Abstract
Malignant ascites (MA) is a pathological condition due to a variety of primary abdominal and extra-abdominal neoplasms. It is a primary cause of morbidity and presents many difficulties in evaluation and treatment. In this study we used dendritic cell vaccines combined with cytokine-induced killer (CIK) cells intraperitoneal injected in patients with MA, and evaluated the safety and efficacy of this treatment. The results showed that the percentage of CD3+ CD56+ CIK cells after treatment increased significantly while the percentage of CD4+ CD25+ Treg cells decreased (P < 0.05). The clinical response rate (RR) was 40.9% and disease control rate (DCR) was 77.3%. We then studied and identified the mechanisms of the anti-tumor effects of the vaccines by analyzing a series of cytokines that are commonly involved in tumor progression and ascitic development including granulocyte macrophage colony stimulating factor (GM-CSF), interleukin-10 (IL-10), interferon-γ (IFN-γ), tumor necrosis factor-α (TGF-α), tumor necrosis factor-β (TGF-β), Vascular endothelial growth factor (VEGF) and monocyte chemotactic protein-1 (MCP-1). These data demonstrated that intraperitoneal injection with DC vaccines combined with CIK cells in patients with malignant peritoneal effusion is safe and feasible. This therapy modality can achieve a certain clinical benefit even in patients resistant to conventional treatments.
Keywords: Dendritic cell, cytokine-induced killer cell, immunotherapy, cytokines, malignant ascites
Introduction
Malignant ascites (MA) is a manifestation of end stage events in a variety of primary abdominal and extra-abdominal neoplasms. It accounts for about 10% of all cases of ascites usually caused by ovarian, endometrial, breast, oesophageal, gastric, colorectal, lung, pancreatic, hepatobiliary and primary peritoneal carcinomas [1-3]. However, in up to 20% of all patients with MA, the primary tumor site remains undiagnosed [4].
The progression of MA is associated with deterioration in quality of life (QoL) and a poor prognosis. It accounts for a variety of symptoms, leading to significant reduction in patients’ QoL: respiratory distress and dyspnea, abdominal tenderness and pain, nausea, anorexia, fatigue and impaired movement. MA is associated with significant morbidity and presents many difficulties or treatment challenges. MA treatment includes a variety of different palliative options with limited efficacy and certain degree of risk, which is from symptomatic relief with simple drainage procedures to chemotherapy and surgery aimed at treating the underlying cancer [5]. Medical options represented by paracentesis and diuretics are first-line treatments, but their efficacy is often partial and time limited. Intraperitoneal chemotherapy (IC), Laparoscopic HIPEC, targeted therapy, immunotherapy and radioisotopes are promising medical options but their clinical application is not yet completely elucidated and further investigations are necessary. There are, however, no generally accepted evidence-based guidelines for evaluation and treatment of this condition.
Immunotherapy has recently become the fourth important treatment modality for malignant tumors, ranked after surgery, radiotherapy, and chemotherapy [6-8]. The peritoneal membrane lines the largest cavity of the human body. Anatomic structures of the peritoneal cavity and the peritoneal immune system which may be stimulated by intraperitoneal administration of autoimmune cells play an important role in the control of tumor progression [9]. It is a great possibility that the intraperitoneal immunotherapy may be used for palliation of MA. Over the past decade, the immune function of cytokine-induced killer (CIK) cells has been studied extensively [10-15]. CIK cells are a unique population of cytotoxic T lymphocytes (CTL) with the characteristic CD3+ CD56+. At present, CIK cells have been recognized as a new type of antitumor effector cells, which can proliferate rapidly in vitro, with stronger antitumor activity and broader spectrum of targeted tumor than other reported antitumor effector cells [7]. Moreover, CIK cells can regulate and generally enhance the immune functions in patients with cancer [11]. Dendritic cells (DCs) are considered to be superior in their antigen-presenting ability, compared with both macrophages and B lymphocytes. They can efficiently prime T cells during development of T cell-mediated immunity and stimulate adaptive immune responses [9]. And the possibility to enhance the cytotoxic activities of CIK cells by DC was studied as reported [16,17]. Thus, in this study we used dendritic cell vaccines combined with CIK cells as a treatment for MA.
The purpose of this study was to evaluate the safety and efficacy of the vaccines of mixed cells intraperitoneal injected in patients with malignant peritoneal effusion who had failed to improve with other conventional treatments.
Patients and methods
Patients’ selection
Criteria for entry into this study included: (1) pathologically confirmed cancers, (2) ascites confirmed by cytology and imaging, (3) Karnofsky performance status (KPS) more than 50%, (4) age between 18 and 75 years, (5) expected survival duratison of more than 3 months and (6) tolerance of hemapheresis. In all patients treated, the treatments before had been failed for at least 2 weeks and no chemotherapy or immunomodulatory treatment had been conducted during the previous 4 weeks. Pregnant and lactating women, autoimmune disease patients, infection patients and severe disorders of hemostasis patients were excluded. The present study was approved by the ethical committee of 81 Hospital of PLA, Nanjing, China, according to the guidelines of the Declaration of Helsinki and informed consent was obtained from all patients before their entry into the study.
