Abstract
Objective: Adiponectin is a hormone that is mainly secreted by fat cells. Adiponectin has anti-inflammatory and anti-atherosclerotic effects, and a protective effect against ischemic brain injury, but the level of expression of adiponectin in brain tissue is unknown. In the current study, a mouse model of transient cerebral ischemia was used to determine the level of expression of adiponectin in ischemic brain tissue. Methods: Sixty CD-1 mice underwent transient middle cerebral artery occlusion. The level of expression of adiponectin in mouse brain tissues 1 hour, 4 hours, 1 day, 3 days, and 7 days, after cerebral ischemia/reperfusion injury were determined using a real-time quantitative polymerase chain reaction, Western blot, and immunohistochemistry. Results: The level of expression of adiponectin in mouse ischemic brain tissues increased after cerebral ischemia/reperfusion injury and was higher in the central area of ischemia than in the peripheral area. The level of expression of adiponectin occurred only in vascular endothelial cells. There was no significant change in the level of expression of adiponectin mRNA in brain tissue pre- and post-ischemia/reperfusion injury. Conclusion: After cerebral ischemia/reperfusion injury, adiponectin accumulated in the vascular endothelial cells of ischemic brain tissues, and non-endogenous adiponectin was generated. Circulating adiponectin accumulated in ischemic brain tissues through its role in adhering to damaged vascular endothelial cells.
Keywords: Adiponectin expression, adiponectin receptors, cerebral ischemia, reperfusion injury, vascular endothelial cells
Introduction
In recent years, the relationship between adiponectin and ischemic cerebrovascular disease has received significant attention. Studies have shown that hypoadiponectinemia is an independent risk factor for ischemic cerebrovascular diseases [1,2]. A lower level of adiponectin level can increases the mortality rate of patients after ischemic stroke [3]. In mouse models of cerebral ischemia/reperfusion injury, the cerebral infarction area and degree of neurologic damage to mice in the adiponectin treatment group have been reported to be significantly less than those of mice in the non-adiponectin treatment group [4]. The cerebral infarction area in adiponectin knockout (APN-KO) mice has been shown to be significantly greater than wild-type (WT) mice, while the cerebral infarction area in APN-KO mice is significantly reduced after adiponectin treatment [5]. These findings strongly suggest that adiponectin has a protective effect against ischemic cerebrovascular diseases.
However, few studies have focused on changes in adiponectin expression in brain tissue during cerebral ischemia/reperfusion injury, and these data are extremely important in understanding the pathological process of brain injury during ischemic stroke. Therefore, in the current study, we measured changes in the expression time course and location of adiponectin expression in mouse brain tissue during cerebral ischemia/reperfusion injury, described the relationship between adiponectin expression and ischemic brain injury, and provided a theoretical basis for future use of adiponectin in the treatment of ischemic stroke and treatment time targets.
Subjects and methods
Sixty healthy 4-week-old CD-1 male mice (25-30 g) were raised under specific pathogen-free conditions. The breeding room temperature was controlled at 20-22°C, with 12-hour light and dark cycles. The mice had free access to food and water. All operations on animals were performed in accordance with requirements of the Animal Use and Care Committee of Shanghai Jiaotong University.
The following primers were used: adipoq, Forward 5’ TGTTCCTCTTAATCCTGCCCA 3’, Reverse 5’ CCAACCTGCACAAGTTCCCTT 3’; GAPDH, Forward 5’ AGGTCGGTGTGAACGGATTTG 3’, Reverse 5’ TGTAGACCATGTAGTTGAGGTCA 3’.
The mice underwent transient middle cerebral artery occlusion (tMCAO) surgery and were sacrificed for brain tissues 1 hour, 4 hours, 1 day, 3 days, and 7 days after reperfusion, with 12 mice randomly selected at each time point. Brain tissue slices were stained with hematoxylin and eosin, 3, 3’-diaminobenzidine (DAB), and immunofluorescence. The protein concentration was determined by Western blot analysis, and the RNA level was determined using a real-time polymerase chain reaction (PCR).
