Abstract
Selective antegrade cerebral perfusion (SACP), which was adopted by many surgical groups for complex neonatal cardiac surgery, especially aortic arch repair, is a proven adjunct for neuroprotection during deep hypothermic circulatory arrest (DHCA). Several recent studies suggest that SUMO2/3 modification of proteins is markedly activated during deep hypothermia and believed to be an endogenous neuroprotective stress response. Here, we report that SACP reduces the increasing degree of SUMO2/3 conjugation following DHCA. Piglets were subjected to 1 h SACP and/or 1 h DHCA. DHCA was sufficient to markedly increase in protein SUMOylation by SUMO2/3 both in the hippocampus and cerebral cortex. SACP, especially at flow rate of 50 ml/kg/min, reduces the increasing degree of SUMO2/3 conjugation and also reduces levels of pro-apoptotic factors, Bax and Caspase 3, and increases levels of antiapoptotic factors, Bcl-2, following DHCA both in the hippocampus and cerebral cortex. This suggests that SACP at flow rate of 50 ml/kg/min is more appropriate for neuroprotection during DHCA in the pig model and level of protein SUMO2/3-ylation maybe an indicator of the degree of brain injury.
Keywords: Selective antegrade cerebral perfusion, SUMO2/3, deep hypothermic circulatory arrest, piglet, apoptosis, brain injury
Introduction
Global cerebral ischemia related to cardiopulmonary bypass (CPB) and deep hypothermic circulatory arrest (DHCA), used in the repair of the cardiovascular defects, appears to play an important role in the neurologic morbidity [1]. Neurologic morbidity afflicts 5-40% of survivals and manifests as seizures, altered tone, diminished cognition, impaired coordination, language delays and/or attention deficit hyperactivity disorder. Selective antegrade cerebral perfusion (SACP) is thought to minimize ischemic brain injury by providing adequate cerebral blood flow and proposed to avoid neurologic complications. SACP has advantages of less cerebral edema, better clearance of metabolic waste, and lower levels of biomarkers of injury, otherwise its capacity for energy preservation, maintenance of cellular integrity, and protection against apoptosis was better than that of DHCA [2]. Although the protective potential of SACP is unquestionable, little is known about the mechanisms how to maximize its efficacy to protect cerebral injury. Elucidating the mechanisms underlying protection of cerebral by SACP is of tremendous clinical interest.
Small ubiquitin-like modifier is a group of proteins that act by covalent attachment to lysine residues on target proteins [3] and thereby modulate various processes that play important roles in key cellular functions under normal and pathological conditions. The functions include maintenance of genome integrity, cell signaling, plasma membrane depolarization, signal transduction, proteasomal degradation of proteins, transcriptional regulation, nuclear transport, protein quality control and DNA-damage repair [4-9]. Four SUMO proteins have been identified which were SUMO1-4. SUMO-4 is mainly localized in the kidney [10] while the other forms in brain. SUMO-2 and SUMO-3 differ from only four N-terminal amino acids and no difference in their functional roles has yet been reported [11]. SUMO-2/3 shares about 50% similar sequence with SUMO-1. In contrast to SUMO1, both SUMO2 and SUMO3 contain a consensus SUMO conjugation site and can form polymeric chains [12], which attach to target proteins. The ability of SUMO2 and SUMO3 to form polymeric chains could be the key significance in pathological states of the brain.
The process of SUMO conjugation is reversible, highly dynamic and involves four different classes of enzymes. In the first step, SUMO is activated in an ATP-dependent reaction by thioester bond formation with the activating enzyme E1 (AOS1-UBA2). Next, the SUMO is transferred to the E2 conjugation enzyme Ubc2. Then Ubc2 transfers SUMO to its target protein, which generally facilitated by a diverse class of SUMO E3 ligases. Sumo is removed from target proteins by isopeptidases known as SUMO-specific proteases (SENPs) [13].
A number of recent publications have suggested that the SUMO conjugation of target proteins plays a role in the cellular response to ischemia and is markedly activated in the brain during deep to moderate hypothermia. It was first shown in hibernating arctic ground squirrels that protein sumo-2/3-ylation massively increased in the torpor state [14], suggested a neuroprotective role of protein SUMOylation to make cells of hibernating animals tolerant to the otherwise lethally low levels of blood flow reduction. The research using a clinically relevant animal model of deep hypothermic cardiopulmonary bypass (DHCPB) provided evidence that deep hypothermia markedly activates the small ubiquitin-like modifier conjugation pathway and triggered a nuclear translation of SUMO2/3-conjugated proteins. Activation of the SUMO conjugation pathway is believed to protect neurons from damage caused by low blood flow [15]. Deep hypothermia induced nuclear translocation of the SUMO-conjugating enzyme Ubc9, suggesting that the increase in nuclear levels of SUMO2/3-conjugated proteins observed in brains of hypothermic animals is an active process [16]. Levels of SUMO2/3-conjugated proteins are massively increased after transient cerebral ischemia and during deep hypothermia, but are very low in the intact brain [14-20]. The post-ischemic activation of SUMO2/3 conjugation is believed to be an endogenous neuroprotective stress response [21].
