Abstract
White adipose tissue (WAT) is a heterogeneous tissue composed of lipid-filled adipocytes and several non-adipocyte cell populations, including endothelial, blood, uncharacterized stromal, and adipocyte precursor cells. Although lipid-filled adipocytes account for the majority of WAT volume and mass, non-adipocyte cell populations have critical roles in WAT maintenance, growth and function.
As mature adipocytes are terminally-differentiated post-mitotic cells, differentiation of adipocyte precursors is required for hyperplastic WAT growth during development and in obesity. In this chapter, we present methods to separate adipocyte precursor cells from other non-adipocyte cell populations within WAT for analysis by flow cytometry or purification by fluorescence-activated cell sorting (FACS). Additionally, we provide methods to study the adipogenic capacity of purified adipocyte precursor cells ex vivo.
Keywords: Adipocyte precursor, adipogenesis, stromal vascular, preadipocytes, stem cell, progenitor, adipose tissue
1.0 Introduction
Distinct white adipose tissue (WAT) depots are distributed throughout the body and include subcutaneous (inguinal) and visceral (epigonadal, retroperitoneal, omental, mesenteric) depots (Cinti, 2007; 2012). While the vast majority of WAT mass is comprised of lipid-filled mature adipocytes, the mature adipocytes account for less than half of the cells in WAT (Hirsch, 1979; Hirsch & Batchelor, 1976; Eto et al, 2009). Several stromal cell populations comprise the non-adipocyte cell populations, including endothelial, blood and mesenchymal cell populations.
As mature adipocytes are terminally differentiated post-mitotic cells, the differentiation of adipocyte precursors is necessary for the establishment of adipocyte number during development and expansion in obesity (Herberg, Döppen, Major, & Gries, 1974; Hirsch & Batchelor, 1976). In most WAT depots, the number of adipocytes is established during childhood and adolescence, with production of new adipocytes from adipocyte precursors in adulthood occurring to replace the dying adipocytes and maintain adipocyte number (Arner et al., 2010; Spalding et al., 2008).
It has long been known that adipocyte precursors reside within WAT depots as culturing of the heterogeneous mixture of WAT resident stromal cells, termed the stromal vascular fraction (SVF), results in the generation of lipid filled adipocytes (Ng et al., 1971). However, until recently, methodology did not allow for the purification of the adipogenic stromal cells from non-adipogenic cells within the SVF, and therefore the identity of adipocyte precursors was unknown.
Currently, the separation of adipocyte precursor populations from non-adipogenic stromal cells using a single marker is not possible (Berry & Rodeheffer, 2013), and thus the separation of these precursor cells requires multi-color flow cytometry (Rodeheffer et al., 2008) to exclude non-adipogenic endothelial and blood lineage cells, and enrich for mesenchymal cell populations. Recent studies identified early adipocyte progenitors, with a specific cell surface marker profile (Lin−: CD29+: CD34+: ScaI+: CD24+), that are capable of differentiating into a functional WAT depot in vivo that rescues hyperglycemia in a lipodystrophic AZIP mouse model (Rodeheffer et al., 2008). Moreover, cells with more limited adipogenic capacity, termed preadipocytes, are enriched in the Lin−: CD29+: CD34+: ScaI+: CD24− population. These cells are enriched for adipogenic expression markers, such as Pparγ2, and are capable of forming adipocytes when transplanted outside of the WAT microenvironment, suggesting further commitment to the adipocyte lineage (Berry & Rodeheffer, 2013),
In this chapter, we provide an overview of methods utilized to identify, analyse and isolate these adipocyte precursor cell populations from murine WAT. We also detail protocols for isolation by fluorescent activated cell sorting (FACS) or analysis by flow cytometry. Finally, we provide a description of the methods and conditions routinely used for the culture and adipogenic differentiation of these cell populations.
