Abstract
The present paper describes a rapid method for identification and characterization of human metallothionein (MT) isoforms in complex cell cultures using high resolution Matrix-assisted laser desorption/ionization time-of-flight/time-of-flight mass spectrometry (MALDI-TOF/TOF). In the proposed method, the sample preparation of MTs from cultured cells is both simple and fast. It is accomplished by trypsin cleavage of cell proteins into small peptide species, majority of which are subsequently removed by gel filtration using beads with an exclusion limit of 4000 Da. In contrast to most cell proteins, MTs remain intact (undigested) upon treated with trypsin, being excluded by the gel beads and thus recovered by low speed centrifugation. To identify the protein constitutes of the MT preparation, the MT sample is divided into two parts, one for intact protein accurate mass measurement; the other for tryptic digestion followed by MS and MS/MS analyses. In the latter case, the MT proteins are denatured by the addition of EDTA which strips of heavy metals from MTs and render them susceptible to tryptic digestion. The obtained accurate mass with the unique peptide sequences of each MT isoform allows for unambiguous identification of MT isoforms in the prepared mixture. The method has been applied to RWPE cells derived from normal human prostate epithelium. Four MT isoforms, 1E, 1G, 1X and 2A, have been confidently identified, being primarily acetylated at N-termini. These results are in agreement with the expression of MT mRNAs in RWPE cells determined by real-time reverse-transcription polymerase chain reaction.
Keywords: metallothionein, isoforms, identification, prostate cells, MALDI-TOF/TOF
Introduction
There are presently no methods that can qualify or quantify all the individual protein isoforms of the human metallothionein (MT) gene family. This is in spite of the fact that the MTs are consistently accepted by the scientific community as a major weapon in the cell's armamentarium for protection against and recovery from environmental insult, particularly those associated with heavy metals.1, 2 The MTs have also been shown to be overexpressed in numerous human cancers with overexpression frequently being related to poor patient prognosis.3 The major reason analytical methods have not been developed to determine the expression of the individual isoforms of the human MT proteins are problems associated with the high sequence homology that is present among the family members, which ranges from a low of 70% to a high of over 90%.4 This high degree of homology arose from a gene duplication event in the MT gene family, render human MT gene organization much more complex than that found in rodent animal models. The MTs are a class of low-molecular weight (Mr = 6,000-7,000), cysteine-rich, inducible, intracellular stress proteins that can be subdivided into 4 classes, MT designated MT-1 through 4, based on small differences in sequence and charge characteristics.1, 2 Despite the central role that MT plays in cellular defense against stress, it is not widely recognized that a gene duplication event occurred in the MT gene family between the evolutionary time scale of rodents and humans, rendering human MT gene organization much more complex. The mouse has four major isoforms of MT, designated 1 - 4, and all are single copy genes with no pseudogenes. The genes coding the MT-1 and MT-2 isoforms are located approximately 6kb apart on mouse chromosome 8 and are coordinately regulated and thought to be functionally equivalent.5 They were the only known rodent MT genes prior to 1992. Subsequently, two additional members of the MT gene family were identified and designated as MT-3 and MT-4; closely linked to, but not coordinately regulated with, the other MT genes on mouse chromosome 8.6, 7 Thus, the molecular organization of the mouse MT locus is comprised of 4 single gene MT isoforms located on mouse chromosome 8.
In contrast to the mouse, the organization of the human MT gene family is far more complex. First, while the human possesses the four major isoforms of MT, the human MT-1 locus possess numerous MT-1 isoforms that are not present in the mouse.8, 9 In humans, the MTs are encoded by a family of genes located at 16q13 that contains 10 functional and 7 non-functional MT isoforms. The gene sequence and expression of all of the human functional and non-functional MT genes have been reported previously. The functional genes were identified as: MT-2A; MT-3; MT-4; MT-1A; MT-1B; MT-1E; MT-1F; MT-1G; MT-1H; and, MT-1X. The non-functional (pseudo) genes were: MT-2B, MT-1C, MT-1D, MT-1I, MT-1J, MT-1K, and MT-1L. All the MT pseudogenes, except the MT-2B gene, possess introns, 3′ UTRs, and promoter regions suggesting that the multiple gene duplication event originated from DNA and not from mRNA. It can be concluded from these findings that the MT gene locus underwent a multiple duplication event in the evolutionary time frame between the mouse and human that preserved both functional and regulatory regions of the original genes. The major question posed by this gene duplication event is if these new human MT genes have gained new functional responsibilities. Primers have been developed that can detect the mRNA's from each of the individual isoforms of the MT gene family.10, 11 The analysis of human MT isoform specific mRNA expression in many systems and cells has allowed evidence to accumulate that the human MT genes can have unique expression profiles that are organ, inducer, and developmentally specific. However, for the findings at the mRNA level to be fully interpreted, they have to be confirmed by the determination of MT isoform specific protein expression. The goal of the present study was to develop the methodology to qualify the expression of the individual protein isoforms of the human MT gene family.
Analyses of MT proteins have been mainly conducted in species other than human, particularly rabbit, horse and sheep.12-20 The reported methods typically involve extensive chromatographic fractionation or isolation of MT proteins prior to detection, e.g. by anion-exchange chromatography, size-exclusion chromatography, reverse phase chromatography, and/or their combinations. Among the variety of detection methods employed, mass spectrometry (MS) is of particular interest as it can measure the molecular weights as well as the metal components of MT proteins.13, 15-18, 21 Further studies demonstrate the utility of MS, especially tandem mass spectrometry (MS/MS), in characterization of MT's structures, like metal binding,22 drug-induced modifications,23, 24 and recently intra-MT disulfide bounds.25 A recent study even demonstrated the ability of MS/MS in direct sequencing of intact MT-2A prepared from rabbit liver.26 These pioneering works encourage the authors to further exploit MS(/MS) methods for identification and characterization of MT isoforms in humans. In addition, a rapid and simple method for sample preparation of MTs from complex cultured cells is proposed.
Experimental Section
Reagents
Human MT-3 (Zn7-MT-3) and apoMT-3 (free of metals) were obtained from Bestenbalt LLC (Tallinn, Estonia). Cd7-MT-3 was reconstructed from apoMT-3. Bio-Gel P-4 polyacrylamide gel of medium size was purchased from Bio-Rad Laboratories (Hercules, CA). Chemicals including ammonium bicarbonate (AMBIC), Tris(2-carboxyethyl)phosphine hydrochloride (TCEP) and ethylenediaminetetraacetic acid disodium salt (EDTA·Na2) were purchased from Sigma (City, State). Trypsin (gold grade for mass spectrometry) was purchased from Promega (Madison, WI). Ziptip micropipette tips were acquired from Millipore (Billerica, MA). The MALDI matrix, alpha-cyano-4-hydroxycinnamic acid (CHCA), was supplied by Applied Biosystems (Foster city, CA). All other reagents, such as trifluoroacetic acid (TFA), water, and acetonitrile (ACN) were obtained from Fisher Scientific (Hampton, NJ).