Whether SACP is associated with a change of protein SUMO2/3-ylation has never been investigated before. We show here that DHCA was sufficient to markedly increase in protein SUMOylation by SUMO2/3. SACP, especially at flow rate of 50 ml/kg/min, reduces the increasing degree of SUMO2/3 conjugation. Moreover, SACP, especially flow rate at 50ml/kg/min, provides significant neuroprotection from cellular apoptosis, as indicated by lower expression of Bax and Caspase-3. This implies that level of protein SUMO2/3-ylation maybe an indicator of the degree of brain injury.
Materials and methods
Animal experiments
The project was approved by the Animal Care and Use Committee of Beijing Anzhen Hospital, Capital Medical University. All animals received humane care in compliance with the “Guide for the Care and Use of Laboratory Animals” published by the National Institute of Health (NIH Publication No. 88.23, revised 1996).
Twenty-two healthy piglets, about 4 weeks of age of either gender, weighing approximately 10 kg, were randomly divided into four groups: Control group (n = 4), anaesthetized, ventilated and intubated but not underwent CPB for 60min that served as normal control group; DHCA group (n = 6), underwent hypothermic circulatory arrest at 18°C for 60 min; SACP 25 group (n = 6), underwent SACP for 60 min at a flow rate of 25 ml/kg/min during DHCA and SACP 50 group (n = 6), underwent SACP for 60 min at a flow rate of 50 ml/kg/min during DHCA.
Deep hypothermic circulatory arrest model was induced in piglets (Chinese minipigs) as described elsewhere [22-24]. The animals were fasted overnight but allowed free access to water. The pigs were pre-medicated with intramuscular ketamine hydrochloride (15 mg/kg) and atropine (0.05 mg/kg) before surgery, and anesthesia was induced with a bolus dose of propofol (1-2 mg/kg IV) and sufentanil (0.3 μg/kg IV). Catheters were inserted in an ear vein and the left femoral artery for monitoring purposes and withdrawal of blood samples. After endotracheal intubation, the animals were ventilated mechanically with 100% oxygen. The ventilator rate and the tidal volume were adjusted to maintain normocapnia (Pco2 35 to 45 mmHg). Arterial oxygen tension was maintained at greater than 100 mmHg. Anesthesia was maintained with a continuous infusion of propofol (6 to 8 mg/kg/h) and sufentanil (0.3 μg/kg/h); muscle relaxation was provided by a continuous infusion of pancuronium (0.2 mg/kg/h). Lactated Ringer’s solution (10 ml/kg/h) was administered throughout the preparation phase.
The circuit was primed with Plasmalyte-A and 25% albumin. Donor whole blood was added to maintain a hematocrit of 25% to 30%. For CPB, a median sternotomy was performed. After heparinization (450 IU/kg), a 10F arterial and a 20F venous single stage cannula were inserted into the ascending aorta and the right atrium, respectively. Using alpha stat blood gas management, nonpulsatile CPB was initiated at a flow rate of 100 ml/kg/min and then adjusted to maintain a minimum arterial pressure of 50 mmHg. All animals were cooled to a nasopharyngeal temperature of 18°C over a 30-minute period and then pump flow was shut off followed by a 60 min interval of DHCA at 18°C, or SACP with the specified flow rate at 18°C, respectively. For SACP, both ascending and descending aorta were cross-clamped proximal and distal to the aortic cannula. Isolated perfusion of the brain was then established via the bicarotid trunk at either 25 or 50 ml/kg/min according to the same protocol as described for global CPB. After 90 min, the aortic clamp was removed and global CPB was reconstituted at a flow rate of 100 ml/kg/min with warming to a brain temperature of 36°C over 30 min. Internal defibrillation was performed. During weaning from CPB, inotropic support and control of vascular resistance was performed if necessary. At 37°C, CPB was discontinued. After finishing the experimental protocol, the animals were euthanized with an overdose of potassium chloride. Brains were quickly removed, the hippocampus and cortex dissected out, and samples were immediately frozen and stored at -80 C until being used for analyses.