2. Adipose Tissue Depots and Cell populations
White adipose tissue depots are composed of a heterogeneous mixture of cell populations including terminally differentiated lipid-filled adipocytes and a stromal vascular fraction (SVF) that contains blood lineage cells, endothelial cells, immune cells, other uncharacterized stromal cells and adipocyte precursor cells. Specific cell populations can be identified and purified based on the expression of cell surface markers (Table 1) and subsequently analysed by flow cytometry or purified by fluorescent-activated cell sorting (FACS). Flow cytometry allows for the single cell analysis of extracellular and intracellular protein expression in complex samples in any tissue, for any markers/species for which antibodies are available. FACS allows the isolation of live cells from specific populations for ex vivo analysis. Adipocyte precursors can be identified by the lack of expression of lineage cell surface markers such as CD45 (blood) and CD31 (endothelial) and enriched for expression of mesenchymal stem cell markers including CD29, CD34, PdgfRα, Sca-1, and CD24 (to distinguish progenitors; Table 1). Additionally, antibodies recognizing cell surface markers of specific hematopoietic cell populations, such macrophages, B-cells, T-cells, can be added to assess changes in multiple SVF populations simultaneously.
Table 1.
Commonly Used Antibody Clones and Dilutions for the Identification and Isolation of Adipose Tissue Cell Populations.
| Antibody | Gene Name | Cell Population | Supplier | Clone | Flurochrome | Dilution* | Excitation (nm) | Bandpass Filter |
|---|---|---|---|---|---|---|---|---|
| Longpass Filter | ||||||||
| Detected Wavelengths | ||||||||
| CD45 | Leukocyte common antigen | Blood | eBioscience | 30-F11 | APC-eFluor e780 | 1:5000 | 640nm (Red) | 780/60 |
| 755 LP | ||||||||
| 755–810 nm | ||||||||
| CD31 | Pecam1; platelet/endot helial cell adhesion molecule 1 | Endothelial | eBioscience | 390 | PE-Cy7 | 1:1200 | 532nm (Green) | 780/60 |
| 735 | ||||||||
| 750 – 810 nm | ||||||||
| CD34 | cluster differentiation hematopoietic progenitor cell antigen | Mesenchymal stem cells | Biolegend | MEC 14.7 | Alexa Fluor 647 | 1:200 | 640nm (Red) | 660/20 |
| N/A | ||||||||
| 650–670 nm | ||||||||
| CD29 | Integrin β1 | Biolegend | HMB eta1-1 | Alexa Fluor 700 | 1:400 | 640nm (Red) | 710/50 | |
| 690 LP | ||||||||
| 690 – 735 nm | ||||||||
| ScaI | Stem cell antigen-1 or Ly6A/E | BD Horizon | D7 | V450 | 1:1000 | 405nm (Violet) | 450/50 | |
| N/A | ||||||||
| 425–475 nm | ||||||||
| CD24 | Heat-Stable Antigen, HSA or HsAg | BD Bioscience | M1/69 | PE | 1:100 | 532nm (Green) | 575/26 | |
| N/A | ||||||||
| 562–588 nm | ||||||||
| PerCP-Cy5.5 | 1:100 | 532nm (Green) | 710/50 | |||||
| 685 LP | ||||||||
| 685–735 nm | ||||||||
| PDGFRa | Platelet derived growth factor receptor alpha; CD140a, | Biolegend | APA 5 | PE | 1:200 | 532nm (Green) | 575/26 | |
| N/A | ||||||||
| 562–588 nm |
Note: Dilutions shown should be used as a guideline for initial control experiments. The correct dilution needs to be determined for each antibody, fluorochrome, and the specific cytometer/sorter used for analysis, as laser power and the filters can influence the signal versus noise.
3. Digestion of Whole WAT for Isolation of SVF
Individual mouse adipose depots are carefully excised and thoroughly minced with scissors (1–2 mm pieces), using sterile techniques.
The minced adipose tissue is digested in 0.83mg/ml collagenase type 2 (Worthington Biochemical Corporation NJ, USA; LS004174) in sterile Hank’s Balanced Salt Solution (HBSS; diluted from 10× stock no Calcium, no Magnesium, no Phenol Red; available from Life Technologies CA, USA; product number 14185-052) containing 3% bovine serum albumin (BSA), 1.23mM calcium chloride, 1.03mM magnesium chloride and 0.83mM zinc chloride for 753minutes in a shaking water bath (120–140 rpm) including vigorous shaking by hand (for 10–20 seconds) after 60 minutes of incubation. Approximately 5 ml of digestion buffer is used to digest one WAT depot from one mouse.
