Abstract
Background
Anaesthetic postconditioning (APoC) attenuates myocardial injury following coronary ischaemia/reperfusion. We hypothesised that APoC at the initiation of cardiopulmonary resuscitation (CPR) will improve post resuscitation myocardial function along with improved mitochondrial function in a pig model of prolonged untreated ventricular fibrillation.
Methods
In 32 pigs isoflurane anaesthesia was discontinued prior to induction of ventricular fibrillation that was left untreated for 15 min. At the initiation of CPR, 15 animals were randomised to controls (CON), and 17 to APoC with 2 Vol% sevoflurane during the first 3 min CPR. Pigs were defibrillated after 4 min of CPR. After return of spontaneous circulation (ROSC), isoflurane was restarted at 0.8-1.5 Vol% in both groups. Systolic and diastolic blood pressures were measured continuously. Of the animals that achieved ROSC, 8 CON and 8 APoC animals were randomised to have their left ventricular ejection fraction (LVEF%) assessed by echocardiography at 4 hrs. Seven CON and 9 APoC were randomised to euthanasia 15 min after ROSC to isolate mitochondria from the left ventricle for bioenergetic studies.
Results
ROSC was achieved in 10/15 CON and 15/17 APoC animals. APoC improved haemodynamics during CPR and post-CPR LVEF%. Mitochondrial ATP synthesis, coupling of oxidative phosphorylation and calcium retention capacity were improved in cardiac mitochondria isolated after APoC.
Conclusions
In a porcine model of prolonged untreated cardiac arrest, APoC with inhaled sevoflurane at the initiation of CPR, is associated with preserved mitochondrial function and improved post resuscitation myocardial dysfunction.
Keywords: Cardiac Arrest, Cardiopulmonary resuscitation, Ischaemia reperfusion injury, Mitochondria, Postconditioning, Return of spontaneous circulation, Sevoflurane
INTRODUCTION
With an estimated 350,000 patients per year in the United States alone1 and a survival rate of only 3 to 16%,2 out-of-hospital cardiac arrest (OHCA) continues to be a significant cause of neurologic3 and cardiac4 morbidity and mortality. We have recently shown that ischaemic postconditioning at the initiation of standard5 or sodium nitroprusside-enhanced cardiopulmonary resuscitation (CPR)6 significantly improved neurologically intact survival following 15 min of untreated ventricular fibrillation (VF) and concomitant global ischaemia in a porcine model of cardiac arrest. CPR was augmented by the use of an active compression/decompression (ACD) device7 and an impedance threshold device (ITD).8 Postconditioning was achieved by three to four 20-sec pauses during the first 3 min of CPR. Alternatively, myocardial postconditioning can also be achieved by pharmacological means. Volatile anaesthetics have been shown to attenuate myocardial injury following coronary ischaemia/reperfusion (IR) in isolated hearts9 as well as in vivo,10 and can be administered by ventilation. In contrast, an intravenous (IV) drug would require establishing IV access and could therefore be administered only significantly after initiation of CPR in the OHCA setting as delaying CPR to reach a therapeutic level at the initiation of CPR is not feasible.
In this investigation we tested the hypotheses that the volatile anaesthetic sevoflurane given for 3 min immediately at the initiation of CPR can a) improve early post-resuscitation cardiac mitochondrial function, and b) improve post-resuscitation left ventricular function in a pig model of prolonged untreated VF.
MATERIALS AND METHODS
This study conformed to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (8th edition, National Academies Press 2011) and was approved by the Institutional Animal Care Committee of the Minneapolis Medical Research Foundation of Hennepin County Medical Center (protocol number 11-05). All experiments were performed on isoflurane-anaesthetised female Yorkshire farm pigs weighing an average of 38.2 ± 2.1 kg.
PREPARATION
Our protocol has been described in detail.5 After endotracheal intubation, an anaesthesia machine (Narkomed 4A, Dräger, Telford, PA, USA) was used to ventilate the animals with a tidal volume of 10 ml kg−1, starting at a respiratory rate of 10-18 min−1 and on room air supplemented with O2, all titrated to achieve normocapnia and an O2 saturation ≥ 95%. Until induction of VF, general anaesthesia was maintained with inhaled isoflurane 0.8-1.5 Vol% end-tidal measured by a gas analyser (Datex-Ohmeda Capnomac; GE Healthcare, Waukesha, WI, USA) and at a fresh gas flow of 2 l min−1; isoflurane was restarted after return of spontaneous circulation (ROSC) following VF and CPR. A warming blanket (Bair Hugger, Augustine Medical, Eden Prairie, MN, USA) was used to maintain body temperature at 37.5 ± 0.5°C. Arterial blood was sampled for blood gas analysis (Gem 3000, Instrumentation Laboratories, Lexington, MA, USA) at five time points: at baseline, at the end of CPR, and 5 min, 15 min and 1 hr after ROSC. Micromanometer-tipped catheters (Mikro-Tip Transducer, Millar Instruments, Houston, TX, USA) were used to continuously record central aortic (AoP) and right atrial (RAP) pressures. During CPR, coronary perfusion pressure (CPP) was calculated as the difference between AoP and RAP during diastole (spontaneously beating) or decompression (CPR). Systolic (SBP) and diastolic (DBP) blood pressure was derived from AoP. CPR compression force, rate, and depth were controlled and continuously recorded during all experiments to assure that all groups received identical CPR quality.