The SPSS13.0 statistical package was used for statistical analysis. All statistical tests were performed using 2-sided tests; P < 0.05 was considered statistically significant. Data normality was ascertained with the Kolmogorov-Smirnov statistical test. Quantitative variables with a non-normal distribution were log-transformed to achieve a normal distribution. Mea-surement data were expressed as the mean ± standard deviation (x̅ ± s). Mean values were compared between groups using an unpaired Student’s t-test. Single-factor analysis of variance was used to compare the mean values in more than three groups.
Results
Mouse models undergoing tMCAO
The mice had varying degrees of signs of neurologic damage in the left middle cerebral artery territory after tMCAO, demonstrated as a narrowed left palpebral fissure, weakened right forelimb muscle strength, rotation to the right when walking, and uncoordinated movement. Most mice exhibited symptoms, such as listlessness, darkened fur, reduced food intake, and weight loss. Three days later, the symptoms gradually improved to preoperative levels. Eight mice died after surgery, and new samples were supplied.
Western-blot semi-quantitative analysis of expression of adiponectin in mouse brain tissue after ischemia/reperfusion injury
The level of expression of adiponectin proteins in mouse brain tissues 1 hour, 4 hours, 1 day, 3 days, and 7 days after cerebral ischemia/reperfusion injury was measured. As shown in Figure 1, adiponectin proteins were strongly expressed in the ischemic hemisphere and weakly expressed in the non-ischemic hemisphere. The intensity of the adiponectin band in the ischemic hemisphere increased 1 hour after reperfusion, peaked on day 3, and was maintained at a higher level on day 7. No significant change in the level of adiponectin expression in the non-ischemic hemisphere was observed compared with the pre-operative value, and the expression of adiponectin did not change with time during reperfusion.
Figure 1.
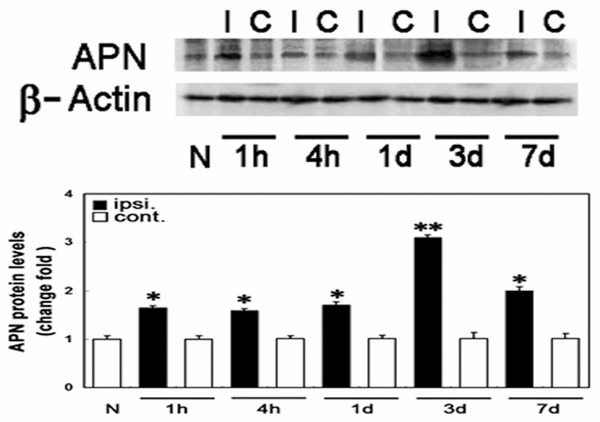
Serial changes in adiponectin protein expression in mouse brain tissues before and after transient middle cerebral artery occlusion surgery. N, normal brain tissue; I, ischemic hemisphere; C, contralateral non-ischemic hemisphere *P < 0.05, **P < 0.01.
Immunohistochemical analysis of expression of adiponectin in mouse brain tissue after ischemia/reperfusion injury
The expression and distribution of adiponectin in mouse ischemic brain tissues were observed by DAB immunohistochemical staining. Figure 2 shows the time course of adiponectin expression in mouse brain tissue; adiponectin was minimally expressed in brain tissues before MCAO, while adiponectin expression was not observed in the non-ischemic brain hemisphere at different time points after reperfusion. In the ischemic hemisphere, expression of adiponectin in either the central or peripheral area of ischemia began to increase 1 hour after reperfusion, peaked on day 3, and maintained at a high level on day 7. Expression of adiponectin in the central area of ischemia was higher than the peripheral area of ischemia at each time point.
Figure 2.
