Abstract
There are abundant sympathetic nerve fibers in cervical posterior longitudinal ligament (PLL). The aim of this study was to investigate the role of PLL in the occurrence of sympathetic symptoms. Ten healthy adult beagles were selected and anesthetized to establish a PLL compression model by C4/5 discectomy, nucleus pulposus tissue replantation, and plate internal fixation. The middle cervical ganglia (MCG) activities were recorded before modeling, shortly after modeling, and two months after modeling. The waveform parameters and spectral densities of autonomic discharge of MCG among the three periods were compared. There was significant difference only in terms of the area of waveform per unit time between before and shortly after modeling. Abnormal discharge waveforms of MCG were detected in two months after modeling. The wave amplitude and waveform area per unit time in two months after modeling were increased significantly compared with those in shortly after modeling. Functional spectral decomposition found a significant increase in 100-250 Hz in two months after modeling. In conclusion, abnormal discharge of MCG caused by chronic compression of PLL may be one of the pathological basis of sympathetic nervous symptoms.
Keywords: Posterior longitudinal ligament, cervical spondylosis, middle cervical ganglia, sympathetic nervous symptom, anterior cervical discectomy and fusion
Introduction
Cervical spondylosis is one of the most common degenerative conditions of the spine characterized by a loss of disc height, end plate sclerosis and formation of osteophytes. It is a natural aging process that occurs in 95% of individuals by the age of 65 years [1]. Sympathetic symptoms of cervical spondylosis, such as vertigo, headache, heart throb, hypomnesia, tinnitus, nausea, vomiting, and gastroenterologic discomfort, are common clinical manifestations. Clinical manifestations of cervical spondylosis associated with sympathetic nervous symptoms are complicated and varied. Patients have diverse subjective symptoms but less objective signs, which bring great difficulties in disease diagnosis and treatment.
Some hypotheses were proposed, such as mechanical compression, cervical spine instability, atlas vertebral artery sulcus ring malformation, vertebral artery sympathetic stimulation [2-5], but they only partially explain the pathogenesis of cervical sympathetic symptoms. It has been demonstrated that sympathetic nerve fibers are abundant in cervical posterior longitudinal ligament (PLL) [6,7]. Therefore, anterior cervical discectomy and fusion (ACDF) combined with removal of the posterior longitudinal ligament can alleviate nerve compression symptoms as well as the sympathetic nervous symptoms to a certain extent in clinical treatment for spinal cord compression combined with sympathetic symptoms in patients with obvious cervical disc prolapse [8-12]. Sympathetic nerve fibers in cervical PLL are considered to be related to sympathetic symptoms [10,12].
The correlation between cervical PLL and sympathetic symptoms has not been confirmed through animal experiments, until now. Based on the hypothesis of sympathetic factors of cervical PLL, the present study aimed to confirm the presence of neural pathways between PLL and cervical sympathetic nerves by using male beagles as experimental subjects.
Materials methods
Animal
Ten healthy adult male beagles aged 16 to 22 months (average weight 13 Kg, range 9-15 Kg) were provided by the Experimental Animal Center of Second Military Medical University. Anesthesia was conducted by intraperitoneal injection of 3% pentobarbital at 1.5-2 ml/kg. The protocol was approved by the Ethic Committee of Changzheng Hospital.
Modeling of PLL compression
The beagles were fixed at supine position after anesthesia, and longitudinal incisions were made along the right edge of trachea to separate subcutaneous tissues. The spaces between sternohyoideus and sternothyroideus were separated through the medial approach of sternocephalicus muscle to expose the carotid sheath. Vagosympathetic trunk concomitant with carotid was separated and marked using a silk suture. Middle cervical ganglion (MCG) was exposed in front of transverse process of C7. The prevertebral fascia was then cut open at carotid sheath medialis at C4/5 level. The longus colli was separated from the interspaces of both ends to expose the anterior border of vertebral body. Prevertebral muscles were cut open along the anterior median line to the extent of one vertebral body around C4/5 interspace, whereas the attachment site of vertebral muscles were cut using a tissue scissor. The muscle was stripped from the middle to both sides under the assistance of a bone knife, to expose the intervertebral space and intervertebral disc. A triple joint rongeur was used to gradually remove the anulus fibrosus in front of the C4/5 disc. Nucleus pulposus clamp and spatula were both used to successively take out the nucleus pulposus and preserve the posterior terminal plate and longitudinal ligament. A rongeur was used to remove the protruding osteophytes on the upper and lower edges of the intervertebral spaces. A bone wax was then used to stop the bleeding. The obtained nucleus pulposus was stuffed within the intervertebral space of C4/5 to compress the posterior longitudinal ligament backward as far as possible. In order to reconstruct the cervical stability, a special titanium alloy steel anterior cervical plate was used to fix C4 and C5 vertebral bodies.