EXPERIMENTAL PROTOCOL
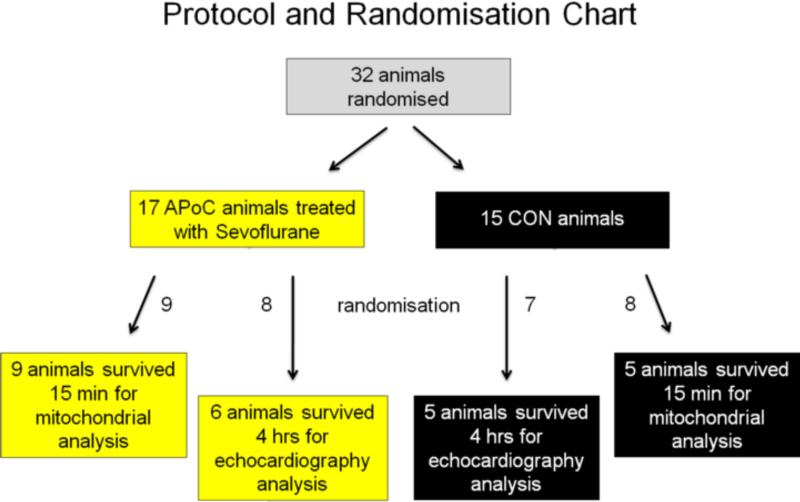
When arterial O2 saturation on room air was ≥ 95%, and endtidal CO2 was stable between 35 and 42 mmHg for 5 min, a direct intracardiac current was used to induce VF. Thirty-two (32) animals were used (Figure 1). Seventeen (17) animals were randomised to the APoC group and received inhaled sevoflurane for the first 3 min of CPR at an endtidal concentration of 2.0 ± 0.2 Vol% (1 minimal alveolar concentration), a clinically relevant concentration shown to significantly improve haemodynamic outcome in a rat model of cardiac arrest and resuscitation.11 Fifteen (15) animals were randomised to the control (CON) group and did not receive any anaesthetic during CPR.
Figure 1.
Protocol and randomisation chart. Animals were initially randomised to either be treated as controls (CON) or to receive inhaled 2 Vol% sevoflurane at the initiation of CPR for 3 min with each breath (anaesthetic postconditioning, APoC). Animals were further randomised to either 15 min survival before euthanasia for mitochondrial analysis or to 4 hrs survival for left ventricular function by echocardiography and serum biomarkers of myocardial injury assessment. The number of surviving animals is stated in each box.
Within the APoC group, 9 of 9 randomised animals were euthanised 15 min after ROSC in order to assess mitochondrial function and reperfusion injury, and 8 animals were randomised to be kept alive for 4 hrs to echocardiographically assess left ventricular function (see below) and biomarkers of cardiac injury. Only 6 animals of the latter group survived the full 4 hrs. These 6 and the 9 animals in the mitochondrial group constituted 15 animals that achieved ROSC out of 17 in the APoC group. The same post-ROSC protocol was used for the 15 animals in the CON group; 7 animals were randomised to harvest tissue 15 min post ROSC, and 8 animals were randomised to survive up to 4 hrs. Five animals in each CON subgroup reached the time endpoints for data analysis (10 in total, Figure 1). All assignments were predetermined at the initial point of randomisation between APoC and CON groups.
In all animals, ACD CPR was performed with a pneumatically driven automatic piston (Pneumatic Compression Controller, Ambu International, Glostrup, Denmark). Uninterrupted chest compressions with a rate of 100 min−1, a 50% duty cycle and a compression depth of 25% of the antero-posterior chest diameter were delivered for 4 min prior to defibrillation. Ventilation parameters remained unchanged from before induction of VF. Return of blood flow from the inferior and superior vena cava to the heart was enhanced in both groups by the use of an ITD (ResQPOD™, Advanced Circulatory Systems Inc, Roseville, MN) during CPR. If ROSC was not achieved at the first cycle, hearts were defibrillated every 2 min thereafter during CPR. Resuscitation efforts continued until ROSC or for a total of 15 min maximum. IV adrenaline was administered in a 13 μg kg−1 bolus at min 3 of CPR, 60 sec before the first defibrillation, and repeated every 3 min if ROSC was not achieved. Animals were ventilated with room air during CPR.
ECHOCARDIOGRAPHY
Transthoracic echocardiography (parasternal long and short axis view) was used to assess left ventricular function starting 15 min after ROSC. Left ventricular ejection fraction (LVEF%) was estimated by two independent clinical echocardiographers blinded to the treatment. For an individual animal the mean of the two ejections fractions reported by the cardiologists was used for group calculations. If a discrepancy of more than 10% was present then LVEF% was reported based on the calculation using the Simpson's method of volumetric analysis.12
CARDIAC INJURY MARKERS
Serum samples for biomarkers of cardiac injury (Troponin I and CK-MB) were obtained 4 hrs after ROSC and were analysed with standard human techniques in the core laboratories of the University of Minnesota Hospital.
MITOCHONDRIAL EXPERIMENTS
All mitochondrial experiments were performed in freshly isolated cardiac mitochondria. Unless otherwise stated, all necessary chemicals were obtained from Sigma, St. Louis, MO, USA.
ISOLATION OF MITOCHONDRIA
Hearts were excised 15 min after ROSC. This shorter duration post ROSC was chosen to test for functional differences in mitochondrial function without the loss of non-viable mitochondria as it would be the case with longer reperfusion times.13 An approximately 2 g myocardial piece from the mid left anterior descending coronary artery region was immediately placed into ice-cold isolation buffer containing (in mM) 200 mannitol, 50 sucrose, 5 KH2PO4, 5 3-(nmorpholino)propanesulfonic (MOPS), 1 EGTA, and 0.1% bovine serum albumin (BSA), pH adjusted to 7.15 with KOH. The tissue was then minced into 1-mm3 pieces and its suspension homogenised (homogeniser 60404-01, Ingenieurbüro Zipperer, Staufen, Germany) for 30 sec in the presence of 5U ml−1 protease (Bacillus licheniformis), followed by another 30 sec after 10-fold dilution of the protease. Mitochondria were then isolated by differential centrifugation. The suspension was first centrifuged for 10 min at 8,000 g to remove the protease. After resuspension of the pellet in 25 ml isolation buffer, it was centrifuged for 10 min at 750 g to remove cellular debris. The resultant supernatant with the mitochondrial fraction was centrifuged for another 10 min further at 8,000 g. The final mitochondrial pellet was resuspended in 500 μl isolation buffer and kept on ice. All isolation procedures were conducted at 4°C whereas all experiments were conducted at room temperature. After determination of mitochondrial protein concentration by the Bradford method,14 mitochondria were diluted in experimental buffer to a final concentration of 0.5 mg protein ml−1. The experimental buffer contained (in mM) 130 KCl, 5 K2HPO4, 20 MOPS, 0.001 Na4P2O7, and 0.1% BSA (pH adjusted to 7.15 with KOH). Dilution in experimental buffer ensured minimal (40 μM) carry-over of EGTA from the isolation buffer.
