Abstract
The self-renewal and differentiation properties of cancer stem cells (CSCs) are regulated and maintained by the CSC niche. However, the mechanism of this maintenance, especially the maintenance contributed by differentiated cancer cells, remains to be fully elucidated. Recently, we have established a model of CSCs, miPS-LLCcm, from mouse induced pluripotent stem cells (miPSCs). In vitro cultured miPS-LLCcm cells were autonomously balanced with stem-like cells and differentiated cells including vascular endothelial cells. Under these conditions, the CSC properties appeared to be stable in the presence of the factor(s) secreted by the differentiated cells. The factor(s) activated Notch signaling and promoted self-renewal of CSCs. In addition, the secreted factor(s) appeared to regulate the differentiation lineage of CSCs. Our results indicate that the differentiated progenies of CSCs containing vascular endothelium play important roles for regulating the CSC's properties. Therefore, miPS-LLCcm cells create their own in vitro niche to maintain themselves in the hierarchy of differentiating CSCs.
What's new?
Cancer stem cells wreak their devastation by taking root in a supportive microenvironment that provides needed factors for both self-renewal and differentiation. But how does the microenvironment, or niche, sustain the stem cells? To investigate, these authors established a CSC system in vitro and assessed whether the progeny cells of CSCs need to stay nearby to create the stem cell niche. They found that the differentiated progeny cells do release factors that maintain the balance between self-renewal and differentiation in the stem cells, in part through the Notch signaling pathway. Understanding this dynamic will help researchers develop strategies to hinder cancer stem cells' ability to take hold.
Keywords: cancer stem cell, niche, self-renewal, differentiation, vascular endothelium
Cancer stem cells (CSCs) are unique subpopulation of tumor cells that possess self-renewal and differentiation capacity, that can give rise to the entire heterogeneous population of the tumor tissue.1–3 With regard to differentiation capacity, three independent groups have demonstrated that vascular endothelial cells are one of the types of cells that differentiate from CSCs.4–6 For example, CD133-positive (CD133+) glioblastoma (GBM) stem-like cells were found to differentiate into vascular endothelial cells that could contribute to tumor angiogenesis in vivo. Interestingly, the capacity to differentiate into CD133+/vascular endothelial cadherin (VE-cadherin, also known as CD144+) endothelial progenitors from CD133+/VE-cadherin-negative(VE-cadherin−) GBM stem-like cells was acquired during co-culture with tumor cells.4 This suggested that CSCs possess plasticity which could be regulated by cell-to-cell interaction or environmental factors within the differentiated component of the tumor tissue.
Similar to normal stem cells, CSCs reside in unique microenvironment called the niche. The fate of stem cells appears to be regulated by the communication between adjacent cells and components within the niche.7–9 It has also been shown that vascular endothelial cells within GBM tumor tissue can activate the Notch signaling pathway in GBM stem-like cells and that this activation of canonical Notch signaling was required for self-renewal of GBM stem-like cells.10,11 Given the fact that GBM stem-like cells differentiate into vascular endothelial cells, it raises the possibility that these differentiated cells which arise from CSCs might generate a CSC niche. However, direct contribution of the differentiated cells from CSCs to regulate the property of CSCs has not been reported.
Recently, we have established a model of CSCs, miPS-CSCs, which were derived from mouse induced pluripotent stem cells (miPSCs) by culturing miPSCs in conditioned medium prepared from the cultures of several different types of mouse cancer cell lines.12 One of the miPS-CSC, miPS-LLCcm cells, was established from miPSCs that had been treated with the conditioned medium from Lewis lung carcinoma (LLC) culture produce highly angiogenic and malignant tumors when transplanted into nude mice. In this study, we assessed whether the progeny cells of CSCs are physiologically essential for the maintenance of self-renewal and differentiation of the CSCs.
Material and Methods
Cell culture
miPS-LLCcm cells12 were maintained in DMEM containing 15% fetal bovine serum (FBS), 0.1 mM nonessential amino acids (NEAA), 2 mM l-Glutamine, 0.1 mM 2-mercaptoethanol, 50 U/mL penicillin, and 50 U/mL streptomycin. For selection of a cancer stem cell-like population, cells were cultured in medium containing 1 µg/mL puromycin for 7 days. Medium was changed every 24 hr during the selection. For preparation of conditioned medium from miPS-LLCcm cells, cells were cultured with or without puromycin until 80% confluency, and then the culture medium was replaced with serum-free culture medium containing Insulin-Transferrin-Selenium-X (ITS-X, Life Technologies). Conditioned medium was collected at 20 hr after the medium replacement and filtered using 0.45 µm filter (Millipore). The conditioned medium was centrifuged at 100,000g for 16 hr at 4°C using Himac CP70MX ultracentrifuge (Hitachi) to remove the microvesicles/exosomes and then supernatant was collected.
Tube formation assay
miPS-LLCcm cultured in various conditions were suspended in complete EGM-2 medium (Takara) or EGM-2 medium without vascular endothelial growth factor (VEGF) and seeded on Matrigel (Becton Dickinson) coated 96-well plates. After 24 hr, images of the cells were taken by using inverted light microscope (IX-80, Olympus).
Flow cytometry analysis, cell sorting
Adherent cells were collected by using 5 mM EDTA (pH 8.0) and stained with the following primary antibodies and secondary antibody. Primary antibodies: phycoerythrin (PE) labeled anti-VEGFR2 rat IgG (1:200; Becton Dickinson) and anti-VE-cadherin (VE-cad) rat IgG (1:100; Becton Dickinson). Secondary antibody: PE labeled anti-rat IgG goat IgG (1:200; Abcam). Cells were then analyzed on a FACS Calibur flow cytometer (Becton Dickinson). To separate GFP positive and negative population, adherent cells were prepared as described above and sorted using FACSAria cell sorter (Becton Dickinson).