Sample preparation and western blotting analysis
Frozen brain regions were crushed on dry ice to make powder, added to 50 mM Tris HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% Triton X-100, 1% mammalian protease inhibitor and 20 mM NEM, and homogenized on ice [18]. The homogenates were sonicated for 10 to 15 s, boiled for 5 min at 95°C and centrifuged at 15,000g for 10 min at 4°C. After the protein concentration was measured, the supernatant was boiled again with 5% β-mercaptoethanol and 2% glycerol, and subjected (30 μg/lane) to SDS-PAGE (4% to 20%). Western blot analyses were performed using the following antibodies: rabbit polyclonal anti-SUMO-2/3 antibodies (1:1000) (Cell Signaling Technology, Beverly, MA, USA), rabbit polyclonal anti-human SENP antibodies (1:1000) (Cell Signaling Technology, Beverly, MA, USA), mouse monoclonal anti-Ubc9 (1:500) (Santa CruzBiotechnology, SantaCruz, CA, USA), rabbit polyclonal anti-Bax (1:1000), anti-Bcl-2 (1:1000) and anti-Cleaved Caspase-3 (1:1000) antibodies (Cell Signaling Technology, Beverly, MA, USA) and mouse monoclonal β-actin antibodies (1:1000) (ABCAM, Cambridge, MA, USA). The band intensity was quantified by densitometry using ImageJ (NIH). For SUMO conjugate band analysis, the entire lane in the range (11 to 250 kDa) was sampled. For each structure (cortex and hippocampus), SACP groups (flow rate at 20 ml/kg/min and 50 ml/kg/min) were compared to control group not exposed to DHCA and SACP on the same Western blot to avoid any differences in signal intensity due to exposure times, etc. The same blots were then re-probed with β-actin antibody as an internal control to ensure equal protein loading in all lanes.
Statistical analysis
Data are presented as Means ± SD. Statistical analysis was performed using Statistical Package for the Social Science (SPSS, version 17.0). Statistical significant differences among groups were evaluated by analysis of variance (ANOVA), followed by Fisher’s protected least-significant differences test. The p values < 0.05 was considered significant.
Results
Effects of SACP on total levels of protein SUMO2/3 conjugation
SACP-induced changes in SUMO2/3 conjugation and free SUMO2/3 are shown in Figure 1A and 1B. A moderate increase in SUMO2/3 conjugation (higher molecular weight area above 50 kDa region) occurred in the SACP animals and a marked increase in SUMO2/3 conjugation occurred in the DHCA animals (Figure 1A and 1B). Level of SUMO2/3-conjugated proteins increased approximately 2.8 ± 0.4-fold and 3.5 ± 0.6-fold in the hippocampus and cortex in DHCA group, respectively, but in SACP 25 and SACP 50 group, the degree of increase of SUMO2/3 conjugation is moderate, just 2.4 ± 0.4-fold and 2.5 ± 0.7-fold, 1.8 ± 0.2-fold and 1.9 ± 0.5-fold in the hippocampus and cortex, respectively, compared to control group (Figure 1C and 1D). Free SUMO2/3 molecular (molecular weight area about 11kDa) levels decrease markedly after DHCA, to 31.4 ± 5.4% and 38.3 ± 5.6% in the hippocampus and cortex, but in SACP 25 and SACP 50 group, the depleting degree of free SUMO2/3 is moderate, just 58.9 ± 6.1% and 52.4 ± 8.9%, 65.3 ± 6.3% and 66.2 ± 6.1% in the hippocampus and cortex, respectively, compared to control group (Figure 1E and 1F). These data indicate that SUMO2/3 conjugation was activated even after DHCA, and the degree of increase of SUMO2/3 conjugation was reduced in SACP group for providing the regional cerebral blood flow during DHCA, especially at flow rate of 50 ml/kg/min.