MITOCHONDRIAL ATP SYNTHESIS
Rate of mitochondrial adenosine triphosphate (ATP) synthesis was determined by chemiluminescence measured in a Modulus luminometer (Turner Biosystems, Sunnyvale, CA, USA) utilising the reaction of firefly luciferase and luciferin with ATP. The solution contained experimental buffer, 0.2 μM diadenosine pentaphosphate, 30 μM ADP, 10 μg ml−1 mitochondria, 0.1 mg ml−1 luciferin, and 1.25 μg ml−1 luciferase.15 Addition of pyruvate/malate or succinate (5 mM each) initiated the reaction that was measured for 120 sec. Defined ATP concentrations were used to obtain the standard curve.
MITOCHONDRIAL OXYGEN CONSUMPTION
Mitochondrial O2 consumption was measured polarographically using a Clark-type O2 electrode (Model 1302; Strathkelvin Instruments, Glasgow, Scotland) in a water-jacketed 500-μl chamber (Model MT200A; Strathkelvin Instruments), equipped with a Teflon-coated magnetic stirring bar and monitored by an O2 meter (Model 782; Strathkelvin Instruments).16 State 2 respiration was initiated 60 sec after sealing the chamber by adding 10 mM of the complex I substrates pyruvate and malate or a combination of the complex II substrate succinate with the complex I blocker rotenone (10 μM, dissolved in dimethyl sulfoxide). Addition of 250 μM adenosine diphosphate (ADP) at 120 sec initiated state 3 respiration, until complete phosphorylation of ADP to ATP led to state 4 respiration. Chamber O2 concentration in μM was monitored for 60 sec after state 4 respiration was achieved or until the O2 concentration was 0. The respiratory control index (RCI) was calculated as the ratio of the rate of state 3 to state 4 respirations. All individual results are the average of duplicate runs.
MITOCHONDRIAL CALCIUM RETENTION AND MPTP OPENING
Mitochondria were suspended in respiration buffer inside a cuvette-based spectrofluorometer (LS 55, Perkin Elmer, Waltham, MA, USA) containing 10 mM of the complex I substrates pyruvate/malate or 10 mM of the complex II substrate succinate. Extra-mitochondrial (em)[Ca2+] was monitored using the fluorescent probe CaGreen-5N hexapotassium salt (100 nM; Life Technologies, Grand Island, NY, USA) at excitation and emission wavelengths of 503 and 532 nm, respectively.17 After a 1-min stabilisation period, CaCl2 (25 mM) was infused at a rate of 10 μl min−1 to add 25 μM min−1 until em[Ca2+] reached a steady state (equilibrium between Ca2+ infusion and mitochondrial Ca2+ uptake). With this continuous infusion of CaCl2, a sudden increase in em[Ca2+] is due to release of mitochondrial Ca2+ indicating mitochondrial permeability transition pore (mPTP) opening. The amount of CaCl2 infused until mPTP opening indicated calcium retention capacity.
STATISTICAL ANALYSIS
All results are expressed as mean ± standard deviation (SD) or as percentages. The haemodynamics during CPR, blood gases and echocardiographic data up to 15 min include all surviving animals randomised to the two groups. Echocardiographic data at 1 and 4 hrs include only data from animals that were randomised to those endpoints for each group, had successful ROSC and survived to this point (Figure 1). The number (n) of animals in each group and at each time point is provided in parentheses. Unpaired student t-tests, Mann-Whitney U tests and Chi-square tests were used to calculate differences between the two experimental groups for parametric, non-parametric and categorical data, respectively. Statistical significance (*) was assumed when P<0.05 (two-tailed).
RESULTS
There were no significant differences between treatment groups in baseline parameters (Tables 1-3) or isoflurane concentrations before and after CPR.
Table 1.
Haemodynamics and success of resuscitation.
| CPR method | Parameter | Baseline | 2 min CPR | 4 min CPR (after adrenaline) | 15 min ROSC | 1 hr ROSC† | Number of shocks to initial ROSC | Total adrenaline dose (mg) | ROSC |
|---|---|---|---|---|---|---|---|---|---|
| CON | SBP | 104.0±15.5 (15) | 42.1±13.9 (15) | 57.8±13.9 (15) | 87.0±20.6 (10) | 92.7±19.7 (5) | 6.7±4.9 (10) | 1.4±1.2 (15) | 10/15 |
| DBP | 73.1±12.4 (15) | 23.0±7.4 (15) | 26.6±7.7 (15) | 43.7±7.6 (10) | 53.4±11.2 (5) | ||||
| RAP | 3.3±2.3 (15) | 3.6±1.9 (15) | 3.6±1.5 (15) | 7.8±3.2 (10) | 6.6±1.3 (5) | ||||
| CPP | 71.6±12.8 (15) | 13.9±7.0 (15) | 21.7±6.2 (15) | 38.1±6.6 (10) | 46.8±11.2 (5) | ||||
| APoC | SBP | 108.0±32.6 (17) | 65.7±15.7* (17) | 92.0±23.5* (17) | 96.8±27.1 (15) | 100.1±22.0 (6) | 3.5±2.1* (15) | 0.7±0.4* (17) | 15/17 |
| DBP | 80.0±16.5 (17) | 27.1±8.2 (17) | 44.7±9.1* (17) | 59.5±18.2* (15) | 54.6±7.3 (6) | ||||
| RAP | 3.5±2.1 (17) | 2.2±2.1 (17) | 2.9±2.9 (17) | 7.7±4.6 (15) | 4.4±2.2 (6) | ||||
| CPP | 77.1±17.3 (17) | 25.0±7.4* (17) | 41.9±8.2* (17) | 52.8±18.1* (15) | 50.3±14.7 (6) |
Cardiopulmonary resuscitation (CPR) was performed with either with ACD and ITD alone (CON) or with anaesthetic postconditioning (APoC) in addition. Values are shown as mean ± SD (n). Pressures are given in mmHg, flows in ml min−1. SBP = systolic blood pressure, DBP = diastolic blood pressure, RAP = right atrial pressure, CPP = coronary perfusion pressure, ROSC = return of spontaneous circulation.
