Abstract
Background
Ebstein’s malformation is a rare congenital cardiac anomaly. Available data are limited to individual reports demonstrating highly variable approaches. We sought to understand the spectrum of surgical treatment of Ebstein’s anomaly across institutions.
Methods
A retrospective review of surgical procedures performed on patients with primary diagnosis of Ebstein’s malformation (2002 through 2009) in The Society of Thoracic Surgeons Congenital Heart Surgery Database (STS-CHSD) was conducted.
Results
A total of 595 operations on 498 patients with Ebstein’s anomaly were included: 116 on neonates (19%), 122 on infants (21%), 264 on children (44%), and 93 on adults (16%). Average annual institutional case volumes were low (median, 1 per year; range, 0 to 8 per year). Neonates had a high rate of palliative procedures: systemic-to-pulmonary artery shunts with or without tricuspid valve closure (43; 37.1%) and tricuspid valve closure (10; 8.6%); Ebstein’s repair or tricuspid valvuloplasty was performed in 32%. The most common procedures among infants were superior cavopulmonary connections (62; 50.8%) and systemic-to-pulmonary shunt (10; 8.2%). Among older patients, procedures were primarily in three categories: tricuspid valve surgery (children, 54.5%; adults, 68.8%), arrhythmia procedures (children, 8.7%; adults, 17.3%), and Fontan (children, 14.8%). In-hospital mortality was high in neonatal patients (23.4%) in comparison with infants (4.1%), children (0.7%), and adults (1.1%).
Conclusions
Surgery for Ebstein’s anomaly consists of a wide range of procedures, with low individual institutional volumes. Mortality is highest among neonates. A prospective multicenter inception cohort study would be valuable to better define indications for specific strategies of surgical management.
Ebstein’s anomaly is a disorder of tricuspid valve development in which the valve leaflets fail to delaminate properly from the ventricular wall [1]. The extent of this failure results in a spectrum of disease characterized by both tricuspid valve (TV) regurgitation and variable reduction in the size of the functional right ventricle [1]. Patients with the most severe forms of Ebstein’s anomaly present in the neonatal period in severe heart failure, often with hypoxemia and consequent high morbidity and mortality [2, 3]. The subset with later presentation typically manifests right heart failure, arrhythmias, or the physiologic consequence of associated anomalies, and generally has a lower overall risk than the neonatal group.
Several management strategies have been advocated for neonatal patients [3–5]. These include isolated valve repairs and systemic-to-pulmonary shunts with or without TV closure. Management decisions ultimately lead to either univentricular or biventricular circulation Because of the rarity and variability of the disease and the possibility of institutional preferences for specific management strategies, series from individual centers are limited in their ability to correlate outcomes with management strategy [4–8]. Although specific aspects of morphology or clinical presentation may be associated with better outcomes using one strategy or another, there is insufficient evidence at present to guide these choices. Management of patients who present later in life is less controversial, but still includes choosing among a variety of types of TV repairs or TV replacement. To achieve a better understanding of the contemporary spectrum of surgical management, we undertook an examination of The Society of Thoracic Surgeons Congenital Heart Surgery Database (STS-CHSD) to evaluate the overall national experience with surgery for Ebstein’s anomaly.
Material and Methods
This study was approved by the Access and Publications Committee of the STS Workforce for National Databases, the Duke University Institutional Review Board, and the Nemours/A.I. duPont Hospital for Children Institutional Review Board.
Data Source
This is a retrospective study of data collected in the STS-CHSD. This database is a voluntary registry that collects operative and perioperative data on patients undergoing operations for congenital cardiac anomalies at more than 100 participating centers. It currently includes more than 85% of pediatric heart surgery programs within the United States [9]. Collected data include basic demographic information, anatomic diagnosis, associated noncardiac and genetic anomalies, STS-CHSD–defined preoperative factors, intraoperative details, surgical procedures performed, postoperative complications incurred, and inhospital mortality [10]. Diagnoses and procedures were coded by clinicians and affiliated data managers using the contemporary version of the STS-CHSD Data Collection Form (version 2.3 before July 10, 2006, and version 2.5 after July 10, 2006) [11]. All STS codes are based on the International Pediatric and Congenital Cardiac Code [12]. Procedures and their respective codes include TV valvuloplasty (STS code = 460), TV replacement (STS code = 470), Ebstein’s repair (STS code = 465), and TV closure (STS code = 480), in addition to the general list of codes for palliative procedures. The specifications accompanying the code for Ebstein’s repair (465) include the following: “repair of Ebstein’s anomaly may include, among other techniques, repositioning of the tricuspid valve, plication of the atrialized right ventricle, or right reduction atrioplasty” [10].