Cell Culture
The RWPE cell line was obtained from the American Type Culture Collection (ATCC). The cell line was derived from human prostate epithelium and has been shown to retain the differentiated features expected of cells from the prostate epithelium.27, 28 The RWPE-1 cells were grown in keratinocyte serum free medium (K-SFM) containing 50 μg/mL bovine pituitary extract and 5 ng/mL epidermal growth factor. Cultures were incubated at 37 °C in a humidified atmosphere containing 5% CO2. The cells were fed fresh growth medium every 3 days and at confluence the cells were subcultured at a 1:4 ratio using trypsin-EDTA (0.05%, 0.02%). For use as a source of total RNA and protein, the cells were subcultured at a 1:4 ratio in T-75 flasks, allowed to reach confluence, and then exposed to 75 μM zinc sulfate for 4 days. The cells were fed fresh medium containing zinc on day 3 and harvested for total RNA and protein on day 4.
Total RNA and Protein Isolation from RWPE-1 Cells
Total RNA was purified from RWPE-1 cells using TRI REAGENTTM (Molecular Research Center, Inc., Cincinnati, OH) as described previously by this laboratory.29, 30 For the isolation of protein, the cell monolayers were washed three times with phosphate buffered saline (PBS), detached from the growth surface by trypsinization, and collected as a pellet by centrifugation at 500 × g for 5 minutes. The cell pellet was resuspended in 1.0 ml of hypotonic buffer containing 50 mM AMBIC and 5 mM TCEP (pH ∼ 8.0) and homogenized using 30 strokes of a dounce homogenizer. The homogenate was transferred to a 1.5-mL microcentrifuge tube and subjected to centrifugation at 16 000 × g for 20 minutes to pellet unbroken cells or cell debris. The supernatant containing the MT's (called the “MT crude fraction”) was collected, divided into 100 μl aliquots, and either used immediately or stored at -80 °C.
Real Time Analysis of MT Isoform mRNA Expression
The measurement of MT isoform mRNA expression was assessed with real time RT-PCR utilizing previously described MT isoform-specific primers.11 Total RNA was purified from RWPE-1 cells and 1 μg was subjected to cDNA synthesis using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules CA) in a total volume of 20 μl. Real time PCR was performed utilizing the SYBR Green kit (Bio-Rad Laboratories) with 2 μl of cDNA, 0.2 μM primers in a total volume of 20 μl in an iCycler iQ real-time detection system (Bio-Rad Laboratories). Amplification was monitored by SYBR Green fluorescence and compared to that of a standard curve of each MT isform gene cloned into pcDNA3.1/hygro (+) and linearized with Fsp I. Cycling parameters consisted of denaturation at 95 °C for 30 sec and annealing at 65 °C for 45 sec which gave optimal amplification efficiency of each standard. The level of MT isoform expression was normalized to that of glyceraldehyde-3-phosphate dehydrogenase assessed by the same assay with the primer sequences being sense: TCCTCTGACTTCAACAGCGACAC and antisense: CACCCTGTTGCTGTAGCCAAATTC with a product size of 126 base pairs.
Preparation of the Lysate for MT Protein Analysis
A 100 uL aliquot of the “MT crude fraction” was subjected to proteolytic digestion using 0.5 μg trypsin under microwave irradiation at maximum power output of 1100 W for 30 sec using a Sharp R-405K domestic microwave (Sharp Corporation, Mahwah NJ). A flask containing 1.0 liter of tap water was inside the microwave during all digestion procedures to absorb excessive microwave energy. The sample was placed in room temperature tap water following irradiation to prevent overheating. This procedure was repeated for 10 times and the reaction stopped by the addition of 0.5% TFA. The total irradiation time was 5 min. The resulting preparation was subjected to gel filtration with Bio-Gel P-4 polyacrylamide beads (exclusion limit, 4000 Da) that were conditioned before use with 100 mM ammonium acetate and packed into a 2-ml bed cartridge. The preparation was allowed to remain in contact with the beads for 2 min to ensure sufficient interaction of sample with the beads. The cartridge was then centrifuged for 1 min using a bench top mini centrifuge (Fisher, Hampton, NJ) and the flow through collected and designated as the “MT isolate”.
Tryptic digestion of the “MT Isolate”
Approximately 100 μl of the above “MT isolate” was subdivided into two parts, one for intact mass measurement and the other for further tryptic digestion and subsequent peptide MS and MS/MS measurements. In the latter case, 5 mM EDTA was added to 50 μl of the “MT isolate” to denature the MTs and render them susceptible to trypsin digestion. The EDTA-treated, denatured MTs were then digested by addition of 0.1 μg trypsin under the conditions of microwave irradiation as described above.
MLADI-TOF/TOF Mass Spectrometric Analysis
The undigested (intact) or tryptically digested MT samples from above were enriched and desalted with ZipTip micropipette tips that were packed with 0.2 μl bed C18 material, according to the manufacturer's User Guide. Briefly, a 10 μL of each sample was drawn into and dispensed for the ZipTip 5×, the ZipTip washed, and the bound peptides were eluted with 5 μL of 70% ACN containing 0.1% TFA. An aliquot, typically 0.4 μL, of the eluted protein/peptide sample was mixed on a MALDI plate with 0.4 μL of matrix solution (50 mM CHCA in 50% ACN containing 0.1% TFA). The MS and MS/MS measurements were carried out on a 4800 MALDI-TOF/TOF mass spectrometer (Applied biosystems, Foster city, CA) equipped with an Nd:YAG 200 Hz laser. The instrument was operated with delayed extraction in reflector-positive ion mode. A mixture of standard peptides (Applied biosystems, Foster city, CA) was used to externally calibrate the instrument. The MS and MS/MS spectra were typically acquired by averaging 15 sub-spectra from total 300 shots of laser with intensity set at 2950 and 3800 (the maximum intensity allowed is 8000), respectively. Both post-source decay (PSD) and collision-induced dissociation with air as collision gas were employed for peptide fragmentation. The MS/MS spectra obtained by these two approaches showed almost no difference.
