable cells.
indicates a significant difference in the value of MF compared to the solvent control group.
Statistical significance was set as P<0.05.
Pivaloylacylation-7ADCA failed to induce chromosome aberration in CHL cells
In vitro mammalian chromosome aberration assay was used to evaluate the genotoxicity of Pivaloylacylation-7ADCA on chromosome level. The result was presented in Table 3. The positive control drugs, MMS and CP, both did induce marked chromosomal aberration. The frequency of aberrant chromosomes of the positive control groups ranged from 28.5%-32.5%, both in short-term exposure (24 h) and continuous exposure (48 h), confirming the sensitivity and stability of the test system. Either with or without S9 mixture, the frequency of aberrant chromosomes of all pivaloylacylation-7ADCA groups remained at low level, ranging from 1.5% to 3.5%, significantly lower than those of the positive groups. The result indicated that pivaloylacylation-7ADCA fail to induce chromosome aberration in CHL cells.
Table 3.
The effects of pivaloylacylation-7ADCA on CHL cell chromosome aberration
| Dose (μg/ml) | -S9 | +S9 | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| 24 h | 48 h | 24 h | 48 h | |||||
|
|
|
|
|
|||||
| Number of distortion | Frequency of aberrant chromosomes % | Number of distortion | Frequency of aberrant chromosomes % | Number of distortion | Frequency of aberrant chromosomes % | Number of distortion | Frequency of aberrant chromosomes % | |
| 1250 | 4 | 2 | 4 | 3 | 4 | 2 | 5 | 2.5 |
| 2500 | 3 | 1.5 | 3 | 1.5 | 6 | 3 | 4 | 2 |
| 5000 | 7 | 3.5 | 6 | 2.0 | 3 | 1.5 | 5 | 2.5 |
| DMSO | 2 | 1b | 4 | 2b | 3 | 1.5b | 3 | 1.5b |
| Positive control | 61 | 30.5a | 59 | 29.5a | 65 | 32.5a | 57 | 28.5a |
In vitro mammalian chromosome aberration assay was further used to evaluate the genotoxicity of Pivaloylacylation-7ADCA on chromosome level. Cells were exposed to a range of dosages of pivaloylacylation-7ADCA (1250, 2500 and 5000 μg/ml). Cells treated with DMSO alone were employed as the solvent control. In the absence of S9 mixture, cells treated by 0.1 μg/ml of mitomycin C (MMC) were used as positive control; while in the presence of S9, cells treated by 20 μg/ml of cyclophosphamide (CP) was employed as the positive control.
denotes a significant difference in the frequency of aberrant chromosomes (%) compared to the DMSO control group.
Statistical significance was set as P<0.05.
Pivaloylacylation-7ADCA did not induce chromosome aberration in in mice bone marrow cells
The genotoxicity of Pivaloylacylation-7ADCA on chromosome level was further assessed using the in vivo mouse micronucleus test. No abnormal signs in general appearance were observed in mice between the first and final administrations in all pivaloylacylation-7ADCA groups, along with the positive control group and solvent control group. The result of the in vivo mouse micronucleus test was shown in Figure 1. No significant differences were observed in the PCE/NCE ratio among all pivaloylacylation-7ADCA groups and the CP group (positive group) (P>0.05), indicating that the cytotoxicity in each group was kept in the same level (Figure 1A). The frequency of micronucleated cells of the positive control group was nearly 7-fold higher than that of the solvent control group (P<0.05) (Figure 1B), suggesting this test system is sensitive and stable. However, pivaloylacylation-7ADCA treatments did not induce remarkable increases in the frequency of micronucleated cells at all test dosages, compared with solvent control group (Figure 1B) (P>0.05), further suggesting that pivaloylacylation-7ADCA may not cause chromosomal aberration.
Figure 1.
