Abstract
Uncertainty about a possible future threat disrupts our ability to avoid it or to mitigate its negative impact, and thus results in anxiety. Here, we focus the broad literature on the neurobiology of anxiety through the lens of uncertainty. We identify five processes essential for adaptive anticipatory responses to future threat uncertainty, and propose that alterations to the neural instantiation of these processes results in maladaptive responses to uncertainty in pathological anxiety. This framework has the potential to advance the classification, diagnosis, and treatment of clinical anxiety.
The human brain, it has been written, is an “anticipation machine, and ‘making future’ is the most important thing it does”1. The ability to use past experiences and information about our current state and environment to predict the future allows us to increase the odds of desired outcomes, while avoiding or bracing ourselves for future adversity. This ability is directly related to our level of certainty regarding future events – how likely they are, when they will occur, and what they will be like. Uncertainty diminishes how efficiently and effectively we can prepare for the future, and thus contributes to anxiety.
Although this relationship between uncertainty about future negative events and anxiety makes intuitive sense, there has been a disconnect between this conceptualization of anxiety and most neuroimaging investigations of clinical anxiety disorders. The predominant focus of this research has been on heightened emotional reactivity to aversive events; however, the tasks commonly used in this research might not fully engage the psychological processes that are at the heart of anxious pathology – that is, the anticipatory cognitive, affective, and behavioral processes executed to avoid or reduce the impact of a potential threat. These anticipatory processes serve an adaptive function when executed at a level commensurate with the likelihood and severity of threat, but can be maladaptive when conducted excessively2. Comprehensive information about the probability, timing, and nature of a future negative event promotes more efficient allocation of these resources, but such information is rarely available owing to the inherent uncertainty of the future.
Here, we argue that a common feature across anxiety disorders is aberrant and excessive anticipatory responding under conditions of threat uncertainty. This perspective has historical roots in animal research on stress responding and fear learning, as well as previous influential models of anxious pathology. We integrate and expand upon this research in our new Uncertainty and Anticipation Model of Anxiety (UAMA), which emphasizes five processes involved in responding to threat uncertainty that function maladaptively in anxiety. We illustrate neural mechanisms associated with each of these five processes, and review evidence linking anxious pathology to disturbances in a distributed set of brain regions, including the amygdala, bed nucleus of the stria terminalis (BNST), ventromedial prefrontal cortex (vmPFC), orbitofrontal cortex (OFC), anterior mid-cingulate cortex (aMCC), and anterior insula.
Anxiety and uncertainty
What is anxiety?
The word “anxiety” can refer to a range of related phenomena: a class of psychiatric disorders, particular patterns of behavior in animal models, and trait-like negative affect (Box 1). Another perspective on anxiety specifies a future-oriented emotional state experienced by all humans to varying degrees:
It is quite likely that the summed frequency and intensity of the fear responses of any given individual to clear and imminent physical or psychological threat … would lag far behind the summed amount of fear in response to the anticipation of such events and the myriad anxious “What if …” mental representations of possible future events that are common in daily life3.
Box 1. Trait anxiety and relationships between anxiety disorders and depression.
The phrase “trait anxiety” is a bit of a misnomer, as the measure to which it most commonly refers – Spielberger’s State-Trait Anxiety Inventory (STAI)195 – shows an equally close association with anxiety and depression196,197. Trait anxiety may thus be better described as negative affect (also indexed by other commonly used instruments198,199), and probably reflects a general risk factor for emotional disorders. Although other self-report scales can distinguish anxiety disorders from depression200–203, the STAI is used in most studies that investigate anxious characteristics in non-clinical samples. Despite its lack of specificity, the relevance of research using the STAI is underscored by its sensitivity as a marker of risk for anxiety disorders.
The six anxiety disorders and depression have both shared and unique characteristics204,205. Some research has questioned whether generalized anxiety disorder aligns more closely with anxiety disorders or depression206,207. The positioning of obsessive-compulsive disorder and posttraumatic stress disorder within a broad diagnostic class labeled “Anxiety Disorders” has also been challenged208. Alternatives to the DSM-IV classification of anxiety and depression have been proposed209,210, but controversy remains about the optimal nosology.