Database Search and Protein Identification
The identification of the individual MT proteins was performed by searching both the MS and MS/MS spectra obtained from the MT digests against the Swiss-Prot protein database using online Mascot (http://www.matrixscience.com/). The mass tolerance was set at 100 ppm for peptide TOF-MS and 0.15 Da for fragment ions in MS/MS spectra. Other parameters were typically set as follows: taxonomy, Homo sapiens; trypsin up to 6 miss cleavages; variable modification including acetyl (N-term) and oxidation (Met); instrument, MALDI-TOF-TOF; and, report AUTO hits which displays only the significant match with a probability value of < 0.05. A protein was considered as identified if a probability value p < 0.05 and two or more peptide hits were found. All of the MS/MS spectra of the identified MT isoforms have been manually inspected to ensure the correct sequence assignment. Custom synthesized peptides (see Table 2) were made by Elim Biopharmaceuticals, Inc. (Hayward, CA) to further validate the MS/MS spectra. The accurate masses of the intact MTs as measured by MALDI-TOF-MS provided supporting evidence for the MT identification.
Table 2.
The identified unique peptides of individual MT isoforms in RWPE cells by MS/MS sequencing.
| Precursor ionX | Accuracy
(ppm)Y |
Identified peptide sequence | Mascot score | MT isoform | ValidatedZ |
|---|---|---|---|---|---|
| 1937.58 | 43 | -.(Acetyl)MDPNCSCATGGSCTCAGSCK.C | 127 | 1E | Yes |
| 2050.66 | 40 | -.(Acetyl)MDPNCSCAAAGVSCTCASSCK.C | 86 | 1G | Yes |
| 1961.76 | 29 | -.(Acetyl)MDPNCSCSPVGSCACAGSCK.C | 111 | 1X | Yes |
| 1780.62 | 21 | K.CAQGCICKGTSDKCSCCA.- | 87 | 1X | - |
| 1965.58 | 40 | -.(Acetyl)MDPNCSCAAGDSCTCAGSCK.C | 154 | 2A | Yes |
| 2556.82 | 34 | -.MDPNCSCAAGDSCTCAGSCKCKECK | 69 | 2A | - |
| 1750.59 | 33 | K.CAQGCICKGASDKCSCCA.- | 91 | 2A | - |
| 1160.35 | 44 | K.SCCSCCPVGCAK.C | 54 | 1E / 1X / 1G / 2A | - |
| 1810.63 | 34 | K.CTSCKKSCCSCCPVGCAK.C | 83 | 1E / 1X / 1G / 2A | - |
| 2401.84 | 41 | K.CKECKCTSCKKSCCSCCPVGCAK.C | 49 | 1E / 1X / 1G / 2A | - |
precursor ion as indicated by arrows in the MS spectrum in Figure 5A;
mass accuracy was calculated by dividing the theoretical mass (Mt) of the identified peptide sequence by the delta mass between observed mass (Mo) and theoretical mass as described below: Accuracy = |(Mo - Mt) |/ Mt × 106.
The MS/MS spectra of the identified peptides were validated with the respective custom synthesized peptides (see the supporting Figure S1).
Results and Discussion
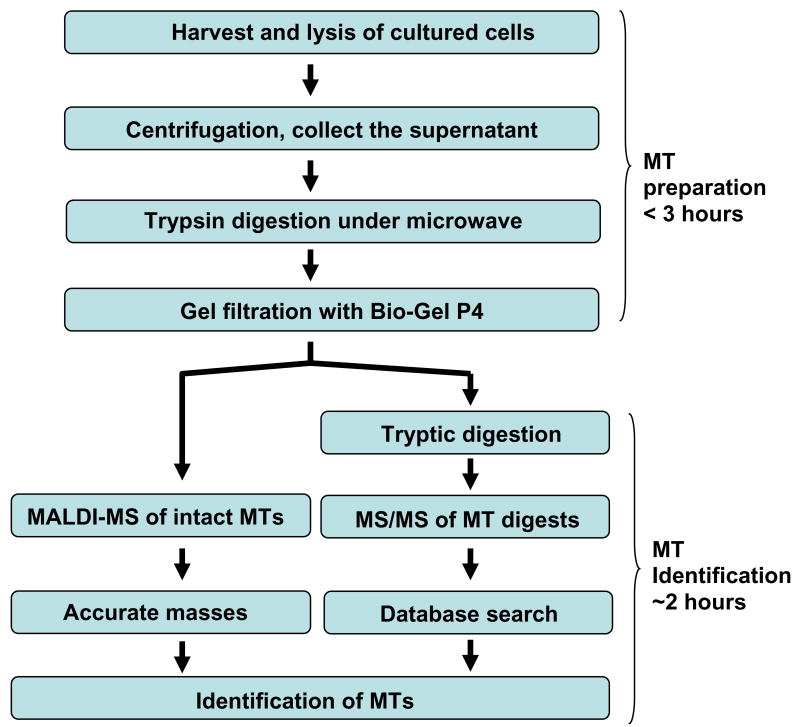
Figure 1 shows a schematic illustration of the proposed method for rapid identification of MTs from complex cell cultures. As can be appreciated, the procedures for sample preparation of MTs from cultured cells and the subsequent protein (MT) identification by MALDI-TOF/TOF mass spectrometry are typically accomplished within three and two hours, respectively.
Figure 1. The work flow of the proposed MALDI-TOF/TOF-MS method for rapid identification of MTs in cultured cells.
MT preparation
The preparation of the samples for the analysis of the MT isoforms expressed in cultured cells required no extensive chromatographic steps that involved fractionation or separation, rendering the procedure to be both simple and fast. The strategy was based on previous reports showing that mammalian MT's when complexed with metals, most often Zn(II), Cd(II) and/or Cu(I), are completely resistant to various proteases, such as subtilisin and protease K.31-33 The strategy employed was to perform an initial isolation step where the cell lysate was digested with trypsin to cleave the majority of cell proteins into small peptides which could be isolated free from the undigested MT's by simple gel filtration. The validity of this strategy was confirmed in this work by demonstrating that the incubation of Zn7-MT-3 and Cd7-MT-3with trypsin failed to produce any detectable peptides by MS analysis following microwave irradiation or incubation at 37 °C overnight. Furthermore, it was shown that increasing the amount of trypsin (e.g. 1/5 ratio of trypsin to proteins) or increasing the digestion time of the trypsin incubation (e.g. 24 hours) did not produce any MT digestion products. This allowed the metal-bound, undigested (intact) MT's, which have a molecular weight of approximately 6500 Da, to be recovered by fractionation using gel beads that had an exclusion limit of 4000 Da. Thus, this strategy allowed an initial step for MT isoform analysis that consisted on cell harvest, lysis, and trypsin digestion. An average harvest of one 75 cm2 T-flask of RWPE cells at approximately 90% confluence (∼ 5 × 106 cells) resulted in sufficient material for multiple (> 5 times) experiments on the MT preparation and MALDI-TOF/TOF analysis. These results are summarized in Figure 2.
