Abstract
Insomnia is commonly seen in elderly populations and is associated with numerous individual and socioeconomic consequences. Elderly patients are more likely to suffer from chronic insomnia characterized by difficulty maintaining sleep than difficulty initiating sleep. Management of insomnia in these patients requires very careful evaluation and exclusion of an underlying medical or psychiatric condition. Nonpharmacologic interventions in elderly patients, especially use of behavioral therapy, have demonstrated some success. Commonly prescribed medications have also been effective, though they have limitations. Newer agents currently under investigation for insomnia hold promise for good efficacy and safety in the elderly population. The following review presents clinical studies, survey results, and guidelines retrieved from peer-reviewed journals in the PubMed database using the search terms elderly, temazepam, trazodone, zolpidem, zaleplon, insomnia, and prevalence and the dates 1980 to 2003. In addition, newer research with emerging agents has been included for completeness.
Insomnia is a condition that is underrecognized, underdiagnosed, and undertreated in the general population.1 Despite being a common complaint among elderly people (aged 65 years and older), sleep disorders are rarely systematically diagnosed and treated, even by geriatric specialists.2 Insomnia is a serious problem among older individuals because of its widespread prevalence and because poor sleep can have detrimental consequences for many of the aspects of vitality and resilience required for successful aging.3 Sleep disturbances among the elderly are associated with significant morbidity and mortality and increase the risk for nursing home placement.4,5 Insomnia is also correlated with risk for falls.6 Sleep maintenance, rather than sleep initiation, is the most commonly reported problem among older people with sleep disturbance2,7,8 and can have serious consequences.8,9 However, while a range of treatment options exists, there is currently a lack of pharmacologic agents that provide an optimum combination of therapeutic benefits. Ideal pharmacologic outcomes would include improved sleep initiation, sleep maintenance without next-day residual effects, and, ideally, improved next-day functioning.
EPIDEMIOLOGY OF INSOMNIA IN THE ELDERLY
In 1982, the National Institute on Aging conducted a multicenter, epidemiologic study to assess the prevalence of sleep complaints among more than 9000 non-institutionalized elderly persons aged 65 years and older. Over half (57%) of these elderly people reported some form of chronic disruption of sleep, while only 12% reported no sleep complaints.7 Among all participants (N = 9282; mean age = 74 years), the prevalence of chronic sleep complaints included difficulty in initiating or maintaining sleep (43%), nocturnal waking (30%), insomnia (29%), daytime napping (25%), trouble falling asleep (19%), waking too early (19%), and waking not rested (13%).7 A 3-year follow-up study reported an annual incidence rate of approximately 5%, with roughly 15% of elderly insomniacs resolving their symptoms each year.10 Chronic insomnia is also more common in this population. A 1991 National Sleep Foundation poll of a representative sample of 1000 Americans aged 18 years or older, who were divided by age into 6 groups (18–24, 25–34, 35–44, 45–54, 55–64, and ≥ 65), found that 9% of the sample reported chronic insomnia, while 20% in the group ≥ 65 years reported chronic insomnia, the highest among all age groups.11
BURDEN OF INSOMNIA IN THE ELDERLY
Insomnia incurs a significant direct and indirect burden on society. Direct economic costs of insomnia were calculated to be $13.9 billion in 1995,12 and a 1996 review indicated that total direct, indirect, and related costs may run as high as $30 to $35 billion annually.13
While the overall economic costs of insomnia specifically in the elderly population have not been assessed to date, several studies14,15 have provided data on segmented direct and indirect costs and on adverse effects on quality-of-life parameters in the elderly. Insomnia may precipitate injuries, such as falls, and aggravate existing health conditions. In a survey of 1526 community-dwelling older adults aged 64 to 99 years, difficulties with “falling asleep at night,” “waking during the night,” and “waking up in the morning” were significantly related to the number of reported falls.6 Subsequent fall-related injuries are an important factor for nursing home placement.4 Estimates indicate that of the $158 billion of lifetime economic costs of injury in the United States, fall-related injuries will contribute a total of $10 billion.6
A 1995 assessment of health care service costs found that nursing home care related to insomnia in the elderly amounted to $10.9 billion (91% of all health care services related to insomnia, across all age groups).12 Sleep disturbances in the elderly, and the subsequent disruption of caregivers' sleep, exact a toll on family support. Insomnia has been cited as a primary factor in caregivers' decisions to institutionalize an elder, with 20.4%12 and 52%16 of admissions to long-term care directly attributable to elderly sleep disturbances. A survey of 1855 elderly urban residents found that insomnia was the strongest predictor among males for both mortality and nursing home placement.5 Insomnia may also contribute to cognitive decline,17 and insomnia-induced cognitive impairments can confound accurate dementia diagnoses and lead to suboptimal and delayed treatment.4
FOCUS ON SLEEP MAINTENANCE
Insomnia is associated with difficulty in initiating sleep (i.e., is a problem of sleep onset), maintaining sleep, or obtaining restorative sleep18; however, the elderly spend more time awake after initially falling asleep than their younger counterparts < 65 years, and sleep maintenance problems are therefore the primary symptoms in this age group.2,8,19 Foley et al.7 reported that 49% of elderly patients experienced sleep maintenance symptoms (30% complained of waking during the night; 19% complained of waking too early), compared with only 19% who experienced the sleep-onset symptom—difficulty falling asleep.
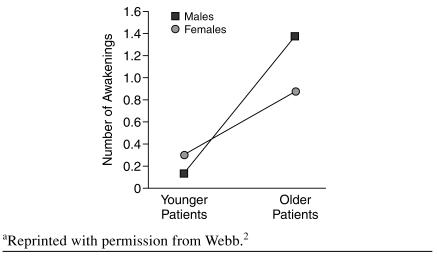
These findings have been confirmed in a study that utilized objective measures. Webb2 compared electroencephalograph measures of 80 healthy older adults (aged 50–60 years) and a control group of 32 younger adults (aged 20–30 years). Sleep in the older group was characterized by more frequent and prolonged awakenings (Figure 1). Among older men, wake after sleep onset, a robust measure of poor sleep maintenance, defined in this study as time (in minutes) awake after sleep onset/time asleep and expressed as a percentage, was increased approximately 8-fold over that of younger men (8.1 vs. 1.2, respectively; p < .01). Number of awakenings lasting 5 minutes or longer were more frequent and of longer duration in the older groups (1.4 in men and 0.9 in women, aged 50–60 years; 0.1 in men and 0.3 in women, aged 20–30 years; p < .01 for comparison among older and younger groups in both genders).2
Figure 1.
Number of Awakenings in Men and Women as a Function of Agea
Sleep maintenance dysfunction contributes to the daytime fatigue and napping common among elderly people and can have negative repercussions on next-day functioning.8,9 Therefore, there is a special need for agents that target both sleep initiation and sleep maintenance without residual next-day effects. An improvement in next-day functioning could have a substantial positive impact on patients and their caregivers.
ASSESSMENT AND DIAGNOSIS OF INSOMNIA IN ELDERLY PATIENTS
There are currently no guidelines that indicate how much sleep is normal for elderly people; however, changes that occur and progress gradually as we age are well documented. Healthy elderly people are prone to spending more time in bed, with no additional time spent asleep.20 They are also more likely to spend more time in stage 1 (light) sleep and less time in deep or slow-wave sleep.20 Abnormal breathing events21 and leg movements22 are also more common in the elderly (≥ 65 years) than in younger adults.
Appropriate recognition of insomnia in elderly patients is vital, as evidenced by the high use of nonpre-scription remedies in this population. A recent survey administered to ambulatory elderly subjects found that 48% had used one or more therapies for sleep within the past year; 50% of those therapies were nonprescription products. Of the 27% of subjects who used such products, 19% used acetaminophen, 15% used diphenhydramine, and 13% used alcohol.23
A few probing questions should be sufficient to detect insomnia (Table 1), followed by queries into the chronicity and causality of the insomnia complaint. Transient insomnia is defined by problems lasting just a few nights, while chronic insomnia is defined as persistent problems lasting at least 1 month.18
Table 1.