The Duke Clinical Research Institute serves as the data coordinating and analytic center for all of the STS National Databases, including the STS-CHSD.
Inclusionary Criteria
All patients undergoing surgical procedures between 2002 and 2009 (inclusive) with the primary diagnosis of Ebstein’s anomaly (STS code = 370) were included in the analysis. For records harvested with STS-CHSD version 2.3, the first diagnosis that is entered in the sequence of diagnoses was treated as the primary diagnosis.
Exclusionary Criteria
Patients with a secondary diagnosis of Ebstein’s anomaly with a different primary diagnosis were excluded from the analysis. This was done to focus the analysis on patients undergoing procedures related to Ebstein’s anomaly, as opposed to patients undergoing procedures primarily directed at another aspect of their congenital heart disease.
Study Population
For the purpose of this analysis patients were stratified into four age categories: neonates (age 0 to 30 days), infants (31 to 365 days), children (1 to 18 years), and adults (≥18 years).
Data Management and Statistical Analysis
All data in the STS-CHSD are deidentified, warehoused, retrieved, and analyzed by the Duke Clinical Research Institute. Patient and operative characteristics and outcomes were described using standard summary statistics. Frequencies are reported along with percentages, means are reported with standard deviation, and medians with interquartile ranges. Owing to the descriptive nature of these analyses, formal statistical comparisons were not made. Denominators may vary, as patients with missing data were excluded from analyses involving that variable.
Results
Overall Experience
A total of 595 operations were performed on 498 patients with a primary diagnosis of Ebstein’s anomaly at 82 centers. This represents 0.48% of the 104,288 patient records (and 0.39% of the 152,484 operations) contained in the STS-CHSD during the same period. Patients were distributed across age groups as follows: neonates 116 (19.5%), infants 122 (20.5%), children 264 (44.4%), and adults 93 (15.6%).
Neonates and Infants
Neonates undergoing operations commonly had at least one STS-CHSD defined preoperative risk factor (73 of 111; 65.8%; Table 1). In particular, they frequently required mechanical ventilation (61 of 111; 54.9%), had acidosis (21 of 111; 18.9%), or had preoperative arrhythmias (24 of 111; 21.6%). However, few patients were in shock (3 of 111; 2.7%) or had preoperative neurologic deficits (1 of 111; 1.8%). Neonates also commonly had noncardiac congenital anomalies (30 of 112; 26.8%). Neonates had a high rate of palliative procedures (Table 2), the most prevalent being systemic-to-pulmonary artery shunts (43 of 116; 37.0%), with (11 of 116; 9.5%) or without (32 of 116; 27.6%) TV closure. A smaller proportion underwent Ebstein’s repair (29 of 116; 25.0%) or TV repair procedures (8 of 111; 6.9%).
Table 1.
Preoperative Data in Those Undergoing Primary Procedures for Ebstein’s Anomaly
| Variable | Overall (n = 595) |
Neonates (n = 116) |
Infants (n = 122) |
Children (n = 264) |
Adults (n = 93) |
|---|---|---|---|---|---|
| Noncardiac congenital abnormality | 81 (13.6%) | 30 (25.9%) | 16 (13.1%) | 26 (9.9%) | 9 (9.7%) |
| Previous cardiothoracic surgery | 209 (35.1%) | 3 (2.6%) | 70 (57.4%) | 104 (39.4%) | 32 (34.4%) |
| Preoperative risk factora | |||||
| Any | 196 (32.9%) | 73 (62.9%) | 41 (33.6%) | 53 (20.1%) | 29 (31.2%) |
| AV block | 9 (1.6%) | 1 (0.9%) | 1 (0.9%) | 5 (2.1%) | 2 (2.3%) |
| Acidosis | 27 (4.8%) | 21 (18.9%) | 5 (4.3%) | 1 (0.4%) | 0 (0.0%) |
| Arrhythmia | 86 (15.4%) | 24 (21.6%) | 10 (8.6%) | 33 (13.6%) | 19 (21.4%) |
| Neurologic deficit | 7 (1.3%) | 2 (1.8%) | 2 (1.7%) | 3 (1.2%) | 0 (0.0%) |
| Shock | 7 (1.3%) | 4 (3.6%) | 2 (1.7%) | 1 (0.4%) | 0 (0.0%) |
| Mechanical ventilatory support | 77 (13.8%) | 61 (55.0%) | 13 (11.2%) | 3 (1.2%) | 0 (0.0%) |
| Mechanical circulatory support | 4 (0.7%) | 2 (1.8%) | 1 (0.9%) | 1 (0.4%) | 0 (0.0%) |
Includes those collected and defined in the Society of Thoracic Surgeons Congenital Heart Surgery Database.