Between July 2011 and January 2013, 22 patients with MA were intraperitoneal injected with dendritic cell vaccines combined with CIK cells in this clinical trial (Table 1). There were 10 male patients and 12 female patients. The mean age of the patients was 55 years (range, 27 to 69 years). Karnofsky performance status (KPS) was 50%-80% before treatment. The patients were treated with paracentesis, diuretics and intraperitoneal chemotherapy (IC) before intraperitoneal administration of autoimmune cells.
Table 1.
Clinical characteristics of 22 patients with malignant ascites
| N | Gender | Age | Diagnose | Previous treatment of MA | Number of cells for injection | KPS | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| DC (×107) | CIK (×109) | ||||||
| 1 | Female | 44 | Colon cancer | P+D+IIE+IIEE | 2.72 | 3.49 | 70 |
| 2 | Female | 56 | Ovarian cancer | P+D+IIE+IIEE | 2.05 | 1.81 | 50 |
| 3 | Male | 58 | Colon cancer | P+D+IIE+IIEE | 0.98 | 1.54 | 60 |
| 4 | Female | 47 | Clear cell cancer | P+D+IIE | 1.81 | 1.77 | 70 |
| 5 | Female | 56 | Colon cancer | P+D+IIE+IIEE | 1.70 | 2.82 | 60 |
| 6 | Female | 63 | Gastric cancer | P+D+IIE+IIEE | 2.32 | 2.41 | 50 |
| 7 | Female | 57 | Ovarian cancer | P+D+IC | 2.02 | 2.55 | 70 |
| 8 | Male | 54 | Rectal cancer | P+D+IIEE | 1.87 | 2.03 | 70 |
| 9 | Male | 47 | Colon cancer | P+D+IIE+IIEE | 1.79 | 1.38 | 70 |
| 10 | Female | 66 | Ovarian cancer | P+D+IIE+IIEE | 0.78 | 3.08 | 60 |
| 11 | Female | 47 | Hepatocellular carcinoma | P+D+IIE+IIEE | 1.13 | 2.08 | 70 |
| 12 | Female | 27 | Rectal cancer | P+D+IIE+IIEE | 1.26 | 3.97 | 60 |
| 13 | Male | 59 | Intrahepatic cholangiocarcinoma | P+D+IIE+IIEE | 2.39 | 2.29 | 70 |
| 14 | Female | 52 | Endometrial cancer | P+D+IIEE | 1.98 | 3.65 | 70 |
| 15 | Male | 46 | Pancreatic adenocarcinoma | P+D+IIE+IIEE | 2.56 | 0.96 | 70 |
| 16 | Male | 69 | Rectal cancer | P+D+IIE+IIEE | 1.88 | 1.86 | 60 |
| 17 | Female | 57 | Cervical cancer | P+D+IIE+IIEE | 3.05 | 2.44 | 70 |
| 18 | Male | 68 | Colon cancer | P+D+IIEE | 3.11 | 2.06 | 80 |
| 19 | Female | 65 | Uterine sarcoma | P+D+IIEE | 2.45 | 1.79 | 70 |
| 20 | Male | 43 | Pancreatic adenocarcinoma | P+D+IIE+IIEE | 1.65 | 2.35 | 70 |
| 21 | Male | 39 | Hepatocellular carcinoma | P+D+IIEE | 1.89 | 2.02 | 50 |
| 22 | Male | 45 | Colon cancer | P+D+IIEE | 1.94 | 3.01 | 70 |
Note: P, Paracentesis; D, Diuretics; IC, Intraperitoneal chemotherapy; IIE, Intraperitoneal Injection of Endostar; IIEE, Intraperitoneal Injection of Elemene Emulsion.
Preparation of dendritic cells and CIK cells
Preparation of dendritic cells
peripheral blood mononuclear cells (PBMCs) of patients collected was isolated and purified by Ficoll density gradient centrifugation and plated to a 75 cm2 culture flask maintained in X-VIVO medium (LONZA, USA) to a concentration of 2×106/ml at 37°C in an atmosphere of 5% CO2 for 1-2 hours. The suspension cells were obtained for CIK cell culture. The adherent cells in the culture flask were maintained with X-VIVO medium supplemented with recombinant human granulocyte macrophage colony stimulating factor (rhGM-CSF) (Peprotech, USA) (500 U/ml) and recombinant human interleukin-4 ( rhIL-4) (Peprotech, USA) (10 ng/ml) in incubator. The culture media was refreshed at day 3 and day 5. It was added into antigen load (allogeneic tumor cell lysate histopathologically similar to patients’) at day 6, tumor necrosis factor-α (TNF-α) (Peprotech, USA) (500 u/ml) at day 7 and harvested tumor antigen pulsed DC vaccines at day 8 and then stored at -80°C.
Preparation of CIK cells
The suspension cells obtained before were plated to a culture flask suspended in GT-T551 medium (TaKaRa, Japan) supplemented with recombinant human interferon-γ (rhIFN-γ) (Peprotech, USA) of a final concentration of 1000 U/ml. After 24 hours it was added into recombinant human interleukin-2 (rhIL-2) (Peprotech, USA) (500 U/ml) and anti-CD3 mAb (DAKO, USA) (50 ng/ml). The static culture flask was fed every 2 days and the volume was increased as needed to maintain cell density at 1~2×106/ml. The cells were harvested at day 9-21 according to the amounts, status and maturity of the cells. At day 14, CIK cells were harvested and analyzed for phenotype and cytotoxicity. Safety tests were performed during the course of cell culture. All products were free of bacterial, mycoplasma, or fungal contamination. The endotoxin was less than 5 EU. The frozen DC vaccines were resuscitated and mixed with CIK cells in 50ml saline for prepare.