Questions to Include When Taking a Sleep History in Elderly Patientsa

Because sleep complaints are fairly common in this population, it is important for the physician to consider 2 major factors: the real nature of the complaint and the presence of underlying medical or psychiatric causes. Some patients attain adequate amounts of total sleep, but they do so by napping during the day or at other unconventional times. Such patients may have advanced or delayed sleep phase syndrome,8 rather than insomnia, and in order to establish a complete picture of a patient's sleep complaint, a thorough sleep history should be taken24 (examples of important questions are listed in Table 1). If there is no or minimal impairment in daytime functioning (such as impaired memory, sudden unplanned napping, mood changes, sleepiness, or falling asleep while driving), patients may simply need to be reassured that their symptoms are part of normal aging. It is often useful to ask that the patient maintain a 2-week sleep diary so that an accurate account of sleeping habits can be recorded.8 Bed partners are useful sources of information and can often shed light on diagnoses that would otherwise not be elicited and give more detailed and reliable accounts of sleep and waking habits. A careful review of medical/ sleep history and medication records should also be conducted to ensure proper diagnosis and treatment and to exclude non-insomnia sleep disorders such as sleep apnea, restless legs syndrome, or periodic leg movements. Sleep histories should always include specific questions about illnesses if they are suspected; for example, asking about snoring may elicit a sleep apnea diagnosis and questions regarding leg discomfort should help elicit the diagnosis of restless legs syndrome.
Referral and consultation should be considered in cases of suspected primary sleep conditions and sleep apnea, which could be better managed by a sleep disorder expert. Surprisingly, many persons with insomnia deny daytime sleepiness. A complaint of daytime sleepiness, coupled with other symptoms, should trigger consideration of a primary sleep disorder such as sleep apnea. The Epworth Sleepiness Scale25 is a well-validated, self-administered test for excessive sleepiness that the patient can complete in the office in less than 2 minutes. A score above 10 indicates excessive sleepiness and warrants further investigation, such as referral to a sleep specialist (Table 2).
Table 2.
Example of the Epworth Sleepiness Scalea

As sleep disturbances among the elderly are often secondary to existing chronic disease, overall poor physical health, and psychosocial morbidity, it is important for practitioners to assess if insomnia is a primary or secondary condition.9 The principal medical or psychiatric condition, as well as medications used to treat it (e.g., cardiovascular medication), may cause sleep disruption and contribute to daytime sleepiness.4,10,26,27 Common causes of insomnia in the elderly are listed in Table 3.
Table 3.
Common Causes of Insomniaa

Longitudinal data have consistently established depression as one of the strongest correlates of insomnia,10,28,29 and while the causal relationship between insomnia and depression remains unclear, it is important to exclude the possibility of a depressive episode in an elderly patient who presents with symptoms of insomnia. The Beck Depression Inventory30 is one of many useful, well-validated, self-administered scales to detect depression symptoms. A score above 10 suggests clinical depression might be present and warrants a more thorough investigation of whether it is, in fact, depression (Table 4).
Table 4.
The 21-Item Beck Depression Inventorya,b,c

Due to the high prevalence of comorbidities and use of concomitant prescription medication in the elderly population, it is imperative that the primary treatment goal be to identify and address any underlying condition, as well as the potential for drug-drug interaction with the patient's existing medication regimen. Although limited research demonstrates that treating the insomnia symptom improves the primary condition, renewed interest exists in this area, with some indication that addressing secondary insomnia may have adjunctive benefits in treating depression31 and may improve quality of life in dementia disorders and Parkinson's disease.32
MANAGEMENT OF INSOMNIA
Nonpharmacologic Therapies
Most treatment guidelines recommend that nonpharmacologic approaches to insomnia control, including sleep hygiene and behavioral methods, be used as supportive therapies.3,4,33,34 Sleep hygiene rules are listed in Table 5.3,4,35,36,37 Behavioral therapy techniques, such as cognitive-behavioral therapy, may be used either alone or in combination with pharmacotherapy and may aid in long-term management of insomnia following medication discontinuation.33 Stimulus control, progressive muscle relaxation, and paradoxical intention meet American Academy of Sleep Medicine criteria for empirically supported psychological treatments for insomnia; sleep restriction, biofeedback, and multifaceted cognitive-behavioral therapy were the treatments considered most likely to be efficacious.38 There are very few data regarding behavioral therapy in primary care.33,39 The effectiveness and extent of use of this treatment in the primary care setting has yet to be determined.
Table 5.
Sleep Hygiene Rulesa

Pharmacologic Therapies
Pharmacologic therapy should take into consideration the pharmacokinetic and pharmacodynamic changes in drug metabolism that typically accompany the aging process. Caution should be exercised in selecting appropriate medication and medication dosages for treatment of insomnia in elderly patients.35 Medications that impair cognitive and psychomotor function can have serious consequences for elderly patients who are institutionalized and for those living in the community.
Use of benzodiazepines has been correlated with an increased risk of falling,40–43 and a higher serum concentration of benzodiazepines has been noted in those who fall compared with those who do not fall.44 Falls appear to be associated with the use of both short- and long-term benzodiazepines.45 Cumming and Klineberg46 found that use of the relatively short-acting temazepam, the most commonly used of all the benzodiazepines for insomnia treatment,47 increased risk of falls compared with nonusers.46 Diazepam was also found to be a risk factor for multiple falls in one study (odds ratio = 3.7, 95% CI = 1.5 to 9.3).41
More recent studies have indicated that excessive benzodiazepine dosage may be a more salient factor than drug half-life.48 According to Beers' 1997 criteria for determining potentially inappropriate medication use in the elderly, short- to intermediate-acting benzodiazepines (e.g., temazepam) and zolpidem are to be considered inappropriate if maximum recommended doses are exceeded.49 Although very little research has been conducted to evaluate the use of agents like temazepam and zolpidem in the naturalistic setting, a recent review50 of the pharmacy profiles of 2193 homebound people older than age 60 years was conducted. Of these people, 285 patients were prescribed excessive doses of temazepam and zolpidem. It was determined that 28% of short- to intermediate-acting benzodiazepine prescriptions and 60% of zolpidem prescriptions exceeded recommended dosing limitations,50 which suggests that excessive dosing does indeed occur.
Despite the increased risk for falls, benzodiazepines and generic antidepressants (such as trazodone) are among the most popular classes of medications prescribed for elderly patients.47,51 Benzodiazepines were second only to cardiac medications in frequency of prescription, and there was a high prevalence of antidepressant drugs.50 An analysis of inappropriate (risk > benefit) psychotropic prescribing, using data from the 1996 National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey, determined that antidepressant agents, antianxiety drugs, and sedative-hypnotics were the drug classes most frequently prescribed to ambulatory elderly patients.52
Consensus guidelines regarding the treatment of insomnia established in 198453 and now considered obsolete by the National Institutes of Health (NIH) appear to have influenced the U.S. Food and Drug Administration's (FDA) decision to restrict prescription of hypnotic medications to a maximum of 1 month.54 This restriction may have contributed to increased utilization of antidepressants for treating insomnia among physicians, as there is evidence that chronic insomnia frequently persists far beyond 1 month, especially in elderly patients.55 These guidelines were formulated, in part, on the basis of the dearth of research exploring longer-term safety and efficacy of these agents. Recently, however, longer-term double-blind56 and open-label57,58 studies have demonstrated the safety of nonbenzodiazepine agents in adults and elderly patients with chronic insomnia. In the future, such findings may contribute to increased confidence for longer-term usage of these medications. So, in the absence of widely accepted algorithms for the use of hypnotics, common sense dictates that hypnotics are justified for short-term, symptomatic relief of transient insomnia, and for short-term relief of chronic insomnia in patients who are frantic and in crisis about their condition. Anxious patients may not be willing to wait for the delayed onset of behavioral therapy for primary insomnia or the delayed onset of treatments for secondary insomnia (i.e., antidepressant treatment of major depression). Hypnotics may be avoided, at least initially, in patients with chronic insomnia who are not anxious and are willing to wait a few weeks to see if alternative treatments work. Hypnotics could be judiciously added later if the initial approach is not fruitful.