Figure 1.
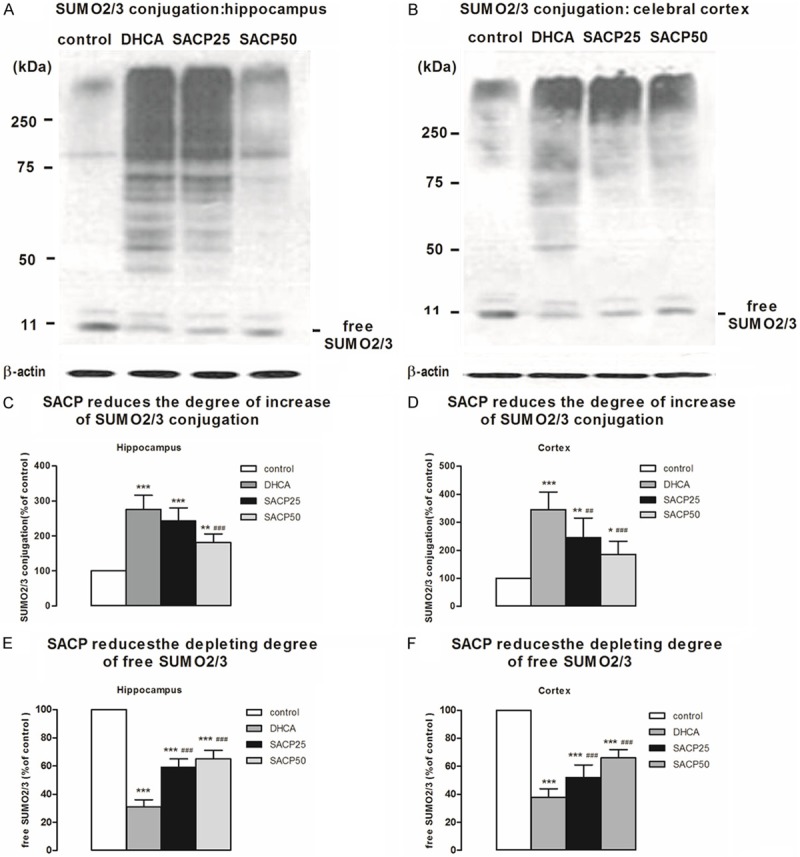
Marked activation of small ubiquitin-like modifier (SUMO) 2/3 conjugation during deep hypothermia circulatory arrest and marked reduction of the degree of increase of SUMO2/3 conjugation in selective antegrade cerebral perfusion, especially at flow rate of 50 ml/kg-1/min-1. The pattern of protein SUMOylation was evaluated by Western blot analysis in samples taken from the hippocampus and cerebral cortex, as described in Materials and methods. A. SACP-induced changes in the pattern of protein modification with SUMO2/3 in samples derived from the hippocampus. β-Actin served as loading control. B. SACP-induced changes in the pattern of protein modification with SUMO2/3 in samples derived from the cerebral cortex. C. Quantification of SUMO2/3 conjugation intensities of the higher molecular weight area above 50 kDa region after SACP in the hippocampus. D. Quantification of SUMO2/3 conjugation intensities of the higher molecular weight area above 50 kDa region after SACP in the cerebral cortex. E. SACP induces a moderate depletion of free SUMO2/3 protein levels in the hippocampus comparing to DHCA. F. SACP induces a moderate depletion of free SUMO2/3 protein levels in the cerebral cortex comparing to DHCA. Quantification data are presented as means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001 compared to control group, ##p < 0.01, ###p < 0.001 compared to DHCA group (ANOVA, followed by Fisher’s protected least-significant difference (PLSD) test).
Effects of SACP on total levels of Ubc9 and SENP
We observed that DHCA induced a marked increase in Ubc9 protein levels in the hippocampus (296.3% ± 20.2% of control; p < 0.001) and cortex (231.5% ± 13.3% of control; p < 0.001) (Figure 2C and 2D) and a marked decrease in SENP levels in the hippocampus (46.3% ± 8.4% of control; p < 0.001) and cortex (39.9% ± 7.2% of control; p < 0.001) (Figure 2E and 2F). SACP at flow rate 50 ml·kg-1·min-1, in contrast, induced moderate increase in Ubc9 protein levels in the hippocampus (135.7% ± 19.7% of control; p < 0.001) and cortex (113.5% ± 15.8% of control; p < 0.001) (Figure 2C and 2D) and a moderate decrease in SENP levels in the hippocampus (88.4% ± 5.2% of control; p < 0.001) and cortex (87.2% ± 9.2% of control; p < 0.001) (Figure 2E and 2F).
Figure 2.
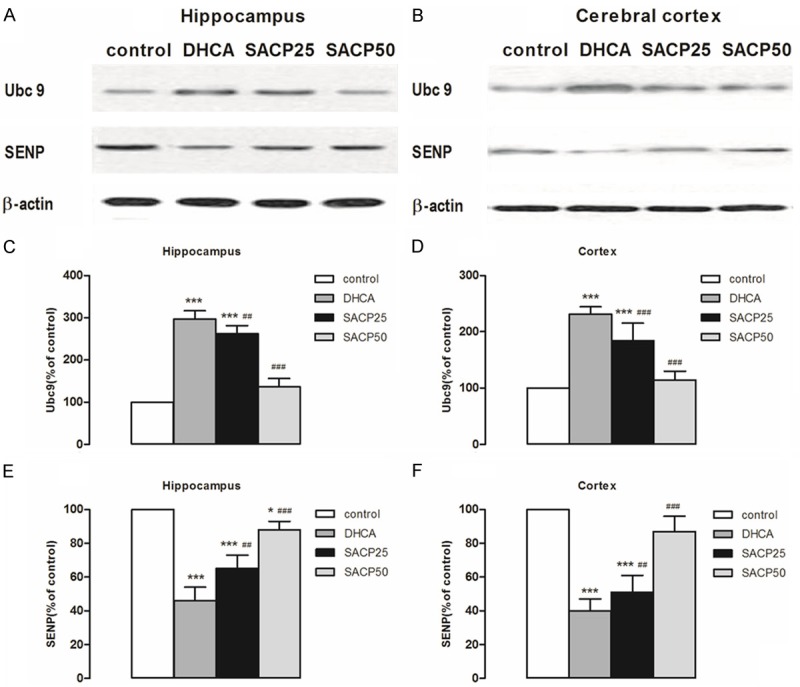
Expression levels of Ubc9 and SENP. The pattern of Ubc9 and SENP was evaluated by Western blot analysis in samples taken from the hippocampus and cerebral cortex, as described in Materials and methods. A, B. SACP-induced changes in the pattern of Ubc9 and SENP in samples derived from the hippocampus and cortex. β-Actin served as loading control. C, D. Quantification of Ubc9 intensities after SACP in the hippocampus and cerebral cortex. E, F. Quantification of SENP intensities after SACP in the hippocampus and cerebral cortex. Quantification data are presented as means ± SD. *p < 0.05, ***p < 0.001 compared to control group, ##p < 0.01, ###p < 0.001 compared to DHCA group (ANOVA, followed by Fisher’s protected least-significant difference (PLSD) test).