Treatment
The mixed cells were transferred to patients every other day for 3 times. The cell amounts of DC vaccines were 1.97×107 (range, 0.78~3.11×107) and CIK cells were 2.34×109 (range, 0.96~3.97×109). Ascites should be drained out as much as possible before intraperitoneal injection.
Detecting the phenotype of CIK cells using flow cytometry analysis
Before transferring, specimen of the cells should be phenotype identified by flow cytometry. CIK cells, (5×105) were stained with flow cytometry antibodies which were CD3-ECD and CD56-PE (Beckman Coulter, USA) or with isotype controls for 15 minutes at 4°C in the dark. Excess antibody was removed from the cells with 3 washes of phosphate-buffered saline. Then the cells were analyzed by Beckman Coulter XL flow cytometry (Beckman Coulter, USA). Bright CD45+ and low side-scattered cells were set to identify cells co-expressing CD3 and CD56. Ten thousand events were then analyzed to determine the proportion of cells co-expressing CD3 and CD56.
Detecting cytotoxicity of CIK cell using a lactate dehydrogenase-based assay
The cytotoxicity of CIK cells was analyzed using a CytoTox 96-well non-radioactive cytotoxicity assay according to the manufacturer’s instructions (Promega, USA). The target cells used for this assay included the lung cancer cell line A549, breast cancer cell line MCF-7, colon cancer cell line HCT-8 and chronic granulocytic leukemia cell line K562. These cell lines were obtained from the American Type Culture Collection. Briefly, 1×105 cells/mL target cells were incubated for 4 h in triplicate with effector cells (CIK cells and normal peripheral blood mononuclear cells) at a ratio of effector to target cells of 20:1. After incubation, 50 μL culture supernatant were transferred to a new, flat 96-well plate, and incubated with 50 μL lactate dehydrogenase (LDH) substrate mixture (for detecting LDH released upon cell lysis) at room temperature for 30 min in the dark. Then 50 μL stop solution were added to each well. Absorbance was measured at 490 nm using a 96-well plate reader. Specific cytotoxicity was calculated as: % specific cytotoxicity=[(experimental counts-effector spontaneous counts-target spontaneous counts)/(target maximal counts-target spontaneous counts)] ×100.
Analysis of lymphocyte subpopulation
Lymphocyte subpopulation cell phenotypes were assessed by flow cytometry (Beckman Coulter, USA). The peripheral blood of patients added heparin was obtained and divided into 3 groups. The 3 groups of blood were added into flow cytometry antibodies which were CD3-ECD, CD4-PC5, CD8-FITC and CD3-ECD, CD19-FITC, CD56-PE and CD4-PC5 and CD25-PE, respectively. After incubation of 20 min, the blood was added into red blood cell lysate and incubated for another 10 min. Then it was centrifuged at 3000 r/min for 5 min and washed with PBS twice to obtain single cell suspension. The singal cell suspension was tested by flow cytometry. The results were analysed by CXP (v2.1) software. Different lymphocyte subpopulation cell markers were as follows: total T cells (CD3+), helper T cells Th (CD3+ CD4+), cytotoxic T cells Tc (CD3+ CD8+), CIK cells (CD3+ CD56+), regulatory T cells Treg (CD4+ CD25+), B cells (CD19+).
Immunologic assessment
Cytokines secretion of granulocyte macrophage colony stimulating factor (GM-CSF), IL-10, IFN-γ, TGF-α, TGF-β, Vascular endothelial growth factor (VEGF) and monocyte chemotactic protein-1 (MCP-1) were analyzed by ELISA (BD Biosciences, USA).
Patients and clinical evaluation
The changes between pre- and one month post-treatment of blood routine examination, hepatorenal function, peripheral blood lymphocyte subsets and serum tumor markers were evaluated. All patients were monitored clinically using image analysis, i.e., ultrasonography. The maximum depth of ascites detected by ultrasonography was baseline prior to treatment. Short-term clinical efficacy was evaluated according to WHO cancerous effusion criteria: complete response (CR), defined as the disappearance of all measurable ascitic fluid for at least 1 month; partial response (PR), defined as a more than 50% decrease of all measurable ascitic fluid; stable disease (SD), defined as a reduction of less than 50% or an increase of less than 25%; and progressive disease (PD), defined as an increase of more than 25%. Response rate (RR) was calculated by CR plus PR and disease control rate (DCR) was calculated by CR plus PR plus SD. Clinical safety was evaluated according to NCI-CTCAE v4.0 version criteria.
The changes of patients’ quality of life (QOL) were evaluated according to karnofsky score. Improvement is defined as an increase of more than 10% of KPS. Stability is defined as a less than 10% variation of KPS. Deterioration is defined as a reduction of more than 10% of KPS.
Statistical methods
All calculations were conducted using the SPSS 19.0 Software. Categorical data was estimated by using χ2 test. X̅±s was used for quantitative data and comparison between two groups was estimated by using t test (Gaussian distribution) or Wilcoxon rank-sum test (abnormal distributions). A P value less than 0.05 using two-sided tests indicates statistical significance.