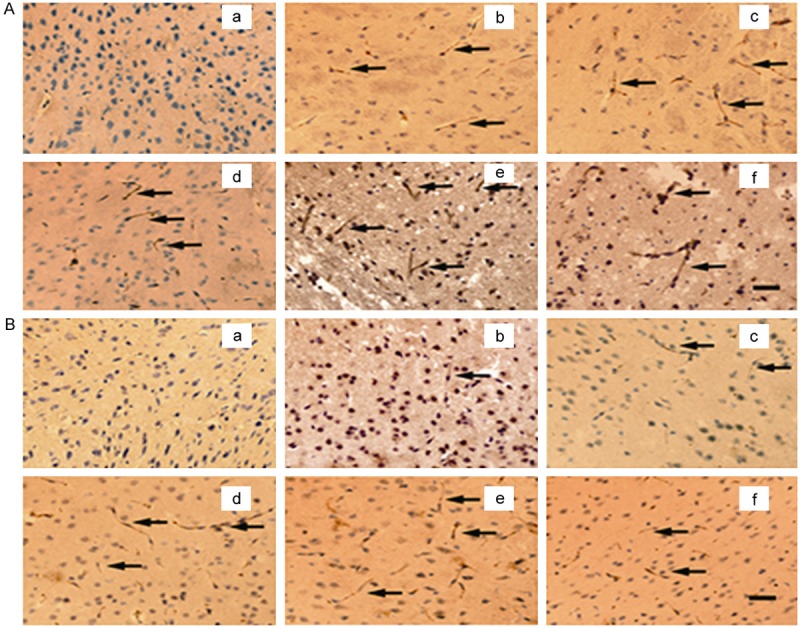
3, 3’-diaminobenzidine immunohistochemistry photomicrographs of expression time course of adiponectin in ischemic mouse brain tissue. A. Expression time course of adiponectin in central area of ischemia; B. Expression time course of adiponectin in peripheral area of ischemia; A-a and B-a. Before ischemia; A-b, B-b. 1 hour after reperfusion; A-c, B-c. 4 hours after reperfusion; A-d, B-d. 1 day after reperfusion; A-e, B-e. 3 days after reperfusion; A-f, B-f. 7 days after reperfusion; bar = 50 μm.
Double immunofluorescence staining was used to observe the expression of adiponectin in various cells in brain tissue (Figure 3). Cell markers, such as von Willebrand factor (VWF), glial fibrillary acidic protein (GFAP), and neuron-specific protein (NeuN), were used to label vascular endothelial, astroglia, and neuronal cells. The results showed that adiponectin was co-expressed with VWF, but not with GFAP or NeuN. suggesting that adiponectin expression occurred only in vascular endothelial cells in ischemic brain tissue.
Figure 3.
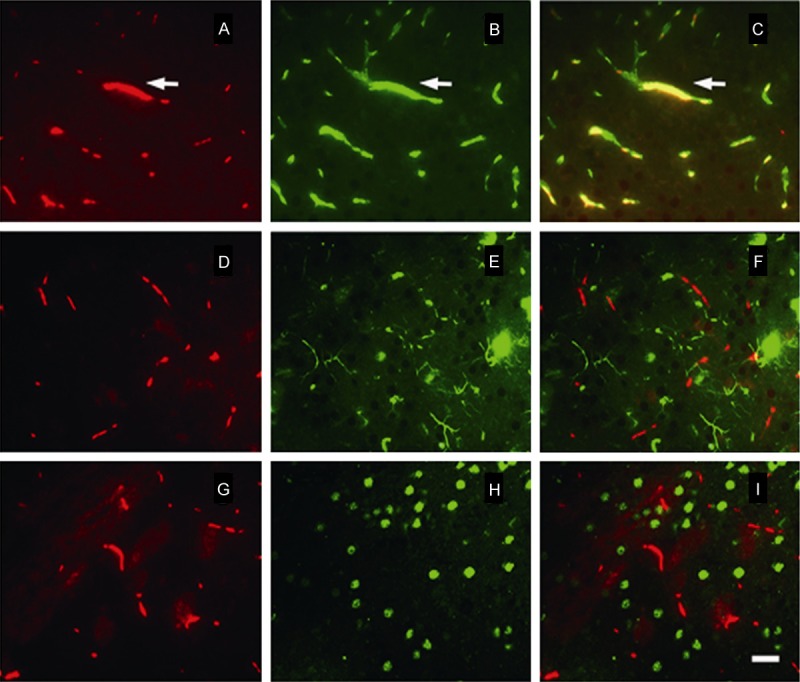
Photomicrographs of double-immunofluorescence staining of adiponectin expression in ischemic brain tissue. Red represents adiponectin; the green represents VWF, GFAP, and NeuN; orange represents the overlapping of red and green; A, D, G. Adiponectin-specific immunoreactive signals; B. VWF immunoreactive signal; E. GFAP immunoreactive signal; H. NeuN immunoreactive signal; bar = 50 μm.