The striking comorbidity between depression and anxiety disorders, as well as their shared features and genetic basis209, raises the question of how they are similar or different with regard to uncertainty and uncontrollability. Helplessness models of depression11 have emphasized a lack of control over stressful events as a precipitating and maintaining factor in depression. It has also been suggested that uncontrollability is a shared feature of anxiety and depression, and that the two are differentiated by predictions about negative events211: anxiety is accompanied by uncertainty about future negative events, whereas depression is accompanied by the perception that negative events are unavoidable, leading to hopelessness212. Mixed anxiety and depression is characterized by uncertainty about the occurrence of negative events and feelings of helplessness regarding control over those events213.
This quote underscores two critical aspects of anxiety. First, heightened anxiety in anticipation of aversive events might be more important than exaggerated responses to those events for understanding the neurobiological and psychological basis of anxiety disorders. Second, anxiety is related to anticipatory representations of possible (that is, uncertain) future events.
Fear and anxiety can be distinguished according to how much certainty one has regarding the likelihood, timing, or nature of future threat2,4–8. Decades of research in rodent models have provided tremendous insight into hierarchically organized defense systems, the underlying neurobiology, and the circumstances under which different defensive responses are recruited6,9,10. Environmental cues indicating the unambiguous presence of immediate threat give rise to intense “fearful” defensive behaviors (that is, “fight or flight”), whereas more diffuse, distal, or unpredictable threat cues produce “anxious” risk assessment behavior11 that is likely to persist until such uncertainty is resolved. Gray’s influential theory of anxiety6, which was grounded in the specific effects of anxiolytics on anxious but not fearful behavior12, posited a central role for a behavioral inhibition system in responding to uncertainty or conflict by increasing the negative valence of stimuli and promoting avoidance behavior. More recent translational research using fear-potentiated startle in rats and humans has provided persuasive evidence for neuropharmacological and neuroanatomical differences between short-lived, “fearful” responses to discrete threat and sustained, “anxious” responses to unpredictable threats4,13. Motivated by this previous work, we define anxiety here as anticipatory affective, cognitive, and behavioral changes in response to uncertainty about potential future threat.
This view of anxiety is more circumscribed than that reflected in the literature on trait anxiety (Box 1), but is highly relevant to each of the six major anxiety disorders specified in the DSM-IV14 (generalized anxiety disorder (GAD), panic disorder (PD), social anxiety disorder (SAD), posttraumatic stress disorder (PTSD), specific phobias, and obsessive-compulsive disorder (OCD)). Despite controversy about the classification of anxiety pathology (Box 1), the experience of anxiety as defined here is central to the distress of all these disorders. Accordingly, an increased focus on neural and psychological mechanisms associated with maladaptive anticipatory responses under conditions of threat uncertainty is essential if we are to better understand clinical anxiety.
Uncertainty, unpredictability, and uncontrollability
Unpredictability and uncertainty are highly similar and are often used interchangeably, but have slightly different connotations. Unpredictability is often used in a sense that is more quantitative and amenable to experimental manipulation to describe aspects of the environment or features of a particular stimulus, such as its probability of occurring, when or where it may occur, or how intense it will be. A rich body of research in rodent models has shown that organisms consistently prefer predictable shocks and associated contexts15–17, and that predictability ameliorates the negative effects of stress18. Uncertainty is a broader and more diverse construct; in the domain of decision-making (Box 2), for example, uncertainty can be decomposed into distinct levels including sensory uncertainty, state uncertainty, rule uncertainty, and outcome uncertainty19. Uncertainty better captures subjective aspects of one’s internal state, and thus appears more frequently in the literature on human anxiety disorders, whereas unpredictability is used more frequently in laboratory studies with controlled conditions. While we discuss both constructs, our primary focus is on uncertainty, which is inextricably linked to the phenomenological experience of anxiety arising from unpredictable future events.
Box 2. Uncertainty in neuroeconomics and decision-making.
The investigation of neural responses to uncertainty is not confined to research on anxiety. Investigations of uncertainty in behavioral economics and neuroeconomics distinguish between decision-making under conditions of risk (when one faces multiple potential outcomes of known probabilities) and ambiguity (when one faces multiple potential outcomes of unknown probabilities). These studies typically emphasize explicit, cognitive calculations related to different outcomes and their expected utilities, with a heavy emphasis on choices individuals make when faced with different kinds of uncertainty. By contrast, the mechanisms discussed here largely involve responses to uncertainty in the absence of explicit decision-making. Additionally, the neuroeconomics literature includes many studies investigating uncertainty about financial and other rewards, which recruit distinct neural mechanisms from those involved in responses to uncertainty about threat. Our perspective primarily relates to research on the anticipation of threat uncertainty in the absence of decision-making, such as reinforcement learning models of fear conditioning. Others have highlighted the potential of applying neuroeconomics frameworks to the study of anxiety disorders and other psychiatric conditions19,60,214.
