Abstract
Cutaneous malignant melanoma represents the major cause of mortality among skin cancers. Metastasis-associated protein CD24 is a small, heavily glycosylated cell surface protein that is overexpressed in various human malignancies. The present study was designed to determine the roles of CD24 in cutaneous malignant melanoma. The levels of CD24 mRNA and protein in cutaneous malignant melanoma tissues were detected by RT-PCR, Western blot and IHC. In patient samples, the levels of CD24 mRNA and protein were higher in cancer tissues than that in normal tissues. CD24 expression decreased the survival time of the patients with melanoma. Taken together, these results suggest that CD24 may be used as a new drug target for cutaneous malignant melanoma.
Keywords: CD24, melanoma, prognosis
Introduction
Cutaneous malignant melanoma represents the major cause of mortality among skin cancers and its incidence rate is increasing during last years [1]. Several histological factors have been shown to correlate with the survival of melanoma patients [2].
Metastasis-associated protein CD24 is a small, heavily glycosylated cell surface protein that is attached to the cell membrane by a glycosyl-phosphatidylinositol anchor [3,4]. CD24 is overexpressed in various human malignancies and has been described as a prognostic marker for breast [5], colon [6], stomach [7], prostate [8] and non-small cell lung cancers [9]. It has been demonstrated that CD24 is involved in cell adhesion and tumor metastasis [9]. However, although increased CD24 has been detected in many human cancers, the role of CD24 in cutaneous malignant melanoma remains unclear. FDA has approved few biological agents and conventional chemotherapeutic agents for metastatic melanoma treatment [10,11]. However, none of the currently available FDA approved therapies clearly alter the natural history of the disease for the population as a whole [12]. So, new targeted agents are needed to be developed and tested alone or in combination with conventional chemotherapies.
In order to discover a new targeted protein to more effectively treat cutaneous malignant melanoma, here we detected CD24 expression in 33 tumor tissue samples taken from patients with melanoma. In addition, we correlated CD24 expression with clinicopathologic variables of cutaneous malignant melanoma.
Method and materials
Subjects
A total of 33 patients with cutaneous malignant melanoma were obtained from the First Hospital of China Medical University (Jun. 2003 to Jun. 2008). All patients underwent standard laboratory tests (cytology and histology). None of the patients underwent radiotherapy or chemotherapy before the operation. Informed consent was provided by all patients according to the Helsinki Declaration.
RNA isolation and reverse transcriptase-polymerase chain reaction (RT-PCR)
In this work, RNA was extracted from tissues using TRIzol solution (Invitrogen Life Technologies, Carlsbad, CA, USA). First strand cDNA was reverse transcribed with 1 μg total RNA, using the TaKaRa Reverse Transcription Kit (TaKaRa, Dalian, China) and oligo (dT)-15 primers (TaKaRa) according to the manufacturer’s instructions. The CD24 primers used were: 5’-ACCTGTTTCCATTCAACAAGAGCAC-3’ (sense) and 5’-TCTGAGATCGCACCACTGCAC-3’ (antisense). The housekeeping gene, GAPDH, was used as an internal normalization control. The GAPDH primers used were: 5’-GAAGGCTGGGGCTCATTT-3’ (sense) and 5’-GGGGCCATCCACAGTCTT-3’ (antisense). PCR amplification of cDNA was performed in 15 μl mixtures. Finally, products were resolved by 1% agarose gel electrophoresis, and visualized by ethidium bromide staining and a UV imaging system (UVP, Upland, CA, USA).
Western blot analysis
Tissues were lysed in lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 1% Triton-X100) containing a protease inhibitor cocktail (Sigma-Aldrich, Saint Louis, MO, USA). Cell extract protein amounts were quantified using the BCA protein assay kit. Equivalent amounts of protein (30 μg) were separated using 12% SDS-PAGE and transferred to PVDF membrane (Millipore Corporation, Billerica, MA, USA). Western blot was performed using primary antibodies: CD24 (sc-7034, Santa Cruz Biotechnology, Santa Cruz, CA), and β-actin (sc-47778, Santa Cruz). Each specific antibody binding was detected with horseradish peroxidase (HRP)-conjugated respective secondary antibodies (Amersham Biosciences, UK) and ECL solutions (Amersham Biosciences).