Real-time PCR analysis of adiponectin mRNA expression in mouse ischemic brain tissue
Changes in the level of expression of adiponectin mRNA expression in mouse brain tissues 1 hour, 4 hours, 1 day, 3 days, and 7 days after cerebral ischemia/reperfusion injury were measured using real-time quantitative PCR (Figure 4). MCAO before and after operation there is no significantly difference at each time point after cerebral ischemia/reperfusion injury compared with normal brain tissue, and there was no significant difference in the expression of adiponectin mRNA in the ischemic and non-ischemic hemispheres.
Figure 4.
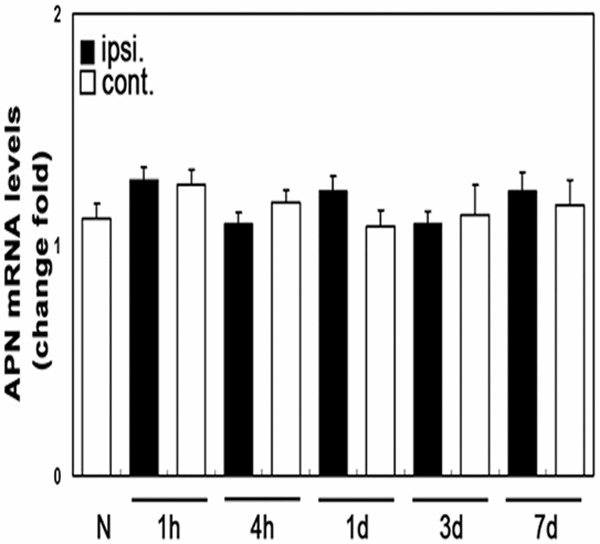
Serial changes in adiponectin mRNA expression in ischemic brain tissue. N, normal brain tissue; ipsi, ischemic hemisphere; cont, contralateral non-ischemic hemisphere.
Discussion
Adiponectin is a bioactive protein hormone secreted by fat cells [6]. Adiponectin has many important physiologic functions,including protection against the initiation and progression of atherosclerosis [1]. Studies have shown that a low level of adiponectin in circulating blood is associated with atherosclerotic diseases, such as acute coronary syndrome and ischemic stroke [7,8]. Numerous studies have demonstrated that patients with coronary heart disease have significant hypoadiponectinemia, and patients with acute coronary syndrome have lower plasma adiponectin levels [9]. Plasma adiponectin levels in patients with coronary heart disease decrease progressively with the exacerbation of coronary artery atherosclerosis. The plasma adiponectin levels in patients with complex coronary lesions is significantly lower than patients with simple lesions, and the low level of adiponectin may increase vulnerability of the plaques [10]. In recent years, it has also been reported that hypoadiponectinemia is associated with incidence of ischemic stroke, and lower plasma adiponectin levels can increase the mortality of patients after ischemic stroke [3].
Chen measured plasma adiponectin levels in 534 type 2 diabetic and non-diabetic subjects with or without cerebral infarctions, and reported that the mean plasma adiponectin level in patients with cerebral infarctions was significantly lower than patients without cerebral infarctions, after correcting for patients’ age, gender, body mass index, the waist-to-hip ratio, diabetes, and smoking status, and that the plasma adiponectin level was negatively correlated with the cerebral infarction area [1]. In mouse models of cerebral ischemia/reperfusion injury, the cerebral infarction area and degree of neurologic damage in mice in the globular adiponectin treatment group were significantly lower than the control group [4]. These results confirmed that adiponectin is important in anti-atherosclerosis and protection of heart and cerebral vessels.