Immunofluorescence microscopy
Cells were seeded onto the Matrigel (Becton Dickinson) coated imaging chambers (Nunc). After 24 hr of culture, the cells were fixed with 4% paraformaldehyde for 20 min at room temperature and then incubated with blocking solution containing 1% bovine serum albumin (BSA) in phosphate buffer saline (PBS) at room temperature for 1 hr. Chambers were then incubated overnight at 4°C with rat anti-CD31 primary antibodies (Santa Cruz) in blocking solution. After wash with PBS, chambers were incubated with Texas Red conjugated goat anti-rat IgG secondary antibodies (Life Technologies) in blocking solution at room temperature for 30 min. After wash in PBS, chambers were mounted with Vectashield mounting medium with 4',6-diamidino-2-phenylindole (DAPI, Vector). Images were taken using an inverted light microscope (IX-80, Olympus) or a confocal microscope equipped with a light fluorescence device (LSM510META, Carl Zeiss).
RNA extraction and quantitative real-time PCR
Total RNA was isolated using RNeasy Mini Kit (QIAGEN) or TRIzol (Invitrogen). Total RNA (3 µg) was then reverse transcribed using SuperScript II Reverse Transcriptase kit (Invitrogen). Quantitative real-time PCR was performed with a Lightcycler480 System II (Roche Applied Science) by using SYBR Green II (Molecular Probes). Primers: Nanog (Forward: 5′-CAG GTG TTT GAG GGT AGC TC-3′ Reverse: 5′-CGG TTC ATC ATG GTA CAG TC-3′), Oct3/4 (Forward: 5′-TCT TTC CAC CAG GCC CCC GGC TC-3′ Reverse: 5′-TGC GGG CGG ACA TGG GGA GAT CC-3′), Klf4 (Forward: 5′-GCG AAC TCA CAC AGG CGA GAA ACC-3′ Reverse: 5′-TCG CTT CCT CTT CCT CCG ACA CA-3′), Sox2 (Forward: 5′-TAG AGC TAG ACT CCG GGC GAT GA-3′ Reverse: 5′-TTG CCT TAA ACA AGA CCA CGA AA-3′), VEGFR2 (Forward: 5′-TAG CTG TCG CTC TGT GGT TCT G-3′ Reverse: 5′-GTC TTT CTG TGT GCT GAG CTT GG-3′), VE-cad (Forward: 5′-CGC ACC AGG TAT TGA ACG CAT C-3′ Reverse: 5′-GGC ATC TTG TGT TTC CAC GAC G-3′), GAPDH (Forward: 5′-AAC GGC ACA GTC AAG GCC GA-3′ Reverse: 5′-ACC CGT TTG GCT CCA CCC TT-3′), DLL1 (Forward: 5′-AAC CAT GAA CAA CCT AGC CAA TT-3′ Reverse: 5′-CAT GGT CCC CGT GAA AGT C-3′), DLL3 (Forward: 5′-GTG AAA CCT CTG GCT CCT TTG AAT G-3′ Reverse: 5′-AAC CAG GTG GGC AAT GAC AGA C-3′), DLL4 (Forward: 5′-GCA CCA ACT CCT TCG TCG TC-3′ Reverse: 5′-GTT TCC TGG CGA AGT CTC TG-3′), jagged1 (Forward: 5′-GAT GCA AAT GAG TGC GAG GCC AAA C-3′ Reverse: 5′-CCA TTA ACC AAA TCC CGA CAG GAG G-3′), jagged2 (Forward: 5′-GAC AAT GAC ACC ACT CCA GAT GAG G-3′ Reverse: 5′-GTT GCA GGT GGC ACT GTA GTA GTT C-3′)
TCA precipitation
Conditioned medium from adherent cells (CM-ad), from puromycin treated cells (CM-sp), or microvesicles/exosomes-free CM-ad (CM-ad–mv/ex) were collected into microtube and ice-cold TCA was added. Proteins were precipitated 15 min at 4°C. Then the suspension was centrifuged at 13,000 rpm for 15 min at 4°C. Supernatant was discarded and the pellet was resuspended with acetone containing 5% TCA. The suspension was centrifuged at 13,000 rpm for 15 min at 4°C and the pellet was dried. Resultant pellet was resuspended with SDS sample buffer.
Western blotting
Whole cell lysates of miPS-CSC spheroid cells cultured in various conditions and proteins concentrated from CM-ad, CM-ad–mv/ex, or CM-sp were extracted and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were electrically transferred to polyvinylidene difluoride (PVDF) membrane and probed with following primary antibodies and secondary antibody. Primary antibodies: rabbit anti-Notch intracellular domain 1 (NICD1) antibody (1:500, Abcam), rabbit anti-β actin antibody (1:1,000, Cell signaling), rabbit anti-DLL1 antibody (1:200, Santa cruz), rabbit anti-DLL4 antibody (1:500, Abcam), rabbit anti-jagged1 antibody (1:500, Abcam), and rabbit anti-jagged2 antibody (1:200, Santa cruz). Secondary antibody: horseradish peroxidase (HRP)-labeled goat anti rabbit IgG (1:2,000–1:5,000, Cell signaling). Immunoreactive signals were developed with an ECL kit (GE healthcare) and detected by Light Capture II (ATTO).
Sphere formation assay and inhibition studies
Single cells were seeded on low attachment dishes and grown in serum-free culture medium with or without CM-ad, CM-sp, CM-ad–mv/ex or conditioned medium from adherent cells after the third round puromycin selection cycle (CM-3rdad) at a density of 5 × 104 cells/mL to generate spheroids. The number and the size of spheroid cells were measured at day 4.
For inhibition studies, miPS-LLCcm cells were seeded on low attachment dishes and grown in serum-free culture medium containing 5, 10, or 20µM N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT, Sigma-Aldrich) with various conditioned medium at a density of 5 × 104 cells/mL. The number and the size of spheroid cells were measured at day 4.
siRNA transfection
Cells were seeded on 60 mm dish and treated with 40 nM of Flexitube siRNA premix for Notch1 or control siRNA (QIAGEN) using HiPerFect Transfection Reagent (QIAGEN) for 24 hr. After incubation, cells were seeded on low attachment dishes and grown in serum-free culture medium with CM-ad at a density of 5 × 104 cells/mL to generate spheroids. The number and the size of spheroid cells were measured at day 4.