In terms of overall trends in insomnia treatment, a 10-year analysis of pharmaceutical data from the National Disease and Therapeutic Index (NDTI) indicated a dramatic decrease in overall pharmacologic treatment of insomnia from 1987 to 1996.51 The NDTI provides descriptive information on disease and treatment patterns in U.S. private medical practices and includes 2790 office-based physicians drawn from 24 medical specialties. Hypnotic drug mentions decreased 53.7%, while antidepressant mentions for insomnia treatment increased 146%. There was a substantial shift away from the use of benzodiazepines and toward the prescribing of antidepressants and nonbenzodiazepine hypnotics. In 1996, NDTI data indicated that trazodone and zolpidem were the 2 drugs prescribed most frequently for treatment of insomnia.51 Temazepam is the most commonly prescribed benzodiazepine for insomnia.47 Therefore, based on current utilization patterns, the following section will review pertinent data on temazepam, trazodone, zolpidem, and the newest nonbenzodiazepine—zaleplon—for the treatment of insomnia in elderly patients.
Temazepam
Temazepam is currently the most commonly prescribed benzodiazepine hypnotic for insomnia.47 As a short-acting benzodiazepine, with an elimination half-life of approximately 8 hours, it carries less danger of dependence than ultra–short-acting benzodiazepines.59,60 Temazepam use has, however, been associated with the development of tolerance over time,61 and long-term use exceeding 4 to 5 weeks is not recommended.59 For the elderly, the standard dose of 30 mg/day is reduced to 15 mg; as noted previously, prescribing patterns commonly exceed dosage recommendations and too-high dosage levels may increase the risk for falls.48,50
In adult studies, temazepam has not objectively demonstrated efficacy in maintaining sleep.62–64 Data are limited for benzodiazepine efficacy in the elderly population.59 One large-scale study65 of 335 elderly insomniacs found that temazepam significantly decreased subjective sleep latency at weeks 1, 3, and 4 versus placebo and only significantly increased subjective sleep duration during week 1. Temazepam use produced a significantly higher incidence of daytime drowsiness (temazepam, 11.9% vs. placebo, 3.6%) and fatigue (temazepam, 6% vs. placebo, 1.2%) than placebo.65 Overall, despite its wide prescription usage, temazepam has not been as extensively studied as other benzodiazepines, and conclusions on the basis of the majority of existing studies are hampered by small sample sizes (18–78 subjects).33,66,67 Although it may be unclear how much of the general benzodiazepine data can be extrapolated specifically to temazepam, benzodiazepines as a pharmaceutical class confer a serious risk of adverse effects, including next-day sedation,68 impaired delayed and immediate recall,69–71 cognitive impairment,72–75 and risk for abuse and dependence.62,76 For the elderly, benzodiazepines have been reported to be a major, independent risk factor for falls leading to femur fractures.48 In one population case-controlled study of 416 elderly subjects, temazepam was associated with a nearly 4-fold increase in risk of hip fracture.46 Due to the high risks and consequences faced by this vulnerable population, many researchers warn that caution should be exercised in prescribing benzodiazepines for elderly patients.43,46,48
Trazodone
Trazodone is a triazolopyridine derivative, chemically and pharmacologically distinct from other antidepressants.77 Trazodone's precise mechanisms of action are unclear. Due to its sedating qualities, trazodone has increasingly been prescribed off-label at subtherapeutic antidepressant doses of 100 mg or less for the treatment of insomnia.4,52 Trazodone prescription for antidepressant purposes has decreased, while its use as an agent for insomnia has substantially increased.51 The recommended maximum tolerated doses of trazodone for elderly patients for the treatment of depression are 300 to 400 mg/day.77,78 The pharmacokinetics of trazodone have been shown to be dependent on age, primarily due to decreased oxidative metabolism in older patients.78,79 The half-life of trazodone is significantly longer in patients older than 69 years compared with younger adults (mean age, 24 years) (11.6 vs. 6.4 hours, respectively),78 and clearance rates are also significantly decreased.78 This difference is most likely influenced by the increased presence of chronic illness and debility in elderly patients.
Despite trazodone's wide clinical use, there is a paucity of clinical trials assessing its effectiveness in the treatment of insomnia and, specifically, in the geriatric population. Most studies have examined trazodone's antidepressant efficacy in this population. In one study80 that evaluated the efficacy of trazodone treatment of primary insomnia in adult patients aged 50 to 70 years (mean age, 61 years), 9 patients received trazodone, 150 mg/night, for 3 weeks. There was no control group. Compared with baseline, trazodone improved subjective sleep quality during weeks 1 and 2 (visual analog scale; p < .001), but not during week 3. There was no improvement with trazodone use on objective polysomnographic measures of sleep-onset latency or total sleep time, in spite of reduced wake after sleep onset (p < .05). Rebound insomnia was significant after trazodone discontinuation on the second withdrawal night (p < .05).80 Other studies have consistently shown that trazodone has negative subjective residual effects, including next-day sedation and worsening of feelings on or after awakening.81 Given the pharmacokinetic implications of trazodone use in the elderly, it is conceivable that elderly patients are more likely to suffer from these effects than are younger patients.82,83
Trazodone is associated with a number of side effects and drug-drug interactions, which may have important implications for elderly patients. Commonly reported adverse effects of trazodone based on pooled data in adult populations include gastrointestinal disorders, such as constipation (13.6%), nausea and vomiting (15.7%), headache (10.4%), blurred vision (8.3%), dry mouth (17.7%), and hypotension (10.1%).84 Dizziness and sedation occur in as many as 21.9% of patients.84 Other central nervous system adverse events are also common, such as anxiety and fatigue. Priapism, which is reported to occur at rates between 1 in 10,000 and 1 in 1000, has been documented even at doses of 50 to 100 mg/day.77,85
Several sleep experts, on the basis of this problematic evidence, have expressed reservations about trazodone's widespread usage. In a clinical review, Ancoli-Israel suggests: “The safety and efficacy of antihistamines and trazodone for use as hypnotics in the elderly have not been adequately evaluated and are not recommended for the treatment of insomnia in the elderly.”4(p.S27) Walsh and Schweitzer comment, “The observation that trazodone is used more often than any other prescription medication [for insomnia] is startling given the dearth of hypnotic efficacy data.”51(p.374) In addition, a 1990 NIH Consensus Panel statement concluded that the safety and efficacy of trazodone for use as a hypnotic in the elderly have not been evaluated, and it is not recommended for this population.86 There are no more recent guidelines available.
Nonbenzodiazepine Hypnotics
Because of their shorter half-lives, the nonbenzodiazepine hypnotics zolpidem and zaleplon are effective agents for improving sleep onset, but not sleep maintenance. Shorter half-life may contribute to the reduced evidence of subjective and objective next-day residual effects associated with zolpidem in adult subjects87–89 as compared with benzodiazepines. There is also some experiential evidence that the nonbenzodiazepine hypnotics may be safer than the benzodiazepines in certain patient populations, for example, those at risk for respiratory depression90; more research is needed to confirm this. In addition, given that elderly patients frequently take numerous medications, it is important to consider the risk of drug-drug interactions when treating insomnia. There appears to be a lower risk of drug-drug interactions associated with zaleplon and zolpidem use,91 possibly related to the differences in xenobiotic metabolism.