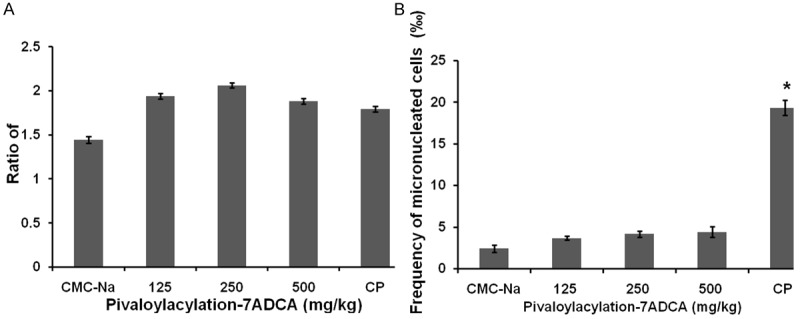
Pivaloylacylation-7ADCA did not induce chromosome aberration in in mice bone marrow cells. The genotoxicity of Pivaloylacylation-7ADCA on chromosome level was assessed using the in vivo mouse micronucleus test. After treatment with different concentrations of pivaloylacylation-7ADCA, along with positive control cyclophosphamide (CP) and solvent control sodium carboxyl methyl cellulose (CMC-Na), mice were sacrificed. Then mice bone marrow smears were prepared, fixed, air dried and examined as described in the “Materials and Method” section. A. Illustrates the ratio of PCE/NCE. B. Illustrates the frequency of micronucleated cells. Data were obtained from three independent experiments and Poisson distribution was used to analyze the data. “a” denotes a significant difference in the frequency of micronucleated cells compared to the DMSO control group. Statistical significance was set as P<0.05.
Pivaloylacylation-7ADCA failed to induce DNA damage in L5178Y mouse lymphoma cells
The effect of pivaloylacylation-7ADCA on DNA damage was assessed using the single cell gel electrophoresis assay. As shown in Figure 2A-E. There was a statistically significant difference (P<0.05) in the tail intensity and OTM between the positive control group (MMS) and the solvent control group (DMSO), which confirmed the sensitivity and stability of the test system (Figure 2A-E). However, pivaloylacylation-7ADCA did not increase the tail intensity and the OTM value even at the highest concentration (5000 μg/plate) (P>0.05) (Figure 2A-E), suggesting that pivaloylacylation-7ADCA can not induce DNA strand breaks in L5178Y mice lymphoma cells.
Figure 2.
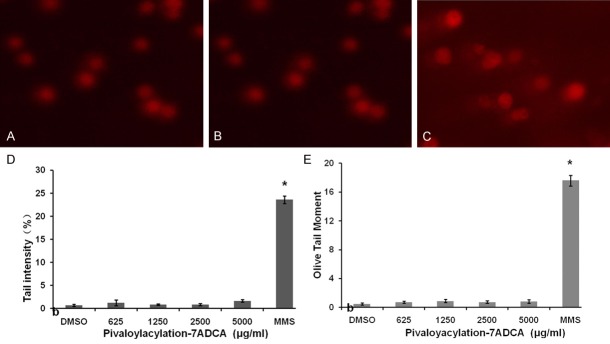
Pivaloylacylation-7ADCA failed to induce DNA damage in L5178Y mouse lymphoma cells. L5178Y mouse lymphoma cells were treated with pivaloylacylation-7ADCA, along with positive control MMS and solvent control DMSO. The effect of pivaloylacylation-7ADCA on DNA damage was assessed using the single cell gel electrophoresis assay. Tail intensity (% of tail DNA) and olive tail moment (OTM) were used as the qualitative and quantitative measurement of DNA damage. A. Represents the DMSO control. B. Represents the cells treated by 5000 μg/ml pivaloylacylation-7ADCA. C. Illustrates the cells treated by positive control methyl methane sulphonate (MMS, 5.0 μg/ml). D. Illustrates the tail intensity (% of tail DNA). E. Illustrates the OTM value. Data were obtained from three independent experiments and and were expressed as mean ± standard deviation (S.D.) Statistical significance was set as P<0.05.