Results
miPS-LLCcm cells differentiate into vascular endothelial like cells
We have previously established a model of CSCs which was derived from miPSCs that had been treated with conditioned medium prepared from the cultures of various types of cancer cell lines.12 In the tumors that were derived from miPS-LLCcm cells, we observed frequent CD31+ vessels indicative of tumor angiogenesis.12 This observation prompted us to investigate the capacity of miPS-LLCcm cells to differentiate into vascular endothelial cells in vitro as had previously been shown for GBM stem-like cells.4–6
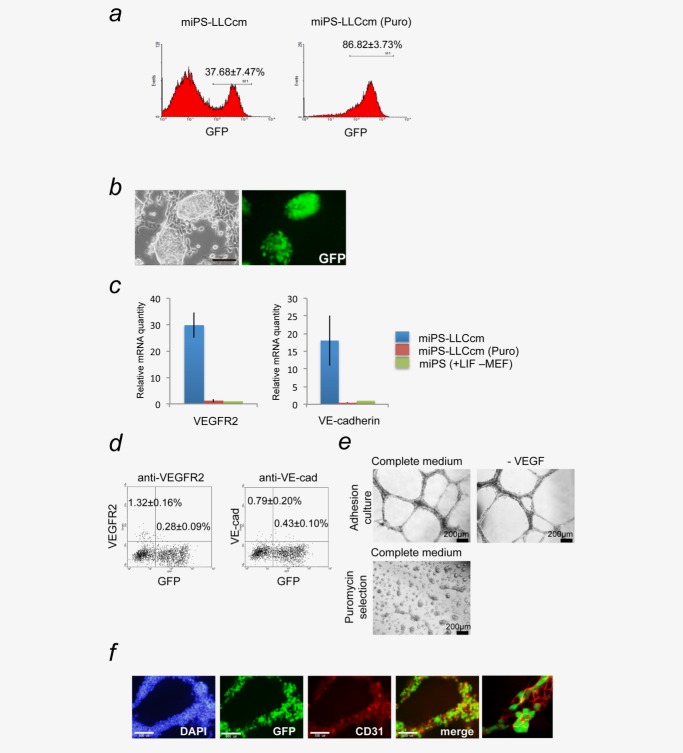
The expression of Green Fluorescent Protein (GFP) and puromycin resistant genes reflects Nanog gene expression in miPS-LLCcm cells.12,13 GFP+ cells should therefore represent undifferentiated CSCs while the GFP- cells should indicate more differentiated cells.12,13 GFP+ undifferentiated cells were successfully selected in the presence of puromycin in this system (Fig. 1a, right). We observed that under adherent culture conditions, GFP+ miPS-LLCcm cells were found to be autonomously maintained without leukemia inhibitory factor (LIF), being surrounded by GFP− cells (Figs. 1a left, 1b).
Figure 1.
miPS-LLCcm cells have potential to differentiate into vascular endothelium-like cell, which exhibited VEGF-independent vascular-like tube formation in vitro. (a) miPS-LLCcm cells were cultured in the medium with or without 1 µg/mL puromycin for 1 week to eliminate differentiated cells. The populations of GFP-positive cells were measured by flow cytometry. (b) Under the adherent condition, the miPS-LLCcm culture consist of GFP-positive cells with stem-like morphology and GFP-negative differentiated cells (scale bar 100 µm). (c) The expression levels of VEGFR2 and VE-cadherin were quantified by real-time RT-PCR. Values were normalized against GAPDH mRNA quantity. The level of each gene in miPS cells was set as one. (d) The population of VEGFR2- and VE-cadherin positive cells in miPS-LLCcm cells cultured under the adherent condition without LIF and puromycin were quantified by flow cytometry. (e) miPS-CSCs were seeded onto BD Matrigel™ and evaluated the ability of vascular-like tube formation in the presence or absence of VEGF (upper panel). The stem cell-enriched population selected by puromycin could not form the tubular structure in vitro (lower panel) (scale bar 200 µm). (f) Immunofluorescence staining was performed using anti-CD31 antibody (red) in tubular structure derived from miPS-LLCcm cells. Nuclei were counterstained with DAPI (blue). A typical image captured by confocal microscopy is presented (right). Data are the results of three independent experiments. Bars indicate SD (scale bar 100 µm). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
We first evaluated the expression of VEGF receptor 2 (VEGFR2) and VE-cadherin in miPS-LLCcm cells (Fig. 1c). Relatively high expression of both genes were only found in miPS-LLCcm cells that were grown under adhesive culture conditions without LIF or puromycin, which allowed the differentiation of these GFP+ cells into GFP− cells. When the cells were cultured in the presence of puromycin, the expression levels of VEGFR2 and VE-cadherin mRNA were similar to those in miPSCs. These results indicated that a subpopulation of cells in the GFP+ miPS-LLCcm cells could differentiate into a vascular endothelial cell lineage. Flow cytometric analysis for VEGFR2 and VE-cadherin proteins also supports the presence of cells that had differentiated into a vascular endothelial lineage (Fig. 1d). Furthermore, the cells were confirmed to functionally develop into vascular tube-like structures on Matrigel (Fig. 1e). These structures contained at least three different phenotypes of cells: CD31+/GFP+, CD31+/GFP−, and CD31−/GFP+, indicating that miPS-LLCcm cells could give rise to a heterogeneous population of cells even in a vascular structure (Figs. 1e and 1f). Interestingly, this in vitro tube formation capacity did not depend on exogenous VEGF-A, which was consistent with the results shown in GBM.6 In addition, the population of CSCs enriched in the presence of puromycin failed to form vascular-like structures, implying that differentiated cells are necessary for tube formation. Collectively, these data demonstrate that miPS-LLCcm cells could differentiate into vascular endothelial-like cells exhibiting heterogeneous phenotypes of cells in the vascular tube-like structures (Fig. 1f).
Auto/paracrine regulation of the self-renewal in miPS-LLCcm cells
Since the vascular endothelial cells could be a component of a stem cell niche,10,11 we hypothesized that differentiated cells including vascular endothelial-like cells that arise from the miPS-LLCcm cells might contribute to the maintenance of a CSC subpopulation in the miPS-LLCcm cells in vitro. To assess this possibility, we examined the effect of different types of conditioned medium from miPS-LLCcm cells for their effect on spheroid formation and growth of CSCs under suspension culture conditions to evaluate the self-renewal capacity of the CSCs.14,15 The number and size of miPS-LLCcm spheroids dramatically increased when the spheroids were cultured in conditioned medium that had been prepared from adherent cultures of miPS-LLCcm (CM-ad), which should contain both differentiated and undifferentiated cell types (Fig. 2a). In contrast, the conditioned medium that was obtained from miPS-LLCcm cells that had been grown in the presence of puromycin (CM-sp) still induced fewer spheroids that were smaller in size as compared to spheroids grown in CM-ad. From these observations, we inferred that both differentiated cells and CSCs might secrete factor(s) that would enhance the self-renewal of CSCs.