Uncertainty makes it difficult to prepare properly for future events: one must strike a balance between preparatory actions that are more efficient (but potentially inadequate) and those that are more effective (but potentially unnecessary). As posited below for UAMA, clinical anxiety disorders are associated with disruptions to a number of processes that bias one toward overly conservative (that is, effective but not efficient) preparatory behavior in the face of unpredictable threat.
Also relevant to uncertainty is uncontrollability (Box 1). According to one definition, uncontrollability is present when the probability or nature of a given event remains unchanged irrespective of any actions an individual may take11,18. Controllability over future events generally implies certainty about their occurrence, whereas the opposite need not be true. Control can also be thought of as “the belief that one has at one’s disposal a response that can influence the aversiveness of an event”20. Thus, increased certainty about future events is an antecedent to control, not necessarily of the occurrence of events, but of adaptive anticipatory responses that can mitigate these events’ negative impact. Uncertainty, on the other hand, precludes one from exercising this form of control, and leads to preparations that are “diffuse, psychologically expensive, and of questionable effectiveness”21.
Responses to uncertainty in anxiety
To understand why uncertainty about future threat is so disruptive in anxiety, we propose five processes involved in maladaptive responses to such conditions: inflated estimates of threat cost and probability, increased threat attention and hypervigilance, deficient safety learning, behavioral and cognitive avoidance, and heightened reactivity to threat uncertainty. Each process can serve an adaptive role in responding to and reducing uncertainty about threat (Box 3). A central tenet of UAMA is that disruptions to the neural circuitry that promote these adaptive responses underlie maladaptive responses to uncertainty in pathological anxiety2. It is unclear whether these neural disruptions cause anxiety disorders. There is much evidence that anxiety disorders are multiply determined and involve genetic and early environmental factors that predispose individuals to pathological anxiety later in life. In addition, practicing anxious thought and behavioral patterns further strengthens associated neural connections. A patient with an anxiety disorder probably builds up neural pathways of anxiety just as a concert pianist strengthens neural pathways of musicianship, through hours of daily practice. Considered in this light, the successful treatment of so many of these patients is a testament to the amazing neuroplasticity of the human brain.
Box 3. Adaptive and maladaptive responses to threat uncertainty.
To illustrate adaptive and maladaptive manifestations of processes highlighted in UAMA, consider the following vignette, in which each of the five UAMA processes has been indicated by number:
Pete, home alone one night, hears rustling in the bushes and loud banging sounds outside his house. Pete immediately feels uncertain regarding whether these noises are benign (curious raccoons) or threatening (burglars). An adaptive response to this uncertainty begins with a rational assessment of the probability of threat (1). This neighborhood has few burglaries, and similar noises have never turned out to be dangerous before. Pete turns down the TV to give more attention to what may be outside, but this heightened vigilance (2) is balanced by attention to cues that indicate safety (3). Because Pete’s security system is silent and the windows and doors are locked, he has reliable signs that nobody has entered his house. Nevertheless, Pete explores the situation to reduce nagging questions (4). Heading downstairs, he sees trash strewn about the garbage cans, and surmises the likely culprit was a raccoon. Despite some unresolved uncertainty, Pete can calm his racing heart (5) and fall asleep knowing that all signs point toward safety.
Next door lives Paul, a chronic worrier diagnosed with GAD, who hears the same noises and experiences similar uncertainty. Instead of objectively weighing the likelihood of alternative outcomes, Paul immediately imagines burglars entering his home (1). Uncontrollable worries and cascading “what if …” thoughts course through his head, and he generates increasingly elaborate scenarios of what evils may befall him. He becomes increasingly attuned to every movement in the branches or creak in the floorboards of his old house (2). Owing to Paul’s exclusive attention toward potential threat, he never notices that his security system is silent (3). Concerned for his safety, Paul locks his bedroom door instead of investigating (4). Having avoided exploring the situation, Paul is left with greater unresolved uncertainty than Pete about the source of the noises. He tries to sleep but his racing heart and sweaty palms keep him from relaxing (5). Not having learned that the situation was safe, Paul will be more likely to assume the worst the next time he hears a noise in the night.