AV = atrioventricular.
Table 2.
Distribution of Operative Procedures for Ebstein’s Anomaly by Age Group
| Variable | Neonates (n = 116) |
Infants (n = 122) |
Children (n = 264) |
Adults (n = 93) |
|---|---|---|---|---|
| Ebstein’s repair | 31 (27.0%) | 10 (8.2%) | 79 (29.9%) | 43 (46.2%) |
| Tricuspid valve repair | 8 (6.9%) | 8 (6.6%) | 36 (13.6%) | 7 (7.5%) |
| Tricuspid valve replacement | 0 (0.0%) | 3 (2.5%) | 29 (11.0%) | 14 (15.1%) |
| Tricuspid valve closure | 8 (6.9%) | 3 (2.5%) | 2 (0.8%) | 0 (0.0%) |
| Systemic-to-pulmonary artery shunt | 32 (27.6%) | 10 (8.2%) | 1 (0.4%) | 0 (0.0%) |
| Systemic-to-pulmonary artery shunt with tricuspid valve closure | 11 (9.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Superior cavopulmonary connection | 1 (0.9%) | 64 (52.5%) | 16 (6.1%) | 0 (0.0%) |
| Superior cavopulmonary connection with tricuspid valve closure | 0 (0.0%) | 2 (1.6%) | 1 (0.4%) | 0 (0.0%) |
| Fontan | 0 (0.0%) | 0 (0.0%) | 39 (14.8%) | 5 (5.4%) |
| Pacemaker implant or revision | 2 (1.7%) | 1 (0.8%) | 5 (1.9%) | 2 (2.2%) |
| Atrial ablation or maze | 0 (0.0%) | 1 (0.8%) | 18 (6.8%) | 14 (15.1%) |
| Atrial septal defect repair | 0 (0.0%) | 2 (1.6%) | 3 (1.1%) | 3 (3.2%) |
| Heart transplant | 0 (0.0%) | 2 (1.6%) | 4 (1.5%) | 0 (0.0%) |
Among infants, STS-CHSD–defined preoperative risk factors were less common; only 41 of 116 (35.3%) had one or more of the risk factors coded in the STS-CHSD, and only 13 of 116 (11.2%) were on mechanical ventilation preoperatively. Approximately half of infants had undergone a previous cardiothoracic operation (70 of 122; 57.4%). Among the infants, the most common procedure was a superior cavopulmonary connection (63 of 122; 51.6%). This was most often a unilateral bidirectional Glenn anastomosis (58 of 122; 47.5%). Ebstein’s repairs (9 of 122; 8.2%) and tricuspid valve surgery (11 of 122; 9.4%) were uncommon in this age group.
Children
Slightly more than a third of children undergoing surgery for Ebstein’s anomaly had a history of previous cardiothoracic operations (104 of 264; 39.4%); in addition, preoperative arrhythmias were common (33 of 243; 13.6%). Otherwise there were few preoperative risk factors (Table 1). Patients most commonly underwent either Ebstein’s repair (79 of 264; 29.9%) or TV repair or replacement (66 of 264; 25.0%; Table 2). Sixteen (6.1%) children underwent superior cavopulmonary connections; however, whether these were part of a single or one and one-half ventricle repair cannot be determined.
Adults
One third of the adult patients had a previous cardiothoracic operation (32 of 93; 34.4%), and preoperative arrhythmias were common (19 of 89; 21.4%). Otherwise there were few other STS-CHSD–defined preoperative risk factors in this group (Table 1). Most adult patients underwent procedures involving the TV: Ebstein’s repair (43 of 93; 46.2%), TV repair (7 of 93; 7.5%), or TV replacement (14 of 93; 15.1%; Table 2).
Hospital Course
Although neonates had long postoperative lengths of stay (median, 24 days; interquartile range, 10 to 49 days), these were considerably shorter in the older groups (infants, 7 days; interquartile range, 5 to 21 days; children, 6 days; interquartile range, 4 to 9.5 days; adults, 6 days; interquartile range, 4 to 8 days). Similarly, the use of postoperative mechanical circulatory support was relatively high among neonates (11 of 116; 9.5%) and lower in the other age groups (infants, 0, 0.0%; children, 5, 1.9%; adults, 1, 1.1%).