Immunohistochemical studies
Excised tumors were fixed in 4% paraformaldehyde for 24 h then embedded in paraffin. Sections (4 μm thick) were generated for immunohistochemical staining. Endogenous peroxidase activity was blocked by incubating sections in 3% hydrogen peroxide for 30 min. Antigen retrieval was performed in citrate buffer (10 mM, pH=6) for 30 min at 95°C in a pressure cooker (YQ50-90A, PESKOE®, Guangzhou, China). Anti-CD24 (sc-7034, Santa Cruz) was applied, and sections were incubated at 4°C overnight. Sections were washed with PBS and then incubated with a biotinylated secondary antibody at 37°C for 2 h and then exposed to a streptavidin complex (HRP; Beyotime, Beijing, China). Positive reactions were visualized using 3, 3’-diaminobenzidine tetrahydrochloride (DAB; Beyotime), followed by counterstaining with hematoxylin (Beyotime).
Statistical analysis
To verify the association between CD24 expression and the other variables, chi-square tests were performed. Overall survival (OS) was defined as the length of time from the date of operation to death. To study OS, Kaplan-Meier curves and the log rank test were used. The confidence interval was 5%. All the statistical analyses and graphics were performed with GraphPad Prism 5.
Results
CD24 expression in cutaneous malignant melanoma specimens
RT-PCR analysis was performed in order to determine the level of CD24 mRNA in 33 cutaneous malignant melanoma specimens. CD24 mRNA in cancer tissue was significantly higher than in normal tissue (Figure 1A, P<0.05). To determine whether CD24 proteins were also increased, western-blot analysis for CD24 expression was performed. Results show that the levels of CD24 proteins were coincident with the levels of CD24 mRNA (Figure 1B, P<0.05). The immunohistochemical staining results were consistent with the western blot analysis results. The staining pattern includes a membranous staining, cytoplasmic staining, or both (Figure 1C).
Figure 1.
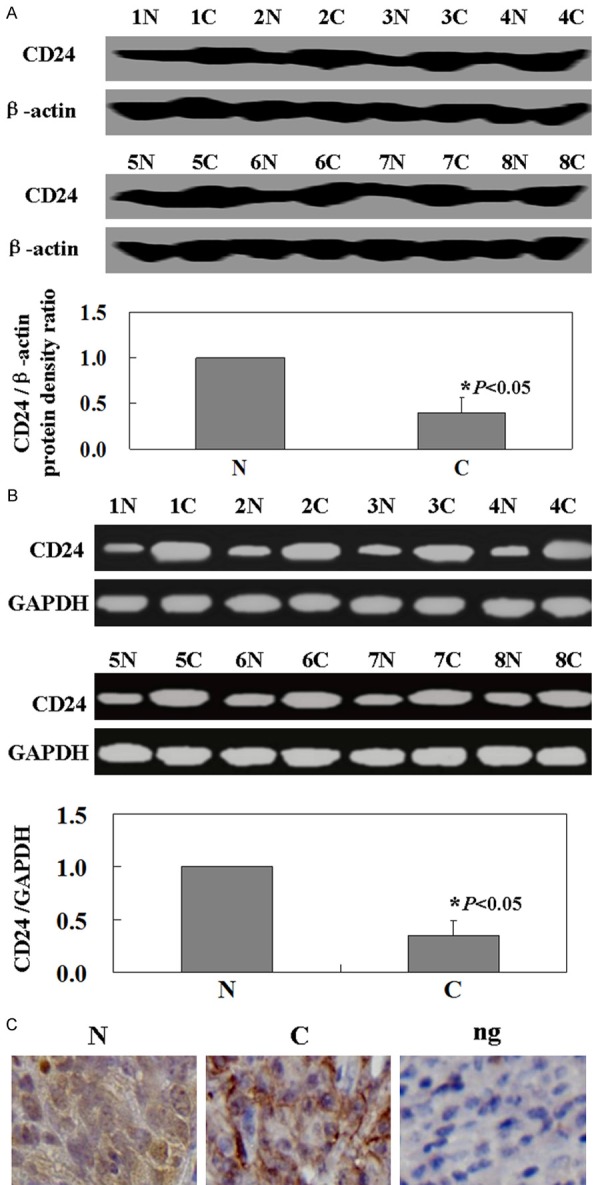