Results
Preparation and identification of dendritic cells and CIK cells
To determine the immunologic effects of CIK cells, the phenotype and cytotoxicity of CIK cells were analyzed using flowcytometry and LDH, respectively before transferring to patients. At day 14, CIK cells were harvested and analyzed for phenotype (data not shown) and cytotoxicity. The cytotoxicity of CIK cells against several cancer cell lines, including the lung cancer cell line A549, breast cancer cell line MCF-7, colon cancer cell line HCT-8 and chronic granulocytic leukemia cell line K562, was detected using the LDH method. The cytotoxic activities of the expanded CD3+ CD56+ CIK cells and normal peripheral blood mononuclear cells against the A549, MCF-7, HCT-8, and K562 cell lines are represented as increases in LDH release above baseline values and are compared in Figure 1. Median lytic activity rates of CD3+ CD56+ cells were 39.5% to 65.4%, a drastic contrast to the median lytic activity rates of normal peripheral mononuclear cells, which were only 7.5% to 9.9% (P < 0.05).
Figure 1.
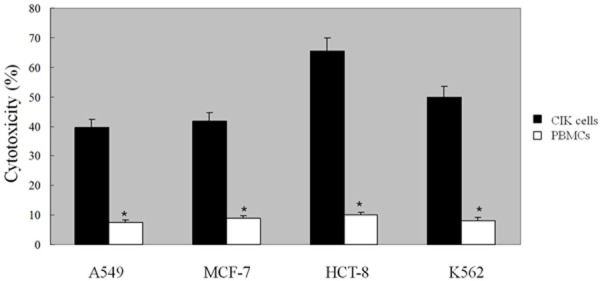
The cytotoxicity of CIK cells were analyzed by the LDH assay. PBMCs were collected from the patients and were cultured in GT-T551 medium supplemented with rhIFN-γ, rhIL-2 and anti-CD3 mAb to obtain CIK cells. After 14 days of culture, CIK cells were harvested and analyzed for the cytotoxicity by the LDH assay. The cytotoxicity of CIK cells and PBMCs against the lung cancer cell line A549, breast cancer cell line MCF-7, colon cancer cell line HCT-8 and chronic myeloid leukemia cell line K562 was detected using the LDH method. The cytotoxicity of CIK cells was significantly increased (P < 0.05) compared with the PBMCs group. Every experiment was repeated three times.
Phenotypic analysis of lymphocyte subpopulation
As reported, marked alterations are found in the immune system of cancer patients. Alterations in both the numbers and function of leukocytes are found in the peripheral blood as well as in the peritoneal cavity of cancer patients. Thus, we studied the lymphocyte subpopulation of peripheral blood of the patients pre- and post-treatment. The Phenotypic changes of pre- and post-treatment of peripheral blood lymphocyte subsets of the patients showed that there was no significant difference in the percentage of CD3+, CD4+, CD8+ T lymphocytes, CD19+ B lymphocytes, and ratio of CD4+/CD8+ between before and after treatment (Table 2). And the percentage of CD3+ CD56+ CIK cells was increased from 11.13% to 16.34% while the percentage of CD4+ CD25+ Treg cells decreased from 7.59±1.7% to 3.29±1.6% significantly after treatment (Table 2) (P < 0.05).
Table 2.
Changes of lymphocyte subsets in peripheral blood of patients pre-therapy and post-therapy (X̅±s, %)
| Group | CD3+ | CD4+ | CD8+ | CD4+/CD8+ | CD19+ | CD3+ CD56+ | CD4+ CD25+ |
|---|---|---|---|---|---|---|---|
| Pre-therapy | 57.97±11.7 | 27.18±8.3 | 23.41±6.2 | 1.27±0.6 | 6.38±4.0 | 11.13±5.9 | 7.59±1.7 |
| post-therapy | 58.80±8.5 | 27.40±7.7 | 23.34±4.5 | 1.23±0.5 | 6.83±3.4 | 16.34±6.5* | 3.29±1.6* |
Treatment response and clinical curative effects
Two patients (9%) achieved a complete res-ponse, seven patients (31.8%) had a partial response, eight patients (36.4%) had a stable disease and five patients (22.7%) had a progressive disease of all twenty-two patients. The overall objective response rate was 40.9% and the disease control rate was 77.3% (Table 3). Two PD patients were accepted other treatments 1 week after intraperitoneal injection with DC vaccines combined with cytokine-induced killer cells because of rapid progress of ascitic fluid. The serum tumor markers of 12 patients were increased (RR, 44.4%) and 10 patients were decreased (RR, 50.0%). There was no statistical significance. Our data showed that the treatments achieve a certain clinical benefits even in patients resistant to conventional treatment.
Table 3.
Treatment response and Clinical curative effects
| patients | CR | PR | SD | PD | RR (CR+PR) | DCR (CR+PR+SD) |
|---|---|---|---|---|---|---|
| 12 | 2 (9%) | 7 (31.8%) | 8 (36.4%) | 5 (22.7%) | 40.9% | 77.3% |
Note: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; RR, response rate ; DCR, disease control rate.