Effect of SACP on expression of Bcl-2 and Bax
Expression of Bax and Bcl-2 in the cortex and hippocampus of piglet brain is shown in Figure 3. As can be seen, SACP, especially at flow rate of 50 ml/kg/min, compared to DHCA group, decreased Bax expression and increased Bcl-2 expression in hippocampus and cortex. In the hippocampus, Bax expression in the SACP 50 group and DHCA group was 127.6 ± 18.1 versus 273.8 ± 25.3 (p < 0.001). In the cortex, Bax expression in the SACP 50 group and DHCA group was 124.1 ± 17.0 versus 261.7 ± 19.4 (p < 0.001) (Figure 3C and 3D). Similarly, in the hippocampus, Bcl-2 expression in the SACP 50 group and DHCA group was 71.0 ± 9.9 versus 35.9 ± 5.7 (p < 0.001). In the cortex, Bcl-2 expression in the SACP 50 group and DHCA group was 57.8 ± 7.7 versus 36.0 ± 4.9 (p < 0.001) (Figure 3E and 3F).
Figure 3.
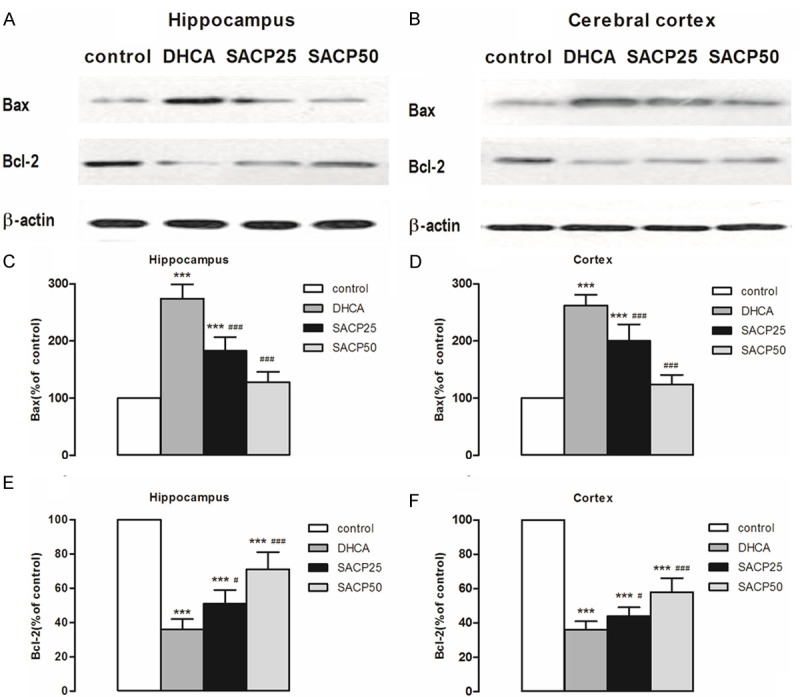
Expression levels of Bax and Bcl-2. The pattern of Bax and Bcl-2 was evaluated by Western blot analysis in samples taken from the hippocampus and cerebral cortex, as described in Materials and methods. A, B. SACP-induced changes in the pattern of Bax and Bcl-2 in samples derived from the hippocampus and cerebral cortex, respectively. β-Actin served as loading control. C, D. Quantification of Bax intensities after SACP in the hippocampus and cerebral cortex, respectively. E, F. Quantification of Bcl-2 intensities after SACP in the hippocampus and cerebral cortex, respectively. Quantification data are presented as means ± SD. ***p < 0.001 compared to control group, #p < 0.05, ###p < 0.001 compared to DHCA group (ANOVA, followed by Fisher’s protected least-significant difference (PLSD) test).
Effects of SACP on expression of caspase-3
Expression of caspase-3 in the hippocampus and cortex of piglet brain is shown in Figure 4. As can be seen, SACP, especially at flow rate of 50 ml·kg-1·min-1, compared to DHCA group, decreased caspase-3 expression in hippocampus and cortex. In the hippocampus, caspase-3 expression in the SACP 50 group and DHCA group was 124.0 ± 18.8 versus 272.9 ± 23.9 (p < 0.001) (Figure 4C). In the cortex, caspase-3 expression in the SACP 50 group and DHCA group was 186.6 ± 29.3 versus 309.2 ± 17.4 (p < 0.001) (Figure 4D).
Figure 4.