Zolpidem.
Zolpidem is a nonbenzodiazepine hypnotic agent approved by the FDA in 1992 for the short-term treatment of insomnia. It is thought to exhibit a more selective binding action than benzodiazepines and may thus avoid some of the side effects associated with benzodiazepine agents.92,93 Zolpidem, in its standard-release form, has a short half-life (2.4 hours in adults; 2.9 hours in the elderly) with no active metabolite, and it does not accumulate during repeated dosing.93 Due to age-associated decreased clearance rates and volumes of distribution, the recommended dosing for elderly patients is reduced from 10 mg/day to 5 mg/day, with an increase to 10 mg/day in more severe cases of insomnia.92,93 In practice, however, most elderly patients are prescribed a dose above the 5-mg daily limit.50 Golden et al.50 found that 75 (60%) of the 125 elderly patients whose medication profiles they reviewed were prescribed zolpidem at doses above 5 mg/night.
As shown in Table 6, many of the zolpidem studies in the elderly population have been conducted with doses that are 2- to 4-fold higher than the recommended doses, which may make these studies less informative from a clinical perspective and may promote higher than recommended dosing in elderly patients.
Table 6.
Clinical Trials of Zolpidem in the Elderly
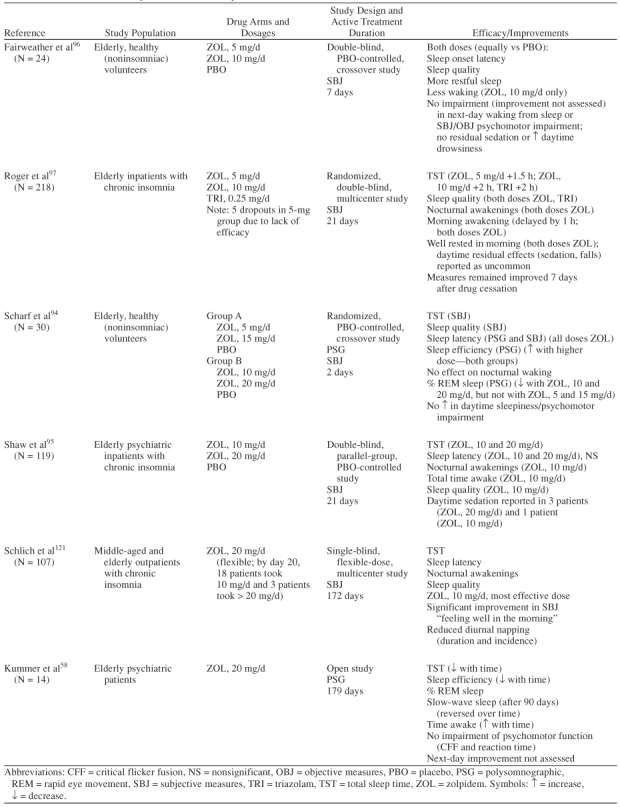
In addition, use of higher doses of zolpidem increases the potential for adverse events, as demonstrated here. While efficacy at the 5-mg dosage has been demonstrated for sleep-onset measures, there is less convincing evidence (either objective or subjective) of improvement in sleep-maintenance measures or absence of next-day impairment. Four studies, which included a total of 391 patients, were identified in a MEDLINE search, using the terms randomized controlled trial, zolpidem, and elderly, as randomized, controlled trials that focused on sleep effects of zolpidem in the elderly (Table 6).94–97 In the 1 randomized, controlled (and short-term [2-night]) crossover study that included objective measures, polysomnographic recordings showed dose-response effects for sleep latency and sleep efficiency with greater improvement at higher dosages (15 or 20 mg) than lower dosages (5 or 10 mg).94 However, zolpidem showed no objective effect on the number of nocturnal awakenings, a measure of sleep maintenance.94 Two studies conducted in adult patients that included objective measures have borne out that zolpidem does not appear to be effective in sleep maintenance measures (number of nocturnal awakenings and wake after sleep).89,98
In their review of zolpidem literature, Langtry and Benfield99 note that while zolpidem at a 5-mg dose or higher reduced sleep latency, dosages of 7.5 mg or higher were required to increase total sleep time.99,100 Other studies have failed to find a significant increase in total sleep time with zolpidem, 5 mg.101 Roger et al.97 reported in their subjective measure trial of 221 elderly patients that estimated total sleep time was more improved with zolpidem 10 mg and triazolam 0.25 mg (+2 hours) than with zolpidem 5 mg (+1.5 hours). In addition, 5 patients dropped out of the zolpidem 5-mg group (N = 70) due to a lack of efficacy, compared with 1 each in the zolpidem 10-mg (N = 74) and triazolam 0.25-mg (N = 77) groups.
Zolpidem has a desirable safety profile at recommended doses, with no significant rebound insomnia, withdrawal effects, pharmacologic tolerance, or drug interactions.92,93,99 However, higher doses (≥ 15 mg) of zolpidem increase the risk of adverse events, with overall incidence rates of adverse drug reactions increasing from 13.2% in elderly patients administered less than 15 mg daily compared with 20.3% in those receiving 15 mg or more.99 Postmarketing data from Europe found adverse events in the elderly occurring at a starting dose of 10 mg.93,102 Most adverse events have been reported at doses of 20 mg or higher, although studies in the elderly utilizing such doses are rare. Scharf et al.94 noted adverse events at 20 mg to be primarily associated with central nervous system symptoms (drowsiness, headache, light-headedness, vertigo) and gastrointestinal symptoms (nausea, vomiting). A case of zolpidem-induced psychosis in a 74-year-old woman administered 20 mg has also been reported,103 and effects have been observed on anterograde memory.104
Mental confusion and cognitive impairment are associated with increased risk for falls, and there is some evidence that hip fractures may be a specific risk associated with zolpidem use. Several literature reviews have indicated that higher zolpidem dosage and user age correlate with a significantly increased fall rate.77,101 Although dosing strata were not reported, a recent large-scale study of 1222 cases of hip fracture in elderly patients and 4888 age- and gender-matched controls observed a 90% increased risk of hip fracture in older (≥ 65 years) users of zolpidem.92
Zaleplon.
Zaleplon is a short-acting nonbenzodiazepine hypnotic, with an estimated half-life of 1 hour. Adult studies have demonstrated sleep onset, but not sleep maintenance, efficacy at the recommended 10-mg dose.105,106 It was noted, however, that improvements in total sleep time were not achieved below the 20-mg dose in either study.105,106 The 1 identified randomized, controlled trial of zaleplon in elderly insomniacs was of 2 weeks' duration, and results were based on subjective patient reports.107 Total sleep time and number of awakenings were improved with zaleplon 10 mg at week 1 only, while zaleplon 5 mg had no effect on total sleep time or number of awakenings.107
Over-the-Counter Remedies: Antihistamines
Antihistamines, particularly diphenhydramine, appear to be used frequently among elderly patients,4 especially in nursing home settings.67,108 Beers et al.109 studied medication use among 850 elderly residents of 12 representative intermediate-care facilities. Twenty-eight percent of patients were receiving sedating agents, and 26% of those patients were taking diphenhydramine. In another study of 2193 “homebound older adults” over age 60 years,50 approximately 10% of subjects were taking first-generation antihistamines. This use was despite a 1990 NIH Consensus Development Panel statement that concluded that the safety and efficacy of antihistamines for use as hypnotics in the elderly have not been evaluated, and the practice is not recommended.86 Since that time, little research has been conducted to advance the understanding of the use of such agents in elderly patients.