Postoperative treatment
The incision was rinsed with saline, and the prevertebral musculature, fascia and skin were sutured layer-by-layer. Antibiotics were intravenously administered to prevent infection after the operation. Furthermore, dexamethasone was used to reduce nerve edema. Antibiotics were injected near the wounds for three consecutive days after operation. The beagles were fed for two months.
Record of autonomic discharge signals in MCG
The autonomic discharge signals of MCG were recorded before modeling, shortly after modeling and two months after modeling. MCG was carefully isolated from the muscle tissues by placing an insulation spacer between them. LIFEs electrode that functioned as recording electrode was connected to the input end of BL-420S biological signal acquisition system. The electrode tip was used to puncture MCG epineurium at an angle of 60°, and then embedded in the MCG nerve bundles. Paraffin oil was applied to form oil groove. The waveforms of autonomic discharge signals in MCG were recorded through BL-420S biological signal acquisition system. The waveform shapes of autonomic discharge in MCG were recorded. Parameters among three periods were compared, including the maximum and minimum values of waveform amplitudes, the maximum and minimum rising rates, the waveform area per unit time, and functional spectral densities in three group were compared.
Statistical analyses
The data were presented as mean with standard deviation (mean ± SD) for quantitative variables. The difference between periods was analyzed by standard Student t test. All statistical analyses were carried out with SPSS for Windows (version 18.0; SPSS, Chicago, IL, USA). A P value < 0.05 was considered statistically significant.
Results
Waveform shapes of autonomic discharge in MCG under various statuses
A relatively stable electrical waveform in MCG in beagles was recorded under normal physiological condition. The shape of electrical waveform in MCG was not significantly different from that under normal physiological condition, after the cervical PLL was mechanically compressed. The waveform amplitudes of autonomic discharges of MCG in two months after modeling were elevated, as compared with those under the physiological condition (Figure 1).
Figure 1.
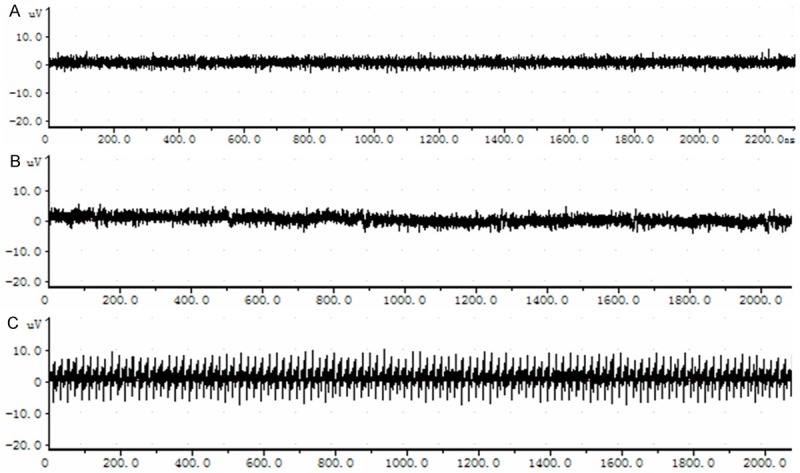
Waveform spectra of autonomic discharge in MCG in three groups. A. Under physiological condition. B. Shortly after modeling. C. Two months after modeling.
Waveform parameters of autonomic discharges in MCG under different statuses
Table 1 summarized the maximum and minimum values of waveform amplitudes, the maximum and minimum rising rates, and the area of waveform per unit time of autonomic discharge in MCG of the three groups. No significant difference in terms of parameters, including the maximum and minimum amplitudes, the maximum and minimum rising rates of the waveforms, was found between under physiological condition and shortly after modeling. The difference in area of waveform per unit time between before and shortly after modeling, however, was statistically significant (P = 0.019). There was significantly difference in all these parameters between shortly and two months after modeling (P < 0.001).
Table 1.