The framework proposed here is not an attempt to reject or replace other models of anxious pathology, but rather incorporates ideas from diverse perspectives and disciplines2,4,5,7,11,15,22–30. In fact, each of the five processes showcased here, their neurobiological correlates, and their relation to anxious pathology have been previously discussed to varying degrees. The UAMA diverges from other models in placing the five processes on equal footing, rather than focusing on a single, primary process. Similarly, a wide network of brain areas are featured rather than singling out one that is especially prominent in anxiety. The primary focus of this review is on research from the past decade that has used increasingly sophisticated imaging methods and experimental paradigms for examining anxiety in the human brain. Nonetheless, the UAMA is heavily informed by decades of research in animal models and humans emphasizing the disruptive and stressful impact of uncontrollable and unpredictable aversive events2,4,15,16,18,31.
It is important to note that there are processes beyond the five proposed here that have received attention in prior work, most notably disrupted fear learning. Fear conditioning has been a cornerstone of translational research that has contributed monumentally to our understanding of fear and anxiety and their neurobiology. Influential learning models propose that environmental or interoceptive cues are more readily associated with threat in anxious individuals22,30,32,33, or that impaired discriminative fear learning results in a state of internal uncertainty even when the environment is objectively predictable, thus resulting in anxiety7,34,35. Aberrant learning is a critical factor in anxious pathology that contributes to or interacts with several UAMA processes.
Ultimately, the theoretical advance of this paper is not in the definition of new processes that are critical for anxious pathology, but rather in the consolidation and integration of multiple perspectives and areas of research typically considered in relative isolation from one another. By focusing this body of work through the common lens of uncertainty, we provide a unifying theme around which an integrated neurobiological and psychological model of anxious pathology can be constructed.
Inflated estimates of threat cost and probability
Adaptive responses to uncertainty about potential future threats rely on accurate estimates of the probability and cost of such events. Highly anxious individuals show neural alterations that contribute to biased assessments of the probability or cost of uncertain negative events, resulting in overly pessimistic expectations. When presented with hypothetical scenarios about negative events, whether common or rare, highly anxious individuals frequently show “judgment bias” – that is, elevated estimates of the cost or probability of such events. Such biases are seen in high trait anxiety36–38 (Box 1) and in individuals with GAD39,40, SAD41,42, and elevated PTSD symptoms43. There is evidence that elevated cost estimates contribute more to anxious pathology than do elevated probability estimates38,41. Combined with the universal tendency to overestimate very small probabilities44, this judgment bias could result in substantial anticipatory distress when anxious individuals face even the slightest possibility of a negative outcome45 (Box 4).
Box 4. Threat assessment: “cold cognition” vs. “subjective feelings”.
There is an important distinction between “cold, cognitive” estimates of probability and cost, and subjective estimates or “feelings” about potential threat45. Anxious individuals predominantly display elevated subjective predictions or feelings about threat. For example, whereas judgment biases in high trait anxiety are observed when subjects report probability estimates using verbal labels (that is,“not at all likely” to “very likely”), the few published studies that did not find such biases asked subjects to report probability estimates using precise numeric anchors38,215. Similarly, individuals with GAD reported higher subjective feelings about the likelihood of negative events than their logical, objective estimates39. These data are corroborated by clinical observations of patients who persistently worry about the potential occurrence of negative outcomes, despite being aware that those outcomes, objectively speaking, are highly unlikely24.
Through the simulation of future events (or “prospection”), humans can generate embodied predictions of events’ emotional impacts before their occurrence154. The “risk-as-feelings” hypothesis45 proposes that anticipatory emotions frequently lead to choices and behaviors that diverge from those considered objectively “optimal” in terms of maximizing benefits and minimizing harm. Predictions stemming from these anticipatory emotions are probably implicitly generated and may not reach conscious awareness, although they can still exert a powerful influence on one’s preparations for the future. The medial OFC and anterior insula are involved in assessing the subjective value of potential events and relaying this information to other regions to influence subsequent choice and action152,153. Disruptions to this circuitry may lead to more vivid or visceral simulations of potential events, and bias anxious individuals’ feelings about threat under conditions of uncertainty.
