Abstract
The study aims to determine the relation between the effects of mouse nerve growth factor (mNGF) and nerve regeneration after corneal surgery nerve damage. Mechanical nerve injury animal model was established by LASIK (the excimer laser keratomileusis) surgery in 12 Belgian rabbits. mNGF and the balanced salt solution (BBS) were alternatively administered in the left and right eye two times every day for 8 weeks. The morphous and growth of the sub-basal nerve plexus and superficial stroma were observed by in vivo confocal microscopy at the end of weeks 1, 2, 4 and 8 after the surgery. The animal model is successfully established. The morphology and density of corneal nerve have been observed and demonstrated by confocal microscopy. A systematic administration of mNGF can significantly promote the nerve regeneration at the end of weeks 1, 2, 4 and 8, which comparing to the administration of balanced salt solution (P < 0.05). mNGF has effect on sub-basal nerve plexus and superficial stroma after corneal nerve damage which is caused by LASIK. The experimental results suggested that the mNGF may solve the problem of dry eye after LASIK.
Keywords: mNGF, LASIK, confocal microscopy, dry eye
Introduction
In recent years, excimer laser corneal refractive surgery developed rapidly due to its good safety, efficacy and predictability. However, complications have also attracted wide attention. Dry eye is the most common complication and dissatisfaction after the excimer laser keratomileusis (LASIK). Main reason of dry eye is the mechanical nerve injury by making the corneal flap caused by LASIK surgery. Since the corneal flap cuts off all the nerves outside the pedicle, which results in corneal sensitivity significantly diminished and directly blocks the reflex tear secretion which leads to dry eye.
The human cornea is most densely innervated. Corneal epithelial innervation density of about 300 to 600 times that of skin and 20-40 times of pulp. Its density decreases gradually from the central cornea to the periphery [1]. The sensory nerves of the cornea have a very important role in corneal nutrition, the integrity of the corneal epithelium, stimulating tear secretion, and adjust the blink reflex [2]. Cornea is dominated by the long ciliary nerve which is the terminal branches of eye branch of trigeminal nerve. Long ciliary nerve enters into the sclera though the suprachoroidal cavity near the limbus from where it is issued a small branch. Once getting into the cornea, the branches walk in the upper 1/3 of stroma and anatomy to form the subcutaneous plexus [3,4]. Recent studies have found that a relatively uniform distribution of the nerve bundles along the limbus and get into the corneal randomly extend to the central cornea [3]. Bazan’s research team has recently drawn a three-dimensional map of corneal nerve structure for the first time through an improved immunofluorescence method. The study found that the number of nerve endings in the central cornea was significantly higher than that of the limbus [5].
LASIK is a current widely accepted corneal refractive surgery by doctors and patients all over the world, which is safe, predictability, light postoperative response [6]. But the central corneal nerves are almost cut off in the process of making the corneal flap. And almost 50 percent of cases within six months after LASIK complain the symptoms of dry eye [2,7,8], with varying degrees, affecting satisfaction of the patient with the surgery even with excellent vision. If some way could be explored to bring opportunities to promote the healing of corneal nerves, it can provide new hope to obtain better clinical results after LASIK [9,10].
On the ocular surface, corneal epithelial cells, endothelial cells, stromal cells, and limbal stem cells are able to synthesis and secret of NGF [11,12]. NGF plays an important role in the development and progression of sympathetic and sensory nerves. It promotes cell differentiation, determines the elongation direction of the axons. At the same time it promotes a variety of non-neuronal cells such as erythropoietin EPO clone, promotes growth and differentiation of alkaline granulocytes and B lymphocytes [13,14]. In the process of wound healing, NGF is able to promote regeneration and functional recovery of the optic nerve, sciatic nerve, the facial nerve, stimulate the body’s stem cell proliferation and differentiation, and regulate the local inflammatory response to promote wound healing [14,15]. NGF has been attracted increasing attention in recent years on the protection of the eye nerve.
mNGF is found in earlier research is most clearly, the most important known neurotrophic factor. The aim of this study is to determine the relationship among mNGF and nerve regeneration after corneal surgery nerve damage.