Immunologic responses
We then studied the mechanisms of the anti-tumor effects exerted by DC vaccines combined with CIK cells. Cytokines play a crucial role in the host’s immune response against tumor growth and the development of ascitic fluid. We analyzed a series of cytokines that are commonly involved in tumor growth and progression and the development of ascitic fluid including GM-CSF, IL-10, IFN-γ, TGF-α, TGF-β, VEGF and MCP-1, using cytokine ELISA. To identify the relationship between serum levels of cytokines and clinical outcomes, we chose 17 patients with CR, PR and SD (group 1) and 5 patients with PD (group 2), and detected serum cytokine levels before and after treatments with DC vaccines combined with CIK cells. Our results showed that the DC vaccines combined with CIK cells treatment increased IFN-γ, GM-CSF, and TNF-α levels while decreased (P < 0.05) or no change in TNF-β, IL-10, VEGF and MCP-1 levels in the 17 patients who had CR, PR and SD (Figure 2A). There were increased IL-10, VEGF and MCP-1 levels (P < 0.05) in 5 patients with PD and no significantly increased in IFN-γ, GM-CSF, and TNF-α levels (Figure 2B). As the Th1 cytokines, such as IFN-γ and TNF-α have significant anti-tumor effects, our data suggest that up-regulation of cytokine and chemokine production may serve as one mechanism by which the DC vaccines combined with CIK cells treatment exert their anti-tumor effects.
Figure 2.
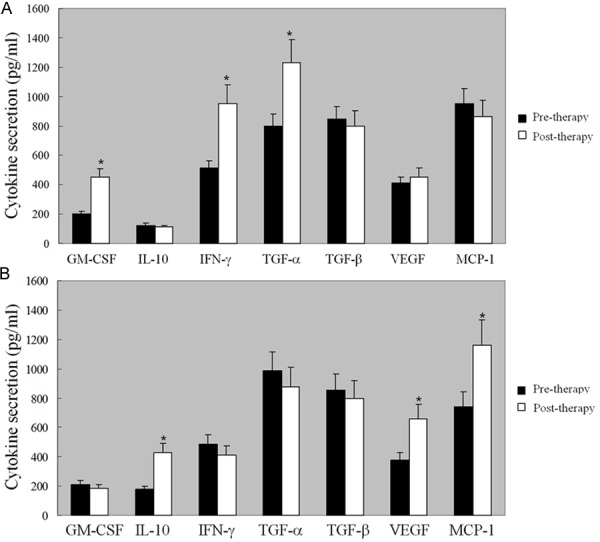
The cytokines analysis of the patients before and after the treatment with DC vaccines combined with CIK cells. The peripheral blood of 17 patients with CR, PR and SD (group 1) and 5 patients with PD (group 2) were collected. The serum cytokine secretion levels were detected by ELISA before and after treatments with DC vaccines combined with CIK cells. A. In group 1, there was significant increase in the secretion of IFN-γ, GM-CSF, and TNF-α levels while decrease or no change in TNF-β, IL-10, VEGF and MCP-1 levels in the 17 patients who had CR, PR and SD; B. In group 2, there were increased IL-10, VEGF and MCP-1 levels in 5 patients with PD and no significantly increased in IFN-γ, GM-CSF, and TNF-α levels. Every experiment was repeated three times. *P < 0.05.
Quality of life (QOL) of the patients
The KPS score of the patients pre- and post- treatment were 65.5±8.00 and 72.7±9.35, respectively (P < 0.05). There were thirteen cases with QOL improvement (59.1%), five cases with QOL stability (22.7%), four cases with QOL deterioration (18.2%) after treatment.
Safety analysis of the treatment
There was no obvious change between pre- and one month post-treatment of blood routine examination, hepatorenal function and serum electrolytes (data not shown). 3 patients had chilly and fever after intraperitoneal injection whose body temperature was not in excess of 39°C. These 3 patients were treated symptomatically. 2 patients had a mild lack of strength and were recovered without any treatments. All adverse events were under grade 3-4.
Discussion
The peritoneal cavity is a unique immunological microenvironment. There is now extensive evidence demonstrating the significance of peritoneal immune mechanisms in the control of metastatic spread. For instance, the omentum studded with a high number of immune aggregates can respond strongly to antigen, suggesting an important role in generating potent immune responses and metastatic spread [18,19]. However, high proportions of T-cells with markers of activation have been demonstrated in the peritoneal cavity of patients with ovarian carcinoma that are capable of reacting with tumor cells [20]. Higher numbers of CD4+ CD25+ T-cells has been demonstrated in the ascites of patients [21,22] which demonstrates a poor immune status. Despite the fact that there is some evidence of a difference between the leukocytes isolated from solid tumor and ascites [23], all major leukocyte populations are represented in the malignant ascites. Thus, due to the ease of injections and the ability to induce potent immune responses, the peritoneal cavity is often used as a site for immunizations. This peritoneal immunotherapy may be used for palliation of malignant ascites as well. In our study, we treated patients with malignant peritoneal effusion who had failed to improve with other conventional treatments with intraperitoneal injection of dendritic cell vaccines combined with CIK cells.