Figure 2.
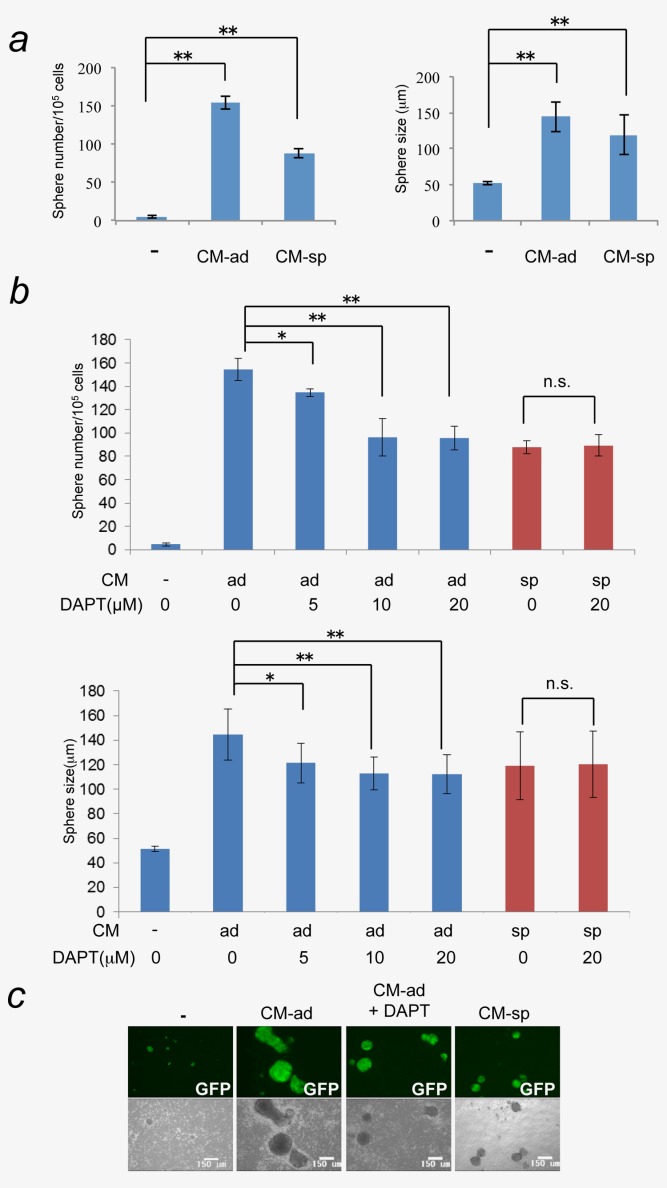
Spheroid formations of miPS-LLCcm cells were induced in the suspension culture with the conditioned medium of miPS-LLCcm cells in adherent culture (CM-ad) or the conditioned medium of puromycin treated-miPS-LLCcm cells (CM-sp). miPS-LLCcm cells were seeded on non-coated dish and grown in serum-free culture medium with/without CM-ad or CM-sp. (a) The number and the size of spheroids were measured at day4 (*p < 0.05; **p < 0.01, Student's t-test). (b) The spheroids formation of miPS-LLCcm cells treated with CM-ad (blue) or CM-sp (red) was assessed in the presence of a γ-secretase inhibitor, DAPT, at indicated concentrations (*p < 0.05; **p < 0.01, Student's t-test). (c) Images of typical spheroids formed under various conditions are shown (scale bar 150 µm). Data are the results of three independent experiments. Bars indicate SD. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Since Notch signaling is known to enhance the self-renewal of CSCs,16–18 we evaluated the self-renewal of miPS-LLCcm cells using N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT), an inhibitor of γ-secretase. DAPT slightly but significantly decreased the number of spheres in a dose-dependent manner with minor effect on sphere size when added to CM-ad (Figs. 2b and 2c). In contrast, DAPT had no effect on sphere number or size that were generated in CM-sp (Fig. 2b, red columns). The Notch intracellular domain (NICD), an indicator of Notch activation, was detected in the spheroids that were treated with CM-ad, but not in the spheroids without conditioned media (Fig. 3b). We confirmed that the production of NICD in spheroids with CM-ad was suppressed in the presence of DAPT (Fig. 3b).
Figure 3.