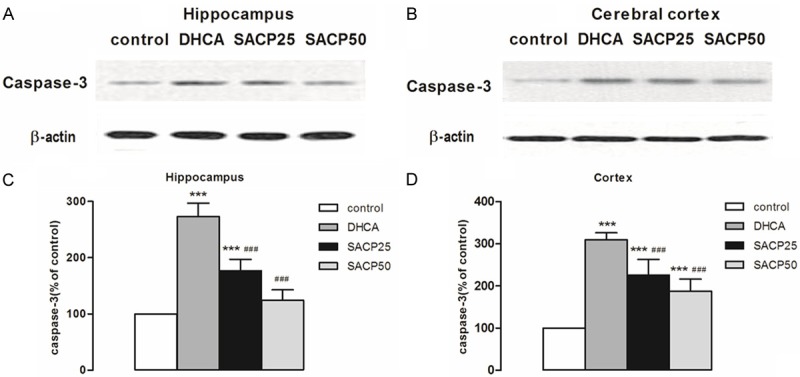
Expression levels of caspase-3. The pattern of caspase-3was evaluated by Western blot analysis in samples taken from the hippocampus and cerebral cortex, as described in Materials and methods. A, B. SACP-induced changes in the pattern of caspase-3 in samples derived from the hippocampus and cerebral cortex, respectively. β-Actin served as loading control. C, D. Quantification of caspase-3 intensities after SACP in the hippocampus and cerebral cortex respectively. Quantification data are presented as means ± SD. ***p < 0.001 compared to control group, ###p < 0.001 compared to DHCA group (ANOVA, followed by Fisher’s protected least-significant difference (PLSD) test).
Discussion
The potential of selective antegrade cerebral perfusion to protect brain from damage induced by deep hypothermic and circulatory arrest is well established. However, the mechanisms underlying brain protection by selective antegrade cerebral perfusion still need to be established. John Hallenbeck and his colleagues had first reported that deep hypothermia induced a massive activation of SUMO conjugation during hibernation in ground squirrels. They concluded that activation of protein SUMOylation may help brain cells to survive the otherwise lethally low levels of blood flow reduction during the state of hibernation. This assumption was proved by the observation that SUMO over-expression made SHSY5Y cells in culture more tolerant to transient oxygen/glucose deprivation [14]. To investigate the effects of deep hypothermia on the small ubiquitin-like modifier (SUMO) conjugation pathway, Wulf Paschen using a clinically relevant animal model of deep hypothermic cardiopulmonary bypass found that a dramatic nuclear translocation of Ubc9 and activation of nuclear SUMO2/3 conjugation induced by deep hypothermia [15]. Recent study found that the post-ischemic activation of SUMO2/3 conjugation is believed to be an endogenous neuroprotective stress response [21]. So we studied the neuroprotective role of selective antegrade cerebral perfusion on SUMO2/3 conjugation events.
Our results demonstrate that 1 h deep hypothermic circulatory arrest induced a marked activation of the SUMO2/3 conjugation, and 1 h selective antegrade cerebral perfusion, especially at flow rate of 50 ml/kg/min providing a continuous cerebral blood flow during circulatory arrest, induced less-activation of the SUMO2/3 conjugation. Free SUMO2/3, in contrast, markedly decreased after deep hypothermic circulatory arrest when SUMO2/3 conjugation was massively increased. But in SACP group, free SUMO2/3 moderately decreased when SUMO2/3 conjugation was moderately increasing. These results are broadly consistent with studies indicating that SUMO-2/3 conjugation to proteins is massively increased in the brains of rats during deep hypothermia (18°C) for 1 h [15] and free SUMO2/3 is markedly decreased after ischemia [19,20].
SUMO2/3 conjugation is considered playing an important role in the cellular response to the metabolic stress induced by interruption of the blood supply. Preconditioning, as a sublethal insult protecting brain from a subsequent, normally harmful, ischemia event, block the SUMO2/3 conjugation following harmful ischemia [25]. It suggests that protective factor, such as preconditioning ischemia and hypothermia pre-conditioning, protecting the brain from ischemic injury could reduce SUMO2/3 conjugation level. In other words, the degree of SUMO2/3 conjugation maybe correlated with the degree of brain injury. In our study, we found that selective antegrade cerebral perfusion, as a neuroprotective method, providing adequate cerebral blood flow during DHCA also blocks the SUMO2/3 conjugation following circulatory arrest, especially SACP at flow rate of 50 ml/kg/min. Our results imply that SUMO2/3 conjugation level in SACP 50 group is less than that in SACP 25 group. It has been known that activation of the apoptotic cascade plays an important role in DHCA neuronal death [26]. In this piglet model of brain injury, we found that SACP, especially at flow rate of 50 ml/kg/min, reduces pro-apoptotic factors, Bax and Caspase 3, and increases anti-apoptotic factors, Bcl-2, both in the hippocampus and cerebral cortex. So SACP at flow rate of 50 ml/kg/min is more appropriate for neuroprotection during DHCA in the pig model.