Immunotherapy using effector cells has been widely used for cancer patients in the last two decades [24-26], but has failed to obtain public support as a standard therapy for cancer patients because the effect has been limited. However, pilot studies in the past couple of years have had promising results, which lead to increased interest and dozens more clinical trials investigating the therapy technique [27]. It has been early reported that different populations of leukocytes may serve as effectors in adoptive immunotherapy, including Tumorin-filtrating lymphocytes (TIL) [28], lymphokine-activated killer (LAK) cells [29,30], and monocytes [31]. Adoptive immunotherapy using CIK cells that constitutes a substantial number of the CD3+ CD56+ cells has shown significant anti-tumor activity in pre-clinical experiments and animal tumor models [32,16]. It has stronger antitumor activity and broader spectrum of targeted tumor than other reported antitumor effector cells, moreover, CIK cells can regulate and generally enhance the immune functions in patients with cancer [7,11]. These expanded human CD3+ CD56+ cells have certain desirable features; in particular, they have minimal cytotoxicity against normal hematopoietic cells, do not suppress bone marrow progenitor activity, and are resistant to Fas-mediated apoptosis [33]. Thus, it appears that the CIK cell would be a choice candidate for the eradication of tumor cells. There is evidence that dendritic cells in malignant ascites under the influence of the tumor microenvironment, are characterized by a less mature phenotype which may have disturbed antigen presenting functions [34,35] and result in less effective T-cell stimulation. In some studies, it’s reported that Ag-loaded DC enhance the cytotoxicity of CIK cells against tumor cells. DC increased the population of the hallmark effector CD3+ CD56+ cells and decreased the proportion of CD4+ CD25+ cells that had potent immune suppressive function [36]. Thus, the vaccines of dendritic cell combined with CIK cells may serve as a promising treatment to malignant ascites. Our data presented that CD3+ CD56+ CIK cells was increased and the percentage of CD4+ CD25+ Treg cells after treatment decreased significantly (P < 0.05) after treatment. But there was no significant difference in the percentage of CD3+, CD4+, CD8+ T lymphocytes, CD19+ B lymphocytes, and ratio of CD4+/CD8+ between before and after treatment. The immunotherapy was well tolerated by patients. Patients showed a low incidence of tumor progression and high RR and DCR. All the data showed that this therapy modality can achieve a certain clinical benefits even in patients resistant to conventional treatment. Though, our data only evaluated the short-term clinical curative effects, more trials should be investigated regarding long-term disease-free survival (DFS).
As cytokines play a crucial role in the host’s immune response, determining the levels of cytokines before and after immunotherapy in MA patients may provide information regarding the mechanisms of DC vaccines combined with CIK cells. We analyzed the production of cytokines commonly involved in MA formation and progression, including GM-CSF, IL-10, IFN-γ, TGF-α, TGF-β, VEGF and MCP-1. The results showed that patients who benefited from immunotherapy showed an increase in levels of IFN-γ, GM-CSF and TNF-α which were not found in patients who had MA progression. IFN-γ and TNF-α as cytokines of Th1 cells, have been shown to improve cytotoxicity of cytotoxic T lymphocytes against tumor cells and the activity of NK cells. IFN-γ has also been found to exert anti-tumor activity by inducing apoptosis of tumor cells. Moreover, IFN-γ can inhibit angiogenesis and tumor metastasis [37,38]. The serum levels of these two Th1 cytokines were increased after immunotherapy, which indicated the activation of T cells and induction of immune responses by the host. This up-regulation of cytokine and chemokine production may serve as one mechanism by which the DC vaccines combined with CIK cells treatment exert their anti-tumor effects.
High concentrations of IL-10, a cytokine with suppressive activity on T-cells and monocytes, have been reported in the malignant ascites [39]. Among other cytokines, VEGF and MCP-1 was shown to be the cytokine responsible for the increase of the permeability of peritoneal membrane and exudate formation [40,41]. Thus, our data showing increased IL-10, VEGF and MCP-1 levels in 5 patients with PD demonstrated a poor immune status and MA progression.
In summary, we evaluated the safety and efficacy of dendritic cell vaccines combined with cytokine-induced killer cells intraperitoneal injected on patients with MA. Our data showed that the treatment on patients with malignant peritoneal effusion achieved a certain clinical benefit which is safe and feasible. This therapy modality can provided a promising way treating patients resistant to conventional treatment.
Acknowledgements
This work was supported by 81 Hospital of PLA.
Disclosure of conflict of interest
None.