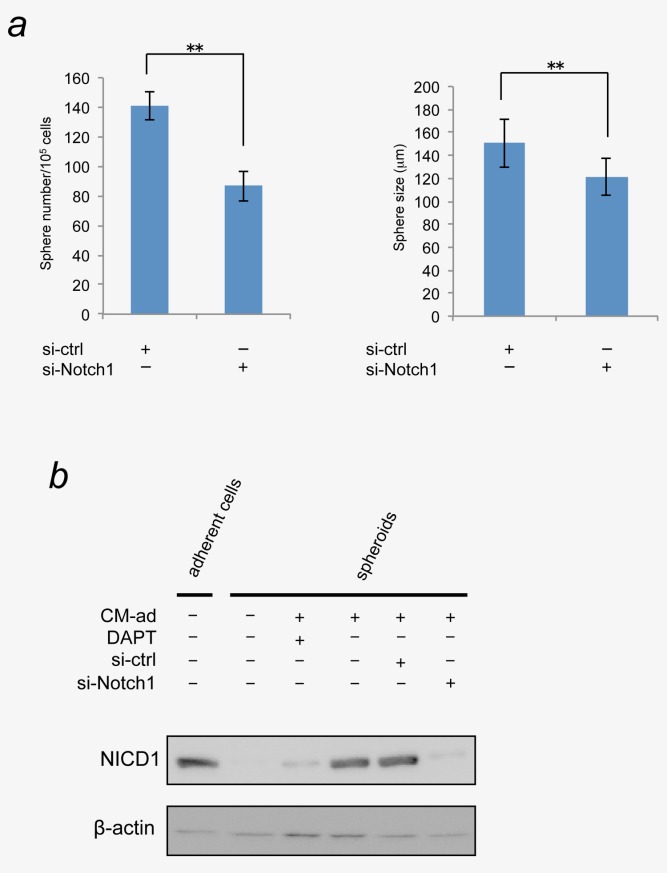
Knockdown of Notch1 significantly reduced sphere-forming ability enhanced by CM-ad. (a) miPS-LLCcm cells were treated with siRNA for Notch1 or control siRNA for 24 hr. Then cells were seeded on non-coated dish and grown in serum-free culture medium with CM-ad. The number and the size of spheroids were measured at day4 (*p < 0.05; **p < 0.01, Student's t-test). Data are the results of three independent experiments. Bars indicate SD. (b) The activation of Notch signaling in the spheroids and adherent cells were evaluated by the detection of Notch intracellular domains, NICD. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The involvement of Notch signaling in the promotion of self-renewal of miPS-LLCcm cells was further confirmed by knockdown experiments (Fig. 3a). The Notch1-specific siRNA significantly reduced sphere number and size as well as DAPT did. The NICD was reduced in the CM-ad spheroids when transfected with Notch1 siRNA (Fig. 3b). These results clearly indicated that the Notch signaling evoked by CM-ad should contribute to the self-renewal of CSCs. Together with this observation, we concluded that activation of Notch signaling promotes the self-renewal of the CSC population by the factor(s) secreted into CM-ad from the differentiated cells. In addition, CSCs themselves also secrete factor(s) to promote self-renewal, which might depend on other signaling pathways such as Wnt and Hedgehog.14,15,19–21
With regard to activation of the Notch signaling pathway, we found that miPS-LLCcm CSCs showed significant mRNA expression of various Notch ligands such as Dll1, Dll4, Jag1, and Jag2 when compared with miPSCs (Fig. 4a and Supporting Information Fig. 1). Notch ligands are membrane-associated proteins that may be present in microvesicles/exosomes22 and that may be secreted from the differentiated cells into CM-ad. However, spheroid formation was still enhanced even after the fraction of microvesicles/exosomes was removed from CM-ad (Fig. 4b). In addition, DAPT similarly reduced the sphere numbers in CM-ad in the presence or absence of microvesicles/exosomes fraction, suggesting the presence of soluble factor(s) or soluble form of Notch ligands that may be responsible for Notch activation in CM-ad. The immune-reactive signals, those apparent molecular weights were smaller than predicted full length of Dll1 and Dll4, were detected in CM-ad with/without microvesicles/exosomes, possibly soluble forms of Dll1 and Dll4 (Fig. 4c).23,24 However, these Notch ligands were also detected in CM-sp, which did not activate the Notch signaling in miPS-LLCcm spheroids (Fig. 2b). Membrane-bound and soluble forms of either Jag1 or Jag2 were not detected (data not shown). Collectively, these results suggested that, together with soluble forms of Notch ligands, additional factor(s) or mechanism(s) should be involved in the Notch signaling activation triggered by CM-ad.
Figure 4.
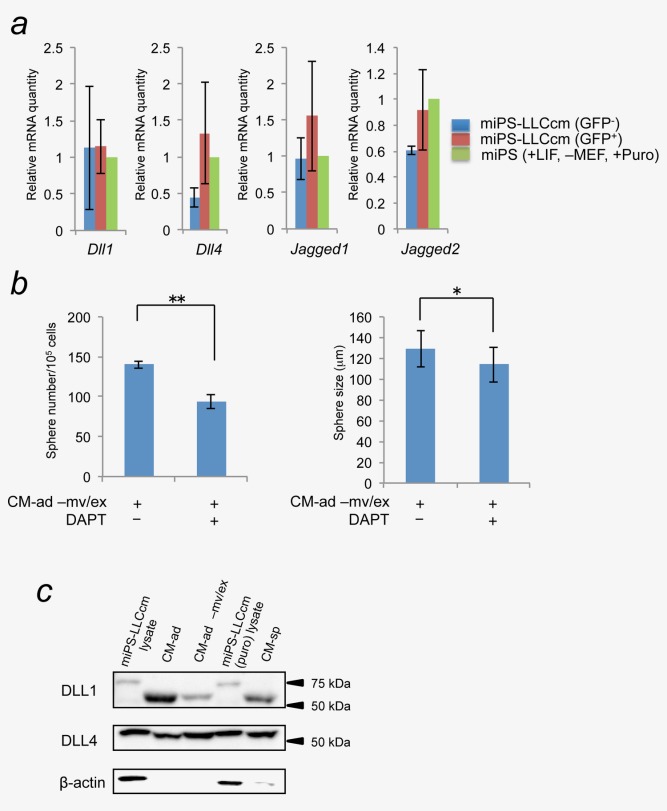
Soluble form of Notch ligands were detected in CM-ad and CM-sp. (a) The expression levels of Notch ligands (Dll1, Dll4, Jag1, and Jag2) in GFP+ and GFP− population of miPS-LLCcm cells sorted by cell sorter were quantified by real-time RT-PCR. The level of each gene in miPS cells was set as one. (b) Sphere-forming ability of microvesicles/exosomes free CM-ad (CM-ad–mv/ex) were analyzed by sphere formation assay with/without 20 µM DAPT (*p < 0.05; **p < 0.01, Student's t-test). Data are the results of three independent experiments. (c) Protein levels of Notch ligands in CM-ad, CM-ad-mv/ex, or CM-sp were analyzed by Western blot. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Regulation of differentiation capacity and lineage of CSCs
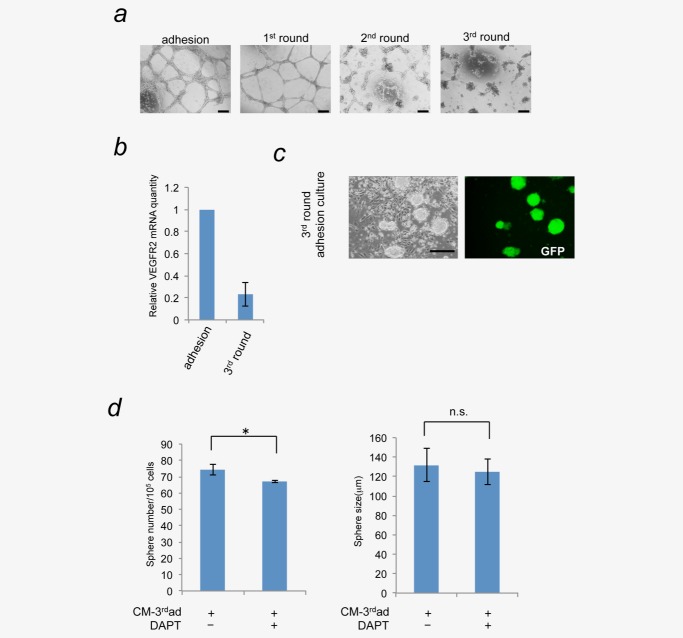