Mean significantly different between groups with P<0.05 (two-tailed).
Data 1 hr after ROSC are derived from surviving animals randomised to the 4-hr echocardiographic endpoint.
Table 3.
Left ventricular ejection fraction.
| CPR method | Baseline | 15 min ROSC | 1 hr ROSC† | 4 hrs ROSC† |
|---|---|---|---|---|
| CON | 61±27 (15) | 37±13 (10) | 36±13 (5) | 35±23 (5) |
| APoC | 63±25 (17) | 53±19* (15) | 58±10* (6) | 63±18* (6) |
Cardiopulmonary resuscitation (CPR) was performed with either with ACD and ITD alone (CON) or with anaesthetic postconditioning (APoC) in addition. ROSC = return of spontaneous circulation. Values are shown as % mean ± SD (n).
Mean significantly different between groups with P<0.05 (two-tailed).
Data at 1 and 4 hrs post ROSC are derived only from surviving animals that were randomised to survive to the 4-hr echocardiographic endpoint.
CPR HAEMODYNAMICS, ROSC AND OUTCOME MEASURES
ROSC was achieved in 10 of 15 CON and 15 of 17 APoC animals (P=0.80). During CPR, DBP, SBP and CPP were improved in APoC vs. CON animals that became non-significant after ROSC (Table 1). Conversely, the adrenaline dose necessary to achieve ROSC was significantly decreased in APoC animals. The number of shocks to achieve ROSC was significantly lower in the APoC group compared to CON, *3.5 ± 2.1 vs. 6.7 ± 4.9, respectively.
ARTERIAL BLOOD GASES
Arterial blood gases showed no relevant differences between the groups (Table 2).
Table 2.
Arterial blood gases and volatile anaesthetic concentrations.
| CPR method | Parameter | Baseline | End of CPR | 5 min ROSC | 15 min ROSC | 1 hr ROSC† |
|---|---|---|---|---|---|---|
| CON | pH | 7.45±0.12 (15) | 7.26±0.23 (15) | 7.24±0.06 (10) | 7.25±0.09 (10) | 7.35±0.11 (5) |
| pCO2 | 39.7±7.4 (15) | 44.7±45.3 (15) | 49.5±5.7 (10) | 43.5±8.2 (10) | 41.0±5.1 (5) | |
| pO2 | 129±104 (15) | 91±50 (15) | 134±66 (10) | 132±51 (10) | 102±27 (5) | |
| HCO3 | 27.4±3.1 (15) | 19.1±8.9 (15) | 21.0±1.6 (10) | 19.0±4.4 (10) | 20.0±2.2 (5) | |
| SaO2 | 99.4±1.2 (15) | 93.7±14.7 (15) | 97.5±3.8 (10) | 97.3±4.1 (10) | 95.0±6.7 (5) | |
| Isoflurane | 1.1±0.2 (15) | 1.0±0.2 (10) | 1.0±0.2 (5) | |||
| APoC | pH | 7.47±0.04 (17) | 7.23±0.16 (17) | 7.26±0.12 (15) | 7.29±0.04 (15) | 7.37±0.07 (6) |
| pCO2 | 37.3±3.7 (17) | 51.8±20.2 (17) | 42.3±7.7* (15) | 38.1±6.6 (15) | 39.5±4.2 (6) | |
| pO2 | 117±91 (17) | 86±33 (17) | 137±112 (15) | 131±70 (15) | 117±54 (6) | |
| HCO3 | 27.5±4.1 (17) | 18.6±1.6 (17) | 20.0±3.9 (15) | 18.1±2.7 (15) | 22.2±1.2 (6) | |
| SaO2 | 99.8±0.4 (17) | 93.5±5.4 (17) | 97.4±6.2 (15) | 97.9±5.8 (15) | 98.0±3.7 (6) | |
| Isoflurane | 1.1±0.2 (17) | 1.0±0.2 (15) | 1.1±0.2 (6) |
Cardiopulmonary resuscitation (CPR) was performed with either with ACD and ITD alone (CON) or with anaesthetic postconditioning (APoC) in addition. Values are shown as mean ± SD (n). Arterial blood gases were measured at baseline, the end of CPR, and 5 min, 15 min and 1 hr after return of spontaneous circulation (ROSC). Partial pressures are given in mmHg. HCO3: bicarbonate in mM; SaO2: percent oxygen saturation. Endtidal isoflurane concentrations are given in Vol%.
Mean significantly different between groups with P<0.05 (two-tailed).
Data 1 hr after ROSC are derived from surviving animals randomised to the 4-hr echocardiographic endpoint.
LEFT VENTRICULAR EJECTION FRACTION AND MYOCARDIAL INJURY MARKERS
The APoC group had a significant increase in post-ROSC LVEF% at 15 min, 1 and 4 hrs (Table 3). CK-MB and troponin levels at 4 hrs were lower in the APoC group compared to CON (*7 ± 9 and *6 ± 11 vs. 37 ± 24 and 31 ± 24 ng/ml, respectively).