Vascular endothelial cells are a target of many cardiovascular risk factors and are closely associated with the development and progression of hypertension, atherosclerosis, and cardiovascular and cerebrovascular events [11]. Vascular endothelial cells can synthesize and secrete a variety of biologically active substances and play important roles in the maintenance of smooth vascular walls and normal vascular contraction and relaxation by maintaining unobstructed blood flow and a dynamic balance between coagulation and fibrinolysis [12]. Impaired vascular endothelial function is important in the pathologic process of atherosclerosis [13]. Recent studies have shown that the antagonistic role of adiponectin in atherosclerosis is associated with its roles in protecting vascular endothelium and inhibiting the inflammatory response.
Ouchi found that, when human blood vessels are damaged with platelet adhesion and thrombus formation, adiponectin is detected in endothelial and subendothelial cells, while adiponectin is not expressed in intact vascular endothelial cells [14]. In a study involving catheter-induced mechanical vascular injury, Okamoto showed that that adiponectin is not expressed in intact vascular cells and accumulates in vascular walls without an endothelial cell layer [15]. In mouse models of heart ischemia/reperfusion injury, expression of adiponectin is detected in the ischemic vascular wall [16]. We found that in mouse models of cerebral ischemia/reperfusion injury, adiponectin accumulated in the vascular walls after ischemia, and the expression of adiponectin protein was higher in the central area of ischemia than in the peripheral area. The results of these studies suggest that adiponectin accumulates in vascular walls with a damaged endothelial barrier. The endothelial cells regulate endothelial function and protect the walls by inhibiting vascular smooth muscle cell proliferation, the inflammatory response, thrombogenesis, and endothelial cell apoptosis.
We found that adiponectin expression only occurs in the vascular endothelial cells of ischemic brain tissues. Expression of adiponectin in the ischemic hemisphere increased 1 hour after reperfusion, peaked on day 3, decreased, and increased again by day 7. The expression of adiponectin in the central area of ischemia was higher than the peripheral area of ischemia at each time point after reperfusion.
In addition to the impairment of endothelial tissue during acute ischemia, the expression of adiponectin in endothelial cells began to increase and the degree of damage to endothelial cells and the expression of adiponectin both peaked. After entering the chronic phase, the endothelial cells were gradually repaired and the adiponectin level decreased. From the site of injury, the degree of damage of endothelial cells and the expression of adiponectin in the central area of ischemia was greater than that the peripheral area. Therefore, the level of adiponectin expression was consistent with the severity of damage to the cerebral vascular endothelial tissues after ischemia/reperfusion injury, while the adiponectin mRNA expression level did not increase after ischemia, indicating that no endogenous adiponectin was produced in ischemic brain tissue. Adiponectin in circulating blood accumulates in ischemic brain tissues by adhering, and thus protecting, the injured vascular endothelial cells.
Conclusion
The mechanism by which circulating adiponectin accumulates in the damaged vascular endothelial cells is not apparent. Some studies have shown that adiponectin is highly homologous with collagen VIII and X in terms of structure and can be combined with collagen I, III, and V. These collagens are components of vascular endangium; circulating adiponectin accumulates in injured vascular walls through combination with these collagen molecules in ischemic and injured endothelial cells [15]. After adhering to endothelial cells, adiponectin inhibits the production of oxygen-free radicals to reduce stress injury, suppress the generation of inflammatory cytokines and adhesion molecules, and decrease the inflammatory response after ischemia. Adiponectin can also induce phosphorylation of endothelial nitric oxide synthase (eNOS) in ischemic tissues, increase nitric oxide levels in blood vessels, dilate blood vessels [17], and protect endothelial function.
Acknowledgements
This study is supported by grant named The Young Scientists Fund of the National Natural Science Foundation of China Project # 81100633 (LHS).
Disclosure of conflict of interest
None.