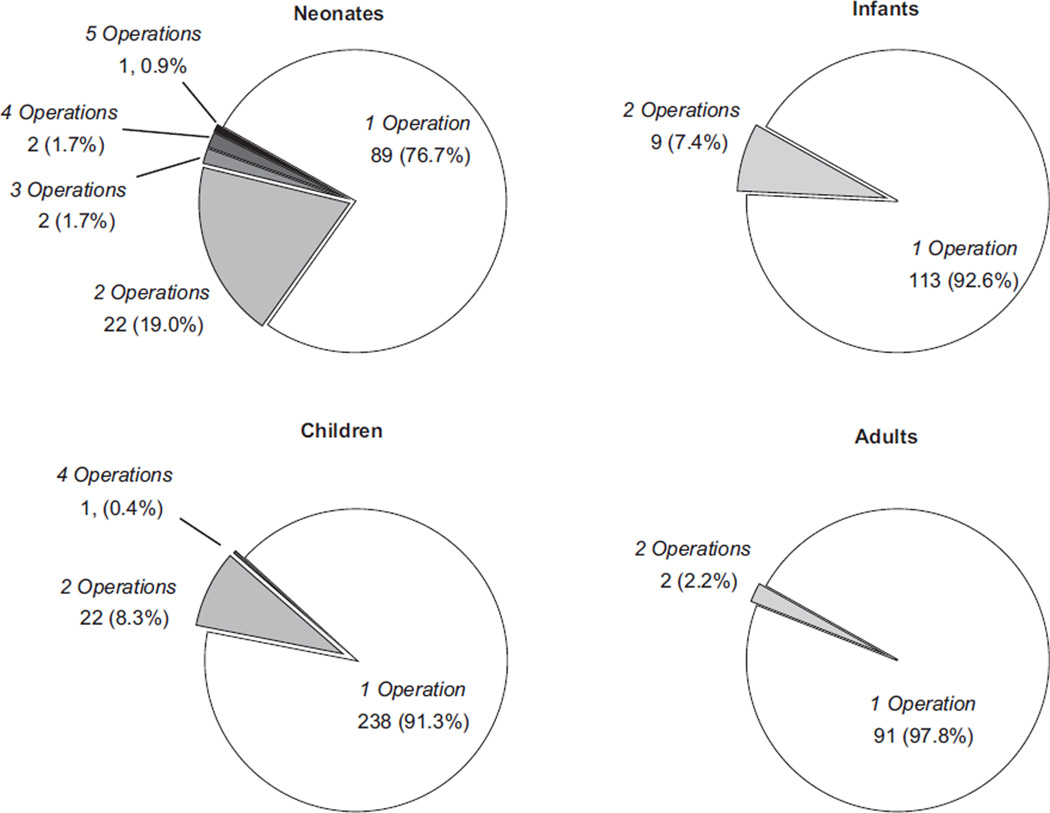
A total of 61 patients (10.3%) had one or more subsequent cardiovascular procedures during the same admission after the initial procedure. This was most common among neonates (Fig 1). The second procedure followed the index procedure at a median of 9 days (interquartile range, 3 to 17 days) after the initial procedure. The most common types of second procedures within each age group are shown in Table 3; many involve reintervention on the TV after the initial procedure.
Fig 1.
Number of separate operative procedures performed during the admission by age group.
Table 3.