Discussion
The β-lactam antibiotics, one of the most consumed antibiotics in clinical application in China, are also among the most easily contaminated by impurities due to the instability of their molecular structures as well as the complex fermentation and chemosynthesis processes. Impurities in β-lactam antibiotics can be grouped into two categories [27]. One category includes the extrinsic impurities synthesized in fermentation process, i.e. protein, polypeptide and polysaccharide, and the conjugations formed by antibiotics and the protein, polypeptide or polysaccharide, e.g. penicilloyl-protein, penicilloyl-polypeptide, etc. The other category involves endogenous impurities formed during production, transportation and storage, i.e. antibiotic self-polymerizations such as dimers, trimers and so on. Mixed in antibiotics products, the impurities not only decrease the antimicrobial activity, but also cause varieties of adverse reactions, e.g. anaphylactic reactions, immune hemolysis, etc., which severely affect drug efficiency and safety [28]. Therefore, impurities from preclinical antibiotics are required to be subjected to safety evaluation on acute/chronic toxicity and genotoxicity. Unfortunately, due to the restriction on drug production technique, previous drug safety evaluation can only test the antibiotics combined with impurities as a whole sample [29]. This can only assess the safety of the mixture while the detailed safety information of specific impurities cannot be acquired, which largely limit the preciseness and reliability of the results. A new lead could be the inquest for detailed safety information of specific impurities. Thus, specific assessment on the safety of purified impurity, as we did in this study, is of considerable significance for the overall drug safety evaluation of β-lactam antibiotics.
The Guiding Principles of Genetic Toxicity of drugs, published by China Food and Drug Administration (CFDA), points out that, besides holding a close connection with other toxicological tests, genotoxicity assays themselves are an important part of non-clinical drug safety evaluation, along with subsequent pre-clinical tests and post-market surveillance. Though genotoxicity assays have been developing rapidly in recent years, with over 200 kinds of short-term test methods being created to detect different genetic endpoints including gene mutation, DNA damage and repair, chromosomal breakage, etc., none of the current genotoxic assays can afford to test all these genetic endpoints. The combination of a group of assays covering three genetic endpoints, namely gene mutation, chromosome aberration or breakage, and DNA damage, is usually utilized in genotoxicity evaluation [30].
As is recommended in 1997 by the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), bacterial gene mutation test (i.e. Ames test), in vitro mammalian cells assays (in vitro chromosome aberration assay or the mouse lymphoma cell TK gene mutation test), and in vivo rodent hematopoietic cells chromosome damage test (chromosome aberration or micronucleus test) should be taken as the standard composed test in genotoxicity assessment. In China, the combination of a group of genotoxic assays, namely microbial reverse mutation test, mammalian cell chromosome aberration assay and rodent micronucleus test is conducted as present convention [20]. In this study, we designed a combination of Ames assay, TK gene mutation test, in vitro chromosome aberration assay, micronucleus test of mouse bone marrow cell and comet assay so as to achieve a more comprehensive evaluation on genotoxicity of the test impurity, for this group of assays was combined with different species, three major genetic endpoints and in vivo/vitro tests.
Ames assay is a classical method of testing gene mutation. In this study, the most commonly used bacteria Salmonella typhimurium strains, TA97, TA98, TA100 and TA102 containing defined mutations in the histidine operon, were employed as test strains. When the dosage of pivaloylacylation-7ADCA ranged from 8 μg/plate to 5000 μg/plate, the bacterial reverse mutation colonies remained similar to that of the 0 μg/plate group and the DMSO control group, indicating that pivaloylacylation-7ADCA has no obvious effect on gene mutation induction. Mouse lymphoma TK gene mutation test is another traditional approach to detect gene mutation. Using eukaryotic L5178 cells as test model, TK gene mutation assay presents more advantages than Ames assay that employs the prokaryotic organism. Tennant RW and his group reported that, when Ames assay and TK gene mutation test being applied contrastively to evaluate 73 kinds of compounds, the sensitivity of TK gene mutation test (70%) can be evidently higher than Ames assay (45%), whereas the specificity of Ames assay (86%) is higher than TK gene mutation test (45%) [31]. Therefore, combining Ames assay with TK gene mutation test to screen genotoxicity at the same time could reduce the probability of false-negative results. Both Ames assay and Mouse lymphoma TK gene mutation test in this study demonstrated that pivaloylacylation-7ADCA shows no potential in gene mutation induction. Both chromosome aberration and micronucleus assay are traditional methods to determine chromosome damages by representing the changes of chromosomal structure and number, and the abnormities in chromosome segregation [32]. We chose to combine chromosome aberration assay with micronucleus test to assess the genotoxicity of pivaloylacylation-7ADCA on chromosome level, for this combination can eliminate the differences in absorption, metabolism and excretion of the same chemical between cultured cells and animal bodies, which indicates an ideal strategy in jointing in vivo and in vitro studies. In chromosome aberration assay, the frequency of aberrant chromosomes in the positive control group was over 10-fold higher than the negative control group, while the frequency of aberrant chromosomes among the test groups and negative control group showed no significant differences. Similarly, in micronucleus test, the frequencies of micronuclear cells in test group and negative control group did not show significant difference: both were evidently lower than that of positive control group (19.70%). The results of these two experiments are consistent, indicating that pivaloylacylation-7ADCA may not induce chromosome aberration.