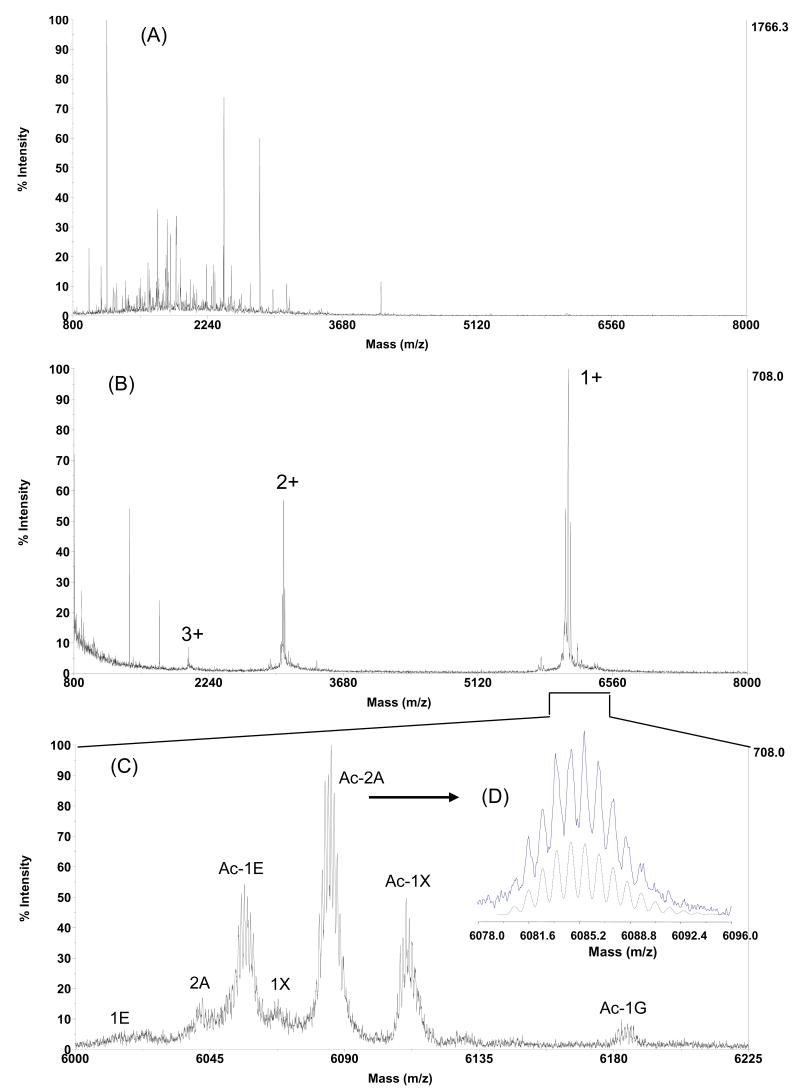
Figure 2. Detection of MTs in RWPE cells by MALDI-MS following sample preparation by trypsin digestion and subsequently gel filtration.
Diagram (A) shows the tryptic digest of the “MT crude fraction” prepared from RWPE cells was comprised of a large number of peptide species majority of which were below 4000 Da; (B) MTs in the same sample became readily detectable as 1+, 2+ and 3+ ions after gel filtration where the interfering peptide species were effectively removed; (C) The enlarged spectrum shows the observed masses representing four MT isoforms, 1E, 1G, 1X and 2A, in modified (N-term acetylated) and unmodified forms (see text at page 15 for more details). (D) The isotopes of MT-2A were well resolved (solid line), matching well to the theoretical isotope profile (dash line). The molecular formula of singly charged (protonized) MT-2A is C225H384N71O83S21, and the respective theoretical isotope distribution with a resolution of 10 000 being computed by Data Explorer Version 4.9.
A representative MALDI-TOF-MS spectrum of the resulting tyrptic digests of the soluble fraction prior to gel filtration demonstrates that only background levels of MT's, with a molecular weight between 6000 and 7000 Da, were present in the sample (Figure 2A). This is in marked contrast to the abundant profiles of non-MT peptides. The analysis of the spectrum also shows that majority (> 95%) of the interfering molecules had a mass below 4000 Da. This observation is consistent with the calculated mass distribution of peptides produced by in silico trypsin digestion of human proteome in non-redundant Swiss-Prot protein database (Version 2007.01.09) with restriction that allows for one miss cleavage (see the supporting Figure S2). Peptides with two or more miss cleavages are only occasionally observed in experiments compared to those having no or only one miss cleavage, being of no or only minimal effect on the overall mass distribution of the theoretical tryptic peptides.