Cognitive side effects associated with antihistamine use are well documented and include next-day sedation110,111 and impaired psychomotor and cognitive function.111–114 Diphenhydramine, the most commonly used antihistamine for insomnia, is associated with toxicity and numerous drug-drug interactions.115 Other side effects include urinary retention and blurred vision,116 orthostatic hypotension, dizziness, and palpitations.110,116
CONCLUSIONS AND FUTURE DIRECTIONS
The elderly population in the United States is growing. The 2000 U.S. Census counted 35 million people over age 65 years, a 12% increase since 1990.117 As the elderly population grows, more attention is being placed on ensuring that quality of life is maintained during the aging process. Insomnia is a condition that disproportionately affects the elderly, as seen by high prevalence (57%) and incidence (5%) rates, and it carries a substantial personal, caregiver, and societal burden. Appropriate recognition and diagnosis of insomnia are thus extremely important. Office consultation should include questions regarding sleep history, and adequate attempts should be made to exclude other primary sleep conditions and underlying medical and psychiatric conditions so that these may be adequately treated.
The goal of insomnia treatment in the elderly is to improve sleep onset and maintenance, ideally with next-day benefits rather than residual effects, with attention to the risk of drug-drug interactions and safety profiles. Currently, commonly used available agents are associated with advantages and limitations, and much work needs to be done in order to understand the problems of elderly insomnia and the utility of various agents for insomnia in this population. Efficacy for sleep maintenance should be considered, along with safety and next-day performance effects. Clinical pharmacotherapy guidelines for geriatric patients recommend using the lowest effective dose, agents with shorter elimination half-lives, short-term treatment (3–4 weeks), gradual discontinuation, and monitoring for rebound insomnia.3,9
At present, there are no clear guidelines that direct the choice and utilization of pharmacologic and nonpharmacologic therapies, and trial and error is often used to determine the proper course of action. There is a clear need for prospective, controlled, randomized data of the safety and efficacy of anti-insomnia agents assessed specifically in elderly populations. Fortunately, 2 investigational agents in development have been prospectively assessed in this setting. Indiplon, a nonbenzodiazepine, has been assessed in 2 trials in the elderly. One 60-patient trial demonstrated sleep onset and maintenance over 2 nights of treatment, as determined by polysomnography.118 The other indiplon trial evaluated 42 patients and demonstrated sleep-onset efficacy and improved total sleep time over 2 nights of treatment, as determined by polysomnography. Wake after sleep onset was not reported.119 Eszopiclone, also a nonbenzodiazepine sedative hypnotic, has been assessed in a prospective, controlled trial of 231 elderly patients treated for 2 weeks.120 This study demonstrated improvements in patient-reported sleep onset, maintenance, total sleep time, sleep quality and depth, measures of next-day function, and several quality-of-life parameters. Eszopiclone also showed statistically significant decreases in the number and duration of naps. A similar 2-week prospective, randomized, placebo-controlled trial evaluating polysomnographic endpoints in 264 elderly patients receiving eszopiclone has completed enrollment. To date, data on both of these agents have been reported only at medical meetings and must be considered preliminary. As these trials represent some of the largest controlled data in this setting, they will add important clinical perspective on the treatment of insomnia in the elderly.
Drug names: acetaminophen (Phrenilin and others), albuterol (Ventolin), dextroamphetamine (Dexedrine and others), diazepam (Diastat and Valium), diphenhydramine (Ambenyl and others), ephedrine (Semprex-D, Trinalin, and others), methyldopa (Aldumet and others), methylphenidate (Ritalin, Concerta, and others), pemoline (Cylert), phenylephrine (Cyclomydril, Prometh VC, and others), phenytoin (Dilantin and others), quinidine (CinQuin and others), temazepam (Restoril), theophylline (Aerolate, Theolair SR, and others), trazodone (Desyrel), triazolam (Halcion), zaleplon (Sonata), zolpidem (Ambien).
Acknowledgments
The author acknowledges Sepracor Inc. (Marlboro, Mass.), Beatrice Mendez, Julia Goldrosen, and Jacqui Brooks, M.B.B.Ch., M.R.C.Psych., for assistance in the preparation of this article.
Footnotes
Dr. McCall has served as a consultant for Searle, Sanofi, Wyeth, and Sepracor; has received grant/research support from Sepracor, Sanofi, Searle, Wyeth, and Takeda; has received honoraria from Wyeth, Sepracor, and King; and has served on the speakers or advisory boards of Wyeth.
REFERENCES
- Roth T. Introduction: new developments for treating sleep disorders. J Clin Psychiatry. 2001 62suppl 10. 3–4. [PubMed] [Google Scholar]
- Webb WB. Sleep in older persons: sleep structures of 50- to 60-year-old men and women. J Gerontol. 1982;37:581–586. doi: 10.1093/geronj/37.5.581. [DOI] [PubMed] [Google Scholar]
- Reynolds CF III, Regestein Q, and Nowell PD. et al. Treatment of insomnia in the elderly. In: Salzman C, ed. Clinical Geriatric Psychopharmacology. 3rd ed. Baltimore, MD: Williams & Wilkins. 1998 395–416. [Google Scholar]
- Ancoli-Israel S. Insomnia in the elderly: a review for the primary care practitioner. Sleep. 2000 23suppl 1. S23–S30. [PubMed] [Google Scholar]
- Pollak CP, Perlick D, and Linsner JP. et al. Sleep problems in the community elderly as predictors of death and nursing home placement. J Community Health. 1990 15:123–135. [DOI] [PubMed] [Google Scholar]
- Brassington GS, King AC, Bliwise DL. Sleep problems as a risk factor for falls in a sample of community-dwelling adults aged 64–99 years. J Am Geriatr Soc. 2000;48:1234–1240. doi: 10.1111/j.1532-5415.2000.tb02596.x. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Monjan AA, and Brown SL. et al. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995 18:425–432. [DOI] [PubMed] [Google Scholar]
- McCall WV. Management of primary sleep disorders among elderly persons. Psychiatr Serv. 1995;46:49–55. doi: 10.1176/ps.46.1.49. [DOI] [PubMed] [Google Scholar]
- Martin J, Shochat T, Ancoli-Israel S. Assessment and treatment of sleep disturbances in older adults. Clin Psychol Rev. 2000;20:783–805. doi: 10.1016/s0272-7358(99)00063-x. [DOI] [PubMed] [Google Scholar]
- Foley DJ, Monjan A, and Simonsick EM. et al. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999 22suppl 2. S366–S372. [PubMed] [Google Scholar]
- Ancoli-Israel S, Roth T. Characteristics of insomnia in the United States: results of the 1991 National Sleep Foundation Survey, 1. Sleep. 1999 22suppl 2. S347–S353. [PubMed] [Google Scholar]
- Walsh JK, Engelhardt CL. The direct economic costs of insomnia in the United States for 1995. Sleep. 1999 22suppl 2. S386–S393. [PubMed] [Google Scholar]