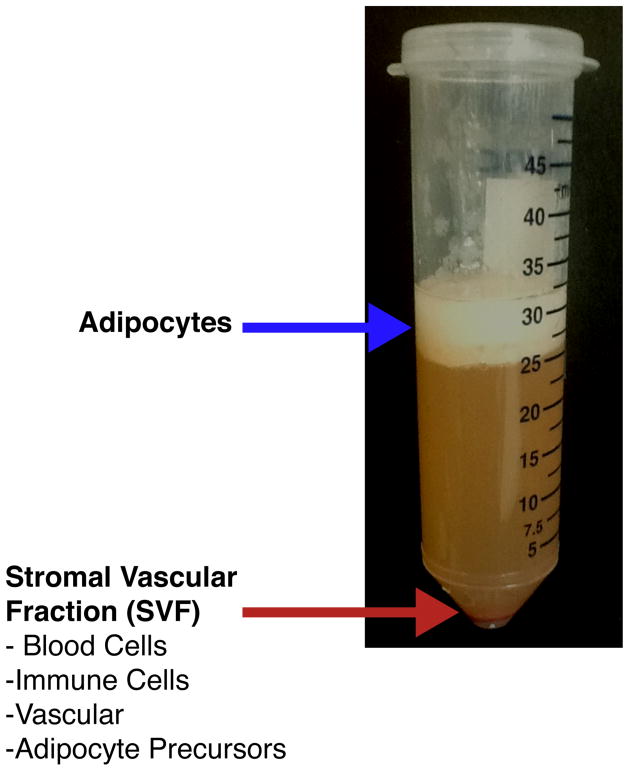
Floating adipocytes are separated from the SVF by centrifugation at 300 × g for 3 3minutes (Figure 1). Note: This digestion protocol results in a low percentage of intact mature adipocytes. Intact mature adipocytes can be isolated through a modified digestion protocol (Section 6).
The floating adipocyte fraction and supernatant is removed and the SVF pellet is re-suspended in HBSS 3% BSA wash buffer and sequentially filtered through sterile 70 μm (BD Biosciences CA, USA; product number 352350) and 40 μm (BD Biosciences CA, USA; product number 352340) nylon mesh filters before antibody staining.
Figure 1.
Separation of SVF from Adipocytes
4. Flow Cytometry and FACS
4.1 Antibody Staining
-
Antibodies (Table 1; user determined antibody concentrations) are diluted in HBSS with 3% BSA and the SVF is re-suspended in antibody staining solution and placed on ice in the dark for 20 minutes. The quantity of antibody, volume of staining solution and incubation period should be optimized for each antibody and sample amount (for example approximately 500,000 cells in 100 μl of antibody staining solution).
Notes:
Depending on the source of tubes used for staining, it may be necessary to coat all tubes in HBSS with 3% BSA overnight at 4 °C to maintain cell viability throughout the procedure.
For maximal recovery of SVF cells through all steps, wing-bucket style centrifuges should be used to pellet the cells at the bottom of the tube.
An excess of HBSS 3% BSA is added to wash and then the SVF is centrifuged at 300 × g for 3 minutes. The wash buffer is carefully removed and the SVF pellet is re-suspended in HBSS with 3% BSA and subsequently filtered through a 40 μm nylon filter prior to analysis by flow cytometry or purification by FACS.
For FACS purification, the SVF is re-suspended in FACS buffer (PBS with 0.5% BSA) with 0.5 g/ml propidium iodide (Sigma-Aldrich MO, USA; P4170) - a fluorescent, plasma membrane impermeant molecule that intercalates into DNA - to identify and exclude dead cells. The cells are then filtered through a 40 μm filter several times until they flow easily through the filter to reduce clogging the cell sorter lines.
FACS purified cell populations are collected in 1.5mL tubes that have been coated with HBSS 3% BSA (1.5 mL tubes are coated by filling the tubes with HBSS 3% BSA and incubating them at 4 °C for greater than 24 hours). The buffer is removed from the tubes prior to cell collection. Live cells can be used for in vivo transplantation to assess lineage commitment and differentiation (Berry & Rodeheffer, 2013; Rodeheffer et al., 2008) and in vitro differentiation (Section 5). Additionally, cells may be sorted directly into TRIzol® LS Reagent (Life Technologies CA, USA; product number 10296) for RNA extraction and subsequent gene expression studies, which differs from the standard TRIzol® reagent in concentration and permits processing of larger samples.