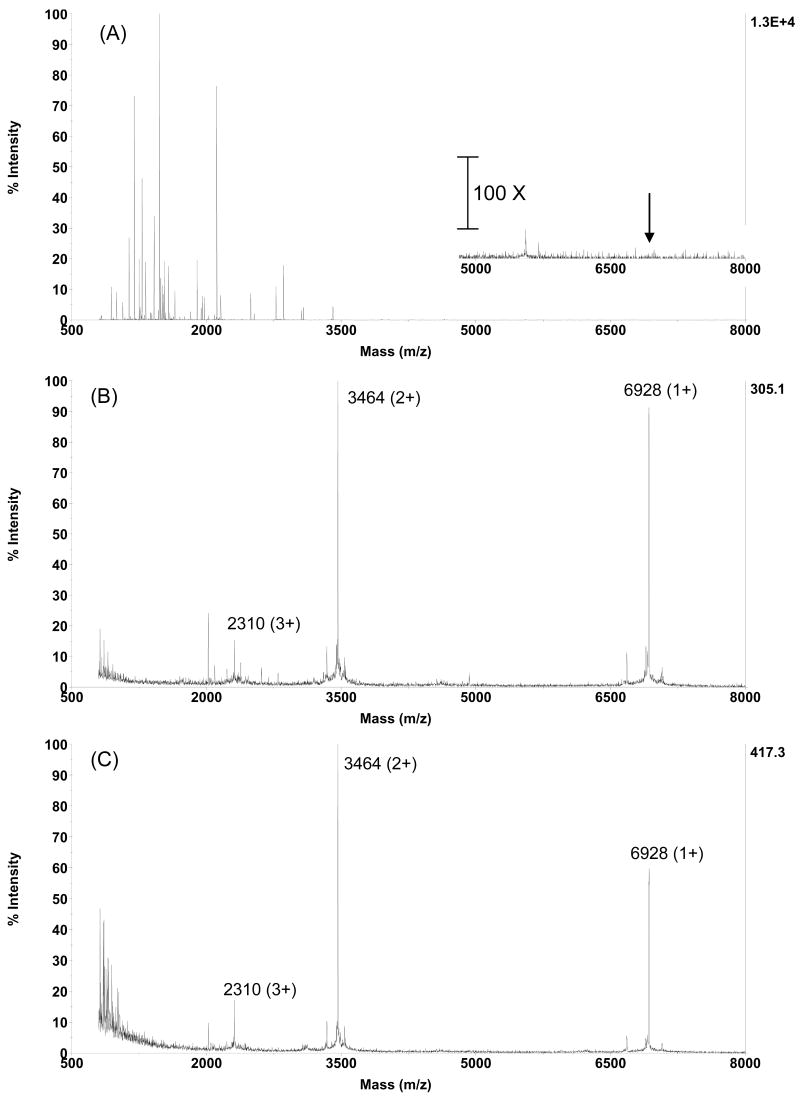
The above observations suggested that the MT-containing soluble fraction should be able to be isolated away from the digested peptides using gel filtration beads having a 4000 Da exclusion limit. This was confirmed by the observation that all but a minor background of interfering peptides was removed by size exclusion using Bio-Gel P4 (Figure 2B). Furthermore, the MT's become readily detectable, appearing as both singly, doubly and triply charged ions. An advantage is that this experimental step is both simple and rapid, with the sample being loaded onto a home-packed cartridge that contains 2 mL-bed Bio-Gel P4 beads, followed by a 2 minute incubation and mini-centrifugation for 60 seconds to collect the MT-containing effluent. The specificity and validity of the gel filtration method has also been assessed using a standard commercially available MT preparation, human Zn7-MT-3, and a tryptic digest of transferrin. Transferrin was chosen in addition to MT-3 because it is known to yield more than 20 tyrptic peptides, several of which have mass close to 4000 Da, the exclusion limit of the gel beads employed (Figure 3A). To test the gel filtration step, a mixture of 0.5 g/L (∼ 6 μM) transferrin digest and 0.01 g/L (∼ 1.4 μM) MT-3 was subjected to gel filtration. It was demonstrated that prior to (or without) gel filtration no MT-3 could be detected in the presence of the transferrin peptides (Figure 3A). In contrast, following gel filtration, it was shown that almost all of the transferrin peptides were removed from the mixture and MT-3 can be easily detected in the resulting preparation (Figure 3B). Furthermore, the intensity of the MT-3 is comparable to that of the original MT-3 standard before it was mixed with the transferrin digest or subjected to filtration (Figure 3C). These results show that this step of the analysis procedure retains peptides around 4000 Da or smaller, while effectively excluding the MT proteins for further analysis.
Figure 3. Gel filtration of human MT-3 in the presence of an excessive amount of tryptic peptides of transferrin.
The diagrams show representative MS spectra of mixed MT-3 with transferrin digest prior to gel filtration (A); after filtration (B) with Bio-Gel P4 beads; and MT-3 alone (C).
MT analysis by MALDI-TOF/TOF
Identification of individual MT isoforms whose sequences are highly conserved requires detection of the unique polypeptide sequence of each isoform. Direct sequencing by MS/MS is one alternative and has been reported for intact rabbit liver MT-2A,26 but this would be difficult to apply to a mixture of MT isoforms. The other alternative is the tryptic peptide mapping of the sequences of the 10 functional human MT isoforms since, as shown in Table 1, each has at least one unique tryptic peptide that falls within the generally used mass range of MALDI-TOF-MS (800 to 4000 Da). Overall, it would appear that trypsin digestion followed by peptide MS (mass mapping) and MS/MS (peptide sequencing) measurements would be the best for the unambiguous identification of MT isoforms. However, the measured accurate masses of intact MTs are supportive evidence of MT isoform identity. Therefore, in the present study the MT preparations were divided into two parts, one for trypsin digestion and subsequent MS and MS/MS measurements, and the other for intact mass measurement by MS. In the former case, EDTA was added to the MT samples to remove the bound metals and render the MT susceptible to tryptic digestion. The MS and MS/MS experiments were performed on a high resolution MALDI-TOF/TOF mass spectrometer which was operated in reflector positive ion mode. Under these conditions it is able to achieve high mass accuracy, e.g. < 100 ppm for intact MTs and < 50 ppm for MT peptides (< 4000 Da). The resulting MS and MS/MS spectra were searched against Swiss-Prot protein database using the peptide fingerprinting and MS/MS ion search modes, respectively. The search results were combined which promotes confident protein identification with high sequence coverage.
Table 1.
The theoretical unique tryptic peptide(s) of each human MT isoform a
| MT isoforms | Residue position | Unique peptide(s) | Mass (monoisotopic) |
|---|---|---|---|
| 1A | 1-20 | MDPNCSC ATG

|
1924.65 |
| 1B | 1-20 | MDPNCSC

|
1894.64 |
| 1E | 1-20 | MDPNCSC ATG GSCTCAGSCK | 1894.64 |
| 1F | 1-20 | MDPNCSC

|
1906.68 |
| 1G | 1-21 |
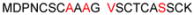
|
2007.73 |
| 1H | 1-20 | MDPNCSC

|
1892.63 |
| 1X | 1-20 | MDPNCSC

|
1918.68 |
| 2A | 1-20 | MDPNCSC

|
1922.64 |
| 3 | 1-21 |

|
2090.72 |
| 4 | 5-21 |

|
1751.59 |
| 1A | 32-43 |
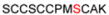
|
1221.37 |
| 1B | 32-43 |
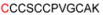
|
1175.37 |
| 1E/1G/1X/2A | 32-43 | SCCSCCPVGCAK | 1159.40 |
| 1F | 32-43 |
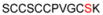
|
1175.39 |
| 1H | 32-43 |

|
1173.41 |
| 4 | 33-44 |
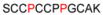
|
1167.40 |
| 3 | 53-63 | GGEAAEAEAEK | 1060.47 |
The table lists only the tryptic peptides with no miss cleavages and within the generally used mass range of MALDI-TOF-MS, i.e. 800 to 4000 Da. Trypsin digestion may also produce peptides with one or more miss cleavages, covering the unique sequence of each MT isoform listed in the table. The red color marks the unique amino acid residues compared to the consensus sequence of MT.