SUMO modification is a dynamic process involving both conjugation and deconjugation enzymes. SUMO modification also is a complex reversible process, thus increased SUMOylation occurs from either acceleration (activation) of the conjugating process or inhibition of the deconjugating process [27]. Ubc9 is the only SUMO conjugating enzyme and deconjugation of SUMO from sumoylated proteins are carried out by the SENP. So when SUMO conjugation increases, level of the SUMO-conjugating enzyme, Ubc9, should increase and level of the SUMO-deconjugating enzyme, SENP, should decrease. In our study, we found that SUMO2/3 conjugation is massively increased induced by DHCA both in the hippocampus and cortex coinciding with an increased in Ubc9 protein levels and a decreased in SENP protein levels. In SACP group, especially at flow rate of 50 ml·kg-1·min-1, the increasing degree of SUMO2/3 conjugation and Ubc9 and the decreasing degree of SENP is more moderate both in the hippocampus and cortex compared to DHCA group. These results are similar to the previous study [14]. They found that the expression levels of Ubc9 are closely correlated with SUMO conjugation levels in the brain during hibernation (an environmental chamber maintained at 4°C to 5°C) in ground squirrels. But the study found that after transient cerebral ischemia, protein SUMOylation by SUMO2/3 both in the hippocampus and cerebral cortex is massive increased, but Ubc9 protein levels do not change in the hippocampus and transiently decrease in the cortex [13]. One plausible explanation for these different observations could be because of the use of different factors resulting in the cerebral injury.
A number of recent publications have suggested that the sumo conjugation of target proteins plays a role in the cellular response to ischemia and is markedly activated in the brain during deep to moderate hypothermia. In our study, we find that DHCA markedly activates apoptotic cascade and results in massive SUMO2/3 conjugation, but in SACP group, these events more moderately. SUMOylation changes the stability, localization, and activity of proteins. Many of the SUMO2/3 conjugation target proteins identified so far are transcription factors and other nuclear proteins. It is clear that many proteins, such as ion channel, receptor, signal pathway, etc. involve in the mechanism of brain injury and brain protection during DHCA and/or SACP. We speculate that comparing the different level of proteins SUMO2/3-ylation between DHCA and SACP could help to find out the target proteins of SUMO2/3 modification. To identify the SUMO2/3 conjugation target proteins could help to design new strategies for preventive and therapeutic interventions to reduce neurologic morbidity induced by DHCA.
Acknowledgements
This research was supported by the national natural science funds (No. 81371443) and Beijing Natural Science Foundation (NO. 7152045). We are grateful to Dr. Yingping Liang, the Second Affiliate Hospital to Nanchang University, for anesthesia.
Disclosure of conflict of interest
None.
References
- 1.Mahle WT, Tavani F, Zimmerman RA, Nicolson SC, Galli KK, Gaynor JW, Clancy RR, Montenegro LM, Spray TL, Chiavacci RM, Wernovsky G, Kurth CD. An MRI study of neurological injury before and after congenital heart surgery. Circulation. 2002;106:109–114. [PubMed] [Google Scholar]
- 2.Liang MY, Tang ZX, Chen GX, Rong J, Yao JP, Chen Z, Wu ZK. Is selective antegrade cerebral perfusion superior to retrograde cerebral perfusion for brain protection during deep hypothermic circulatory arrest? Metabolic evidence from microdialysis. Crit Care Med. 2014;42:e319–328. doi: 10.1097/CCM.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 3.Mahajan R, Gerace L, Melchior F. Molecular characterization of the SUMO-1 modification of RanGAP1 and its role in nuclear envelope association. J Cell Biol. 1998;140:259–270. doi: 10.1083/jcb.140.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cubenas-Potts C, Matunis MJ. SUMO: a multifaceted modifier of chromatin structure and function. Dev Cell. 2013;24:1–12. doi: 10.1016/j.devcel.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geoffroy MC, Hay RT. An additional role for SUMO in ubiquitin-mediated proteolysis. Nat Rev Mol Cell Biol. 2009;10:564–568. doi: 10.1038/nrm2707. [DOI] [PubMed] [Google Scholar]
- 6.Heun P. SUMOrganization of the nucleus. Curr Opin Cell Biol. 2007;19:350–355. doi: 10.1016/j.ceb.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Jackson SP, Durocher D. Regulation of DNA damage responses by ubiquitin and SUMO. Mol Cell. 2013;49:795–807. doi: 10.1016/j.molcel.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 8.Prudden J, Pebernard S, Raffa G, Slavin DA, Perry JJ, Tainer JA, McGowan CH, Boddy MN. SUMO-targeted ubiquitin ligases in genome stability. EMBO J. 2007;26:4089–4101. doi: 10.1038/sj.emboj.7601838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheschonka A, Tang Z, Betz H. Sumoylation in neurons: nuclear and synaptic roles? Trends Neurosci. 2007;30:85–91. doi: 10.1016/j.tins.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Bohren KM, Nadkarni V, Song JH, Gabbay KH, Owerbach D. A M55V polymorphism in a novel SUMO gene (SUMO-4) differentially activates heat shock transcription factors and is associated with susceptibility to type I diabetes mellitus. J Biol Chem. 2004;279:27233–27238. doi: 10.1074/jbc.M402273200. [DOI] [PubMed] [Google Scholar]