4.2 FACS and Flow Cytometry
The selection of multi-color fluorochrome combinations for flow cytometry can be challenging and is dependent on the specific flow cytometry system – which can have different laser and optical filter combinations to excite and properly detect a given combination of fluorochromes (Baumgarth & Roederer, 2000; Maecker & Trotter, 2008; Darzynkiewicz, Crissman, & Robinson, 2000; Ormerod, 2000 and Purdue University, 2013). The selection of fluorochrome combinations and filters can be assisted by tools such as the BD Biosciences Spectrum Guide and Fluorescence Spectrum Viewer (BD Biosciences, 2013) or Invitrogen’s Flow Cytometry and data analysis tutorials (Invitrogen; Life Technologies CA, USA 2013). Additionally, multi-color flow cytometry requires compensation between the emission spectra of fluorochromes used in combination due to their potential overlap. Compensation is the mathematical elimination of spectral overlap (Baumgarth & Roederer, 2000; Roederer, 2001) and must be performed during multi-color flow cytometry when any two fluorochromes used have partially overlapping emission spectra. Compensation can be performed before or after data collection manually or using software-based automation. When software automation is used, it is recommended to manually check the compensation settings to ensure that calculated compensation values are correct. This becomes increasingly important as the number of fluorchromes used per sample increases as the likelihood of spectral overlap also increases, as does the risk for compensation errors (Baumgarth & Roederer, 2000). When compensation is not performed correctly, it is possible for a population that is negative for a specific antigen to appear positive for that antigen simply because the fluorescent signal from a different fluorescently conjugated antibody “bleeds” into the filter that is intended to detect the fluorescently conjugated antibody of interest.
To definitively determine if an observed fluorescent signal is derived from the fluorescently conjugated antibody of interest, a fluorescent-minus-one (FMO) control should be performed. In this control, a sample is split into two with one sample being stained with all of the antibodies in the antigen scheme. The second sample, the FMO control, is stained with all of the antibodies except for the antibody that binds to the antigen of interest. Both samples are then analysed using the same compensation settings. If a positive signal is observed for the antigen of interest in the FMO control sample then the observed signal is the result of bleed through from the emission spectrum of a different fluorescently conjugated antibody. In this scenario, compensation settings must be properly adjusted. In a sample that it is compensated correctly, all cells in the FMO control sample will have a negative fluorescence signal for the antigen of interest.
When a sample has been stained with all antibodies and is acquired under the proper compensation settings, any cell that has fluorescent signal greater than the negative population in the FMO control can be considered positively stained for the fluorescently conjugated antibody of interest. In this manner, FMO controls can be used to ensure that any fluorescent signal above background in a multi-color flow cytometry scheme is due to staining by the antibody of interest and not from “bleed through” as a result of spectral overlap. In some cases, particularly intracellular staining, an isotype control may be a more appropriate control. In this case, samples are prepared as described above for the FMO control, but an antibody of the same isotype and conjugated fluorochrome are substituted for the antibody that recognizes the antigen of interest. Alternatively, if cell signalling events or expression repression/induction are being assayed it may be possible to use positive and negative conditions as a biological control.
4.3 Cytometers / Sorters
We routinely sort our SVF samples on a BD FACSAria II and III cell sorters and analyze them on BD LSRII flow cytometers (BD Biosciences CA, USA), each equipped with BD FACSDiva Software. Please see Table 1 for a recommended set up including excitation/emission optical filters based on the BD LSRII instrument.
4.4 Flow Cytometry Data Acquisition
Specific flow cytometers and manufacturers provide software for collecting and analyzing sample data during the acquisition of experiments. We routinely start by selecting cells based on forward scatter area (FSC-A) and side scatter area (SSC-A). Live cells are subsequently gated on both SSC and FSC singlets, ensuring that the staining of individual cells is analyzed.
Note: The FSC and SSC may need to be altered to ensure that the cell population of interest is positioned on scale based on the FSC and SSC. Adipocyte precursor cells may appear larger in size than other SVF populations such as immune cells that are routinely analyzed on flow cytometers. This can be facilitated by back gating - showing the final gated population of interest within the population of its ancestors based on the hierarchy.