Identification of MT isoforms in Zn-Stimulated RWPE cells
The RWPE cell culture was chosen as a human biological model to validate the utility of the present method since ongoing studies in the laboratory have defined the expression of the 10 active MT isoform-specific mRNAs using real time PCR. The expectation from the use of such a model is that the newly developed method would detect MT isoform-specific proteins that would be in agreement with the mRNA expression patterns of the isoforms. The method for the analysis of the MT isoform-specific proteins and identification by MS and MS/MS was performed as detailed in the above analysis scheme.
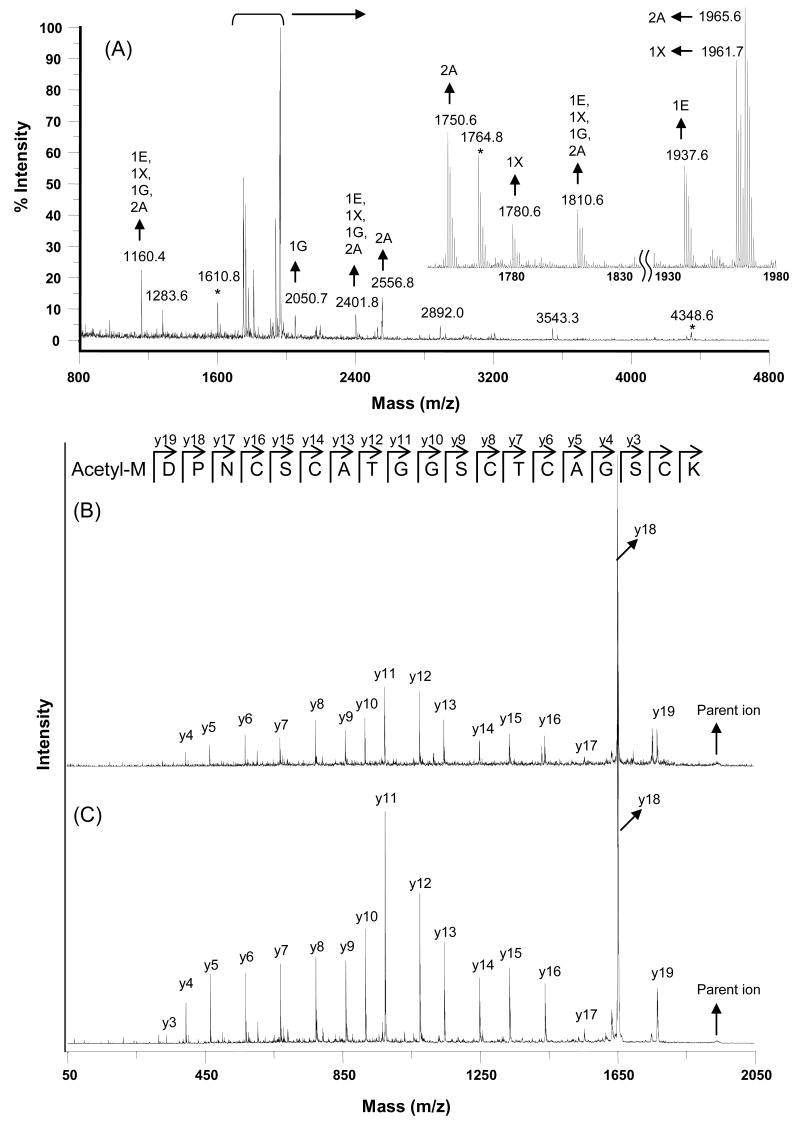
The results of peptide mass mapping analysis tentatively identified five MT isoform-specific proteins in the RWPE cells, MT-1E, MT-2A, MT-1I, MT-1X (or -1M) and MT-1G. These were identified (in order of Mascot score) by searching the MS spectrum (Figure 4A) against Swiss-Prot protein sequence database by Mascot Peptide Mass Fingerprint at a significance p < 0.05. The isoforms were identified with 100% sequence coverage. In order to further exclude the possibility of ambiguous identification, the MT peptide peaks (indicated by arrows in Figure 5A) were selected for further MS/MS sequencing. The acquired MS/MS spectra were pooled together and subjected to Mascot MS/MS ion search. The results from the MS/MS spectra assigned the selected peptides to four MT isoforms, MT-1E, MT-2A, MT-1X, and MT-1G (Table 2). The Mascot scores of the identified peptides were typically 50 or higher which indicates a very low incidence (< 10-5) of random match of the spectra with the peptide sequences. This can be attributed to the acquired high quality MS/MS spectra by the MALDI-TOF/TOF-MS (Figure 4B & 4C). For each of the four identified MT isoforms, custom synthesized peptide was made to validate the MS/MS spectra of the respective identified peptide (Table 2).
Figure 4. Identification and characterization of MTs in RWPE cells by MALDI-TOF/TOF.
Diagram (A) shows a representative MS spectrum of the tryptic digest of MT preparation from RWPE cells; (B) the observed MS/MS spectrum of the peptide precursor ion 1937.6 in (A); and (C) the MS/MS spectrum of synthesized peptide, Acetyl-MDPNCSCATGGSCTCAGSCK (m/z 1937.6) belonging to MT-1E. There is a perfect match between the MS/MS spectra in (B) and (C), indicating the correct sequence assignment for the observed MS/MS in (B).
Information was also obtained regarding the N-terminal sequences of the 4 MT isoform-specific proteins (Table 2 & Fig 4B). For instance, the peptide peak at 1937.6 in Figure 4A was identified as an N-terminal acetylated MDPNCSCATGGSCTCAGSCK sequence which correlates to the first N-terminal 1-20 amino acid sequence of MT-1E. The observed MS/MS spectrum of this peptide exactly matches to that of the respective synthesized peptide sequence (Figure 4B & 4C). Likewise, the N-term acetylated sequences of MT-1G, MT-1X and -2A were also identified by MS/MS and validated with custom synthesized peptides (Table 2). For the 4 identified MT's in the RWPE cells, both acetylated and non-acetylated sequences were obtain for all the isoforms except MT-1G, for which non-acetylated (unmodified) form was not identified in the analysis. The finding of no non-acetylated MT-1G could simply be due to low abundance, since in general the native peptides were in much lower abundance than the modified peptides and the MT-1G isoform had the lowest abundance on initial analysis.