References
- 1.Chen MP, Tsai JC, Chung FM, Yang SS, Hsing LL, Shin SJ, Lee YJ. Hypoadiponectinemia is associated with ischemic cerebrovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:821–826. doi: 10.1161/01.ATV.0000157784.25920.a7. [DOI] [PubMed] [Google Scholar]
- 2.Hung WC, Wang CP, Lu LF, Yu TH, Chiu CA, Chung FM, Lee YJ. Circulating adiponectin level is associated with major adverse cardiovascular events in type 2 diabetic patients with coronary artery disease. Endocr J. 2010;57:793–802. doi: 10.1507/endocrj.k10e-020. [DOI] [PubMed] [Google Scholar]
- 3.Wei CH, Chao PW, Li FL. Plasma adiponectin levels and five-year survival after first-ever ischemic stroke. Endocrine J. 2010;57:793–802. [Google Scholar]
- 4.Chen B, Liao WQ, Xu N, Xu H, Wen JY, Yu CA, Campbell W. Adiponectin protects against cerebral ischemia-reperfusion injury through anti-inflammatory action. Brain Res. 2009;1273:129–137. doi: 10.1016/j.brainres.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Nishimura M, Izumiya Y, Higuchi A, Shibata R, Qiu J, Kudo C, Ouchi N. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation. 2008;117:216–223. doi: 10.1161/CIRCULATIONAHA.107.725044. [DOI] [PubMed] [Google Scholar]
- 6.Wang ZV, Schraw TD, Kim JY, Khan T, Rajala MW, Follenzi A, Scherer PE. Secretion of the adipocyte-specific secretory protein adiponectin critically depends on thiol-mediated protein retention. Mol Cell Biol. 2007;27:3716–3731. doi: 10.1128/MCB.00931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hopkins TA, Ouchi N, Shibata R, Walsh K. Adiponectin actions in the cardiovascular system. Cardiovasc Res. 2007;74:11–18. doi: 10.1016/j.cardiores.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ying ZQ, Zhong DD, Xu G, Chen MY, Chen QY. Adiponectin levels are associated with the number and activity of circulating endothelial progenitor cells in patients with coronary artery disease. J Zhejiang Univ Sci B. 2009;10:368–374. doi: 10.1631/jzus.B0820285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakamura Y, Shimada K, Fukuda D, Shimada Y, Ehara S, Hirose M, Yoshikawa J. Implications of plasma concentrations of adiponectin in patients with coronary artery disease. Heart. 2004;90:528–533. doi: 10.1136/hrt.2003.011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otsuka F, Sugiyama S, Kojima S, Maruyoshi H, Funahashi T, Matsui K, Ogawa H. Plasma adiponectin levels are associated with coronary lesion complexity in men with coronary artery disease. J Am Coll Cardiol. 2006;48:1155–1162. doi: 10.1016/j.jacc.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 11.Luscher TF. Vascular protection: Current possibilities and future perspectives. Int J Clin Pract Suppl. 2001:3–6. [PubMed] [Google Scholar]
- 12.Yue WS, Lau KK, Siu CW, Wang M, Yan GH, Yiu KH, Tse HF. Impact of glycemic control on circulating endothelial progenitor cells and arterial stiffness in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2011;10:113. doi: 10.1186/1475-2840-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekmekci H, Ekmekci OB. The role of adiponectin in atherosclerosis and thrombosis. Clin Appl Thromb Hemost. 2006;12:163–168. doi: 10.1177/107602960601200203. [DOI] [PubMed] [Google Scholar]
- 14.Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, Matsuzawa Y. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class a scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto Y, Arita Y, Nishida M, Muraguchi M, Ouchi N, Takahashi M, Matsuzawa Y. An adipocyte-derived plasma protein, adiponectin, adheres to injured vascular walls. Horm Metab Res. 2000;32:47–50. doi: 10.1055/s-2007-978586. [DOI] [PubMed] [Google Scholar]
- 16.Shibata R, Sato K, Kumada M, Izumiya Y, Sonoda M, Kihara S, Walsh K. Adiponectin accumulates in myocardial tissue that has been damaged by ischemia-reperfusion injury via leakage from the vascular compartment. Cardiovasc Res. 2007;74:471–479. doi: 10.1016/j.cardiores.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Cheng KK, Lam KS, Wang Y. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56:1387–1394. doi: 10.2337/db06-1580. [DOI] [PubMed] [Google Scholar]