Comet assay is so far one of the most sensitive methods to detect DNA damage, i.e. DNA single/double strand breaks and alkaline labile sites, on the single cell level [24]. A number of studies indicated that, in terms of sensitivity, comet assay is much better than chromosome aberration test and micronucleus test, especially in heavy metal detection [33]. Nowadays, comet assay has been highly recommended in genotoxicity assessment to improve the overall detection sensitivity of the test chemical, especially at low dose. In the present study, alkaline comet assay was used to evaluate the genotoxicity of pivaloylacylation-7ADCA, and tail intensity and OTM were utilized as parameters in the assessment of DNA damage. The positive control group showed positive response with significant tail intensity enhancement and OTM elevation, whereas no notable differences were observed among the pivaloylacylation-7ADCA groups and the solvent control group, indicating pivaloylacylation-7ADCA may not induce DNA damage in mouse lymphoma cells.
Conclusion
In the present study, the genotoxicity of newly synthesized pivaloylacylation-7ADCA was firstly systematically assessed, which should be an essential part of the drug safety assessment of β-lactam antibiotics. The results from multiple genetic endpoints consistently showed that no mutagenicity of pivaloylacylation-7ADCA was found in the whole set of assays, suggesting that pivaloylacylation-7ADCA fail to induce genotoxicity. Importantly, in the present study, the genotoxicity assessment was conducted by using purified pivaloylacylation-7ADCA in specific, which could be original tests of the field. Moreover, a series of well-designed in vitro and in vivo assays were combined with different species and different genetic endpoints, which may largely improve the reliability as well as preciseness of the results. Our study is expected to serve as a reference for the genotoxicity assessment of other antibiotic impurities in the future.
Acknowledgements
This work was supported by the Project No. 81172632 from the National Natural Science Foundation of China to Prof. Zunzhen Zhang. We thank Sichuan Industrial Institute of Antibiotics, National Pharmaceutical Group, for providing us with the tested substance (purified pivaloylacylation-7ADCA material). We also thank Ms. Xue Zhao at Foreign Language Institute, Sichuan University, for kindly helping us in reviewing the manuscript critically.
Disclosure of conflict of interest
None.
Abbreviations
- 7-ADCA
7-aminodesacetoxycephalosporanic acid
- DMSO
dimethyl sulfoxide
- CHL cell
Chinese hamster lung cell
- SPF
specific pathogen free
- ABSL-3
animal biosafety level 3
- 2-AF
2-aminofluorene
- 2
4,7-tNFO, 2, 4, 7-trinitrofluoren-9-one
- SA
sodium azide
- MMC
mitomycin C
- MMS
methyl methane sulphonate
- RSG
relative suspension growth
- PE
plating efficiency
- RV
relative viability
- RTG
relative total growth
- MF
mutant frequency
- CP
cyclophosphamide
- CMC-Na
sodium carboxyl methyl cellulose
- PCEs
polychromatic erythrocytes
- NCEs
normochromatic erythrocytes
References
- 1.Elbur AI, M AY, El-Sayed AS, Abdel-Rahman ME. Prophylactic antibiotics and wound infection. J Clin Diagn Res. 2013;7:2747–2751. doi: 10.7860/JCDR/2013/6409.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dumarey M, Sneyers R, Janssens W, Somers I, Vander HY. Drug impurity profiling: Method optimization on dissimilar chromatographic systems: Part I: pH optimization of the aqueous phase. Anal Chim Acta. 2009;656:85–92. doi: 10.1016/j.aca.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Guideline on setting specifications for related impurities in antibiotics. EMA. 2009 [Google Scholar]