Data can be presented in a number of formats including dot plots and histograms that can display fluorescent intensity of one or more fluorochromes simultaneously (for examples of dot plots see Figure 2).
Figure 2. Flow Cytometry Gating of Adipocyte Precursor Cells.
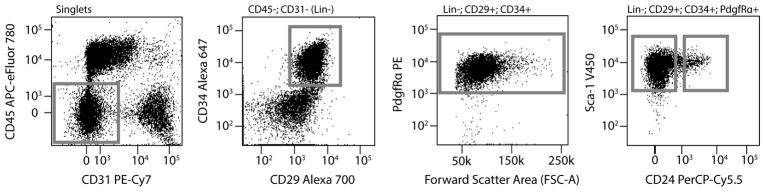
Each plot displays only the cell population from the populations gated in the leftward plot, which is also indicated at the top of each plot.
Minimal Markers for Flow Cytometry
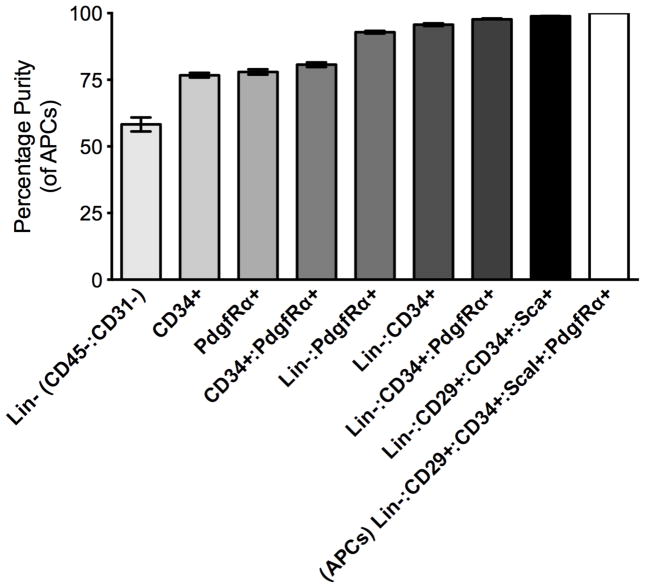
Depending on the limitation of excitation and emission detection on different flow cytometers an alternative minimal set of cell surface markers can be used to analyze and isolate adipocyte precursors from WAT (Figure 3; Table 2). While the use of minimal marker schemes results in reduced adipocyte precursor purity compared to the scheme described above (Table 2), it can still be used to enrich for adipocyte precursors.
Figure 3. Adipocyte Precursor Cell Purity with Minimal Cell Surface Markers.
Data (n=4) are expressed as mean±SEM.
Table 2.
Minimal Cell Surface Markers for the Identification and Isolation of Adipose Tissue Cell Populations
| Antibody Combination | Percentage of Adipocyte Precursor Cells (±SEM; n=4) |
|---|---|
| (CD45−/CD31−/CD34+/CD29+/ScaI+:PdgfRα) | |
| * Lin− (CD45−:CD31−) | 58.27% ±2.6 |
| CD34+ | 76.72% ±0.89 |
| PdgfRα | 77.97% ±1.00 |
| CD34+: PdgfRα | 80.68% ±0.85 |
| * Lin−:PdgfRα | 92.87% ±0.47 |
| * Lin−:CD34+ | 95.68% ±0.49 |
| * Lin−:CD34+:PdgfRα | 97.69% ±0.33 |
| * Lin−:CD34+:CD29+:ScaI+ | 98.42% ±0.25 |
| * Lin− :CD34+:CD29+:ScaI+:PdgfRα | 100% |
The same fluorochrome can be used for the CD45 and CD31 antibodies for exclusion of these ‘lineage’ populations in a single channel.
4.5 Flow Cytometry Software Analysis
Tree Star’s FlowJO software package is commonly used for flow cytometry analysis and can analyze data from any flow cytometer in Windows and MacIntosh operating systems (TreeStar, 2012). FlowJo is based around ‘workspace’ areas that include detailed graphical reports, gating, compensation, tables and statistical analysis of parental populations. Compensation and gating templates can be applied to multiple samples within an experiment or to multiple independent experiments. Additionally, high-resolution, publication quality histograms and dot plots can be exported in multiple image formats.