Waveform parameters of autonomic discharges in MCG in three groups
| Group | Maximum amplitude (μV) | Minimum amplitude (μV) | Maximum rising rate (μV/ms) | Minimum rising rate (μV/ms) | Area (μV × s) |
|---|---|---|---|---|---|
| Before modeling | 4.77 ± 0.323 | -3.49 ± 0.485 | 19.50 ± 0.290 | -20.17 ± 1.476 | 0.95 ± 0.009 |
| Shortly after modeling | 4.74 ± 0.546 | -5.10 ± 0.605 | 18.63 ± 0.414 | -20.01 ± 1.915 | 1.13 ± 0.082 |
| Two months after modeling | 7.50 ± 0.457 | -9.54 ± 0.719 | 22.31 ± 1.397 | -21.68 ± 0.787 | 1.90 ± 0.047 |
The waveform spectra of autonomic discharges in MCG under different statuses
We further analyzed the waveform spectra of autonomic discharges in MCG of the three groups. There was no significant difference between before and short after modeling. The 100~250 Hz composition in two months after modeling was significantly increased compared with that under normal physiological condition (Figure 2).
Figure 2.
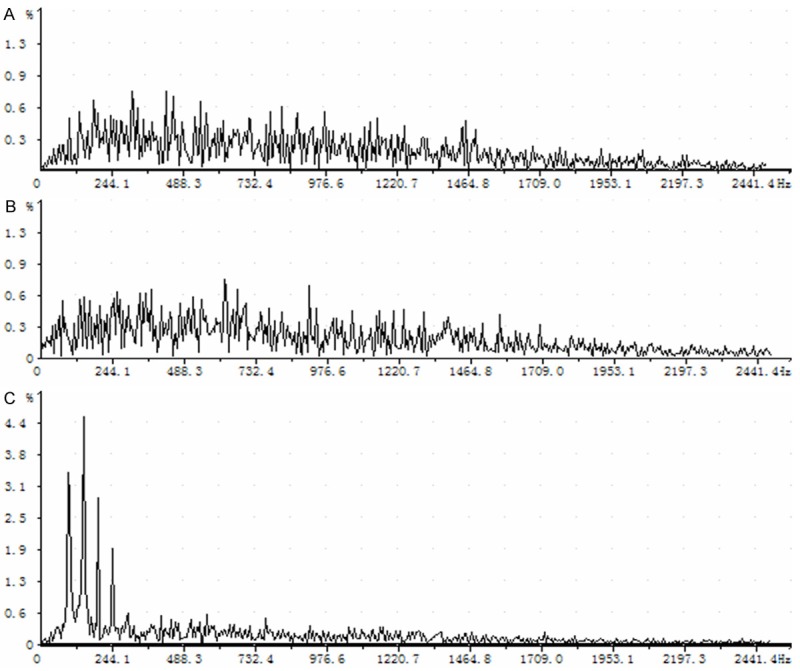
Schematic diagram of autonomic discharge waveforms in MCG in three groups. A. Under physiological condition. B. Shortly after modeling. C. Two months after modeling.
Discussion
Sympathetic nerves are widely distributed within human neck. They are concomitant with the external carotid artery to govern the sweat glands and blood vessels of the face. The branches around the internal carotid artery control brain, fundus, pupils, and arrector pili muscles, whereas the branches around the vertebral artery control blood vessels of brainstem, cerebellum, temporal lobe and inner ear. In addition, the branches are also distributed within the throat and heart. Human cervical sympathetic trunk is distributed at the superficial surface of the longus scapitis and longus colli, deep surface of carotid sheath and prevertebral fascia, and slightly medial to the vagus nerve stem, where there are many branches incorporated into cervical nerves, such as the cervical blood vessels and other structures [13-15]. The abundant sensory nerve fibers and sympathetic fibers on the posterior longitudinal ligament form interactive synapses. Therefore, the sensory nerve stimulation can either enhance or enlarge sympathetic excitation [16]. The sympathetic fibers distributed within cervical PLL may be the physiological basis that sense a series of pathological stimulations such as cervical spine degeneration and nuclear herniation.
In clinical practice, the anterior access intervertebral decompression and fusion, especially the removal of PLL in decompression space, can alleviate sympathetic symptoms to some extent, if three months or longer conservative treatment is ineffective for cervical spondylosis patients with sympathetic symptoms who have radiographic abnormalities such as changes of physiological curvature, intervertebral disc prolapse, and PLL compression caused by thickening of PLL. Patients with either mild intervertebral disc prolapse or only PLL/spinal dura mater compression accompanied by severe sympathetic symptoms are frequently seen in clinical practice. For these patients, where neurological, otorhinolaryngological, and related diseases are excluded, sympathetic blockage through lidocaine injection in PLL by intervertebral space puncture under fluoroscopy can provide references for the selection of diagnosis and treatment protocol, if the sympathetic symptoms are alleviated.