Levels of CD24 in the specimens of patients with melanoma. A. The levels of CD24 mRNA were measured by using RT-PCR. The levels of CD24 mRNA were higher in cancer tissues than matched normal tissues (P<0.05). GAPDH was used as an internal control. B. Representative results of paired of cancer tissue and corresponding normal tissue by western-blot. CD24 proteins were higher in cancer tissues than matched normal tissues (P<0.05). β-actin was used as an internal control. C. Immunohistochemical staining for CD24 protein in specimens. The nuclei were counterstained with hematoxylin. N: normal tissue; C: cancer tissue; Ng: negative control.
Association between CD24 expression and the clinicopathological features of cutaneous malignant melanoma
The association of CD24 expression with the clinicopathological features of patients with cutaneous malignant melanoma was shown in Table 1. We did not find any significant association of CD24 staining with patients’ gender and age, tumor thickness, ulceration, lymph node metastasis and tumor subtype. The association between 5-year survival rate and the expression of CD24 was analyzed using Kaplan-Meier method. The median survival times of patients presenting tumors with and without CD24 expression were 7 and 23 months, respectively. Accordingly, negative CD24 expression was closely correlated with the favorable prognosis of patients with cutaneous malignant melanoma (Figure 2, P<0.05).
Table 1.
Relationship between CD24 expression and clinicopathological parameters of cutaneous malignant melanoma
| Clinicopathological features | n | CD24 expression | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| - | + | ++ | +++ | PR (%) | χ2 | P | ||
| Sex | 0.52 | 0.91 | ||||||
| Female | 14 | 2 | 4 | 6 | 2 | 85.7 | ||
| Male | 19 | 3 | 6 | 6 | 4 | 84.2 | ||
| Age (years) | 3.89 | 0.27 | ||||||
| <65 | 15 | 3 | 2 | 7 | 3 | 80.0 | ||
| ≥65 | 18 | 2 | 8 | 5 | 3 | 88.9 | ||
| Tumour thickness (mm) | 3.83 | 0.28 | ||||||
| <1.0 | 11 | 1 | 3 | 3 | 4 | 90.9 | ||
| ≥1.0 | 22 | 4 | 7 | 9 | 2 | 81.8 | ||
| Ulceration | 0.31 | 0.95 | ||||||
| - | 24 | 4 | 7 | 9 | 4 | 83.3 | ||
| + | 9 | 1 | 3 | 3 | 2 | 88.9 | ||
| Lymph node metastasis | 4.17 | 0.24 | ||||||
| - | 16 | 4 | 4 | 4 | 4 | 75.0 | ||
| + | 17 | 1 | 6 | 8 | 2 | 94.1 | ||
| Tumor subtype | 3.61 | 0.73 | ||||||
| ALM | 20 | 3 | 8 | 7 | 2 | 85.0 | ||
| NM | 7 | 1 | 1 | 3 | 2 | 85.7 | ||
| SSM | 6 | 1 | 1 | 2 | 2 | 83.3 | ||
Abbreviations: ALM: acral lentiginous melanoma; NM: nodular melanoma; SSM: superficial spreading melanoma; PR: positive rate; χ2: Chi-square distribution.
Figure 2.