Judgment biases in anxious individuals suggest abnormalities in the neural circuitry associated with expected value calculation (Figure 1a). Dorsomedial prefrontal regions (including Brodmann areas 8 and 10 and the aMCC) contribute to probability assessment46–48, whereas activity in the orbitofrontal cortex (OFC) reflects the anticipated cost of future events49,50. In addition to showing activation for primary appetitive and aversive reinforcers51, the OFC also represents the integrated value of higher-level, complex outcomes52 or the expected value of future states53. Although it has weak projections to primary motor areas, the OFC influences decision-making processes by relaying information about the expected value of competing alternatives to regions involved in action selection and execution, such as the striatum, lateral PFC, and cingulate cortex54.
Figure 1. Neural regions and circuitry implicated in the UAMA.
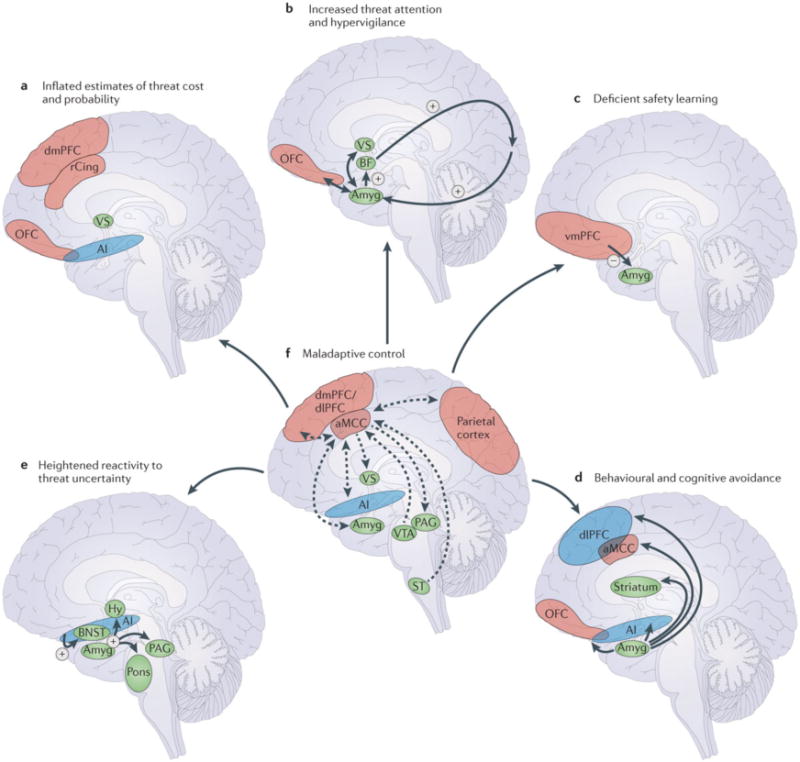
A. Inflated estimates of threat cost and probability reflect disruptions to the dorsomedial prefrontal cortex (dmPFC), rostral cingulate (rCing), orbitofrontal cortex (OFC), ventral striatum (VS), and anterior insula (AI). B. Elevated amygdala (Amyg) activity leads to increased basal forebrain (BF) modulation of visual and other sensory input [Au: could we label the region that the arrow from BF points to ‘sensory cortex’?] and heightened threat attention. Interactions between the amygdala, OFC, and VS further increase threat expectancies and threat attention. C. Deficient safety learning reflects disrupted inhibitory ventromedial PFC (vmPFC)-amygdala circuitry. D. Behavioral and cognitive avoidance reflects interactions between the amygdala and circuitry involved in decision-making and action selection, including the OFC, dorsolateral PFC (dlPFC), striatum, anterior mid-cingulate cortex (aMCC), and anterior insula. E. Hyperactivity of the bed nucleus of the stria terminalis (BNST) and amygdala in response to sustained, unpredictable threat modulate defensive responding as mediated by the hypothalamus (Hy), pons, periaqueductal gray (PAG), and other midbrain/brainstem structures. Anterior insula dysfunction is associated with elevated intolerance of uncertainty and further contributes to BNST and amygdala hyperactivity. F. Dysfunction of the aMCC, or disrupted structural connectivity between the aMCC and interconnected regions, prevents individuals from identifying and executing adaptive responses to uncertainty and contributes to the disruptions highlighted in A–E. Lateral cortical regions are shown in blue, medial cortical regions in green, and subcortical regions in orange. The functional pathways in A–E are indicated with red arrows (excitatory) and blue arrows (inhibitory). The known structural connections in F are indicated with purple arrows (directionality indicated by arrowheads). ST=spinothalamic tract; VTA=ventral tegmental area.