Materials and methods
Materials
12 Belgian rabbits weighing 1.5 to 2.0 Kg were purchased from the West China Experimental Animal Center and were used in this study. All experimental protocols conformed to the guidelines set out in the Association for research in Vision and Ophthalmology (ARVO) Statement for Use of Animals in Ophthalmic and Vision Research and approved by the West China Hospital Cancer Center’s Animal Care Committee.
Methods
Twelve Belgian rabbits underwent LASIK treatment on both eyes. The experimental rabbits were anesthetized with ketamine hydrochloride (Intramuscular 50 mg/kg). Then the topical anesthesia of the surface of the eye was given with 0.4% oxybuprocaine hydrochloride eye drops (Alcon Inc., Hunengerg, Switzerland). The corneal stromal flap in LASIK-treated eyes were created using a 110-um microkeratome (KM-5000A, Kangming Inc., Wuxi, PR China). The flaps were uncovered and covered. The mNGF (100 μg/ml) (provided by a company) and the balanced salt solution (BSS) were alternatively administered in the left and right eye two times every day for 8 weeks. After the surgery, tobramycin eye drops (Alcon Inc., Couvreur, Belgium) and fluorometholone eye drops (Santen Inc., Japan) were applied of both eyes for one week.
The corneal flap was placed on the center of the cornea in vivo. We used corneal confocal microscopy to scanning and imaging and showed corneal nerve fibers in the central portion of the picture selection. The morphous change and growth of the sub-basal nerve plexus and superficial stroma were observed by in vivo confocal microscopy at the end of weeks 1, 2, 4 and 8 after the surgery. Mean values in each group were compared with the normal control values using a 2-tailed independent Student t test. A P value < 0.05 was considered statistically significant. All statistical analysis were performed using the SPSS software package [16].
Results
Normal rabbit corneal nerves
Figure 1A shows the corneal epithelial cells which on the 12 μm layer from the surface of the cornea. The cells are mosaic arranged in clear cell borders with no significant distribution of nerve fibers.
Figure 1.
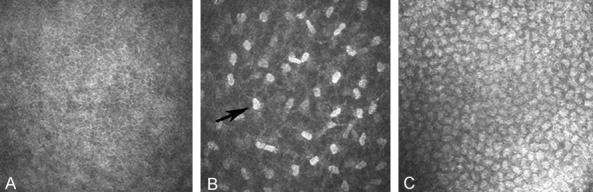
In vivo confocal microscopic image of normal rabbit corneal nerves. A: The corneal epithelial cells which on the 12 μm layer from the surface of the cornea. B: The cells and sensory nerve are shown with the distance from the surface with 50 μm. C: The rabbit corneal endothelial cells (×800).
Corneal stroma is in the corneal intermediate layer, transparent, thick, accounting for 80-90% of the total corneal volume mainly by collagen fibers (Type I collagen). The cells and sensory nerve are shown in Figure 1B with the distance from the surface with 50 μm (shallow). Fibroblast nucleus is egg-shaped corpuscles with the strong reflected light (Corneal fibroblasts nuclei, black arrow). And it is connected by a transparent thin layer (lamellae) of the black area which contains a large number of corneal mesh nerve fibers with the similar distribution position of the human nerve fibers. The number of each image on the corneal fibers is greater than 15.
Below the corneal stroma are corneal endothelial cells. Figure 1C shows the rabbit corneal endothelial cells. The cells are regularly arranged with the six prism monolayer like a honeycomb, without significant nerve fiber distribution. Unlike epithelial cells, endothelial cells undergo apoptosis but not regeneration and the survival endothelial cells t fill the gap left by apoptotic cells by the stretch to others.
Corneal nerve observed by confocal microcopy after LASIK
After 1 week and 2 weeks, mNGF group (Figure 2A, 2B) has significantly more number of nerve fibers than the BSS group (Figure 2C, 2D) in the superficial stroma (50 μm from the surface of the cornea) (800×). The width of the area was central cornea 3 mm. After 1 week, the tiny nerve fibers have been found in both groups. And nerve fibers of mNGF group grow several curved, linear neuroblastoma, and occasionally dendritic branch structure can be seen. After 2 weeks, mNGF group has visible coarse, rod-like nerve fibers, some of which forms sparse mesh.
Figure 2.