- 4.Giordani A, Kobel W, Gally HU. Overall impact of the regulatory requirements for genotoxic impurities on the drug development process. Eur J Pharm Sci. 2011;43:1–15. doi: 10.1016/j.ejps.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Onoue S, Yamada S, Chan HK. Nanodrugs: Pharmacokinetics and safety. Int J Nanomedicine. 2014;9:1025–1037. doi: 10.2147/IJN.S38378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srirangan K, Orr V, Akawi L, Westbrook A, Moo-Young M, Chou CP. Biotechnological advances on penicillin G acylase: pharmaceutical implications, unique expression mechanism and production straegies. Biotechnol Adv. 2013;8:319–322. doi: 10.1016/j.biotechadv.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Paim CS, Nogueira DR, Mitjans M, Ramos Lopez D, de Lapuente Pere J, Steppe M, Schapoval EE, Vinardell MP. Biological safety studies of gemifloxacin mesylate and related substances. Photochem Photobiol Sci. 2013;12:805–812. doi: 10.1039/c3pp25369d. [DOI] [PubMed] [Google Scholar]
- 8.Heo HS, Choi JH, Oh JJ, Lee WJ, Kim SS, Lee DH, Lee HK, Song SW, Kim KH, Choi YK, Ryu KS, Kang BH. Evaluation of general toxicity and genotoxicity of the silkworm extract powder. Toxicol Res. 2013;29:263–278. doi: 10.5487/TR.2013.29.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z, Che W, Liang Y, Wu M, Li N, Shu Y, Liu F, Wu D. Comparison of cytotoxicity and genotoxicity induced by the extracts of methanol and gasoline engine exhausts. Toxicology in vitro. 2007;21:1058–1065. doi: 10.1016/j.tiv.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Bacterial reverse mutation test (test no.471, adopted on July 21, 1997). OECD guidelines for testing of chemicals. OECD. 1997:1997a. [Google Scholar]
- 11.Gatehouse D. Bacterial mutagenicity assays: test methods. Methods Mol Biol. 2012;817:21–34. doi: 10.1007/978-1-61779-421-6_2. [DOI] [PubMed] [Google Scholar]
- 12.Kim BS, Margolin BH. Statistical methods for the Ames Salmonella assay: A review. Mutat Res. 1999;436:113–122. doi: 10.1016/s1383-5742(98)00025-8. [DOI] [PubMed] [Google Scholar]
- 13.Food and Drug Administration, HHS. International Conference on Harmonisation; guidance on S2(R1) Genotoxicity Testing and Date Interpretation for Pharmaceuticals intended for Human Use; availability. Notice. Fed Reqist. 2012;77:33748–33749. [PubMed] [Google Scholar]
- 14.Dobrovolsky VN, Shaddock JG, Heflich RH. Analysis of in vivo mutation in the Hprt and Tk genes of mouse lymphocytes. Methods Mol Biol. 2014;1105:255–270. doi: 10.1007/978-1-62703-739-6_20. [DOI] [PubMed] [Google Scholar]
- 15.Hakulinen P, Yamamoto A, Koyama N, Kumita W, Yasui M, Honma M. Induction of TK mutations in human lymphoblastoid TK6 cells by the rat carcinogen 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone (MX) Mutation Res. 2011;725:43–49. doi: 10.1016/j.mrgentox.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd M, Kidd D. The mouse lymphoma assay. Methods Mol Biol. 2012;817:35–54. doi: 10.1007/978-1-61779-421-6_3. [DOI] [PubMed] [Google Scholar]
- 17.Moore MM, Honma M, Clements J, Bolcsfoldi G, Burlinson B, Cifone M, Clarke J, Delongchamp R, Durward R, Fellows M, Gollapudi B, Hou S, Jenkinson P, Lloyd M, Majeska J, Myhr B, O’Donovan M, Omori T, Riach C, San R, Stankowski LF Jr, Thakur AK, Van Goethem F, Wakuri S, Yoshimura I. Mouse lymphoma thymidine kinase gene mutation assay: follow-up meeting of the International Workshop on Genotoxicity Testing--Aberdeen, Scotland, 2003--Assay acceptance criteria, positive controls, and data evaluation. Environ Mol Mutagen. 2006;47:1–5. doi: 10.1002/em.20159. [DOI] [PubMed] [Google Scholar]