The calculation of expected value is a dynamic process, and heightened threat expectancies in anxious individuals could also reflect disruptions to reinforcement learning processes that are used to update threat expectancies. Prediction error signals generated by midbrain dopaminergic neurons55 reflect a mismatch between predicted and actual outcomes, and result in increasingly accurate future predictions for both rewarding and aversive stimuli56. Reinforcement learning models have been applied to functional magnetic resonance imaging (fMRI) studies of fear conditioning, revealing activity consistent with aversive prediction errors in the ventral striatum, anterior insula, and rostral cingulate cortex57–59. Disrupted aversive prediction error signaling in anxiety disorders results in a failure to appropriately adjust expectancies when predicted negative events do not occur28,60.
Increased threat attention and hypervigilance
Anxiety also involves alterations to attentional processes that facilitate threat detection61, which result in heightened perceptions of harm: “The range of stimuli that can evoke anxiety in generalized anxiety disorder may increase until almost any stimulus is perceived as a danger”62. This tendency to view ambiguous stimuli as threatening, called “interpretation bias,” has been observed when patients with GAD are presented with ambiguously described scenarios or spoken words with multiple meanings40,63,64. Disorder-specific interpretation biases have been reported for ambiguous social scenarios and facial expressions in SAD65,66; ambiguous interoceptive cues in PD67; and sentence stems that could be completed to form combat-related words in veterans with PTSD68. Given these interpretation biases, along with increased attention for objectively threatening stimuli (“attentional bias”)61, elevated estimates of threat under conditions of uncertainty might reflect adaptive anticipatory responses to a world that appears more dangerous to anxious individuals.
Biased threat attention and observations of hypervigilance across anxiety disorders implicate amygdala hyperactivity2 (Figure 1B). Heightened resting metabolic activity and blood flow in the amygdala have been observed in participants with PD69, PTSD70,71 (but see72,73), and SAD74. In non-human primates, resting amygdala metabolism is correlated with a combined behavioral and hormonal assay of anxious temperament75,76. In addition to these alterations at rest, a meta-analysis of functional imaging studies in PD, PTSD, and SAD showed elevated task-induced amygdala activity across diagnoses and paradigms77. In GAD, studies of emotional anticipation78 and implicit emotion regulation79 reported amygdala hyperactivity across experimental conditions, suggesting indiscriminately elevated amygdala activation. Of particular relevance for our emphasis on uncertainty and anticipation, heightened amygdala activity has been reported in socially anxious individuals about to deliver a public speech80 and in clinically anxious children anticipating unknown peer feedback81.
Heightened amygdala activity in anxiety has implications for distinct aspects of fear learning mediated by different amygdala subregions. Influential fear conditioning perspectives22,23,33 emphasize exaggerated associative learning for environmental cues and aversive outcomes35, a process that critically involves the basolateral amygdala (BLA)82. The central nucleus of the amygdala (CeA) has a complementary role in attentional gating that moderates such learning83,84. According to the Pearce-Hall learning model85, environmental cues that have previously been paired with surprising (that is, unpredicted) outcomes demand greater attentional resources, increasing the likelihood for new associations to be formed with these cues (which are thus said to have high associability). In rodents, activity in the CeA reflects a cue’s associability83,84. A study of reversal learning in humans similarly found greater amygdala responses to cues that had been paired with surprising outcomes on recent trials59. The CeA projects heavily to cholinergic basal forebrain structures, which can selectively modulate sensory processing and therefore enhance learning after surprising events through their ascending cholinergic projections to cortical regions86.
In highly anxious individuals, tonic and indiscriminate activation of the amygdala2,69–71,74,75,77–81 results in decreased sensitivity to the associability of cues, inefficient deployment of attentional resources toward the most relevant features of the environment, and impaired learning of stimulus-outcome associations. As a result, the anxious individual is biased to interpret conditions of uncertainty as threatening; moreover, impaired discriminative learning can lead to an internal state of uncertainty about threat despite objectively predictable conditions34. The amygdala has rich, bidirectional connections with the ventral striatum and OFC87,88, which assign subjective value to potential future events. Together, these regions form a network in which increased attention to threat as facilitated by the amygdala is likely to affect the value assigned to future events, and differences in valuation as facilitated by the striatum and OFC are likely to influence attentional deployment. While the amygdala is highlighted in nearly all neurobiological accounts of anxious pathology, emphasis is often placed on its role in the expression of fear2,22. It might be more useful to consider increased amygdala activity as reflecting increased vigilance29 under conditions of uncertainty.