MITOCHONDRIAL PARAMETERS
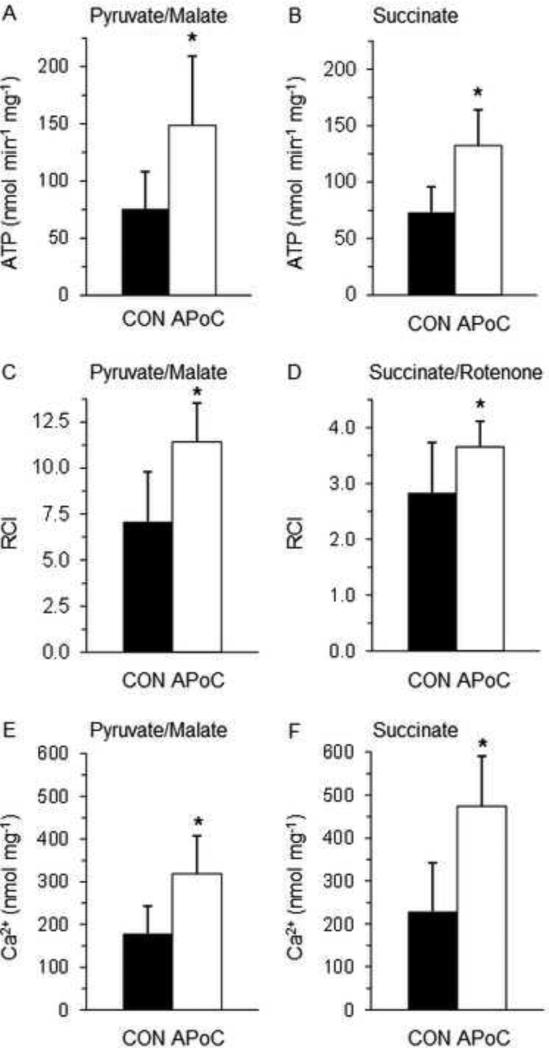
For both complex I and complex II substrates the rates of ATP synthesis and the RCIs as markers of coupling of oxidative phosphorylation were improved, and Ca2+ retention capacity was enhanced in mitochondria isolated from hearts of APoC vs. CON animals (Figure 2).
Figure 2.
Mitochondrial function studies in control (CON, n = 5, black bars) vs postconditioned hearts (APoC, n = 9, white bars). Results for mitochondrial complex I substrates pyruvate and malate are displayed on the left, results for complex II substrate succinate on the right. Panels A and B show a significantly higher rate of ATP synthesis, panels C and D better coupling of oxidative phosphorylation as measured by the respiratory control index (RCI), and panels E and F a higher Ca2+ retention capacity in APoC vs. CON mitochondria. All values are mean ± SD. * Mean significantly different between groups with P < 0.05 (two-tailed).
DISCUSSSION
This is the first report that APoC with the volatile anaesthetic sevoflurane, when given at the initiation of CPR, can improve intra-CPR haemodynamics, post-resuscitation myocardial function up to 4 hrs and preserve mitochondrial function in a preclinical porcine model of prolonged cardiac arrest.
While protection by ischaemic preconditioning, i.e. periods of brief IR before prolonged IR first described in 1986 by Murry and colleagues,18 requires prior knowledge or at least a significant probability of an ischaemic insult to follow, postconditioning has the advantage that it can be readily employed upon reperfusion after ischaemia has already occurred. Vinten-Johansen's group19,20 has first described and compared ischaemic postconditioning with preconditioning one decade ago. Since then, numerous investigations have been devoted to its further characterisation and the elucidation of specific intracellular signalling pathways.21-24
Studies to quantify the success of postconditioning in the IR-treated heart have traditionally revealed a decrease in infarct size of about 20 to 50%19-21,25-27 while post-IR function such as contractility21,26 or biomarker release was improved by no more than 70% compared to control.20,21,26 Consequently, our recent results on the introduction of an ischaemic postconditioning protocol by limited interruptions of chest compressions during the first 3 min of CPR5,6 were of a similar magnitude, with one important exception: neurologically intact survival after CPR, a decisive marker of resuscitation success, was significantly improved after prolonged untreated cardiac arrest,5 indicating a considerable potential to improve cardiac and neurologic outcome and survival after a prolonged global ischaemic insult such as cardiac arrest by VF.
Alternatively to brief periods of IR, pre- and postconditioning can also be accomplished by pharmacological means. Many drugs have been reported to trigger pre- and/or postconditioning of the myocardium, e.g. adenosine and its receptor agonists,28-30 bradykinin,30,31 B-type natriuretic peptide,32 opioids,33 cyclosporine A,34 noble gases,35-39 and volatile anaesthetics.11,40-42 It is commonly believed that not only pre- and postconditioning, but also ischaemic and pharmacological conditioning share the same common pathways,21-24 including the reperfusion injury salvage kinase (RISK) pathway, glycogen synthase kinase (GSK) 3β-phosphorylation and a delay or inhibition of mPTP opening.43
Although application of gases such as volatile anaesthetics or noble gases is not routinely performed in the setting of OHCA, it might display an attractive alternative to IV drug administration as airway management including endotracheal intubation is an integral part of CPR. While previous reports confirmed the feasibility of a syringe pump-based system to deliver volatile anaesthetics even without a dedicated anaesthesia machine44 further studies need to focus on whether these can also be delivered reliably and safely via a bag-mask.