Most Common Subsequent Procedures After Index Operation Among Patients Requiring Multiple Operations During Admission
| Variable | Neonates (n = 27) | Infants (n = 9) | Children (n = 26) | Adults (n = 2) |
|---|---|---|---|---|
| Revision of a systemic-to-pulmonary artery shunt | 4 (14.8%) | 3 (33.3%) | – | – |
| Repair or replace tricuspid valve | 4 (14.8%) | 5 (19.2%) | 1 (50.0%) | |
| Tricuspid valve closure | 3 (11.1%) | 3 (33.3%) | – | – |
| Superior cavopulmonary connection | 2 (7.4%) | – | 2 (7.7%) | |
| Heart transplant | 2 (7.4%) | – | 1 (3.8%) | |
| Pacemaker implantation | – | 2 (22.2%) | 7 (26.9%) |
Mortality
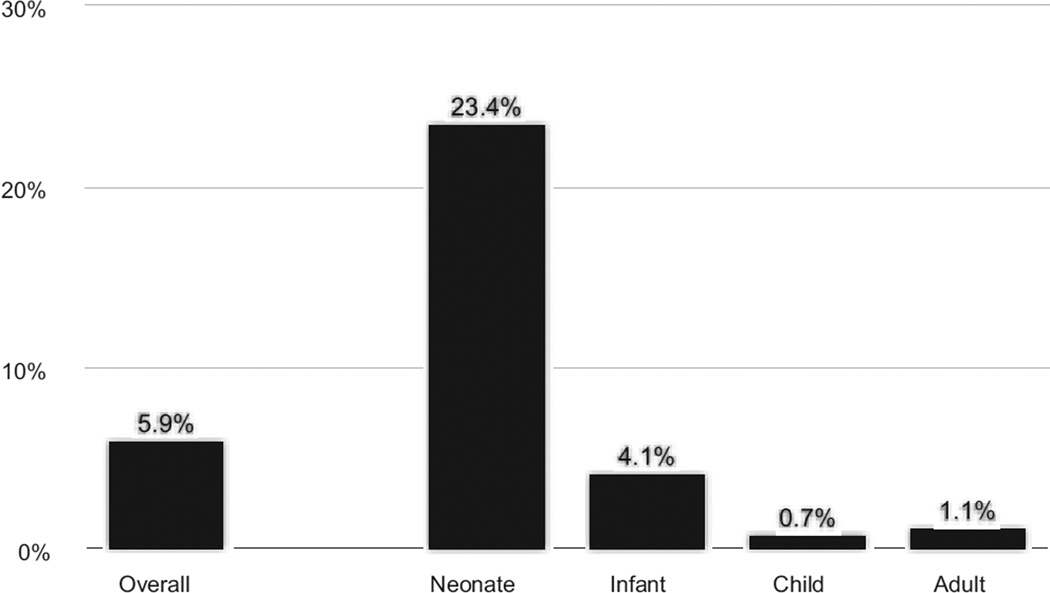
Overall in-hospital mortality was 5.9% (35 of 592), and was highest in the neonatal group (Fig 2). Within the neonatal population, mortality among patients requiring multiple operations (7 of 27; 26.9%) during the same admission was similar to those requiring only a single operation during the admission (20 of 88; 22.7%).
Fig 2.
In-hospital mortality in those undergoing operative procedures for Ebstein’s anomaly by age group.
Center Experience
The 595 procedures were performed at 82 centers. The median annual case volume for Ebstein’s anomaly was 1 case per year (interquartile range, 0.5 to 1.8; maximum, 8.3), whereas the median annual case volume for neonatal Ebstein’s was only 0.5 cases per year (interquartile range, 0.1 to 1.0; maximum, 5.2). Three fourths of centers (n = 63) performed at least one operation on neonatal patient(s) during the study period.
Comment
This analysis provides a contemporary overview of the surgical management of Ebstein’s anomaly across institutions. Our data demonstrate the wide variety of surgical procedures performed on patients with Ebstein’s anomaly. Although these patients may need surgery throughout life, the specific procedures performed differ considerably across age groups. Most importantly, the experience of any individual center is highly circumscribed. In this setting, individual institutional experiences provide limited information to help guide choices that must be made to achieve optimal surgical management of future patients, particularly neonates.
Ebstein’s anomaly is a rare disease of abnormal TV development and morphology, consisting of two components of variable severity: TV regurgitation, and a reduction in the size of the functional right ventricle [13–15]. The variability in severity results in a spectrum of presentation, often described as bimodal. Neonates with severe regurgitation and a functionally smaller right ventricle exhibit decreased effective pulmonary blood flow, leading to severe hypoxia and metabolic acidosis [8].
Neonatal Patients With Ebstein’s Anomaly
Our data demonstrate that neonates requiring surgery have a mortality exceeding 20%. This is consistent with the findings of Goldberg and colleagues [2], who reviewed an administrative data set from 40 institutions participating in the Pediatric Health Information System database, and is similar to the mortality rate among patients who underwent nonoperative management in that study. None of the published surgical series documented significantly better survival in neonatal patients [4, 5, 7, 16].
It is primarily within the group of neonates with significant physiologic embarrassment that an optimal management strategy remains elusive. Given the spectrum of morphologic and physiologic derangement present in Ebstein’s anomaly, it would be logical to assume that a spectrum of management strategies might be appropriate, including both medical therapy and surgical strategies that ultimately lead to either univentricular or biventricular physiology. Although a few management algorithms have been suggested [4, 5, 7], these algorithms are limited by the small number of patients in any individual series. The proposed surgical strategies are based on a variety of anatomic and physiologic factors, including the GOSE score [17], cardiothoracic ratio, presence or absence of antegrade pulmonary blood flow, ductal dependency, anatomic or physiologic pulmonary atresia, and the absolute and relative sizes of the two ventricles. Using these criteria, some institutions have explored the limits of biventricular repairs in neonates, whereas others have favored univentricular palliation with similar outcomes [4, 5, 7].