The above results were further confirmed by determining the accurate masses of the intact (undigested) MT preparations that were measured by MALDI-TOF-MS (Fig 2C & 2D). The results showed four major peaks with apex masses of 6056.80, 6085.72, 6111.78 and 6184.55; all within 100 ppm to the respective theoretical masses (in order of peak mass) of the N-terminal acetylated MT-1E (6057.26, m/z, 1+), MT-2A (6085.27, m/z, 1+), MT-1X (6111.35, m/z, 1+) and MT-1G (6184.44, m/z, 1+). There were also three peaks of lower abundance that represented the masses of the native (unmodified) MT isoforms, 1E, 2A and 1X. A mass resolution of 10 000 or higher for MTs was typically achieved. As a consequence the isotopic peaks of MT isoform are well resolved (Figure 2C & 2D). This not only allows for accurate mass measurement of MT protein, but also provide further evidence of the MT protein identity by matching the observed isotope profile to the theoretical isotope profile (Figure 2D). Thus, the consistent results from intact mass measurement of MT preparation and MS and MS/MS measurements of the tryptic digest of MT preparations, provide compelling evidence that four MT isoform proteins, 1E, 1G, 1X and 2A, are expressed in RWPE cells. Furthermore, all of these isoforms are primarily acetylated at the N-termini.
The finding that the RWPE cells contained the 4 protein isoforms, MT-1E, MT-1G, MT-1X and MT-2A is in agreement with the MT isoform-specific mRNA profile of the cells used in the analysis. The real-time PCR analysis of MT mRNA expression showed the expression of MT-1E, -G, -X and -2A mRNAs (Table 3). Also in agreement with the MT protein analysis, was the finding that the MT-1G isoform was the lowest abundance mRNA among the four identified MT isoforms. To the author's knowledge, this is the first time that the individual human MT isoforms have been detected in human cells simultaneously at both the mRNA and protein levels of examination. It should be pointed out that mRNA expression level does not always correlate with the respective proteins expression level since there are many factors regulating the translation of MT mRNA to proteins. For instance, the mRNA expression level of MT-1E is about 20 and 40 times of that of MT-1X and -2A, respectively, but its protein expression level appears about equal to MT-1X's and lower than MT-2A's (Figure 2C). MT-1F has a similar number of transcripts to MT-1G, but its protein expression level is much lower than MT-1G's.
Table 3.
Expression of MT isoform mRNAs in Zinc Treated RWPE-1 Cellsa
| MT Isoformb | mRNA Expressionc |
|---|---|
| MT-1A | 0.00519 |
| MT-1E | 57.2 |
| MT-1F | 0.534 |
| MT-1G | 0.581 |
| MT-1H | 0.0384 |
| MT-1X | 3.05 |
| MT-2A | 1.57 |
| MT-3 | 0.000189 |
Messenger RNA expression was measure with real time PCR using MT isoform specific primers and total RNA extracted from RWPE-1 cells treated with 75 μM zinc for four days (see the section “Real Time Analysis of MT isoform mRNA Expression” at page 8).
Other isoforms were measured but not detected, MT-1B, -1I, -1J, -1K, -1L, and MT-4. The expression of the rest three MT isoforms, MT-1C, -1D and -1M, was not assessed since their gene sequences are either not available in databases (e.g. NCBI or Swiss-Prot) or of controversy.
Expressed as the number of mRNA molecules normalized to that of the house-keeping gene, glyceraldehyde-3 phosphate dehydrogenase.
Comprehensive profiling of the human MTs is beyond the capability of the current MALDI-TOF-MS method and will need further methodology development. One of the major difficulties is the wide dynamic range of MT expression which is shown in Table 3 varying by 5 orders of magnitude (i.e. from 57 of MT-1E to 0.00019 of MT-3). Detection of the low abundant MT isoforms in the presence of the abundant ones is challenging by a single MALDI-TOF-MS method. Another issue is the employed MALDI-TOF-MS is still not sufficient in resolving all of the human MT isoforms as well as their isotopes. This issue can be to some extent alleviated by using a higher resolution mass spectrometer, e.g. Fourier-transform ion cyclotron resonance mass spectrometry. But such an instrument is expensive and not readily available to most of researchers. Chromatographic separation (e.g. by reverser-phase chromatography) prior to MS analyses is thus of more interest to the authors for further methodology development. Despite these limitations, the current MALDI-TOF-MS method has shown the power of mass spectrometry in identification and post-translational modification characterization of the complex family of human MT isoforms, namely, its speed, selectivity (un-ambiguity) and sensitivity.
Conclusion
A rapid and simple method based on MALDI-TOF/TOF mass spectrometry has been developed for identification and characterization of MT isoforms in cultured cells (Fig 2). As summarized, the preparation of the MT's from cultured cells is accomplished by a simple tryptic digestion of the majority of non-MT proteins into small peptides which are subsequently removed from undigested MTs by gel filtration. This allows the isolation of the MT fraction away from these interfering proteins. Subsequently, the high mass accuracy and high quality MS and MS/MS spectra provided by MALDI-TOF/TOF allows the MT isoforms to be unambiguously identified, complete with information on post-translation modifications (in this work, acetylation at N-terminus). The current MS method allows correlation of isoform-specific protein expression with isoform-specific mRNA expression by providing protein expression information of MT isoforms in a rapid fashion.
Supplementary Material
Figure S1. Trypsin digestion of the MT crude fractions prepared from RWPE assessed by SDS-PAGE. Figure S2 shows the calculated mass distribution of the peptides produced by in silico trypsin digestion of human proteome in non-redundant Swiss-Prot protein database (Version 2007.01.09) with restriction that allows for one miss cleavage. These materials are available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This research was supported by grant R01 ES015100 from the National Institute of Environmental Health Sciences and grant R01 CA098832 from the National Cancer Institute (NCI), NIH. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCI and NIEHS, NIH. The proteomics core facility was supported by NIH Grant Number P20 RR016741 from the INBRE Program of the National Center for Research Resources. Lu is the recipient of a faculty initiation grant from NSF EPSCoR (#: EPS-0447679). The authors thank Dr. Y. Hathout from Children's National Medical Center, Washington DC, for his helpful advice and discussion.