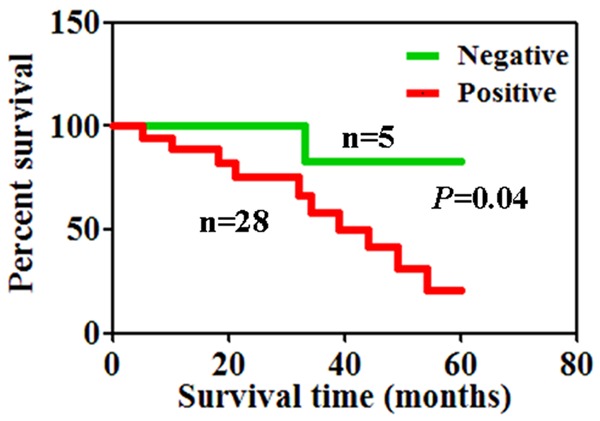
CD24 protein and prognosis of patients with melanoma. Kaplan-Meier curve survival analysis indicating that tumors with CD24 expression had poorer disease specific survival than those without CD24 expression.
Discussion
In this study, we determined the relationships between CD24 expression and melanoma. As noted in Introduction, CD24 is overexpressed in various human malignancies [6-10]. Consistent with previous studies, in this work, we also confirmed CD24 is higher in cancer tissues than that in normal tissues of patients with melanoma. As the results of IHC, we found that CD24 is localized in cytomembrane and cytoplasm. In agreement with the previous study [13], the CD24 protein expression in NSCLC appeared mainly in the cytoplasm and circumferential membrane. However, opposite conclusions is still ambiguous. Mylona et al. [14] considered mainly membrane CD24, whereas Honeth et al. [15] considered CD24 staining at the cytoplasm, possibly explaining. Moreover, the extension of staining in different tissues is needed to carry out in further studies.
Furthermore, we demonstrated that an independent prognostic value of CD24 positive expression for melanoma. Similar results were obtained by Weichert et al. [6] in colorectal adenocarcinoma, and Kristiansen et al. [8] in prostate cancer. However, we didn’t find any associations between CD24 with the clinicopathological features of melanoma. Jacob et al. [16] reported that CD24 was expressed in 71.6% of pancreatic adenocarcinomas and was correlated with higher tumor grades. In pancreatic cancer patients, CD24 expression is correlated with pT status, nodal metastasis, and histologic grade [17]. This discrepancy may be caused by different tissues. Another reason is that surgical resection is not the primary therapy for melanoma. So we didn’t obtain the specimens of the patients with all stage melanoma.
In conclusion, we demonstrated for the first time that CD24 is an important marker of malignancy and poor prognosis in melanoma. CD24 may be used as a new drug target for cutaneous malignant melanoma.
Disclosure of conflict of interest
None.
References
- 1.Garbe C, Leiter U. Melanoma epidemiology and trends. Clin Dermatol. 2009;27:3–9. doi: 10.1016/j.clindermatol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC Jr, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann P, Cremers N, Kroese F, Orend G, Chiquet-Ehrismann R, Uede T, Yagita H, Sleeman JP. CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res. 2005;65:10783–10793. doi: 10.1158/0008-5472.CAN-05-0619. [DOI] [PubMed] [Google Scholar]
- 4.Bircan S, Kapucuoglu N, Baspinar S, Inan G, Candir O. CD24 expression in ductal carcinoma in situ and invasive ductal carcinoma of breast: an immunohistochemistry-based pilot study. Pathol Res Pract. 2006;202:569–576. doi: 10.1016/j.prp.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Kristiansen G, Winzer KJ, Mayordomo E, Bellach J, Schlüns K, Denkert C, Dahl E, Pilarsky C, Altevogt P, Guski H, Dietel M. CD24 expression is a new prognostic marker in breast cancer. Clin Cancer Res. 2003;9:4906–4913. [PubMed] [Google Scholar]