We were surprised to find that mortality was not higher in neonatal patients requiring multiple operations during the same hospital admission. It is important to recognize that a return to the operating room is not associated with worse outcome in these patients.
Beyond The Neonatal Period
Beyond the neonatal period, we found that mortality is low. Older patients undergoing surgery for Ebstein’s anomaly typically have few preoperative risk factors. There are several groups of patients who present throughout their life for surgical repair. Infants younger than 1 year have commonly undergone a previous cardiothoracic procedure. This reoperative group includes patients whose initial palliative procedures are eventually followed by superior cavopulmonary connections and Fontan procedures. In addition, in all of the older groups there are later presenting patients undergoing TV procedures. Among these patients, the functional status and degree of postoperative TV regurgitation are likely to be more appropriate measures of successful outcomes than early in-hospital mortality. Optimal timing of valve repair or replacement (whether initial or reoperative) in the older population remains unclear. Although some published data suggest that earlier repair (before the onset of cardiomegaly or heart failure) results in better outcomes, earlier age at surgery results in a shorter time to reoperation [18]. Thus, specific, reproducible criteria for valve intervention have not been defined.
Several repair techniques have been advocated. These include the cone procedure popularized by da Silva and associates [19], vertical plication as described initially by Carpentier along with its various modifications [20, 21, 22], and TV replacement. It is possible that matching anatomy and physiology to the specific intervention may contribute to significant improvements in outcome. Such a determination would require analysis of a data set rich with detailed information about morphology and surgical procedures. Clearer delineation of the appropriate timing and most effective techniques for valve surgery in this population is necessary.
Limitations of Currently Available Data
Despite the frequent need for surgery in neonates diagnosed with Ebstein’s anomaly [2], our data demonstrate that it represents a tiny fraction of the overall surgical experience within the STS-CHSD. The median individual center experience was less than a single case per year of neonatal Ebstein’s anomaly. Not surprisingly, even relatively large single-center studies have only a limited number of neonatal patients, often operated on over a prolonged period [4, 5, 7, 16]. In older patients, beyond specific institutions with a large referral population [23], the experience with adult repair of Ebstein’s anomaly, both initial and reoperative, is small. Even the center with the largest experience in our series had only 6 operations annually for neonatal Ebstein’s anomaly patients. As a result, analyses of single-center experiences are unlikely to provide sufficient data to define the optimal management strategies for all age groups.
Large database studies such as the current STSCHSD– based study help to characterize the scope of the challenge of improving care for patients with this rare anomaly. But the absence of preoperative hemodynamic and echocardiographic data and long-term follow-up preclude comparisons among different management strategies. Analysis of administrative data from the Pediatric Health Information System database adds an additional dimension because it also includes data pertaining to patients managed without operation [2]. However, as an administrative database it provides very limited data on clinical status and procedures performed [2].
Thus, the management of patients with Ebstein’s anomaly, and neonates in particular, remains controversial. Choice of initial procedures not only has the potential to impact operative survival but also ultimately may determine a therapeutic pathway culminating in either univentricular or biventricular physiology [4, 5, 24]. Data from the European Congenital Heart Surgeons Association suggest that mortality rates are highest among the youngest patients undergoing palliative procedures, but whether this is the result of underlying physiology or a mismatch among anatomy, morphology, and therapeutic decision making cannot be determined [25]. At the present time, there is limited information to guide choices of management strategy.
Although the STS-CHSD provides a unique opportunity to examine the broad spectrum of surgical repair across institutions because it is a general congenital heart surgery rather than an Ebstein’s-specific data set, there is limited information regarding more detailed preoperative echocardiographic or clinical variables, and absence of long-term follow-up of survival, cardiac function, or need for reoperation beyond the initial hospitalization. Fields coded in the STS-CHSD do not distinguish completely among several types of procedures directed primarily at the TV or the TV and atrialized right ventricle.
In some instances, the codes for procedures performed on neonates and young infants are highly suggestive of the surgeon’s intent to pursue a strategy directed toward univentricular physiology, as would most likely be the case when the initial procedure includes closure of the tricuspid valve and placement of a systemic-to-pulmonary artery shunt. In many other instances (including isolated systemic-pulmonary shunt and/or a variety of interventions on the tricuspid valve), surgical intent cannot be reliably inferred from the nature of the initial procedure alone, and in fact the ultimate choice to pursue either biventricular or univentricular physiology in the longer term may not be reached until some later point in time.