The concept of attenuation of IR injury by postconditioning necessitates the stimulus or drug to be applied immediately within the first few minutes of reperfusion.45 This may also be the reason why some previously conducted studies were unable to find an improvement in neurological deficit score or cerebral cellular and molecular pathways when the anaesthetic was given after achievement of ROSC in a pig model of cardiac arrest for only 8 min.37,46 Whether noble gases differ in this context and provide neurologic and myocardial protection when given later is the subject of ongoing research.37,39 Certainly, due to their anaesthetic effects volatile anaesthetics require a tight seal and greater caution and need to be scavenged to avoid environmental contamination and potential danger to the emergency personnel when compared to noble gases. In contrast to IV drugs, however, distributed by circulation only, both types of gases require circulation and – in case of respiratory arrest positive pressure – ventilation. Continued sedation by volatile anaesthetics during therapeutic hypothermia extending their potential postconditioning effects to protect vital organs from further reperfusion injury is also subject of current investigations.44
In this study we show that APoC with sevoflurane, after prolonged untreated cardiac arrest, can significantly improve post resuscitation left ventricular function up to 4 hrs and can decrease myocardial injury based on biomarkers of injury such as troponin and CK-MB. These clinically relevant findings are observed in conjunction with significant improvements in ATP synthesis, coupling of oxidative phosphorylation and delay of mPTP opening in cardiac mitochondria isolated 15 min after ROSC. The effect of sevoflurane on mitochondrial function during early reperfusion may directly contribute to mitochondrial and thereby to tissue protection.43
Some limitations to our study need to be acknowledged. The use of isoflurane as a general anaesthetic before and after VF and CPR in both the APoC and CON group may have contributed to a smaller APoC effect of the sevoflurane as the CON hearts may also have received some degree of cardioprotection by preconditioning;47,48 in the absence of a negative outcome, however, this is of negligible concern. We did not perform a dose response study in the APoC group as these are more difficult in larger vs smaller animals11 due to the large number of subjects needed. Although our porcine model is the closest before clinical trials, a one-to-one translation into clinical practice may be hampered by species- and organ-dependent differences in sensitivity to IR.49 Also, we have only used young adult female and healthy farm pigs whereas patients with VF cardiac arrest are typically of advanced age and have a variety of comorbidities, medications or other confounding factors interfering with cardioprotective strategies.50 Finally, we have not evaluated the effect of sevoflurane on neurological function and survival; ongoing studies will address this question.
CONCLUSION
In summary, we have shown that inhaled sevoflurane when administered for 3 min at the initiation of CPR, after 15 min of untreated cardiac arrest, significantly improved systemic haemodynamics during CPR and myocardial function after ROSC and did so by preserving mitochondrial function in pigs.
Supplementary Material
ACKNOWLEDGMENT
Project-related funding was provided by institutional funds and by the National Institutes of Health (NIH; R01HL108926-01and R01HL123227 to DY). Further research funding was received from the Department of Veterans Affairs (CARA-026-10F to MLR) and the NIH (5R01 HL098490-03 to MB and MLR). None of these entities had a role in the study design; the collection, analysis or interpretation of data; or the manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Received from: TVHS VA Medical Center and Department of Anesthesiology, Vanderbilt University, Nashville, TN; Departments of Emergency Medicine, Anesthesiology, Pharmacology & Toxicology, and Surgery, Medical College of Wisconsin, Milwaukee, WI; Department of Emergency Medicine, University of Michigan Health System, Ann Arbor, MI; and Departments of Medicine - Cardiovascular Division, and Integrative Biology & Physiology, University of Minnesota, Minneapolis, MN.
CONFLICT OF INTEREST STATEMENT
None of the authors has any financial and personal relationships with other people or organisations that could inappropriately have influenced this work.
REFERENCES
- 1.Nichol G, Soar J. Regional cardiac resuscitation systems of care. Curr Opin Crit Care. 2010;16:223–30. doi: 10.1097/MCC.0b013e32833985b5. [DOI] [PubMed] [Google Scholar]
- 2.Nichol G, Thomas E, Callaway CW, Hedges J, Powell JL, Aufderheide TP, Rea T, Lowe R, Brown T, Dreyer J, Davis D, Idris A, Stiell I. Regional variation in out-of-hospital cardiac arrest incidence and outcome. JAMA. 2008;300:1423–31. doi: 10.1001/jama.300.12.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grubb NR. Managing out-of-hospital cardiac arrest survivors: 1. Neurological perspective. Heart. 2001;85:6–8. doi: 10.1136/heart.85.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grubb NR. Managing out-of-hospital cardiac arrest survivors: 2. Cardiological perspective. Heart. 2001;85:123–4. doi: 10.1136/heart.85.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segal N, Matsuura T, Caldwell E, Sarraf M, McKnite S, Zviman M, Aufderheide TP, Halperin HR, Lurie KG, Yannopoulos D. Ischemic postconditioning at the initiation of cardiopulmonary resuscitation facilitates functional cardiac and cerebral recovery after prolonged untreated ventricular fibrillation. Resuscitation. 2012;83:1397–403. doi: 10.1016/j.resuscitation.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yannopoulos D, Segal N, Matsuura T, Sarraf M, Thorsgard M, Caldwell E, Rees J, McKnite S, Santacruz K, Lurie KG. Ischemic post-conditioning and vasodilator therapy during standard cardiopulmonary resuscitation to reduce cardiac and brain injury after prolonged untreated ventricular fibrillation. Resuscitation. 2013;84:1143–9. doi: 10.1016/j.resuscitation.2013.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen TJ, Tucker KJ, Lurie KG, Redberg RF, Dutton JP, Dwyer KA, Schwab TM, Chin MC, Gelb AM, Scheinman MM, et al. Active compression-decompression. A new method of cardiopulmonary resuscitation. Cardiopulmonary Resuscitation Working Group. JAMA. 1992;267:2916–23. doi: 10.1001/jama.267.21.2916. [DOI] [PubMed] [Google Scholar]