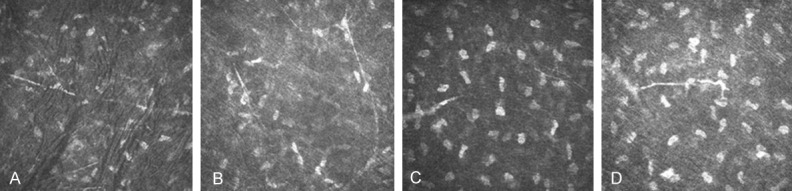
In vivo confocal microscopic image of corneal nerve fibre after 1 week LASIK. A and B: The number of nerve fibers in the superficial stroma (50 μm from the surface of the cornea) of mNGF group after LASIK. C and D: The number of nerve fibers in the superficial stroma of BBS group after LASIK.
Four weeks after the operation, the number of nerve fibers in the superficial stroma of mNGF group (Figure 3A) is increased significantly than the BSS group (Figure 3B), showing that new nerve fibers collateral which is slender than the normal nerve fiber. The branch increased each other to form a collateral anastomosis.
Figure 3.
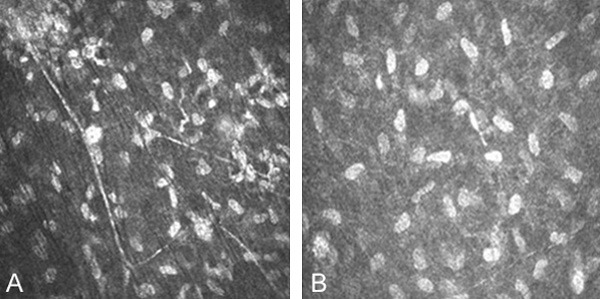
In vivo confocal microscopic image of corneal nerve fibre after 4 weeks LASIK. The number of nerve fibers in the superficial stroma of mNGF group (A) is increased significantly than the BSS group (B). The width of the area was central cornea 3 MM (800×).
After eight weeks, the fiber mesh structure in superficial stroma of mNGF group (Figure 4A) compared to the BSS group (Figure 4B) forms of a high density, number of nerve fibers than normal branch. Nerve fiber quantity of mNGF group is more than BSS group.
Figure 4.
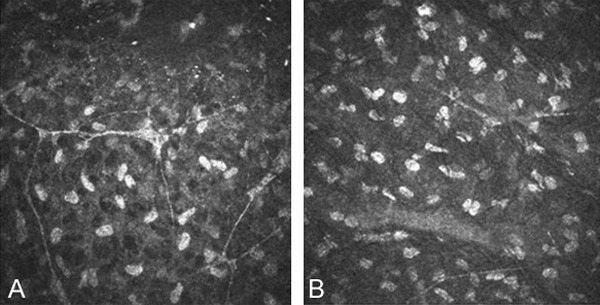
In vivo confocal microscopic image of corneal nerve fibre after 8 weeks LASIK. Nerve fiber quantity of mNGF group (A) is more than BSS group (B). The width of the area was central cornea 3 MM (800×).
Statistical analysis of the number of nerve fibers in the superficial stroma layer
Eight corneal confocal images of each the rabbit are randomly selected. The number of nerve fibers was recorded for each images, paired t test between the BSS the control group and mNGF experimental group has been done. Figure 4A and shown in Table 1, the number of corneal nerve fibers in the control group and the experimental group after 1 week, 2 weeks, 4 weeks and 8 weeks, were statistically significant (P < 0.05). The mNGF group has corneal nerve regeneration promoting effect (Figure 4A).
Table 1.
Superficial stroma nerve fibers after LASIK in mNGF group and BSS group
| Time point | BSS (n = 6) | mNGF (n = 6) | P |
|---|---|---|---|
| 1 week | 2.13±0.64 | 4.40±0.41 | 0.000356656 |
| 2 weeks | 2.93±0.76 | 5.35±0.74 | 0.000028157 |
| 4 weeks | 2.60±0.92 | 4.98±0.90 | 0.004838645 |
| 8 weeks | 3.21±0.45 | 7.00±1.72 | 0.003253497 |
The observation of same eye on different time point is also analyzed by single factor variance analysis. The results is below: (1) After 1 week, 2 weeks, 4 weeks, the changes in the number of corneal nerve fibers in two groups were no statistical significant (P > 0.05). After 8 weeks, in mNGF group, corneal nerve fiber number had statistically significant (P < 0.05). It indicates the natural regeneration progress of the nerve was slow; (2) After 1 week, 2 weeks, 4 weeks, nerve fibers in mNGF group was not statistically significant (P > 0.05). 8 weeks after the surgery, the number changes were significant (P < 0.05). The prompt mNGF corneal nerve regeneration role was no significant difference in 1-4 weeks, after 8 weeks it was significantly enhanced.