For other rare congenital cardiac anomalies such as critical left ventricular outflow obstruction or pulmonary atresia with intact ventricular septum, large multi-institutional prospective cohort studies have shown that patient-related factors (including some details of morphology), discriminated among end-states and that institutions that adopted a balanced approach (both univentricular and biventricular repairs) achieved the highest risk-adjusted prevalence of definitive repair and a lower prevalence of mortality [26, 27]. A prospective investigation of that type may help to define optimal management strategies for patients with Ebstein’s anomaly.
Summary
This study provides an overview of recent surgical experience with Ebstein’s anomaly. It demonstrates that surgical repair of Ebstein’s anomaly is performed infrequently at most centers, with the median number of surgical procedures performed at any individual center being one per year. Management strategies are diverse, culminating in both single- and two-ventricle pathways. At the present time, objective evidence to guide the selection of the best management strategy for individual patients is limited. Patients with Ebstein’s anomaly undergo surgery throughout life, and mortality is highest among those requiring surgery as neonates. Beyond the neonatal period, functional assessments of TV function and quality of life are likely to be important outcome measurements, but this information in not available within the current dataset. A prospective multiinstitutional study would help to define optimal management strategies. It should include operative and nonoperative patients, as well as precise diagnostic information and procedural details, to evaluate long-term outcomes including survival, reoperation, and other reinterventions, as well as neurodevelopmental outcomes, functional health status, and quality of life.
Footnotes
Presented at the Forty-ninth Annual Meeting of The Society of Thoracic Surgeons, Los Angeles, CA, Jan 26–30, 2013. Winner of the Richard E. Clark Award for Congenital Heart Surgery.
References
- 1.Knott-Craig CJ, Goldberg SP. Management of neonatal Ebstein’s anomaly. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2007:112–116. doi: 10.1053/j.pcsu.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg SP, Jones RC, Boston US, et al. Current trends in the management of neonates with Ebstein’s anomaly. World J Pediatr Congenit Heart Surg. 2011;2:554–557. doi: 10.1177/2150135111416016. [DOI] [PubMed] [Google Scholar]
- 3.Barre E, Durand I, Hazelzet T, David N. Ebstein’s anomaly and tricuspid valve dysplasia: prognosis after diagnosis in utero. Pediatr Cardiol. 2012;33:1391–1396. doi: 10.1007/s00246-012-0355-z. [DOI] [PubMed] [Google Scholar]
- 4.Shinkawa T, Polimenakos AC, Gomez-Fifer CA, et al. Management and long-term outcome of neonatal Ebstein anomaly. J Thorac Cardiov Surg. 2010;139:354–358. doi: 10.1016/j.jtcvs.2009.07.062. [DOI] [PubMed] [Google Scholar]
- 5.Reemtsen BL, Fagan BT, Wells WJ, Starnes VA. Current surgical therapy for Ebstein anomaly in neonates. J Thorac Cardiov Surg. 2006;132:1285–1290. doi: 10.1016/j.jtcvs.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 6.McElhinney DB, Salvin JW, Colan SD, et al. Improving outcomes in fetuses and neonates with congenital displacement (Ebstein’s malformation) or dysplasia of the tricuspid valve. Am J Cardiol. 2005;96:582–586. doi: 10.1016/j.amjcard.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 7.Boston US, Goldberg SP, Ward KE, et al. Complete repair of Ebstein anomaly in neonates and young infants: a 16-year follow-up. J Thorac Cardiov Surg. 2011;141:1163–1169. doi: 10.1016/j.jtcvs.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Celermajer DS, Bull C, Till JA, et al. Ebstein’s anomaly: presentation and outcome from fetus to adult. J Am Coll Cardiol. 1994;23:170–176. doi: 10.1016/0735-1097(94)90516-9. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs ML, Daniel M, Mavroudis C, et al. Report of the 2010 Society of Thoracic Surgeons Congenital Heart Surgery Practice and Manpower Survey. Ann Thorac Surg. 2011;92:762–769. doi: 10.1016/j.athoracsur.2011.03.133. [DOI] [PubMed] [Google Scholar]