- Chilcott LA, Shapiro CM. The socioeconomic impact of insomnia: an overview. Pharmacoeconomics. 1996 10suppl 1. 1–14. [DOI] [PubMed] [Google Scholar]
- Stoller MK. Economic effects of insomnia. Clin Ther. 1994;16:873–897. [PubMed] [Google Scholar]
- Zammit GK, Weiner J, and Damato N. et al. Quality of life in people with insomnia. Sleep. 1999 22suppl 2. S379–S385. [PubMed] [Google Scholar]
- Sanford JR. Tolerance of debility in elderly dependants by supporters at home: its significance for hospital practice. Br Med J. 1975;3:471–473. doi: 10.1136/bmj.3.5981.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49:1185–1189. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association, Inc. 2000 [Google Scholar]
- Reynolds CF III. Sleep disorders. In: Sadavoy J, Lazarus LW, Jarvik LF, et al, eds. Comprehensive Review of Geriatric Psychiatry-II. 2nd ed. Washington, DC: American Psychiatric Press, Inc. 1996 693–712. [Google Scholar]
- Hirshkowitz M, Moore CA, and Hamilton CR III. et al. Polysomnography of adults and elderly: sleep architecture, respiration, and leg movement. J Clin Neurophysiol. 1992 9:56–62. [PubMed] [Google Scholar]
- Hoch CC, Reynolds CF III, and Monk TH. et al. Comparison of sleep-disordered breathing among healthy elderly in the seventh, eighth, and ninth decades of life. Sleep. 1990 13:502–511. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Kales A, and Vela-Bueno A. et al. Nocturnal myoclonus and nocturnal myoclonic activity in the normal population. Res Commun Chem Pathol Pharmacol. 1982 36:129–140. [PubMed] [Google Scholar]
- Sproule BA, Busto UE, and Buckle C. et al. The use of non-prescription sleep products in the elderly. Int J Geriatr Psychiatry. 1999 14:851–857. [PubMed] [Google Scholar]
- Doghramji PP. Detection of insomnia in primary care. J Clin Psychiatry. 2001 62suppl 10. 18–26. [PubMed] [Google Scholar]
- Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Whitney CW, Enright PL, and Newman AB. et al. Correlates of daytime sleepiness in 4578 elderly persons: the Cardiovascular Health Study. Sleep. 1998 21:27–36. [DOI] [PubMed] [Google Scholar]
- Asplund R. Sleep disorders in the elderly. Drugs Aging. 1999;14:91–103. doi: 10.2165/00002512-199914020-00002. [DOI] [PubMed] [Google Scholar]
- Morgan K, Clarke D. Risk factors for late-life insomnia in a representative general practice sample. Br J Gen Pract. 1997;47:166–169. [PMC free article] [PubMed] [Google Scholar]
- Chang PP, Ford DE, and Mead LA. et al. Insomnia in young men and subsequent depression: The Johns Hopkins Precursors Study. Am J Epidemiol. 1997 146:105–114. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, and Mendelson M. et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961 4:561–571. [DOI] [PubMed] [Google Scholar]
- Londborg PD, Smith WT, and Glaudin V. et al. Short-term cotherapy with clonazepam and fluoxetine: anxiety, sleep disturbance and core symptoms of depression. J Affect Disord. 2000 61:73–79. [DOI] [PubMed] [Google Scholar]
- Shochat T, Loredo J, Ancoli-Israel S. Sleep disorders in the elderly. Curr Treat Options Neurol. 2001;3:19–36. doi: 10.1007/s11940-001-0021-x. [DOI] [PubMed] [Google Scholar]
- Morin CM, Colecchi C, and Stone J. et al. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999 281:991–999. [DOI] [PubMed] [Google Scholar]
- Pitkala KH, Strandberg TE, Tilvis RS. Inappropriate drug prescribing in home-dwelling, elderly patients: a population-based survey. Arch Intern Med. 2002;162:1707–1712. doi: 10.1001/archinte.162.15.1707. [DOI] [PubMed] [Google Scholar]
- The treatment of sleep disorders of older people. Consens Statement. 1990;8:1–22. [PubMed] [Google Scholar]
- Zarcone VP Jr. Sleep hygiene. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia, Pa: WB Saunders Co. 2000 657–661. [Google Scholar]
- Stepanski EJ. Behavioral therapy for insomnia. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 3rd ed. Philadelphia, Pa: WB Saunders Co. 2000 647–656. [Google Scholar]
- Morin CM, Hauri PJ, and Espie CA. et al. Nonpharmacologic treatment of chronic insomnia: an American Academy of Sleep Medicine review. Sleep. 1999 22:1134–1156. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Wohlgemuth WK, and Radtke RA. et al. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA. 2001 285:1856–1864. [DOI] [PubMed] [Google Scholar]
- Cumming RG. Epidemiology of medication-related falls and fractures in the elderly. Drugs Aging. 1998;12:43–53. doi: 10.2165/00002512-199812010-00005. [DOI] [PubMed] [Google Scholar]
- Cumming RG, Miller JP, and Kelsey JL. et al. Medications and multiple falls in elderly people: the St. Louis OASIS study. Age Ageing. 1991 20:455–461. [DOI] [PubMed] [Google Scholar]
- Luukinen H, Koski K, and Laippala P. et al. Predictors for recurrent falls among the home-dwelling elderly. Scand J Prim Health Care. 1995 13:294–299. [DOI] [PubMed] [Google Scholar]
- Sorock GS, Shimkin EE. Benzodiazepine sedatives and the risk of falling in a community-dwelling elderly cohort. Arch Intern Med. 1988;148:2441–2444. [PubMed] [Google Scholar]
- Ryynanen OP, Kivela SL, and Honkanen R. et al. Medications and chronic diseases as risk factors for falling injuries in the elderly. Scand J Soc Med. 1993 21:264–271. [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Blackwell TL, and Mangione CM. et al. Central nervous system-active medications and risk for falls in older women. J Am Geriatr Soc. 2002 50:1629–1637. [DOI] [PubMed] [Google Scholar]
- Cumming RG, Klineberg RJ. Psychotropics, thiazide diuretics and hip fractures in the elderly. Med J Aust. 1993;158:414–417. doi: 10.5694/j.1326-5377.1993.tb121839.x. [DOI] [PubMed] [Google Scholar]
- IMS Health. National Prescription AuditTM Plus M. 2002 Mar [Google Scholar]
- Herings RM, Stricker BH, and de Boer A. et al. Benzodiazepines and the risk of falling leading to femur fractures: dosage more important than elimination half-life. Arch Intern Med. 1995 155:1801–1807. [PubMed] [Google Scholar]
- Beers MH. Explicit criteria for determining potentially inappropriate medication use by the elderly: an update. Arch Intern Med. 1997;157:1531–1536. [PubMed] [Google Scholar]
- Golden AG, Preston RA, and Barnett SD. et al. Inappropriate medication prescribing in homebound older adults. J Am Geriatr Soc. 1999 47:948–953. [DOI] [PubMed] [Google Scholar]
- Walsh JK, Schweitzer PK. Ten-year trends in the pharmacological treatment of insomnia. Sleep. 1999;22:371–375. [PubMed] [Google Scholar]
- Mort JR, Aparasu RR. Prescribing potentially inappropriate psychotropic medications to the ambulatory elderly. Arch Intern Med. 2000;160:2825–2831. doi: 10.1001/archinte.160.18.2825. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Consensus conference. Drugs and insomnia: the use of medications to promote sleep. JAMA. 1984;251:2410–2414. [PubMed] [Google Scholar]
- Zolpidem, tartate (Ambien). Physicians' Desk Reference. Montvale, NJ: Medical Economics. 2003 2979–2983. [Google Scholar]