- 8.Lurie KG, Coffeen P, Shultz J, McKnite S, Detloff B, Mulligan K. Improving active compression-decompression cardiopulmonary resuscitation with an inspiratory impedance valve. Circulation. 1995;91:1629–32. doi: 10.1161/01.cir.91.6.1629. [DOI] [PubMed] [Google Scholar]
- 9.Schlack W, Hollmann M, Stunneck J, Thamer V. Effect of halothane on myocardial reoxygenation injury in the isolated rat heart. Br J Anaesth. 1996;76:860–7. doi: 10.1093/bja/76.6.860. [DOI] [PubMed] [Google Scholar]
- 10.Schlack W, Preckel B, Barthel H, Obal D, Thamer V. Halothane reduces reperfusion injury after regional ischaemia in the rabbit heart in vivo. Br J Anaesth. 1997;79:88–96. doi: 10.1093/bja/79.1.88. [DOI] [PubMed] [Google Scholar]
- 11.Knapp J, Bergmann G, Bruckner T, Russ N, Böttiger BW, Popp E. Pre- and postconditioning effect of Sevoflurane on myocardial dysfunction after cardiopulmonary resuscitation in rats. Resuscitation. 2013;84:1450–5. doi: 10.1016/j.resuscitation.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Quinones MA, Waggoner AD, Reduto LA, Nelson JG, Young JB, Winters WL, Jr., Ribeiro LG, Miller RR. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64:744–53. doi: 10.1161/01.cir.64.4.744. [DOI] [PubMed] [Google Scholar]
- 13.Gadicherla AK, Stowe DF, Antholine WE, Yang M, Camara AK. Damage to mitochondrial complex I during cardiac ischemia reperfusion injury is reduced indirectly by anti-anginal drug ranolazine. Biochim Biophys Acta. 2012;1817:419–29. doi: 10.1016/j.bbabio.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 15.Pravdic D, Hirata N, Barber L, Sedlic F, Bosnjak ZJ, Bienengraeber M. Complex I and ATP synthase mediate membrane depolarization and matrix acidification by isoflurane in mitochondria. Eur J Pharmacol. 2013;690:149–57. doi: 10.1016/j.ejphar.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riess ML, Camara AK, Heinen A, Eells JT, Henry MM, Stowe DF. KATP channel openers have opposite effects on mitochondrial respiration under different energetic conditions. J Cardiovasc Pharmacol. 2008;51:483–91. doi: 10.1097/FJC.0b013e31816bf4a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aldakkak M, Stowe DF, Dash RK, Camara AK. Mitochondrial handling of excess Ca2+ is substrate-dependent with implications for reactive oxygen species generation. Free Radic Biol Med. 2013;56:193–203. doi: 10.1016/j.freeradbiomed.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 19.Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, Vinten-Johansen J. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285:H579–88. doi: 10.1152/ajpheart.01064.2002. [DOI] [PubMed] [Google Scholar]
- 20.Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera JS, Halkos ME, Kerendi F, Guyton RA, Vinten-Johansen J. Postconditioning attenuates myocardial ischemia-reperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res. 2004;62:74–85. doi: 10.1016/j.cardiores.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 21.van Vuuren D, Lochner A. Ischaemic postconditioning: from bench to bedside. Cardiovasc J Afr. 2008;19:311–20. [PMC free article] [PubMed] [Google Scholar]
- 22.Huffmyer J, Raphael J. Physiology and pharmacology of myocardial preconditioning and postconditioning. Semin Cardiothorac Vasc Anesth. 2009;13:5–18. doi: 10.1177/1089253208330709. [DOI] [PubMed] [Google Scholar]
- 23.Sanada S, Komuro I, Kitakaze M. Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning, and translational aspects of protective measures. Am J Physiol Heart Circ Physiol. 2011;301:H1723–41. doi: 10.1152/ajpheart.00553.2011. [DOI] [PubMed] [Google Scholar]
- 24.Minamino T. Cardioprotection from ischemia/reperfusion injury: basic and translational research. Circ J. 2012;76:1074–82. doi: 10.1253/circj.cj-12-0132. [DOI] [PubMed] [Google Scholar]
- 25.Wu QL, Shen T, Shao LL, Ma H, Wang JK. Ischemic postconditioning mediates cardioprotection via PI3K/GSK-3β/β-catenin signaling pathway in ischemic rat myocardium. Shock. 2012;38:165–9. doi: 10.1097/SHK.0b013e31825b5633. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Ma J, Liu H. Protective effect of ischemic postconditioning against ischemia reperfusion-induced myocardium oxidative injury in IR rats. Molecules. 2012;17:3805–17. doi: 10.3390/molecules17043805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner C, Ebner B, Tillack D, Strasser RH, Weinbrenner C. Cardioprotection by ischemic postconditioning is abrogated in hypertrophied myocardium of spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2013;61:35–41. doi: 10.1097/FJC.0b013e3182760c4d. [DOI] [PubMed] [Google Scholar]
- 28.Thornton JD, Liu GS, Olsson RA, Downey JM. Intravenous pretreatment with A1-selective adenosine analogues protects the heart against infarction. Circulation. 1992;85:659–65. doi: 10.1161/01.cir.85.2.659. [DOI] [PubMed] [Google Scholar]
- 29.Kin H, Zatta AJ, Lofye MT, Amerson BS, Halkos ME, Kerendi F, Zhao ZQ, Guyton RA, Headrick JP, Vinten-Johansen J. Postconditioning reduces infarct size via adenosine receptor activation by endogenous adenosine. Cardiovasc Res. 2005;67:124–33. doi: 10.1016/j.cardiores.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 30.Gross ER, Gross GJ. Ligand triggers of classical preconditioning and postconditioning. Cardiovasc Res. 2006;70:212–21. doi: 10.1016/j.cardiores.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 31.Sun W, Wainwright CL. The potential antiarrhythmic effects of exogenous and endogenous bradykinin in the ischaemic rat heart in vivo. Coron Artery Dis. 1994;5:541–50. [PubMed] [Google Scholar]