References
- 1.Runyon BA. Care of patients with ascites. N Engl J Med. 1994;330:337–342. doi: 10.1056/NEJM199402033300508. [DOI] [PubMed] [Google Scholar]
- 2.Parsons SL, Watson SA, Steele RJC. Malignant ascites (Review) Br J Surg. 1996;83:6–4. doi: 10.1002/bjs.1800830104. [DOI] [PubMed] [Google Scholar]
- 3.Runyon BA, Hoefs JC, Morgan TR. Ascitic fluid analysis in malignancy-related ascites. Hepatology. 1988;8:1104–1109. doi: 10.1002/hep.1840080521. [DOI] [PubMed] [Google Scholar]
- 4.Saif MW, Siddiqui IA, Sohail MA. Management of ascites due to gastrointestinal malignancy (review) Ann Saudi Med. 2009;29:369–377. doi: 10.4103/0256-4947.55167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keen A, Fitzgerald D, Bryant A. Management of drainage for malignant ascites in gynaecological cancer (review) Cochrane Database Syst Rev. 2010;20:CD007794. doi: 10.1002/14651858.CD007794.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 7.Hontscha C, Borck Y, Zhou H, Messmer D, Schmidt-Wolf IG. Clinical trials on CIK cells: first report of the international registry on CIK cells (IRCC) J Cancer Res Clin Oncol. 2011;137:305–310. doi: 10.1007/s00432-010-0887-7. [DOI] [PubMed] [Google Scholar]
- 8.Schwaab T, Schwarzer A, Wolf B, Crosby NA. Clinical and immunologic effects of intranodal autologous tumor lysate-dendritic cell vaccine with Aldesleukin (Interleukin 2) and IFN-{alpha}2a therapy in metastatic renal cell carcinoma patients. Clin Cancer Res. 2009;15:4986–4992. doi: 10.1158/1078-0432.CCR-08-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melichar B, Freedman RS. Immunology of the peritoneal cavity: relevance for host-tumor relation. Int J Gynecol Cancer. 2002;12:3–17. doi: 10.1046/j.1525-1438.2002.01093.x. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume KG, Weissman IL. Use of a SCID mouseihuman lymphoma model to evaluate cytokine-induced killer cells with potent antitumour cell activity. J Exp Med. 1991;174:139–149. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt-Wolf IG, Lefterova P, Mehta BA, Fernandez LP, Huhn D, Blume KG, Weissman IL, Negrin RS. Phenotypic characterization and identification of effector cells involved in tumour cell recognition of cytokine-induced killer cells. Exp Hematol. 1993;21:1673–1679. [PubMed] [Google Scholar]
- 12.Schmidt-Wolf IG, Lefterova P, Johnston V, Scheffold C, Csipai M, Mehta BA, Tsuruo T, Huhn D, Negrin RS. Sensitivity of multidrug-resistant tumour cell lines to immunologic effector cells. Cell Immunol. 1996;169:85–90. doi: 10.1006/cimm.1996.0094. [DOI] [PubMed] [Google Scholar]
- 13.Lu PH, Negrin RS. A novel population of expanded human CD3+ CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153:1687–1696. [PubMed] [Google Scholar]
- 14.Scheffold C, Brandt K, Neubauer V, Lefterova P, Degen B, Schöntube M, Huhn D, Neubauer A, Schmidt-Wolf IG. Potential of autologous immunologic effector cells for bone marrow purging in patients with chronic myeloid leukemia. Bone Marrow Transplant. 1995;15:33–39. [PubMed] [Google Scholar]
- 15.Hoyle C, Bangs C, Mehta B, Chang P, Kamel O, Negrin RS. Expansion of Philadelphia chromosome-negative CD3+ CD56+ cytotoxic cells from chronic myeloid leukemiapatients: in vitro and in vivo efficacy in severe combined immunodeficiency disease mice. Blood. 1998;92:3318–3327. [PubMed] [Google Scholar]
- 16.Marten A, Ziske C, Schöttker B, Renoth S, Weineck S, Buttgereit P, Schakowski F, von Rucker A, Sauerbruch T, Schmidt-Wolf IG. Interactions between dendritic cells and cytokine-induced killer cells lead to an activation of both populations. J Immunother. 2001;24:502–510. doi: 10.1097/00002371-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Ziske C, Marten A, Schottker B, Buttgereit P, Schakowski F, Gorschlüter M, von Rücker A, Scheffold C, Chao N, Sauerbruch T, Schmidt-Wolf IG. Resistance of pancreatic carcinoma cells is reversed by coculturing NK-like T cells with dendritic cells pulsed with tumor-derived RNA and CA 19-9. Mol Ther. 2001;3:54–60. doi: 10.1006/mthe.2000.0230. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen EW, Gerber SA, Sedlacek AL, Rybalko VY, Chan WM, Lord EM. Omental immune aggregates and tumor metastasis within the peritoneal cavity. Immunol Res. 2009;45:185–194. doi: 10.1007/s12026-009-8100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao L, Hu X, Zhang Y, Sun XT. Omental milky spots in screening gastric cancer stem cells. Neoplasma. 2011;58:20–26. doi: 10.4149/neo_2011_01_20. [DOI] [PubMed] [Google Scholar]
- 20.Freedman RS, Kudelka AP, Kavanagh JJ. Clinical and biological effects of intraperitoneal injections of recombinant interferon-gamma and recombinant interleukin-2 with or without tumor-infiltrating lymphocytes in patients with ovarian or peritoneal carcinomatosis. Clin Cancer Res. 2000;6:2268–2278. [PubMed] [Google Scholar]