- 10.STS Congenital Heart Surgery Database Data Specifications. [Accessed Jan 20, 2013];Version 3.0. 2009 Available at: http://www.sts.org/sites/default/files/documents/pdf/CongenitalDataSpecificationsV3_0_20090904.pdf. [Google Scholar]
- 11. [Accessed Jan 20, 2013];STS Congenital Heart Surgery Database, v3.0. Available at: http://www.sts.org/node/518. [Google Scholar]
- 12.International Paediatric and Congenital Cardiac Code. [Accessed Jan 20, 2013]; Available at: http://www.ipccc.net. [Google Scholar]
- 13.Engle MA, Payne TPB, Bruins C, Taussig HB. Ebstein’s anomaly of the tricuspid valve; report of three cases and analysis of clinical syndrome. Circulation. 1950;1:1246–1260. doi: 10.1161/01.cir.1.6.1246. [DOI] [PubMed] [Google Scholar]
- 14.Capozzi G, Caputo S, Pizzuti R, et al. Congenital heart disease in live-born children: incidence, distribution, and yearly changes in the Campania region. J Cardiovasc Med (Hagerstown) 2008;9:368–374. doi: 10.2459/JCM.0b013e3282eee866. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman JIE, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 16.Knott-Craig CJ, Goldberg SP, Overholt ED, Colvin EV, Kirklin JK. Repair of neonates and young infants with Ebstein’s anomaly and related disorders. Ann Thorac Surg. 2007;84:587–593. doi: 10.1016/j.athoracsur.2007.03.061. [DOI] [PubMed] [Google Scholar]
- 17.Celermajer DS, Cullen S, Sullivan ID, Spiegelhalter DJ, Wyse RK, Deanfield JE. Outcome in neonates with Ebstein’s anomaly. J Am Coll Cardiol. 1992;19:1041–1046. doi: 10.1016/0735-1097(92)90291-t. [DOI] [PubMed] [Google Scholar]
- 18.Badiu CC, Schreiber C, Hörer J, et al. Early timing of surgical intervention in patients with Ebstein’s anomaly predicts superior long-term outcome. Eur J Cardiothorac Surg. 2010;37:186–192. doi: 10.1016/j.ejcts.2009.06.052. [DOI] [PubMed] [Google Scholar]
- 19.da Silva JP, Baumgratz JF, da Fonseca L, et al. The cone reconstruction of the tricuspid valve in Ebstein’s anomaly. The operation: early and midterm results. J Thorac Cardiovasc Surg. 2007;133:215–223. doi: 10.1016/j.jtcvs.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Malhotra SP, Petrossian E, Reddy VM, et al. Selective right ventricular unloading and novel technical concepts in Ebstein’s anomaly. Ann Thorac Surg. 2009;88:1975–1981. doi: 10.1016/j.athoracsur.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 21.Palmen M, de Jong PL, Klieverik LMA, Venema AC, Meijboom FJ, Bogers AJJC. Long-term follow-up after repair of Ebstein’s anomaly. Eur J Cardiothorac Surg. 2008;34:48–54. doi: 10.1016/j.ejcts.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 22.Carpentier A, Chauvaud SM, Mace L, Relland J, Mihaileanu S, Marino JP, Abry B, Guibourt P. A new reconstructive operation for Ebstein’s anomaly of the tricuspid valve. J Thorac Cardiovasc Surg. 1988;96:92. [PubMed] [Google Scholar]
- 23.Brown ML, Dearani JA, Danielson GK, et al. Functional status after operation for Ebstein anomaly: the Mayo Clinic experience. J Am Coll Cardiol. 2008;52:460–466. doi: 10.1016/j.jacc.2008.03.064. [DOI] [PubMed] [Google Scholar]
- 24.Knott-Craig CJ, Goldberg SP, Ballweg JA, Boston US. Surgical decision making in neonatal Ebstein’s anomaly: an algorithmic approach based on 48 consecutive neonates. World J Pediatr Congenit Heart Surg. 2012;3:16–20. doi: 10.1177/2150135111425933. [DOI] [PubMed] [Google Scholar]
- 25.Sarris GE, Giannopoulos NM, Tsoutsinos AJ, et al. Results of surgery for Ebstein anomaly: a multicenter study from the European Congenital Heart Surgeons Association. J Thorac Cardiovasc Surg. 2006;132:50–57. doi: 10.1016/j.jtcvs.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 26.Ashburn DA, Blackstone EH, Wells WJ, et al. Determinants of mortality and type of repair in neonates with pulmonary atresia and intact ventricular septum. J Thorac Cardiovasc Surg. 2004;127:1000–1008. doi: 10.1016/j.jtcvs.2003.11.057. [DOI] [PubMed] [Google Scholar]
- 27.Hickey EJ, Caldarone CA, Blackstone EH, et al. Critical left ventricular outflow tract obstruction: the disproportionate impact of biventricular repair in borderline cases. J Thorac Cardiovasc Surg. 2007;134:1429–1437. doi: 10.1016/j.jtcvs.2007.07.052. [DOI] [PubMed] [Google Scholar]