In the present study, we analyzed the waveforms of autonomic discharges in MCG among beagles under physiological status, shortly after modeling of PLL compression, and two months after modeling. Only the areas of waveform in shortly after modeling were significantly increased compared with those under physiological status. This suggests that acute compression on PLL will not cause abnormal discharge in MCG. However, chronic compression on PLL causes abnormal discharges in MCG. Previous studies revealed that some cytokines, such as TGFβ and BMP-2, and Indian hedgehog signaling are involved in the pathogenesis of ossification of PLL [17-19]. Song et al. [20] found that cyclooxygenase 2 (COX2) was expressed in cervical PLL of patients with cervical spondylotic myelopathy, and might be associated with the degeneration of the PLL in cervical spondylotic myelopathy. Cytokines, including COX2, may be present in cervical PLL that is exposed to chronic compression. Therefore, long-term over-stimulation, such as mechanical compression and cytokines, may lead to morphological changes of sympathetic nerve fibers in PLL of patients with cervical disc degeneration and PLL compression. We hypothesize that pathological signals may stimulate the terminals of sympathetic fibers in PLL. The nerve impulses are transmitted to the MCG and then to the sympathetic preganglionic neurons in the lateral horn of T1-T4 through the gray and white communicating branches. Then nerve impulses are transmitted through the white communicating branch from the lower central sympathetic nerves to cervical and thoracic ganglia, where neurons are exchanged and issued to various postganglionic fibers. Finally, the stimulation signals are transmitted to each innervated organs, causing a series of sympathetic symptoms such as dizziness, nausea, blurred vision, tinnitus, and palpitation. The stimulation of pathological factors in PLL may be an important factor that causes sympathetic symptoms. Further studies are required to validate the underlying mechanisms.
In conclusion, our results provided evidence that abnormal autonomic discharge was present in MCG when the PLL was suffered from chronic compression, indicating a functional relationship between PLL and MCG. Abnormal discharge of MCG caused by chronic compression of PLL may be one of the pathological basis of sympathetic symptoms.
Acknowledgements
This study was supported by the National Natural Scientific Foundation of China (grant No. 81472128).
Disclosure of conflict of interest
None.
References
- 1.Mullin J, Shedid D, Benzel E. Overview of Cervical Spondylosis Pathophysiology and Biomechanics. World Spinal Column J. 2011;2:89–97. [Google Scholar]
- 2.Qian J, Tian Y, Hu JH, Qiu GX. Retrospective study of relationship between cervical instability and sympathetic cervical spondylosis. Chin J Spine Spinal Cord. 2009;19:27–29. [Google Scholar]
- 3.Wrisley DM, Sparto PJ, Whitney SL, Furman JM. Cervicogenic dizziness: a review of diagnosis and treatment. J Orthop Sports Phys Ther. 2000;30:755–766. doi: 10.2519/jospt.2000.30.12.755. [DOI] [PubMed] [Google Scholar]
- 4.Olszewski J, Majak J, Pietkiewicz P, Repetowski M. [Analysis of select diagnostic examination results and their connection with cervical vertigo diagnosis] . Pol Merkur Lekarski. 2005;19:393–395. [PubMed] [Google Scholar]
- 5.Cagnie B, Barbaix E, Vinck E, D’Herde K, Cambier D. Extrinsic risk factors for compromised blood flow in the vertebral artery: anatomical observations of the transverse foramina from C3 to C7. Surg Radiol Anat. 2005;27:312–316. doi: 10.1007/s00276-005-0006-7. [DOI] [PubMed] [Google Scholar]
- 6.Yamada H, Honda T, Yaginuma H, Kikuchi S, Sugiura Y. Comparison of sensory and sympathetic innervation of the dura mater and posterior longitudinal ligament in the cervical spine after removal of the stellate ganglion. J Comp Neurol. 2001;434:86–100. doi: 10.1002/cne.1166. [DOI] [PubMed] [Google Scholar]