- Hohagen F, Kappler C, and Schramm E. et al. Prevalence of insomnia in elderly general practice attenders and the current treatment modalities. Acta Psychiatr Scand. 1994 90:102–108. [DOI] [PubMed] [Google Scholar]
- Krystal AD, Walsh JK, and Laska E. et al. Sustained efficacy of eszopiclone over six months of nightly treatment: results of a randomized, double-blind, placebo controlled study in adults with chronic insomnia. Sleep. 2003 26:793–799. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Richardson GS, and Mangano RM. Long-term exposure to zaleplon is safe and effective in younger-elderly and older-elderly patients with primary insomnia. In: 2003 Annual Meeting Abstract Supplement of the 17th Annual Meeting of the Associated Professional Sleep Societies. 3–8June2003 Chicago, Ill. Abstract 0189.C:A77. [Google Scholar]
- Kummer J, Guendel L, and Linden J. et al. Long-term polysomnographic study of the efficacy and safety of zolpidem in elderly psychiatric in-patients with insomnia. J Int Med Res. 1993 21:171–184. [DOI] [PubMed] [Google Scholar]
- Wortelboer U, Cohrs S, and Rodenbeck A. et al. Tolerability of hypnosedatives in older patients. Drugs Aging. 2002 19:529–539. [DOI] [PubMed] [Google Scholar]
- Wheatley D. Prescribing short-acting hypnosedatives: current recommendations from a safety perspective. Drug Saf. 1992;7:106–115. doi: 10.2165/00002018-199207020-00003. [DOI] [PubMed] [Google Scholar]
- Gilbert SS, Burgess HJ, and Kennaway DJ. et al. Attenuation of sleep propensity, core hypothermia, and peripheral heat loss after temazepam tolerance. Am J Physiol Regul Integr Comp Physiol. 2000 279:R1980–R1987. [DOI] [PubMed] [Google Scholar]
- Kales A, Manfredi RL, and Vgontzas AN. et al. Rebound insomnia after only brief and intermittent use of rapidly eliminated benzodiazepines. Clin Pharmacol Ther. 1991 49:468–476. [DOI] [PubMed] [Google Scholar]
- Mitler MM, Seidel WF, and van den Hoed J. et al. Comparative hypnotic effects of flurazepam, triazolam, and placebo: a long-term simultaneous nighttime and daytime study. J Clin Psychopharmacol. 1984 4:2–13. [PubMed] [Google Scholar]
- Roehrs T, Vogel G, and Vogel F. et al. Dose effects of temazepam tablets on sleep. Drugs Exp Clin Res. 1986 12:693–699. [PubMed] [Google Scholar]
- Leppik IE, Roth-Schechter GB, and Gray GW. et al. Double-blind, placebo-controlled comparison of zolpidem, triazolam, and temazepam in elderly patients with insomnia. Drug Dev Res. 1997 40:230–237. [Google Scholar]
- Linnoila M, Viukari M, and Lamminsivu U. et al. Efficacy and side effects of lorazepam, oxazepam, and temazepam as sleeping aids in psychogeriatric inpatients. Int Pharmacopsychiatry. 1980 15:129–135. [DOI] [PubMed] [Google Scholar]
- Meuleman JR, Nelson RC, Clark RL Jr. Evaluation of temazepam and diphenhydramine as hypnotics in a nursing-home population. Drug Intell Clin Pharm. 1987;21:716–720. doi: 10.1177/106002808702100908. [DOI] [PubMed] [Google Scholar]
- Holbrook AM, Crowther R, and Lotter A. et al. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000 162:225–233. [PMC free article] [PubMed] [Google Scholar]
- Juhl RP, Daugherty VM, Kroboth PD. Incidence of next-day anterograde amnesia caused by flurazepam hydrochloride and triazolam. Clin Pharm. 1984;3:622–625. [PubMed] [Google Scholar]
- Ponciano E, Freitas F, and Camara J. et al. A comparison of the efficacy, tolerance and residual effects of zopiclone, flurazepam and placebo in insomniac outpatients. Int Clin Psychopharmacol. 1990 5suppl 2. 69–77. [PubMed] [Google Scholar]
- Scharf MB, Fletcher K, Graham JP. Comparative amnestic effects of benzodiazepine hypnotic agents. J Clin Psychiatry. 1988;49:134–137. [PubMed] [Google Scholar]
- Johnson LC, Chernik DA, Hauri P. A multicenter 14-day study of flur-azepam and midazolam in chronic insomniacs: general discussion and conclusions. J Clin Psychopharmacol. 1990;10:76S–90S. [PubMed] [Google Scholar]
- Judd LL, Ellinwood E, McAdams LA. Cognitive performance and mood in patients with chronic insomnia during 14-day use of flurazepam and midazolam. J Clin Psychopharmacol. 1990;10:56S–67S. [PubMed] [Google Scholar]
- Moskowitz H, Linnoila M, Roehrs T. Psychomotor performance in chronic insomniacs during 14-day use of flurazepam and midazolam. J Clin Psychopharmacol. 1990;10:44S–55S. [PubMed] [Google Scholar]
- Ngen CC, Hassan R. A double-blind placebo-controlled trial of zopiclone 7.5 mg and temazepam 20 mg in insomnia. Int Clin Psychopharmacol. 1990;5:165–171. doi: 10.1097/00004850-199007000-00001. [DOI] [PubMed] [Google Scholar]
- Rickels K, Schweizer E, and Case WG. et al. Long-term therapeutic use of benzodiazepines, 1: effects of abrupt discontinuation. Arch Gen Psychiatry. 1990 47:899–907. [DOI] [PubMed] [Google Scholar]
- Haria M, Fitton A, McTavish D. Trazodone: a review of its pharmacology, therapeutic use in depression and therapeutic potential in other disorders. Drugs Aging. 1994;4:331–355. doi: 10.2165/00002512-199404040-00006. [DOI] [PubMed] [Google Scholar]
- Gerner RH. Geriatric depression and treatment with trazodone. Psychopathology. 1987 20suppl 1. 82–91. [DOI] [PubMed] [Google Scholar]
- Bayer AJ, Pathy MS, Ankier SI. Pharmacokinetic and pharmacodynamic characteristics of trazodone in the elderly. Br J Clin Pharmacol. 1983;16:371–376. doi: 10.1111/j.1365-2125.1983.tb02180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery I, Oswald I, and Morgan K. et al. Trazodone enhances sleep in subjective quality but not in objective duration. Br J Clin Pharmacol. 1983 16:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacker R, Shanks NJ, and Chapman N. et al. The drug treatment of depression in general practice: a comparison of nocte administration of trazodone with mianserin, dothiepin and amitriptyline. Psychopharmacology (Berl). 1988 95(suppl). S18–S24. [DOI] [PubMed] [Google Scholar]
- Davey A. A comparison of two oral dosage regimens of 150 mg trazodone in the treatment of depression in general practice. Psychopharmacology (Berl). 1988 95(suppl). S25–S30. [DOI] [PubMed] [Google Scholar]
- Moon CA, Davey A. The efficacy and residual effects of trazodone (150 mg nocte) and mianserin in the treatment of depressed general practice patients. Psychopharmacology (Berl). 1988 95suppl. S7–S13. [DOI] [PubMed] [Google Scholar]
- Maxmen JS. Antidepressants. In: Psychotropic Drugs: Fast Facts. 1st ed. New York, NY: WW Norton & Co. 1991 57–97. [Google Scholar]
- Carson CC III, Mino RD. Priapism associated with trazodone therapy. J Urol. 1988;139:369–370. doi: 10.1016/s0022-5347(17)42419-0. [DOI] [PubMed] [Google Scholar]
- Consensus Development Panel. National Institutes of Health Consensus Development Conference Statement: the treatment of sleep disorders of older people March 26–28, 1990. Sleep. 1991;14:169–177. doi: 10.1093/sleep/14.2.169. [DOI] [PubMed] [Google Scholar]
- Walsh JK, Erman M, and Erwin CW. et al. Subjective hypnotic efficacy of trazodone and zolpidem in DSM-III-R primary insomnia. Hum Psychopharmacol. 1998 13:191–198. [Google Scholar]
- Walsh JK, Roth T, and Randazzo A. et al. Eight weeks of non-nightly use of zolpidem for primary insomnia. Sleep. 2000 23:1087–1096. [PubMed] [Google Scholar]