Another example of flow cytometry software is the BD Biosciences FACSDiva™ software package used on BD flow cytometers and sorters (BD Biosciences NJ, USA). BD FACSDiva™ is routinely used to establish multi-color analysis templates including compensation parameters necessary for establishing multi-colour templates. Additionally, BD FACSDiva™ software, like FlowJO, allows data to be expressed in detailed graphical reports, tables and statistical population analysis. Data files from BD FACSDiva™ can be exported for further analysis and imported into FlowJo for high quality images.
5. Primary Adipocyte Precursor Cell Culture and Differentiation
5.1 Primary Adipocyte Precursor Cell Culture
Adipocyte precursor cells can be isolated from WAT depots of any mouse model through FACS for further study of adipogenesis in vitro.
Adipocyte precursors from subcutaneous WAT (SWAT) are isolated via FACS and plated onto carboxyl-coated 24-well plates (BD Biosciences CA, USA; 354775) in DMEM (ATCC VA, USA; Catalog No. 30-2002) supplemented with 10% fetal bovine serum (FBS; GIBCO, Life Technologies CA, USA) and maintained in a 5% CO2 atmosphere. Approximately 25,000 cells are plated per well of a 24-well plate. These cells take on a fibroblast-morphology after 3–5 days and are allowed to grow to confluence with DMEM media supplemented with 10% FBS changed every 48 hours.
Once at confluence the cells are held for 48 hours without changing the media.
The media is exchanged with adipocyte differentiation cocktail (MDI; 3-isobutyl-1-methylxanthine, dexamethasone and insulin). DMEM media supplemented with 10% FBS, 1 μg/ml insulin (Sigma-Aldrich MO, USA; I-6634; 10,000 × stock is 10 mg/ml in 0.01 M HCL), 0.25 μg/ml dexamethasone (Sigma-Aldrich MO, USA; D4902; 10,000× stock is 1 mg/ml in 100% ethanol), and 30 μg/ml IBMX (3-isobutyl-1-methylxanthine; Sigma-Aldrich MO, USA; I5879; 500× stock is 15 mg/ml in 0.3 M KOH; made fresh each time).
-
Every 48 hours, media is exchanged with maintenance differentiation media (DMEM supplemented with 10% FBS and 1 μg/ml insulin). Lipid filling is observed within 48–72 hours after the addition of adipocyte differentiation media in step 3. By day 7, lipid filling is complete and cells can be harvested for RNA/protein isolation or stained with Oil Red O (section 5.2).
Note: When isolated from the inguinal subcutaneous WAT, adipocyte precursor cells, positive and negative for CD24 (Rodeheffer et al., 2008) differentiate with the addition of only insulin (1 μg/ml in DMEM supplemented with 10% FBS); however very low rates of differentiation are observed when similar cells are isolated from the visceral epigonadal depot even when induced with MDI adipocyte differentiation cocktail (Figure 4B,C).
Figure 4. Differentiation of Primary Adipocyte Precursor Cells.
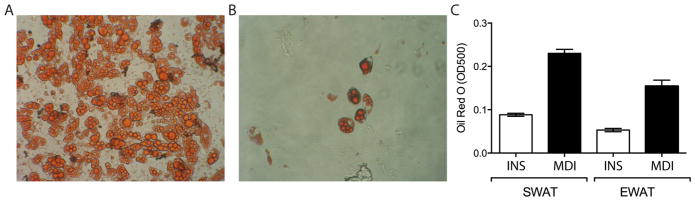
A. Day 7 Subcutaneous WAT (SWAT), B. Day 7 Epigonadal WAT (EWAT), C. Day 7 Oil Red O quantification. INS, insulin only differentiation. MDI (adipogenic cocktail containing IBMX, dexamethasone and insulin).
5.2 Oil Red O Lipid Staining
For lipid staining by Oil Red O, the differentiated cells are fixed with 2% formaldehyde and 0.2% glutaraldehyde in PBS for 15 minutes and then rinsed in PBS for 10 minutes, twice in water for 1 minute, followed by 30 seconds in 60% isopropanol.