- 6.Weichert W, Denkert C, Burkhardt M, Gansukh T, Bellach J, Altevogt P, Dietel M, Kristiansen G. Cytoplasmic CD24 expression in colorectal cancer independently correlates with shortened patient survival. Clin Cancer Res. 2005;11:6574–6581. doi: 10.1158/1078-0432.CCR-05-0606. [DOI] [PubMed] [Google Scholar]
- 7.Chou YY, Jeng YM, Lee TT, Hu FC, Kao HL, Lin WC, Lai PL, Hu RH, Yuan RH. Cytoplasmic CD24 expression is a novel prognostic factor in diffuse-type gastric adenocarcinoma. Ann Surg Oncol. 2007;14:2748–2758. doi: 10.1245/s10434-007-9501-x. [DOI] [PubMed] [Google Scholar]
- 8.Kristiansen G, Pilarsky C, Wissmann C, Kaiser S, Bruemmendorf T, Roepcke S, Dahl E, Hinzmann B, Specht T, Pervan J, Stephan C, Loening S, Dietel M, Rosenthal A. Expression profiling of microdissected matched prostate cancer samples reveals CD166/MEMD and CD24 as new prognostic markers for patient survival. J Pathol. 2005;205:359–376. doi: 10.1002/path.1676. [DOI] [PubMed] [Google Scholar]
- 9.Kristiansen G, Schluns K, Yongwei Y, Denkert C, Dietel M, Petersen I. CD24 is an independent prognostic marker of survival in non-small cell lung cancer patients. Br J Cancer. 2003;88:231–236. doi: 10.1038/sj.bjc.6600702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kristiansen G, Sammar M, Altevogt P. Tumour biological aspects of CD24, a mucin-like adhesion molecule. J Mol Histol. 2004;35:255–262. doi: 10.1023/b:hijo.0000032357.16261.c5. [DOI] [PubMed] [Google Scholar]
- 11.Tarhini AA, Agarwala SS. Cutaneous melanoma: available therapy for metastatic disease. Dermatol Ther. 2006;19:19–25. doi: 10.1111/j.1529-8019.2005.00052.x. [DOI] [PubMed] [Google Scholar]
- 12.Fecher LA, Flaherty KT. Where are we with adjuvant therapy of stage III and IV melanoma in 2009? J Natl Compr Canc Netw. 2009;7:295–304. doi: 10.6004/jnccn.2009.0022. [DOI] [PubMed] [Google Scholar]
- 13.Lee HJ, Choe G, Jheon S, Sung SW, Lee CT, Chung JH. CD24, a novel cancer biomarker, predicting disease-free survival of non-small cell lung carcinomas: a retrospective study of prognostic factor analysis from the viewpoint of forthcoming (seventh) new TNM classification. J Thorac Oncol. 2010;5:649–657. doi: 10.1097/JTO.0b013e3181d5e554. [DOI] [PubMed] [Google Scholar]
- 14.Mylona E, Giannopoulou I, Fasomytakis E, Nomikos A, Magkou C, Bakarakos P, Nakopoulou L. The clinicopathologic and prognostic significance of CD44+/CD24(-/low) and CD44-/CD24+ tumor cells in invasive breast carcinomas. Hum Pathol. 2008;39:1096–2102. doi: 10.1016/j.humpath.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Honeth G, Bendahl PO, Ringner M, Saal LH, Gruvberger-Saal SK, Lövgren K, Grabau D, Fernö M, Borg A, Hegardt C. The CD44+/CD24- phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10:R53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacob J, Bellach J, Grutzmann R, Alldinger I, Pilarsky C, Dietel M, Kristiansen G. Expression of CD24 in adenocarcinomas of the pancreas correlates with higher tumor grades. Pancreatology. 2004;4:454–460. doi: 10.1159/000079824. [DOI] [PubMed] [Google Scholar]
- 17.Ikenaga N, Ohuchida K, Mizumoto K, Yu J, Kayashima T, Hayashi A, Nakata K, Tanaka M. Characterization of CD24 expression in intraductal papillary mucinous neoplasms and ductal carcinoma of the pancreas. Hum Pathol. 2010;41:1466–1474. doi: 10.1016/j.humpath.2010.04.004. [DOI] [PubMed] [Google Scholar]