Discussion
This experiment establishes animal corneal nerves mechanical injury model by doing the LASIK surgery on Belgian rabbit to explore the effect of the mNGF on the nerve regeneration after LASIK surgery. NGF is a kind of secretory protein which plays an important role of regulation function on nerve development, differentiation, survival, functional expression and signal transduction [17]. NGF is one of the members of neurotrophic factor (neurotrophin, NTFS) family, which can prevent the death of nerve cells, with many features of typical neurotransmitter molecules [18]. NGF is synthesized and secreted by corneal epithelial, endothelial, and corneal stromal cells, and it can be up-taken by the sympathetic or sensory nerve ending, then transported retrograde to neuronal cell bodies where it induces activity of the enzyme, thus promotes nerve regeneration. The NGF functional receptor TrkA is widely present in the corneal epithelial cells, endothelial cells, stromal cells, limbal stem cells and conjunctival epithelial cells [19,20]. In this study, exogenous NGF is administrated to observe the role of NGF on corneal nerve regeneration after intervention corneal nerve damage. Current clinical drug on promoting corneal nerve recovery is still limited.
The ingredient used in this experiment is the NGF which is extracted from the mouse submandibular with the molecular weight of 26.5KD. NGF is a kind of biologically active protein with a promoting nerve repairment role. Human corneal nerves come from the ciliary nerves branches near the limbus, issued radial nerve bundles along the limbus into the upper 1/3 central layer of corneal stroma, and perpendicular forwards to the subcutaneous of the Bowman layer to form the subcutaneous plexus. The small branches on the plexus enter the basement membrane of the basal cell and then lose Schwann sheath, located in the corneal epithelial cells [3].
LASIK surgery on corneal nerve damage mainly because. The lamellar microkeratome cuts the subepithelial nerves and superficial stroma nerves in the process of making the corneal flap. And in the deep stroma below the flap, corneal nerve has no change before and after surgery. In addition, nerve damage laser is also caused by ablation of the corneal stroma. The main nerve regeneration on subcutaneous area and superficial stroma after LASIK corneal flap comes from the undamaged subepithelial nerve plexus surrounding the corneal flap and undamaged neural stem in the deep stroma. Linna found that the corneal nerve damage which is simply caused by the corneal flap without laser is similar to LASIK surgery [21]. The rabbit cornea structure and neuromorphic distribution is similar to the human eye, and compared with the mouse, strong refractive index of corneal fibroblasts nucleus can be observed in the rabbit corneal stroma which is very similar to human beings [1,22]. Therefore, this study selects rabbit as an animal model to simulate the human cornea after LASIK nerve regeneration.
Corneal confocal microscopy results show that nerve fiber density in corneal flap local area is significantly reduced after LASIK than normal rabbits. LASIK surgery does cause nerve damage of the subepithelial stroma area within the corneal flap. In the subepithelial stroma (50 μm from the surface of the cornea), the number of nerve fibers in mNGF group was significantly (P < 0.05) more than the BSS group after 1, 2, 4, 8 weeks. Eight weeks after LASIK, fiber mesh structure forms of high density of mNGF group compared to the BSS control group. mNGF promotes the regeneration of nerve fibers. After 1 week, 2 weeks, 4 weeks, the changes in the number of corneal nerve fibers in BSS control group are not statistically significant (P > 0.05). Eight weeks after LASIK compared to 1 week, the changes in the number of corneal nerve fibers in BSS control group are statistically significant (P < 0.05), combined with morphological observations, which suggest that the natural nerve repairment process is slow. After 1 week, 2 weeks, 4 weeks, mNGF group is not statistically significant (P > 0.05). Some papers showed that the bilateral nerve density can affect each other. Yamaguchi et al. showed immediate loss of subbasal nerves in axotomized eyes and decreased subbasal nerves in contralateral eyes after unilateral axotomy [23]. Patients with unilateral herpes zoster ophthalmicus (HZO) demonstrated a profound and significant bilateral loss of the corneal nerve plexus as compared with controls, demonstrating bilateral changes in a clinically unilateral disease. Loss of corneal sensation strongly correlated with subbasal nerve plexus alterations as shown by in vivo confocal microscopy [24].