- 18.In vitro mammalian chromosomal aberration test (test no.473, adopted on July 21, 1997). OECD guidelines for testing of chemicals. OECD. 1997:1997b. [Google Scholar]
- 19.Huang YN, Zhao YL, Gao XL, Zhao ZF, Jing Z, Zeng WC, Yang R, Peng R, Tong T, Wang LF, Cen JQ, Gao H. Intestinal alpha-glucosidase inhibitory activity and toxicological evaluation of Nymphaea stellata flowers extract. J Ethnopharmacol. 2010;131:306–312. doi: 10.1016/j.jep.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 20.Doherty AT. The in vitro micronucleus assay. Methods Mol Biol. 2012;817:121–141. doi: 10.1007/978-1-61779-421-6_7. [DOI] [PubMed] [Google Scholar]
- 21.Mammalian erythrocyte micronucleus test (test no.474, adopted on July 21, 1997). OECD guidelines for testing of chemicals. OECD. 1997 [Google Scholar]
- 22.Kimura A, Miyata A, Honma M. A combination of in vitro comet assay and micronucleus test using human lymphoblastoid TK6 cells. Mutagenesis. 2013;28:583–590. doi: 10.1093/mutage/get036. [DOI] [PubMed] [Google Scholar]
- 23.Hayashi M, Sofuni T, Ishidate MJ. An application of Acridine Orange fluorescent staining to the micronucleus test. Mutat Res. 1983;120:241–247. doi: 10.1016/0165-7992(83)90096-9. [DOI] [PubMed] [Google Scholar]
- 24.Luo Q, Lai Y, Liu S, Wu M, Liu Y, Zhang Z. Deregulated expression of DNA polymerase beta is involved in the progression of genomic instability. Environ Mol Mutagen. 2012;53:325–333. doi: 10.1002/em.21697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burlinson B. The in vitro and in vivo comet assays. Methods Mol Biol. 2012;817:143–163. doi: 10.1007/978-1-61779-421-6_8. [DOI] [PubMed] [Google Scholar]
- 26.Lepailleur A, Bureau R, Halm-Lemeille MP, Bouquet M, Pecquet R, Paris-Soubayrol C, Goff JL, Andre V, Lecluse Y, Lebailly P, Maire MA, Vasseur P. Assessment of the genotoxic and carcinogenic potentials of 3-aminothiophene derivatives using in vitro and in silico methodologies. J Appl Toxicol. 2014;34:775–786. doi: 10.1002/jat.2938. [DOI] [PubMed] [Google Scholar]
- 27.Liu SY, Li YP, Hu CQ. Influence of impurities on the specific optical rotation of cefozopran. Die Pharmazie. 2012;67:590–594. [PubMed] [Google Scholar]
- 28.Kahsay G, Shraim F, Villatte P, Rotger J, Cassus-Coussere C, Van Schepdael A, Hoogmartens J, Adams E. Development and validation of a reversed phase liquid chromatographic method for analysis of oxytetracycline and related impurities. J Pharm Biomed Anal. 2013;75:199–206. doi: 10.1016/j.jpba.2012.11.042. [DOI] [PubMed] [Google Scholar]
- 29.Heo HS, Choi JH, Oh JJ, Lee WJ, Kim SS, Lee DH, Lee HK, Song SW, Kim KH, Choi YK, Ryu KS, Kang BH. Evaluation of general toxicity and genotoxicity of the silkworm extract powder. Toxicol Res. 2013;29:263–278. doi: 10.5487/TR.2013.29.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Judson P. The application of structure-activity relationships to the prediction of the mutagenic activity of chemicals. Methods Mol Biol. 2012;817:1–19. doi: 10.1007/978-1-61779-421-6_1. [DOI] [PubMed] [Google Scholar]
- 31.Tennant RW, Spalding JW, Stasiewicz S, Caspary WD, Mason JM, Resnick MA. Comparative evaluation of genetic toxicity patterns of carcinogens and noncarcinogens: strategies for predictive use of short-term assays. Environ Health Perspect. 1987;75:87–95. doi: 10.1289/ehp.877587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clare G. The in vitro mammalian chromosome aberration test. Methods Mol Biol. 2012;817:69–91. doi: 10.1007/978-1-61779-421-6_5. [DOI] [PubMed] [Google Scholar]
- 33.Dobrovolsky VN, Shaddock JG, Heflich RH. Analysis of in vivo mutation in the Hprt and Tk genes of mouse lymphocytes. Methods Mol Biol. 2014;1105:255–270. doi: 10.1007/978-1-62703-739-6_20. [DOI] [PubMed] [Google Scholar]