- 21.Curiel TJ, Coukos G, Zou L. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 22.Bamias A, Tsiatas ML, Kafantari E. Significant differences of lymphocytes isolated from ascites of patients with ovarian cancer compared to blood and tumor lymphocytes. Association of CD3+ CD56+ cells with platinum resistance. Gynecol Oncol. 2007;106:75–81. doi: 10.1016/j.ygyno.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 23.Kono K, Ichihara F, Izuka H, Sekikawa T, Matsumoto Y. Differences in the recognition of tumor-specific CD8+ T cells derived from solid tumor, metastatic lymph nodes and ascites in patients with gastric cancer. Int J Cancer. 1997;71:978–981. doi: 10.1002/(sici)1097-0215(19970611)71:6<978::aid-ijc12>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Eberlein TJ, Rosenstein M, Rosenberg SA. Regression of a disseminated syngeneic solid tumor by systemic transfer of lymphoid cells expanded in interleukin 2. J Exp Med. 1982;156:385–397. doi: 10.1084/jem.156.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenberg SA, Dudley ME. Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes. Proc Natl Acad Sci U S A. 2004;101(Suppl 2):14639–14645. doi: 10.1073/pnas.0405730101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dudley ME, Wunderlich JR, Yang JC. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humphries C. Adoptive cell therapy: Honing that killer instinct. Nature. 2013;504:S13–5. doi: 10.1038/504S13a. [DOI] [PubMed] [Google Scholar]
- 28.Freedman RS, Tomasovic B, Templin S. Large-scale expansion in interleukin-2 of tumor-infiltrating lymphocytes from patients with ovarian carcinoma for adoptive immunotherapy. J Immunol Methods. 1994;197:145–160. doi: 10.1016/0022-1759(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 29.Stewart JA, Belinson JL, Moore AL. Phase I trial of intraperitoneal recombinant interleukin-2/lymphokineactivated killer cells in patients with ovarian cancer. Cancer Res. 1990;50:6302–6310. [PubMed] [Google Scholar]
- 30.Steis RG, Urba WJ, VanderMolen LA. Intrape-ritoneal lymphokine-activated killer-cell and interleukin-2 therapy for malignancies limited to the peritoneal cavity. J. Clin. Oncol. 1990;8:1618–1629. doi: 10.1200/JCO.1990.8.10.1618. [DOI] [PubMed] [Google Scholar]
- 31.Lopez M, Fechtenbaum J, David B. Adoptive immunotherapy with activated macrophages grown in vitro from blood monocytes in cancer patients: a pilot study. J Immunother. 1992;11:209–217. doi: 10.1097/00002371-199204000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Mac Manus MP, Matthews JP, Worotniuk V, Ball DL. Unexpected long-term survival after low-dose palliative radiotherapy for non-small cell lung cancer. Cancer. 2006;106:1110–1116. doi: 10.1002/cncr.21704. [DOI] [PubMed] [Google Scholar]
- 33.Verneris MR, Kornacker M, Mailander V, Negrin RS. Resistance of ex vivo expanded CD3+ CD56+ T cells to Fas-mediated apoptosis. Cancer Immunol Immunother. 2000;49:335–345. doi: 10.1007/s002620000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gabrilovich DI, Ciernik IF, Carbone DP. Dendritic cells in antitumor immune response. I. Defective antigen presentation in tumor-bearing hosts. Cell Immunol. 1996;170:101–110. doi: 10.1006/cimm.1996.0139. [DOI] [PubMed] [Google Scholar]
- 35.Gabrilovich DI, Nadaf S, Corak J, Berzofsky JA, Carbone DP. Dendritic cells in antitumor responses. II. Dendritic cells grown from bone marrow precursor, but not mature DC form tumor-bearing mice, are effective antigen carriers in the therapy of established tumors. Cell Immunol. 1996;170:111–119. doi: 10.1006/cimm.1996.0140. [DOI] [PubMed] [Google Scholar]
- 36.Gorbachev AV, Kobayashi H, Kudo D, Tannenbaum CS, Finke JH, Shu S. CXC chemokine ligand 9/monokine induced by IFN-gamma production by tumor cells is critical for T cell-mediated suppression of cutaneous tumors. J Immunol. 2007;178:2278–2286. doi: 10.4049/jimmunol.178.4.2278. [DOI] [PubMed] [Google Scholar]
- 37.Wahl SM, Wen J, Moutsopoulos N. TGF-beta: a mobile purveyor of immune privilege. Immunol Rev. 2006;213:213–227. doi: 10.1111/j.1600-065X.2006.00437.x. [DOI] [PubMed] [Google Scholar]
- 38.Galliher AJ, Neil JR, Schiemann WP. Role of transforming growth factor-beta in cancer progression. Future Oncol. 2006;2:743–763. doi: 10.2217/14796694.2.6.743. [DOI] [PubMed] [Google Scholar]
- 39.Gotlieb WH, Abrams JS, Watson JM, Velu TJ, Berek JS, Martinez-Maza O. Presence of interleukin-10 (IL-10) in the ascites of patients with ovarian and other intraabdominal cancers. Cytokine. 1992;4:385–390. doi: 10.1016/1043-4666(92)90082-3. [DOI] [PubMed] [Google Scholar]
- 40.Liss C, Fekete MJ, Hasina R, Lam CD, Lingen MW. Paracrine angiogenic loop between head-and-neck squamous-cell carcinomas and macrophages. Int J Cancer. 2001;93:781–785. doi: 10.1002/ijc.1407. [DOI] [PubMed] [Google Scholar]
- 41.Zebrowski BK, Liu W, Ramirez K, Akagi Y, Mills GB, Ellis LM. Markedly elevated levels of vascular endothelial growth factor in malignant ascites. Ann Surg Oncol. 1999;6:373–378. doi: 10.1007/s10434-999-0373-0. [DOI] [PubMed] [Google Scholar]