- 32.Burley DS, Baxter GF. B-type natriuretic peptide at early reperfusion limits infarct size in the rat isolated heart. Basic Res Cardiol. 2007;102:529–41. doi: 10.1007/s00395-007-0672-1. [DOI] [PubMed] [Google Scholar]
- 33.Gross ER, Hsu AK, Gross GJ. GSK3β inhibition and KATP channel opening mediate acute opioid-induced cardioprotection at reperfusion. Basic Res Cardiol. 2007;102:341–9. doi: 10.1007/s00395-007-0651-6. [DOI] [PubMed] [Google Scholar]
- 34.Huhn R, Heinen A, Hollmann MW, Schlack W, Preckel B, Weber NC. Cyclosporine A administered during reperfusion fails to restore cardioprotection in prediabetic Zucker obese rats in vivo. Nutr Metab Cardiovasc Dis. 2010;20:706–12. doi: 10.1016/j.numecd.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Pagel PS, Krolikowski JG, Shim YH, Venkatapuram S, Kersten JR, Weihrauch D, Warltier DC, Pratt PF., Jr. Noble gases without anesthetic properties protect myocardium against infarction by activating prosurvival signaling kinases and inhibiting mitochondrial permeability transition in vivo. Anesth Analg. 2007;105:562–9. doi: 10.1213/01.ane.0000278083.31991.36. [DOI] [PubMed] [Google Scholar]
- 36.Huhn R, Heinen A, Weber NC, Kerindongo RP, Oei GT, Hollmann MW, Schlack W, Preckel B. Helium-induced early preconditioning and postconditioning are abolished in obese Zucker rats in vivo. J Pharmacol Exp Ther. 2009;329:600–7. doi: 10.1124/jpet.108.149971. [DOI] [PubMed] [Google Scholar]
- 37.Fries M, Coburn M, Nolte KW, Timper A, Kottmann K, Kuru TH, Weis J, Rossaint R. Early administration of xenon or isoflurane may not improve functional outcome and cerebral alterations in a porcine model of cardiac arrest. Resuscitation. 2009;80:584–90. doi: 10.1016/j.resuscitation.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 38.Schwiebert C, Huhn R, Heinen A, Weber NC, Hollmann MW, Schlack W, Preckel B. Postconditioning by xenon and hypothermia in the rat heart in vivo. Eur J Anaesthesiol. 2010;27:734–9. doi: 10.1097/EJA.0b013e328335fc4c. [DOI] [PubMed] [Google Scholar]
- 39.Brücken A, Kurnaz P, Bleilevens C, Derwall M, Weis J, Nolte K, Rossaint R, Fries M. Dose dependent neuroprotection of the noble gas argon after cardiac arrest in rats is not mediated by KATP-channel opening. Resuscitation. 2014;85:826–32. doi: 10.1016/j.resuscitation.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Chiari PC, Bienengraeber MW, Pagel PS, Krolikowski JG, Kersten JR, Warltier DC. Isoflurane protects against myocardial infarction during early reperfusion by activation of phosphatidylinositol-3-kinase signal transduction: evidence for anesthetic-induced postconditioning in rabbits. Anesthesiology. 2005;102:102–9. doi: 10.1097/00000542-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 41.Feng J, Lucchinetti E, Ahuja P, Pasch T, Perriard JC, Zaugg M. Isoflurane postconditioning prevents opening of the mitochondrial permeability transition pore through inhibition of glycogen synthase kinase 3β. Anesthesiology. 2005;103:987–95. doi: 10.1097/00000542-200511000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Ge ZD, Pravdic D, Bienengraeber M, Pratt PF, Jr., Auchampach JA, Gross GJ, Kersten JR, Warltier DC. Isoflurane postconditioning protects against reperfusion injury by preventing mitochondrial permeability transition by an endothelial nitric oxide synthase-dependent mechanism. Anesthesiology. 2010;112:73–85. doi: 10.1097/ALN.0b013e3181c4a607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hausenloy DJ, Ong SB, Yellon DM. The mitochondrial permeability transition pore as a target for preconditioning and postconditioning. Basic Res Cardiol. 2009;104:189–202. doi: 10.1007/s00395-009-0010-x. [DOI] [PubMed] [Google Scholar]
- 44.Hellström J, Öwall A, Martling CR, Sackey PV. Inhaled isoflurane sedation during therapeutic hypothermia after cardiac arrest: a case series. Critical care medicine. 2014;42:e161–6. doi: 10.1097/CCM.0b013e3182a643d7. [DOI] [PubMed] [Google Scholar]
- 45.Huang CH, Tsai MS, Hsu CY, Su YJ, Wang TD, Chang WT, Chen WJ. Post-cardiac arrest myocardial dysfunction is improved with cyclosporine treatment at onset of resuscitation but not in the reperfusion phase. Resuscitation. 2011;82(Suppl 2):S41–7. doi: 10.1016/S0300-9572(11)70150-2. [DOI] [PubMed] [Google Scholar]
- 46.Meybohm P, Gruenewald M, Albrecht M, Müller C, Zitta K, Foesel N, Maracke M, Tacke S, Schrezenmeir J, Scholz J, Bein B. Pharmacological postconditioning with sevoflurane after cardiopulmonary resuscitation reduces myocardial dysfunction. Crit Care. 2012;15:R241. doi: 10.1186/cc10496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riess ML, Camara AK, Chen Q, Novalija E, Rhodes SS, Stowe DF. Altered NADH and improved function by anesthetic and ischemic preconditioning in guinea pig intact hearts. Am J Physiol Heart Circ Physiol. 2002;283:H53–60. doi: 10.1152/ajpheart.01057.2001. [DOI] [PubMed] [Google Scholar]
- 48.Kehl F, Krolikowski JG, Mraovic B, Pagel PS, Warltier DC, Kersten JR. Is isoflurane- induced preconditioning dose related? Anesthesiology. 2002;96:675–80. doi: 10.1097/00000542-200203000-00025. [DOI] [PubMed] [Google Scholar]
- 49.Shen YT, Vatner SF. Differences in myocardial stunning following coronary artery occlusion in conscious dogs, pigs, and baboons. Am J Physiol. 1996;270:H1312–22. doi: 10.1152/ajpheart.1996.270.4.H1312. [DOI] [PubMed] [Google Scholar]
- 50.Riess ML, Stowe DF, Warltier DC. Cardiac pharmacological preconditioning with volatile anesthetics: from bench to bedside? Am J Physiol Heart Circ Physiol. 2004;286:H1603–7. doi: 10.1152/ajpheart.00963.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.