The cells are stained with Oil Red O (0.7% in 60% isopropanol; Electron Microscopy Sciences, PA, USA; CAT # 26503-02.) for 10 minutes and rinsed with 60% isopropanol for 1 minute followed by water.
Oil Red O stained cells are directly visualized and imaged using an inverted microscope (Figure 4A,B).
Quantification of lipid accumulation is achieved by Oil Red O extraction by lysis (100% isopropanol with 4% NP40 substitute IGEPAL CA-630, Sigma-Aldrich MO, USA; I8896; 300 μl per well of a 24 well plate) and gentle agitation for 10 minutes at room temperature.
Following Oil Red O extraction 100 μl is transferred to a 96-well plate and absorbance measured at 490–520 nm using a plate reader or spectrophotometer (Figure 4C).
6. Digestion of Whole WAT for Isolation of Mature Adipocytes
Individual adipose depots are carefully excised and minced into 2–4 mm pieces with scissors.
Minced WAT is digested in 13mg/ml collagenase type 2 (Worthington Biochemical Corporation NJ, USA; LS004174) in Krebs Ringer Buffer (KRB), 1.23mM calcium chloride, 1.03mM magnesium chloride, 0.83mM zinc chloride, 4 mM glucose (Sigma-Aldrich MO, USA; G7021) and 500 nM adenosine (Sigma-Aldrich MO, USA; A9251) for 803minutes in a shaking water bath (less than 110 rpm) including gentle mixing by inversion every 20 minutes. Approximately 5 ml of digestion buffer is used to digest one WAT depot from one mouse.
The tissue digest is passed through a 200 μm nylon filter.
Floating adipocytes are separated from the SVF by centrifugation at 150 × g for 83minutes.
The floating fraction is carefully transferred with a wide opening plastic transfer pipette into KRB containing 4 mM glucose and 500 nM adenosine to wash the adipocyte fraction. Excess KRB buffer is removed from below the adipocyte layer using a glass pasteur pipette.
Isolation of intact adipocytes is verified by staining for plasma membrane with Cell Mask Orange (CMO; Life Technologies CA, USA; C10045) and nuclei with DAPI (Life Technologies CA, USA; D1306).
-
The stained adipocyte solution is transferred to a slide and immediately visualized under a fluorescent microscope for verification of purified intact adipocytes. Intact adipocytes are characterized by a CMO+ plasma membrane that contains a lipid droplet and a single DAPI+ nucleus near the CMO+ membrane (Figure 5A).
The presence of excessive CMO-lipid droplets, free-floating nuclei, adipocyte ghosts (adipocyte-like structures that lack a nucleus; Figure 5B) or cells with multiple nuclei suggest adipocyte lysis and/or SVF contamination. Because of the fragility of lipid-filled mature adipocytes, several isolations may be necessary to acquire a preparation of intact, purified mature adipocytes with minimal stromal cell contamination. The purified adipocyte fraction can be further studied for gene expression and metabolic studies.
Alternatively, the adipocytes can be lysed to yield adipocyte nuclei by re-suspending the adipocyte layer in 2–4 volumes of adipocyte nuclei isolation buffer (KRB containing 0.2% NP40 substitute IGEPAL CA-630 (Sigma-Aldrich MO, USA; I8896), followed by vortexing for 10–20 seconds and placing on ice for 5 minutes.
Adipocyte nuclei are separated from cellular debris by centrifugation at 2000 × g for 53minutes and the careful removal of excess adipocyte nuclei isolation buffer. The adipocyte nuclei pellet is re-suspended in nuclei flow cytometry buffer (KRB containing 0.02% NP40 substitute IGEPAL CA-630; Sigma-Aldrich MO, USA) with DAPI and CMO for flow cytometry analysis. Gating on CMO-: DAPI+ nuclei allows for the analysis of purified adipocyte nuclei.
Figure 5. Isolation of Adipocytes.