As shown in Figure 1, we observed the differentiation of miPS-LLCcm into vascular endothelial lineage. We next addressed whether this differentiation lineage in CSCs was committed or not. To test this, miPS-LLCcm cells were treated with puromycin for 1 week to eliminate differentiated cells and then puromycin was removed for 1 week to allow growth of the differentiated progenies of CSCs. This cycle was repeated three times (Supporting Information Fig. 2). Differentiation capacity into vascular endothelial cells was evaluated by either real-time PCR or in vitro tube formation assay. We found that the in vitro tube forming ability was decreased and nearly lost after the third round culture (Fig. 5a). The expression of VEGFR2 also decreased (Fig. 5b). This observation does not mean the decrease in differentiation capacity of CSCs, because the GFP- cells surrounding GFP+ cells were present even after the third round culture (Fig. 5c). The conditioned medium prepared from the third round culture (CM-3rdad) was applied to the sphere formation assay. Although the CM-3rdad ad increased the sphere number, the effect was as weak as the half of that of CM-ad (Fig. 5d, see also Fig. 2b). Intriguingly, this effect of CM-3rdad was not depending on the Notch signaling since DAPT treatment had little effect. These results suggested the plasticity of CSC differentiation or the heterogeneity of CSCs in miPS-LLCcm. Furthermore, these results implied that the determination of differentiation lineage was affected by the secreted factor(s) from differentiated cells, which was observed in at least into vascular endothelial cells. In addition, these results implicated that the Notch mediated self-renewal of CSCs were promoted in the niche provided by the CSCs with the potential to differentiate into vascular endothelial cells.
Figure 5.
Long-term depletion of differentiation factor(s) secreted from differentiated cancer cells lead to the loss of vascular differentiation properties of miPS-LLCcm cells. (a) miPS-LLCcm cells were cultured in the medium containing puromycin for 1 week to eliminate differentiated cells. Following a week, cells were cultured in puromycin-free medium to allow differentiated cells to grow. This cycle was repeated three times. The ability to differentiate into vascular endothelial cells at the end of each cycle was evaluated by in vitro tube formation assay (scale bar 200 µm). (b) The expression levels of VEGFR2 in indicated cells were quantified by real-time RT-PCR. The ability of differentiation into endothelial cells was deprived. (c) After third round selection and differentiation, the resultant culture consisted of GFP-positive cells with stem-like morphology and GFP-negative differentiated cells, indicating the stem cells still possessed the differentiation capacity (scale bar 200 µm). (d) Sphere-forming ability of conditioned medium from miPS-LLCcm cells After third round selection (CM-3rdad) were analyzed by sphere formation assay with/without 20 µM DAPT (*p < 0.05; **p < 0.01, Student's t-test). Data are the results of three independent experiments. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
We further analyzed the effect of the secreted factor(s) on the differentiation of CSCs into vascular endothelial lineage cells. miPS-LLCcm spheres were formed with CM-ad or CM-sp in the presence or absence of DAPT. After inducing differentiation of miPS-LLCcm spheres using adherent culture conditions, we performed both flow cytometric analysis and in vitro tube formation assays. There was no significant difference in the differentiation into vascular endothelial cells between the spheres that had been pre-treated with various conditioned media (Fig. 6a). Only the sphere group that had previously been treated with CM-ad containing DAPT showed significant increase in a vascular endothelial cell population that was positive for either VEGFR2 and/or VE-cadherin. Reciprocally, there was a significant decrease in GFP+ stem cell population (Figs. 6a and 6b, Supporting Information Fig. 3). In this condition, the most robust tube-like formation was found with cells differentiated from spheres that had been pretreated with CM-ad containing DAPT (Fig. 6c). Thus, suppression of Notch signaling in CSCs can enhance the differentiation of CSCs when grown as spheroids. These results indicate that the secreted factor(s) can regulate the CSC capacity to differentiate into vascular endothelial cells. Therefore, we concluded that the differentiated progenies of CSCs are also required for the regulation of differentiation capacity and lineage of CSCs.
Figure 6.