- Scharf MB, Roth T, and Vogel GW. et al. A multicenter, placebo-controlled study evaluating zolpidem in the treatment of chronic insomnia. J Clin Psychiatry. 1994 55:192–199. [PubMed] [Google Scholar]
- Steens RD, Pouliot Z, and Millar TW. et al. Effects of zolpidem and triazolam on sleep and respiration in mild to moderate chronic obstructive pulmonary disease. Sleep. 1993 16:318–326. [DOI] [PubMed] [Google Scholar]
- Hesse LM, von Moltke LL, Greenblatt DJ. Clinically important drug interactions with zopiclone, zolpidem and zaleplon. CNS Drugs. 2003;17:513–532. doi: 10.2165/00023210-200317070-00004. [DOI] [PubMed] [Google Scholar]
- Salva P, Costa J. Clinical pharmacokinetics and pharmacodynamics of zolpidem: therapeutic implications. Clin Pharmacokinet. 1995;29:142–153. doi: 10.2165/00003088-199529030-00002. [DOI] [PubMed] [Google Scholar]
- Darcourt G, Pringuey D, and Salliere D. et al. The safety and tolerability of zolpidem: an update. J Psychopharmacol (Oxf). 1999 13:81–93. [DOI] [PubMed] [Google Scholar]
- Scharf MB, Mayleben DW, and Kaffeman M. et al. Dose response effects of zolpidem in normal geriatric subjects. J Clin Psychiatry. 1991 52:77–83. [PubMed] [Google Scholar]
- Shaw SH, Curson H, Coquelin JP. A double-blind, comparative study of zolpidem and placebo in the treatment of insomnia in elderly psychiatric in-patients. J Int Med Res. 1992;20:150–161. doi: 10.1177/030006059202000207. [DOI] [PubMed] [Google Scholar]
- Fairweather DB, Kerr JS, Hindmarch I. The effects of acute and repeated doses of zolpidem on subjective sleep, psychomotor performance and cognitive function in elderly volunteers. Eur J Clin Pharmacol. 1992;43:597–601. doi: 10.1007/BF02284957. [DOI] [PubMed] [Google Scholar]
- Roger M, Attali P, Coquelin JP. Multicenter, double-blind, controlled comparison of zolpidem and triazolam in elderly patients with insomnia. Clin Ther. 1993;15:127–136. [PubMed] [Google Scholar]
- Ware JC, Walsh JK, and Scharf MB. et al. Minimal rebound insomnia after treatment with 10-mg zolpidem. Clin Neuropharmacol. 1997 20:116–125. [DOI] [PubMed] [Google Scholar]
- Langtry HD, Benfield P. Zolpidem: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential. Drugs. 1990;40:291–313. doi: 10.2165/00003495-199040020-00008. [DOI] [PubMed] [Google Scholar]
- Merlotti L, Roehrs T, and Koshorek G. et al. The dose effects of zolpidem on the sleep of healthy normals. J Clin Psychopharmacol. 1989 9:9–14. [PubMed] [Google Scholar]
- Hoehns JD, Perry PJ. Zolpidem: a nonbenzodiazepine hypnotic for treatment of insomnia. Clin Pharm. 1993;12:814–828. [PubMed] [Google Scholar]
- Chaumet-Riffaud AE, Desforges C, and Lavoisy J. Review of the post-marketing surveillance experience collected with zolpidem during the first three years after the launch in Europe. J Sleep Res. 1992 1suppl 1. 40. 10607024 [Google Scholar]
- Pitner JK, Gardner M, and Neville M. et al. Zolpidem-induced psychosis in an older woman [letter]. J Am Geriatr Soc. 1997 45:533–534. [DOI] [PubMed] [Google Scholar]
- Wyatt JK, Bootzin RR, and Anthony J. et al. Sleep onset is associated with retrograde and anterograde amnesia. Sleep. 1994 17:502–511. [DOI] [PubMed] [Google Scholar]
- Elie R, Ruther E, and Farr I. et al, for the Zaleplon Clinical Study Group. Sleep latency is shortened during 4 weeks of treatment with zaleplon, a novel nonbenzodiazepine hypnotic. J Clin Psychiatry. 1999 60:536–544. [DOI] [PubMed] [Google Scholar]
- Fry J, Scharf M, and Mangano R. et al. Zaleplon improves sleep without producing rebound effects in outpatients with insomnia. Zaleplon Clinical Study Group. Int Clin Psychopharmacol. 2000 15:141–152. [DOI] [PubMed] [Google Scholar]
- Hedner J, Yaeche R, and Emilien G. et al. Zaleplon shortens subjective sleep latency and improves subjective sleep quality in elderly patients with insomnia. The Zaleplon Clinical Investigator Study Group. Int J Geriatr Psychiatry. 2000 15:704–712. [DOI] [PubMed] [Google Scholar]
- James DS. Survey of hypnotic drug use in nursing homes. J Am Geriatr Soc. 1985;33:436–439. doi: 10.1111/j.1532-5415.1985.tb07155.x. [DOI] [PubMed] [Google Scholar]
- Beers M, Avorn J, and Soumerai SB. et al. Psychoactive medication use in intermediate-care facility residents. JAMA. 1988 260:3016–3020. [PubMed] [Google Scholar]
- Rickels K, Morris RJ, and Newman H. et al. Diphenhydramine in insomniac family practice patients: a double-blind study. J Clin Pharmacol. 1983 23:234–242. [DOI] [PubMed] [Google Scholar]
- Gengo FM, Gabos C, Mechtler L. Quantitative effects of cetirizine and diphenhydramine on mental performance measured using an automobile driving simulator. Ann Allergy. 1990;64:520–526. [PubMed] [Google Scholar]
- Mattila MJ, Mattila M, Konno K. Acute and subacute actions on human performance and interactions with diazepam of temelastine (SK&F93944) and diphenhydramine. Eur J Clin Pharmacol. 1986;31:291–298. doi: 10.1007/BF00981126. [DOI] [PubMed] [Google Scholar]
- Roth T, Roehrs T, and Koshorek G. et al. Sedative effects of antihistamines. J Allergy Clin Immunol. 1987 80:94–98. [DOI] [PubMed] [Google Scholar]
- Witek TJJ, Canestrari DA, and Miller RD. et al. Characterization of daytime sleepiness and psychomotor performance following H1 receptor antagonists. Ann Allergy Asthma Immunol. 1995 74:419–426. [PubMed] [Google Scholar]
- Lessard E, Yessine MA, and Hamelin BA. et al. Diphenhydramine alters the disposition of venlafaxine through inhibition of CYP2D6 activity in humans. J Clin Psychopharmacol. 2001 21:175–184. [DOI] [PubMed] [Google Scholar]
- Katzung BG. Basic and clinical pharmacology. 8th ed. New York, NY: Lange Medical Books/McGraw Hill. 2001 [Google Scholar]
- Census 2000 Brief: The 65 Years and Over Population: 2000. Available at: http://www.census.gov/prod/2001pubs/c2kbr01-10.pdf. Accessed September 10, 2002. [Google Scholar]
- Walsh JK, Lankford DD, and Krystal A. et al. Efficacy and tolerability of four doses of indiplon (NBI-34060) modified-release in elderly patients with sleep maintenance insomnia. In: 2003 Annual Meeting Abstract Supplement of the 17th annual meeting of the Associated Professional Sleep Societies. 3–8June2003 Chicago, Ill. Abstract 0190.C:A78. [Google Scholar]
- Scharf MB, Rosenberg R, and Cohn M. et al. Safety and efficacy of immediate release indiplon (NBI-34060) in elderly patients with insomnia. In: 2003 Annual Meeting Abstract Supplement of the 17th Annual Meeting of the Associated Professional Sleep Societies. 3–8June2003 Chicago, Ill. Abstract 0209.C:A85. [Google Scholar]
- Scharf M, Seiden D, and Erman M. et al. Eszopiclone rapidly induced sleep and provided sleep maintenance in elderly patients with chronic insomnia. Presented at the 11th International Congress of the International Psychogeriatric Association. 17–22August2003 Chicago, Ill. [Google Scholar]
- Schlich D, L'Heritier C, and Coquelin JP. et al. Long-term treatment of insomnia with zolpidem: a multicentre general practitioner study of 107 patients. J Int Med Res. 1991 19:271–279. [DOI] [PubMed] [Google Scholar]