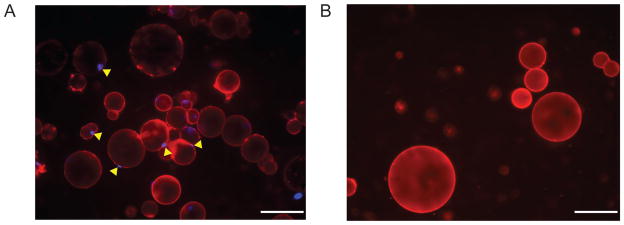
A. Intact adipocytes with single DAPI positive nuclei within a cell membrane B. Lipid ghosts without DAPI positive nuclei. White scale bar represents 100 μm.
Acknowledgments
We thank Elise Jeffery for protocol developments and discussion. This work was supported by American Diabetes Association Award 7-12-JF-46, DERC pilot project grant DK045735 and NIDDK grant DK090489 to M.S.R and EMBO Long-term postdoctoral fellowship (ALTF 132-2011 to CC).
References
- Arner E, Westermark PO, Spalding KL, Britton T, Rydén M, Frisén J, et al. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes. 2010;59(1):105–109. doi: 10.2337/db09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarth N, Roederer M. A practical approach to multicolor flow cytometry for immunophenotyping. Journal of immunological methods. 2000;243(1–2):77–97. doi: 10.1016/s0022-1759(00)00229-5. [DOI] [PubMed] [Google Scholar]
- Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nature cell biology. 2013;15(3):302–308. doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BD Biosciences, NJ, USA. Multicolor Flow Cytometry-Tools. 2013 from http://www.bdbiosciences.com/research/multicolor/tools/index.jsp.
- Cinti S. Adipose Tissue and Adipokines in Health and Disease. 2007. The adipose organ. Nutrition and Health. [Google Scholar]
- Cinti S. The adipose organ at a glance. Disease models & mechanisms. 2012;5(5):588–594. doi: 10.1242/dmm.009662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Crissman HA, Robinson JP. Cytometry. 3. 2000. Methods in Cell Biology; pp. 63–64. [Google Scholar]
- Eto H, Suga H, Matsumoto D, Inoue K, Aoi N, Kato H, et al. Characterization of structure and cellular components of aspirated and excised adipose tissue. Plastic and reconstructive surgery. 2009;124(4):1087–1097. doi: 10.1097/PRS.0b013e3181b5a3f1. [DOI] [PubMed] [Google Scholar]
- Herberg L, Döppen W, Major E, Gries FA. Dietary-induced hypertrophic--hyperplastic obesity in mice. Journal of lipid research. 1974;15(6):580–585. [PubMed] [Google Scholar]
- Hirsch J. Isotopic labeling of DNA in rat adipose tissue: evidence for proliferating cells associated with mature adipocytes. Journal of lipid research 1979. 1979 Aug;20(6):691–704. [PubMed] [Google Scholar]
- Hirsch JJ, Batchelor BB. Adipose tissue cellularity in human obesity. Clinics in Endocrinology and Metabolism. 1976;5(2):299–311. doi: 10.1016/s0300-595x(76)80023-0. [DOI] [PubMed] [Google Scholar]
- Invitrogen, Life Technologies, CA, USA, editor. An Introduction to Flow Cytometry 2013. 2013 from http://www.invitrogen.com/site/us/en/home/support/Tutorials.html.
- Maecker H, Trotter J. Selecting reagents for multicolor flow cytometry with BD™ LSR II and BD FACSCanto™ systems. Application Note. Nature Methods. 2008:5. [Google Scholar]
- Ng CW, Poznanski WJ, Borowiecki M, Reimer G. Differences in growth in vitro of adipose cells from normal and obese patients. Nature. 1971;231(5303):445. doi: 10.1038/231445a0. [DOI] [PubMed] [Google Scholar]
- Ormerod MG. A Practical Approach. Oxford University Press; 2000. Flow Cytometry. Issue 229 of Practical Approach Series. [Google Scholar]
- Purdue University, editor. Purdue University Cytometry Laboratories 2013. 2013 from http://www.cyto.purdue.edu/flowcyt/educate.htm.
- Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135(2):240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Roederer M. Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry. 2001 Nov 1;45(3):194–205. doi: 10.1002/1097-0320(20011101)45:3<194::aid-cyto1163>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- Tree Star. FLOWJO Data Analysis Software for Flow Cytometry. 2012;10:1–32. Retrieved from http://www.flowjo.com/home/manual.html#. [Google Scholar]