Confocal microscopy is a method to observe the cornea nerve in vivo, non-invasive. It can do the observation of dynamic changes at different time points in the different layers of tissue with the advantages of high resolution, large magnification. The resolution is 1 μm and amplification factor is to 1000 times, so that the full layer can be observed at the cellular level after LASIK morphology [25,26]. It has a direct access to investigate vivo corneal epithelium, stroma, nerves and corneal thickness and to provide objective, quantitative measurement and analysis of data. mNGF is able to promote the nerve regeneration after mechanical corneal nerve injury. The duration of NGF administration is a vital determinant of behavioral recovery following corneal nerve injury.
Acknowledgements
This work was supported by grants from Chengdu City Science and Technology Bureau (grant No. ZH13044) and the National Natural Science Foundation of China (NSFC grant No. 30901635).
Disclosure of conflict of interest
None.
References
- 1.Rozsa AJ, Beuerman RW. Density and organization of free nerve endings in the corneal epithelium of the rabbit. Pain. 1982;14:105–120. doi: 10.1016/0304-3959(82)90092-6. [DOI] [PubMed] [Google Scholar]
- 2.Muller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Exp Eye Res. 2003;76:521–542. doi: 10.1016/s0014-4835(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 3.Al-Aqaba MA, Fares U, Suleman H, Lowe J, Dua HS. Architecture and distribution of human corneal nerves. Br J Ophthalmol. 2010;94:784–789. doi: 10.1136/bjo.2009.173799. [DOI] [PubMed] [Google Scholar]
- 4.Benitez-Del-Castillo JM, Acosta MC, Wassfi MA, Díaz-Valle D, Gegúndez JA, Fernandez C, García-Sánchez J. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest Ophthalmol Vis Sci. 2007;48:173–181. doi: 10.1167/iovs.06-0127. [DOI] [PubMed] [Google Scholar]
- 5.He J, Bazan NG, Bazan HE. Mapping the entire human corneal nerve architecture. Exp Eye Res. 2010;91:513–523. doi: 10.1016/j.exer.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomon KD, Fernández de Castro LE, Sandoval HP, Biber JM, Groat B, Neff KD, Ying MS, French JW, Donnenfeld ED, Lindstrom RL Joint LASIK Study Task Force. LASIK world literature review: quality of life and patient satisfaction. Ophthalmology. 2009;116:691–701. doi: 10.1016/j.ophtha.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 7.Darwish T, Brahma A, Efron N, O’Donnell C. Subbasal nerve regeneration after LASEK measured by confocal microscopy. J Refract Surg. 2007;23:709–715. doi: 10.3928/1081-597X-20070901-10. [DOI] [PubMed] [Google Scholar]
- 8.Donnenfeld ED, Solomon K, Perry HD, Doshi SJ, Ehrenhaus M, Solomon R, Biser S. The effect of hinge position on corneal sensation and dry eye after LASIK. Ophthalmology. 2003;110:1023–1029. doi: 10.1016/S0161-6420(03)00100-3. discussion 1029-1030. [DOI] [PubMed] [Google Scholar]
- 9.Patel SV, McLaren JW, Kittleson KM, Bourne WM. Subbasal nerve density and corneal sensitivity after laser in situ keratomileusis: femtosecond laser vs mechanical microkeratome. Arch Ophthalmol. 2010;128:1413–1419. doi: 10.1001/archophthalmol.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konomi K, Chen LL, Tarko RS, Scally A, Schaumberg DA, Azar D, Dartt DA. Preoperative characteristics and a potential mechanism of chronic dry eye after LASIK. Invest Ophthalmol Vis Sci. 2008;49:168–174. doi: 10.1167/iovs.07-0337. [DOI] [PubMed] [Google Scholar]