Abbreviation
- ACN
acetonitrile
- AMBIC
ammonium bicarbonate
- ApoMT
metal free metallothionein
- CHCA
alpha-cyano-4-hydroxycinnamic acid
- EDTA
ethylenediaminetetraacetic acid
- MALDI
matrix-assisted laser desorption/ionization
- MS/MS
tandem mass spectrometry
- MT
metallothionein
- RT-PCR
reverse-transcription polymerase chain reaction
- TCEP
Tris(2-carboxyethyl)phosphine hydrochloride
- TFA
trifluoroacetic acid
- TOF/TOF-MS
tandem time-of-flight mass spectrometry
References
- 1.Andrews GK. Biochem Pharmacol. 2000;59:95–104. doi: 10.1016/s0006-2952(99)00301-9. [DOI] [PubMed] [Google Scholar]
- 2.Hamer DH. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- 3.Theocharis SE, Margeli AP, Klijanienko JT, Kouraklis GP. Histopathology. 2004;45:103–118. doi: 10.1111/j.1365-2559.2004.01922.x. [DOI] [PubMed] [Google Scholar]
- 4.Sewell AK, Jensen LT, Erickson JC, Palmiter RD, Winge DR. Biochemistry. 1995;34:4740–4747. doi: 10.1021/bi00014a031. [DOI] [PubMed] [Google Scholar]
- 5.Searle PF, Davison BL, Stuart GW, Wilkie TM, Norstedt G, Palmiter RD. Mol Cell Biol. 1984;4:1221–1230. doi: 10.1128/mcb.4.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmiter RD, Findley SD, Whitmore TE, Durnam DM. Proc Natl Acad Sci U S A. 1992;89:6333–6337. doi: 10.1073/pnas.89.14.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quaife CJ, Findley SD, Erickson JC, Froelick GJ, Kelly EJ, Zambrowicz BP, Palmiter RD. Biochemistry. 1994;33:7250–7259. doi: 10.1021/bi00189a029. [DOI] [PubMed] [Google Scholar]
- 8.Stennard FA, Holloway AF, Hamilton J, West AK. Biochim Biophys Acta. 1994;1218:357–365. doi: 10.1016/0167-4781(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 9.West AK, Stallings R, Hildebrand CE, Chiu R, Karin M, Richards RI. Genomics. 1990;8:513–518. doi: 10.1016/0888-7543(90)90038-v. [DOI] [PubMed] [Google Scholar]
- 10.Garrett SH, Sens MA, Shukla D, Flores L, Somji S, Todd JH, Sens DA. Prostate. 2000;43:125–135. doi: 10.1002/(sici)1097-0045(20000501)43:2<125::aid-pros7>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 11.Mididoddi S, McGuirt JP, Sens MA, Todd JH, Sens DA. Toxicol Lett. 1996;85:17–27. doi: 10.1016/0378-4274(96)03632-6. [DOI] [PubMed] [Google Scholar]
- 12.Minami T, Ichida S, Kubo K. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781:303–311. doi: 10.1016/s1570-0232(02)00496-8. [DOI] [PubMed] [Google Scholar]
- 13.Prange A, Schaumloffel D. Anal Bioanal Chem. 2002;373:441–453. doi: 10.1007/s00216-002-1350-7. [DOI] [PubMed] [Google Scholar]
- 14.Montes-Bayon M, Profrock D, Sanz-Medel A, Prange A. J Chromatogr A. 2006;1114:138–144. doi: 10.1016/j.chroma.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 15.Infante HG, Van Campenhout K, Blust R, Adams FC. J Chromatogr A. 2006;1121:184–190. doi: 10.1016/j.chroma.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 16.Andon B, Barbosa J, Sanz-Nebot V. Electrophoresis. 2006;27:3661–3670. doi: 10.1002/elps.200500168. [DOI] [PubMed] [Google Scholar]
- 17.Sanz-Nebot V, Andon B, Barbosa J. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;796:379–393. doi: 10.1016/j.jchromb.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Nischwitz V, Michalke B, Kettrup A. Anal Bioanal Chem. 2003;375:145–156. doi: 10.1007/s00216-002-1594-2. [DOI] [PubMed] [Google Scholar]
- 19.Schaumloffel D, Prange A, Marx G, Heumann KG, Bratter P. Anal Bioanal Chem. 2002;372:155–163. doi: 10.1007/s00216-001-1164-z. [DOI] [PubMed] [Google Scholar]
- 20.Prange A, Schaumloffel D, Bratter P, Richarz AN, Wolf C. Fresenius J Anal Chem. 2001;371:764–774. doi: 10.1007/s002160101019. [DOI] [PubMed] [Google Scholar]
- 21.Lobinski R, Schaumloffel D, Szpunar J. Mass Spectrom Rev. 2006;25:255–289. doi: 10.1002/mas.20069. [DOI] [PubMed] [Google Scholar]
- 22.Afonso C, Hathout Y, Fenselau C. Int J Mass Spectrom. 2004;231:5. [Google Scholar]
- 23.Antoine M, Fabris D, Fenselau C. Drug Metab Dispos. 1998;26:921–926. [PubMed] [Google Scholar]
- 24.Wei D, Fabris D, Fenselau C. Drug Metab Dispos. 1999;27:786–791. [PubMed] [Google Scholar]
- 25.Feng W, Benz FW, Cai J, Pierce WM, Kang YJ. J Biol Chem. 2006;281:681–687. doi: 10.1074/jbc.M506956200. [DOI] [PubMed] [Google Scholar]
- 26.Mandal R, Li XF. Rapid Commun Mass Spectrom. 2006;20:48–52. doi: 10.1002/rcm.2268. [DOI] [PubMed] [Google Scholar]
- 27.Bello D, Webber MM, Kleinman HK, Wartinger DD, Rhim JS. Carcinogenesis. 1997;18:1215–1223. doi: 10.1093/carcin/18.6.1215. [DOI] [PubMed] [Google Scholar]
- 28.Webber MM, Bello D, Kleinman HK, Hoffman MP. Carcinogenesis. 1997;18:1225–1231. doi: 10.1093/carcin/18.6.1225. [DOI] [PubMed] [Google Scholar]
- 29.Garrett SH, Somji S, Todd JH, Sens DA. Environ Health Perspect. 1998;106:587–595. doi: 10.1289/ehp.98106587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garrett SH, Somji S, Todd JH, Sens MA, Sens DA. Environ Health Perspect. 1998;106:825–832. doi: 10.1289/ehp.98106825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nielson KB, Atkin CL, Winge DR. J Biol Chem. 1985;260:5342–5350. [PubMed] [Google Scholar]
- 32.Nielson KB, Winge DR. J Biol Chem. 1983;258:13063–13069. [PubMed] [Google Scholar]
- 33.Winge DR, Miklossy KA. J Biol Chem. 1982;257:3471–3476. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Trypsin digestion of the MT crude fractions prepared from RWPE assessed by SDS-PAGE. Figure S2 shows the calculated mass distribution of the peptides produced by in silico trypsin digestion of human proteome in non-redundant Swiss-Prot protein database (Version 2007.01.09) with restriction that allows for one miss cleavage. These materials are available free of charge via the Internet at http://pubs.acs.org.