- 11.Hay RT. SUMO: a history of modification. Mol Cell. 2005;18:1–12. doi: 10.1016/j.molcel.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Vertegaal AC. Small ubiquitin-related modifiers in chains. Biochem Soc Trans. 2007;35:1422–1423. doi: 10.1042/BST0351422. [DOI] [PubMed] [Google Scholar]
- 13.Yang W, Sheng H, Homi HM, Warner DS, Paschen W. Cerebral ischemia/stroke and small ubiquitin-like modifier (SUMO) conjugation--a new target for therapeutic intervention? J Neurochem. 2008;106:989–999. doi: 10.1111/j.1471-4159.2008.05404.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee YJ, Miyake S, Wakita H, McMullen DC, Azuma Y, Auh S, Hallenbeck JM. Protein SUMOylation is massively increased in hibernation torpor and is critical for the cytoprotection provided by ischemic preconditioning and hypothermia in SHSY5Y cells. J Cereb Blood Flow Metab. 2007;27:950–962. doi: 10.1038/sj.jcbfm.9600395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang W, Ma Q, Mackensen GB, Paschen W. Deep hypothermia markedly activates the small ubiquitin-like modifier conjugation pathway; implications for the fate of cells exposed to transient deep hypothermic cardiopulmonary bypass. J Cereb Blood Flow Metab. 2009;29:886–890. doi: 10.1038/jcbfm.2009.16. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Ma Q, Yang W, Mackensen GB, Paschen W. Moderate hypothermia induces marked increase in levels and nuclear accumulation of SUMO2/3-conjugated proteins in neurons. J Neurochem. 2012;123:349–359. doi: 10.1111/j.1471-4159.2012.07916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cimarosti H, Henley JM. Investigating the mechanisms underlying neuronal death in ischemia using in vitro oxygen-glucose deprivation: potential involvement of protein SUMOylation. Neuroscientist. 2008;14:626–636. doi: 10.1177/1073858408322677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cimarosti H, Lindberg C, Bomholt SF, Ronn LC, Henley JM. Increased protein SUMOylation following focal cerebral ischemia. Neuropharmacology. 2008;54:280–289. doi: 10.1016/j.neuropharm.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Yang W, Sheng H, Warner DS, Paschen W. Transient focal cerebral ischemia induces a dramatic activation of small ubiquitin-like modifier conjugation. J Cereb Blood Flow Metab. 2008;28:892–896. doi: 10.1038/sj.jcbfm.9600601. [DOI] [PubMed] [Google Scholar]
- 20.Yang W, Sheng H, Warner DS, Paschen W. Transient global cerebral ischemia induces a massive increase in protein sumoylation. J Cereb Blood Flow Metab. 2008;28:269–279. doi: 10.1038/sj.jcbfm.9600523. [DOI] [PubMed] [Google Scholar]
- 21.Datwyler AL, Lattig-Tunnemann G, Yang W, Paschen W, Lee SL, Dirnagl U, Endres M, Harms C. SUMO2/3 conjugation is an endogenous neuroprotective mechanism. J Cereb Blood Flow Metab. 2011;31:2152–2159. doi: 10.1038/jcbfm.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ananiadou OG, Drossos GE, Bibou KN, Palatianos GM, Johnson EO. Acute regional neuronal injury following hypothermic circulatory arrest in a porcine model. Interact Cardiovasc Thorac Surg. 2005;4:597–601. doi: 10.1510/icvts.2005.112813. [DOI] [PubMed] [Google Scholar]
- 23.Meybohm P, Hoffmann G, Renner J, Boening A, Cavus E, Steinfath M, Scholz J, Bein B. Measurement of blood flow index during antegrade selective cerebral perfusion with near-infrared spectroscopy in newborn piglets. Anesth Analg. 2008;106:795–803. doi: 10.1213/ane.0b013e31816173b4. [DOI] [PubMed] [Google Scholar]
- 24.Schultz S, Antoni D, Shears G, Markowitz S, Pastuszko P, Greeley W, Wilson DF, Pastuszko A. Brain oxygen and metabolism during circulatory arrest with intermittent brief periods of low-flow cardiopulmonary bypass in newborn piglets. J Thorac Cardiovasc Surg. 2006;132:839–844. doi: 10.1016/j.jtcvs.2006.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loftus LT, Gala R, Yang T, Jessick VJ, Ashley MD, Ordonez AN, Thompson SJ, Simon RP, Meller R. Sumo-2/3-ylation following in vitro modeled ischemia is reduced in delayed ischemic tolerance. Brain Res. 2009;1272:71–80. doi: 10.1016/j.brainres.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ditsworth D, Priestley MA, Loepke AW, Ramamoorthy C, McCann J, Staple L, Kurth CD. Apoptotic neuronal death following deep hypothermic circulatory arrest in piglets. Anesthesiology. 2003;98:1119–1127. doi: 10.1097/00000542-200305000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Li SJ, Hochstrasser M. A new protease required for cell-cycle progression in yeast. Nature. 1999;398:246–251. doi: 10.1038/18457. [DOI] [PubMed] [Google Scholar]