- 11.McDonald NQ, Hendrickson WA. A structural superfamily of growth factors containing a cystine knot motif. Cell. 1993;73:421–424. doi: 10.1016/0092-8674(93)90127-c. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y, Chu R, Zhou X, Dai J, Qu X. Determination of the nerve growth factor level in the central cornea after LASIK and Epi-LASIK treatment in a rabbit model system. Cornea. 2009;28:1144–1148. doi: 10.1097/ICO.0b013e3181a2a7e3. [DOI] [PubMed] [Google Scholar]
- 13.Dechant G, Neumann H. Neurotrophins. Adv Exp Med Biol. 2002;513:303–334. doi: 10.1007/978-1-4615-0123-7_11. [DOI] [PubMed] [Google Scholar]
- 14.Gordon T. The physiology of neural injury and regeneration: The role of neurotrophic factors. J Commun Disord. 2010;43:265–273. doi: 10.1016/j.jcomdis.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 16.Cruzat A, Witkin D, Baniasadi N, Zheng L, Ciolino JB, Jurkunas UV, Chodosh J, Pavan-Langston D, Dana R, Hamrah P. Inflammation and the nervous system: the connection in the cornea in patients with infectious keratitis. Invest Ophthalmol Vis Sci. 2011;52:5136–5143. doi: 10.1167/iovs.10-7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiore M, Chaldakov GN, Aloe L. Nerve growth factor as a signaling molecule for nerve cells and also for the neuroendocrine-immune systems. Rev Neurosci. 2009;20:133–145. doi: 10.1515/revneuro.2009.20.2.133. [DOI] [PubMed] [Google Scholar]
- 18.Lambiase A, Manni L, Rama P, Bonini S. Clinical application of nerve growth factor on human corneal ulcer. Arch Ital Biol. 2003;141:141–148. [PubMed] [Google Scholar]
- 19.Lambiase A, Bonini S, Micera A, Rama P, Bonini S, Aloe L. Expression of nerve growth factor receptors on the ocular surface in healthy subjects and during manifestation of inflammatory diseases. Invest Ophthalmol Vis Sci. 1998;39:1272–1275. [PubMed] [Google Scholar]
- 20.Nilsson G, Forsberg-Nilsson K, Xiang Z, Hallbook F, Nilsson K, Metcalfe DD. Human mast cells express functional TrkA and are a source of nerve growth factor. Eur J Immunol. 1997;27:2295–2301. doi: 10.1002/eji.1830270925. [DOI] [PubMed] [Google Scholar]
- 21.Linna TU, Perez-Santonja JJ, Tervo KM, Sakla HF, Alio y Sanz JL, Tervo TM. Recovery of corneal nerve morphology following laser in situ keratomileusis. Exp Eye Res. 1998;66:755–763. doi: 10.1006/exer.1998.0469. [DOI] [PubMed] [Google Scholar]
- 22.Lee HK, Lee KS, Kim HC, Lee SH, Kim EK. Nerve growth factor concentration and implications in photorefractive keratectomy vs laser in situ keratomileusis. Am J Ophthalmol. 2005;139:965–971. doi: 10.1016/j.ajo.2004.12.051. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi T, Turhan A, Harris DL, Hu K, Prüss H, von Andrian U, Hamrah P. Bilateral nerve alterations in a unilateral experimental neurotrophic keratopathy model: a lateral conjunctival approach for trigeminal axotomy. PLoS One. 2013;8:e70908. doi: 10.1371/journal.pone.0070908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamrah P, Cruzat A, Dastjerdi MH, Prüss H, Zheng L, Shahatit BM, Bayhan HA, Dana R, Pavan-Langston D. Unilateral herpes zoster ophthalmicus results in bilateral corneal nerve alteration: an in vivo confocal microscopy study. Ophthalmology. 2013;120:40–47. doi: 10.1016/j.ophtha.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavanagh HD, Jester JV, Essepian J, Shields W, Lemp MA. Confocal microscopy of the living eye. CLAO J. 1990;16:65–73. [PubMed] [Google Scholar]
- 26.Grupcheva CN, Wong T, Riley AF, McGhee CN. Assessing the sub-basal nerve plexus of the living healthy human cornea by in vivo confocal microscopy. Clin Experiment Ophthalmol. 2002;30:187–190. doi: 10.1046/j.1442-9071.2002.00507.x. [DOI] [PubMed] [Google Scholar]