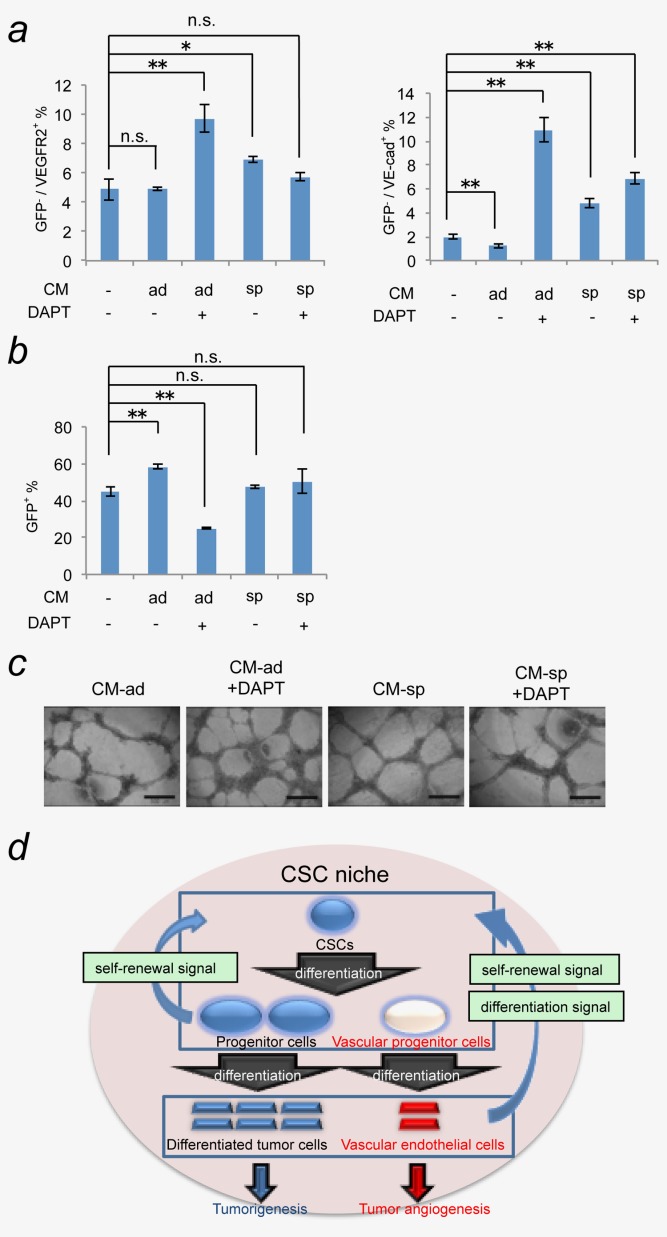
Factor(s) secreted from differentiated cancer cells enhanced differentiation properties of spheroid cells. Spheroids were formed with CM-ad or CM-sp in the presence or absence of 20 µM DAPT. The spheroids were enzymatically dissociated and cultured under adherent condition for 1 week to allow differentiated cells to grow. The potential of the spheroid cells to differentiate into vascular endothelium grown under each condition were assessed by flow cytometry (a), (b) and in vitro tube formation assay (c). Data are the results of three independent experiments (*p < 0.05; **p < 0.01, Student's t-test) (scale bar 500 µm). (d) Model of the CSC niche created by CSCs themselves. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Discussion
The niche for CSCs has been considered to regulate self-renewal and differentiation of CSCs. Thus, blockade of CSC niche should be an attractive approach for cancer treatment. However, the details of the mechanisms involved in this regulation remain to be elucidated. In this work, we investigated the roles of the differentiated cells from CSCs as the microenvironments using a model cell line of CSC, miPS-LLCcm.12 Our results indicate that the self-renewal potential of CSCs can be enhanced by the factor(s) secreted from both undifferentiated CSCs and a differentiated population (Fig. 2). Furthermore, some factor(s) secreted from the differentiated population of CSCs can also maintain the differentiation capacity and lineage of CSCs (Figs. 3 and 4). The level of Notch signaling in CSCs is up-regulated by the factor(s) secreted from differentiated CSC progenies contribute to the maintenance of a balance between self-renewal and differentiation of CSCs. Thus, the differentiated progenies of CSCs play an important role in maintaining CSC properties as a component of CSC niche. In other words, the self-renewal and differentiation capacity and lineage of our miPS-LLCcm cells, as a model of CSCs, are maintained in a niche provided by their differentiated progeny (Fig. 6d).
The secreted factor(s) could promote CSC self-renewal in part, via activation of Notch signaling pathway (Fig. 2). It has been shown that vascular endothelial cells were components of CSC niche for promoting self-renewal of CSCs.10,11 In the case of GBM tumor, Notch signaling in CSCs for self-renewal was activated by endothelial cells.11 The study used “normal” endothelial cells such as HUVEC, as a source of Notch ligand for in vitro analysis. In addition, the report showed the expression of Notch ligands in differentiated GBM cells, and implied the possibility of differentiated cells to be the providers of Notch ligands. Our results clearly indicate that the differentiated tumor cells from CSCs should provide Notch ligands. It should be noteworthy that the Notch ligands were apparently present when miPS-LLCcm could give rise to endothelial cells (Figs. 2, 3 and 5d). CSCs are able to differentiate into various types of differentiated cells including vascular endothelial cells,4–6 thus CSC-derived vascular endothelial cells must be the primary source of Notch ligands.
In our study, the activation of Notch was induced by soluble factor(s), rather than by cell-cell interaction. Canonical Notch ligands are supposed to be trans-membrane proteins, thus these ligands may locate on the surface of microvesicles/exosomes secreted from the differentiated cells. Indeed, one of the Notch ligands, Dll4, is reported to be on exosome.22 However, we showed the microvesicles/exosomes were not involved in this Notch activation in CSCs of miPS-LLCcm (Fig. 4b). Recently, soluble forms of Jag1 that are secreted from liver parenchyma endothelial cells have been shown to promote CSC properties in colorectal cancer.25 Therefore soluble form Notch ligands may also be involved in the Notch activation. Actually, we could detect the gene expression of Notch ligands and also find out soluble forms of Dll1 and Dll4 in CMs (Figs. 4a and 4c). However, the gene expression of canonical Notch ligands in GFP negative population in miPS-LLCcm cells were not prominent in comparison with those in GFP positive cells (Fig. 4a). In addition, soluble forms of Dll1 and Dll4 were also present in CM-sp, which could not activate Notch signaling effectively in miPS-LLCcm spheroids. Therefore, we currently speculate that the factor(s) other than typical Notch ligands should be also secreted from differentiated cells, contributing to trigger the Notch signaling for the self-renewal of CSCs. Factor(s) possessing function similar to Fibulin-3 might be a candidate for the activator of Notch signaling pathway. An antagonizing effect of Fibulin-3 against Dll3, a cis-inhibitor of Notch, has been proposed.26 Similar mechanism might be involved in the Notch activation observed here. Although the factor(s) that are responsible for the promotion of self-renewal of CSCs remain to be identified, these factor(s) might be therapeutic targets to suppress the self-renewal of CSCs. Furthermore, our data also indicate the possibility that other signaling pathways for the activation of CSC self-renewal is regulated in the soluble factor(s)-dependent manner. Wnt and Hedgehog signalings are such candidates of pathways.14,15,19–21 It is important to clarify whether the soluble factor(s) secreted by differentiated progenies of CSCs could also activate these pathways.