- 7.Johnson GM. The sensory and sympathetic nerve supply within the cervical spine: review of recent observations. Man Ther. 2004;9:71–76. doi: 10.1016/S1356-689X(03)00093-6. [DOI] [PubMed] [Google Scholar]
- 8.Hong L, Kawaguchi Y. Anterior cervical discectomy and fusion to treat cervical spondylosis with sympathetic symptoms. J Spinal Disord Tech. 2011;24:11–14. doi: 10.1097/BSD.0b013e3181dd80f5. [DOI] [PubMed] [Google Scholar]
- 9.Liang L, Wang XW, Yuan W, Li J, Chen HJ, Qi M, Wang ZC. Total disc replacement to treat cervical spondylotic myelopathy with sympathetic symptoms. Chin J Orthop. 2012;32:389–392. [Google Scholar]
- 10.Li J, Gu T, Yang H, Liang L, Jiang DJ, Wang ZC, Yuan W, Wang XW. Sympathetic nerve innervation in cervical posterior longitudinal ligament as a potential causative factor in cervical spondylosis with sympathetic symptoms and preliminary evidence. Med Hypotheses. 2014;82:631–635. doi: 10.1016/j.mehy.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 11.Liang L, Wang XW, Yuan W, Wang ZC, Chen DY, Li J, Jiang DJ. Analysis of anterior discectomy and fusion for treating cervical spondylosis accompanied by sympathetic symptoms. Chin J Spine Spinal Cord. 2012;22:14–19. [Google Scholar]
- 12.Wang Z, Wang X, Yuan W, Jiang D. Degenerative pathological irritations to cervical PLL may play a role in presenting sympathetic symptoms. Med Hypotheses. 2011;77:921–923. doi: 10.1016/j.mehy.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Civelek E, Karasu A, Cansever T, Hepgul K, Kiris T, Sabanci A, Canbolat A. Surgical anatomy of the cervical sympathetic trunk during anterolateral approach to cervical spine. Eur Spine J. 2008;17:991–995. doi: 10.1007/s00586-008-0696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saylam CY, Ozgiray E, Orhan M, Cagli S, Zileli M. Neuroanatomy of cervical sympathetic trunk: a cadaveric study. Clin Anat. 2009;22:324–330. doi: 10.1002/ca.20764. [DOI] [PubMed] [Google Scholar]
- 15.Kiray A, Arman C, Naderi S, Guvencer M, Korman E. Surgical anatomy of the cervical sympathetic trunk. Clin Anat. 2005;18:179–185. doi: 10.1002/ca.20055. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed M, Bjurholm A, Kreicbergs A, Schultzberg M. Neuropeptide Y, tyrosine hydroxylase and vasoactive intestinal polypeptide-immunoreactive nerve fibers in the vertebral bodies, discs, dura mater, and spinal ligaments of the rat lumbar spine. Spine (Phila Pa 1976) 1993;18:268–273. doi: 10.1097/00007632-199302000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Uchida K, Nakajima H, Yayama T, Sato R, Baba H. [Updates on ossification of posterior longitudinal ligament. Ossification front of posterior longitudinal ligament and cellular biological assessment of chronic mechanical compressed spinal cord] . Clin Calcium. 2009;19:1472–1479. [PubMed] [Google Scholar]
- 18.Sato R, Uchida K, Kobayashi S, Yayama T, Kokubo Y, Nakajima H, Takamura T, Bangirana A, Itoh H, Baba H. Ossification of the posterior longitudinal ligament of the cervical spine: histopathological findings around the calcification and ossification front. J Neurosurg Spine. 2007;7:174–183. doi: 10.3171/SPI-07/08/174. [DOI] [PubMed] [Google Scholar]
- 19.Sugita D, Yayama T, Uchida K, Kokubo Y, Nakajima H, Yamagishi A, Takeura N, Baba H. Indian hedgehog signaling promotes chondrocyte differentiation in enchondral ossification in human cervical ossification of the posterior longitudinal ligament. Spine (Phila Pa 1976) 2013;38:E1388–1396. doi: 10.1097/BRS.0b013e3182a40489. [DOI] [PubMed] [Google Scholar]
- 20.Song HX, Scarpatetti M, Kreil W, Shen HL, Bodo K, Ebner B, Schrottner H, Mokry M. Quantitative analysis of cyclooxygenase 2 in the posterior longitudinal ligament of cervical spondylotic myelopathy. Chin Med J (Engl) 2011;124:2480–2484. [PubMed] [Google Scholar]