The secreted factor(s) appeared to also regulate the differentiation capacity of CSCs, at least the capacity into vascular endothelial cells (Figs. 5 and 6). Alternatively, our results may suggest the plasticity in differentiation of CSCs and/or the heterogeneity of CSCs in miPS-LLCcm.27,28 Feedback message(s) from the differentiated CSC progeny cells including vascular endothelial cells may regulate gene expression related to particular differentiation lineages in CSCs, or stimulate particular CSCs that have already been committed to differentiate into particular lineage. Either way, the plasticity and/or heterogeneity of CSCs should contribute to comprise overall heterogeneity of the tumor depending on the microenvironment/niche where CSCs reside. Identification of factor(s) that regulate the differentiation properties of CSCs may be contributed to develop differentiation therapies of tumor.29
Acknowledgments
This research has been performed under the Grant-in-Aid for Challenging Exploratory Research No. 23650598 (to M.S.), the Grant-in-Aid for Scientific Research (B) No. 21300179 (to M.S.), the Grant-in-Aid for Scientific Research (C) No. 24501315 (to A.M.), and Advanced Research Training program (to A.S.).
Glossary
Abbreviations:
- CSC
cancer stem cell
- DAPT
N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester
- GBM
glioblastoma
- GFP
Green Fluorescent Protein
- LIF
leukemia inhibitory factor
- LLC
Lewis lung carcinoma
- miPSC
mouse induced pluripotent stem cell
- VE-cadherin
vascular endothelial cadherin
- VEGF
vascular endothelial growth factor
- NICD
Notch intracellular domain
- VEGFR2
vascular endothelial growth factor receptor 2
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information
References
- 1.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15:1010–12. doi: 10.1038/nm0909-1010. [DOI] [PubMed] [Google Scholar]
- 3.Zhao RC, Zhu YS, Shi Y. New hope for cancer treatment: exploring the distinction between normal adult stem cells and cancer stem cells. Pharmacol Ther. 2008;119:74–82. doi: 10.1016/j.pharmthera.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Wang R, Chadalavada K, Wilshire J, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–33. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 5.Ricci-Vitiani L, Pallini R, Biffoni M, et al. Tumor vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–8. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 6.Soda Y, Marumoto T, Friedmann-Morvinski D, et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc Natl Acad Sci USA. 2011;108:4274–80. doi: 10.1073/pnas.1016030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bissell MJ, LaBarge MA. Context, tissue plasticity, and cancer: are tumor stem cells also regulated by the microenvironment? Cancer Cell. 2005;7:17–23. doi: 10.1016/j.ccr.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lobo NA, Shimono Y, Qian D, et al. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–99. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 9.Yi SY, Hao YB, Nan KJ, et al. Cancer stem cells niche: a target for novel cancer therapeutics. Cancer Treatment Rev. 2013;39:290–6. doi: 10.1016/j.ctrv.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Krishnamurthy S, Dong Z, Vodopyanov D, et al. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. 2010;70:9969–78. doi: 10.1158/0008-5472.CAN-10-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu TS, Costello MA, Talsma CE, et al. Endothelial cells create a stem cell niche in glioblastoma by providing notch ligands that nurture self-renewal of cancer stem-like cells. Cancer Res. 2011;71:6061–72. doi: 10.1158/0008-5472.CAN-10-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen L, Kasai T, Li Y, et al. A model of cancer stem cells derived from mouse induced pluripotent stem cells. PloS One. 2012;7:e33544. doi: 10.1371/journal.pone.0033544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 14.Bisson I, Prowse DM. WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res. 2009;19:683–97. doi: 10.1038/cr.2009.43. [DOI] [PubMed] [Google Scholar]
- 15.Song Z, Yue W, Wei B, et al. Sonic Hedgehog pathway is essential for maintenance of cancer stem-like cells in human gastric cancer. PloS One. 2011;6:e17687. doi: 10.1371/journal.pone.0017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan X, Khaki L, Zhu TS, et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28:5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espinoza I, Miele L. Notch inhibitors for cancer treatment. Pharmacol Ther. 2013;139:95–110. doi: 10.1016/j.pharmthera.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capaccione KM, Pine SR. The Notch signaling pathway as a mediator of tumor survival. Carcinogenesis. 2013;34:1420–30. doi: 10.1093/carcin/bgt127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holland JD, Klaus A, Garratt AN, et al. Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol. 2013;25:254–64. doi: 10.1016/j.ceb.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Merchant AA, Matsui W. Targeting Hedgehog—a cancer stem cell pathway. Clin Cancer Res. 2010;16:3130–40. doi: 10.1158/1078-0432.CCR-09-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang SN, Fu J, Nall D, et al. Inhibition of sonic hedgehog pathway and pluripotency maintaining factors regulate human pancreatic cancer stem cell characteristics. Int J Cancer. 2012;131:30–40. doi: 10.1002/ijc.26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheldon H, Heikamp E, Turley H, et al. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood. 2010;116:2385–94. doi: 10.1182/blood-2009-08-239228. [DOI] [PubMed] [Google Scholar]
- 23.Six E, Ndiaye D, Laabi Y, et al. The Notch ligand Delta1 is sequentially cleaved by an ADAM protease and gamma-secretase. Proc Natl Acad Sci USA. 2003;100:7638–43. doi: 10.1073/pnas.1230693100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin G, Zhang F, Chan KM, et al. MT1-MMP cleaves Dll1 to negatively regulate Notch signalling to maintain normal B-cell development. EMBO J. 2011;30:2281–93. doi: 10.1038/emboj.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu J, Ye X, Fan F, et al. Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer Cell. 2013;23:171–85. doi: 10.1016/j.ccr.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu B, Nandhu M, Sim H, et al. Fibulin-3 promotes glioma growth and resistance through a novel paracrine regulation of notch signaling. Cancer Res. 2012;72:3873–85. doi: 10.1158/0008-5472.CAN-12-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lotem J, Sachs L. Epigenetics and the plasticity of differentiation in normal and cancer stem cells. Oncogene. 2006;25:7663–72. doi: 10.1038/sj.onc.1209816. [DOI] [PubMed] [Google Scholar]
- 28.Tang DG. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012;22:457–72. doi: 10.1038/cr.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shekhani MT, Jayanthy AS, Maddodi N, et al. Cancer stem cells and tumor transdifferentiation: implications for novel therapeutic strategies. Am J Stem Cell. 2013;2:52–61. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Information
Supplementary Information
Supplementary Information