Deficient safety learning
Environmental safety signals are reliable indicators that threat will not occur, and thus relieve individuals from a state of anticipatory anxiety18,27. Under conditions of uncertainty, weak or non-existent contingencies between cues and negative outcomes make it difficult to identify safety signals, particularly for highly anxious individuals89 whose biased attention toward threat impedes fine-grained discriminative analysis of environmental cues. Heightened reactivity to objectively safe conditions has been observed across anxiety disorders using discriminative fear conditioning paradigms35. In addition to contingent presentations of a conditioned stimulus (CS+) and unconditioned stimulus (US), these paradigms include another cue (CS−) that is presented in the absence of the US and therefore associated with safety. Failure to show discriminative physiological responses to a CS+ and CS−, reflecting increased fearful responding to the CS−, has been reported in PD90,91, PTSD92,93, and mixed childhood anxiety disorders94. The application of conditional discrimination tasks95 in anxiety disorders may clarify whether these results reflect impaired learning about safety or a failure to inhibit fearful responding following successful safety learning.
Investigations in rodents and humans have identified a ventral PFC–amygdala circuit involved in learning about and responding to safety in potentially threatening contexts (Figure 1C). In rats, electrical stimulation of the infralimbic cortex reduces the expression of amygdala-mediated conditioned fear responses96, and inactivation of this region impairs the acquisition and recall of fear extinction97. Neuroimaging studies in humans have revealed a comparable role for the vmPFC in responding to cues that predict safety58,98–100. In clinical anxiety disorders, altered function and connectivity of the vmPFC and amygdala have been linked to deficient fear extinction, one of the dominant models of PTSD101. Impaired recall of extinction in PTSD was associated with decreased vmPFC activation102, which has also been reported in patients with PTSD exposed to traumatic or aversive stimuli77,103,104. Relative to controls, individuals with GAD showed less discriminant vmPFC activity for cues visually similar to a reinforced CS+, reflecting generalization of learned fear to safe cues105. Indiscriminately elevated amygdala activation during the anticipation of neutral and aversive pictures in GAD78 further suggests a failure of patients to down-regulate amygdala activity in response to safe neutral cues. Notably, patients with heightened anticipatory responses in the pregenual ACC (just superior to the vmPFC) showed the largest decrease in symptoms following venlafaxine treatment78, consistent with other studies of pre-treatment predictors of treatment response in anxiety106 and depression107,108. Thus, some preservation of regulatory function in the ACC/vmPFC has prognostic benefits in anxiety and mood disorders. Complementing this fMRI research, diffusion tensor imaging (DTI) has revealed microstructural alterations to the uncinate fasciculus in individuals with GAD109, SAD110, and elevated trait anxiety111.
Additional findings and anatomical considerations challenge a simple model in which the vmPFC inhibits the amygdala and reduces stress-related responses112. In Vietnam veterans, vmPFC lesions were found to protect against PTSD113. Several studies have reported increased activation of the vmPFC and pregenual ACC in PTSD114,115. In addition, lesions to the macaque OFC (extending laterally from the vmPFC) can reduce anxious behavior, perhaps by altering BNST activity116. Future research is needed to clarify the role of the vmPFC in anxiety, and to investigate whether alterations to specific sectors of the vmPFC or their connectivity with the amygdala explain these disparate findings112.
Behavioral and cognitive avoidance
Avoidant behavior and thoughts, including worry, prevent anxious individuals from being exposed to evidence that might contradict negative predictions about the future25,26,117. According to classic two-process theory23,30, exaggerated fear conditioning to environmental threat cues leads to operant learning of avoidance behavior to reduce fear. Whereas these processes are assumed to operate implicitly in animal models, the extension of this thinking to human anxiety disorders suggests that avoidance may further heighten threat expectancies under conditions of uncertainty. Because events that are avoided or worried about typically fail to occur, behavioral and cognitive avoidance tendencies are negatively reinforced and anxious individuals develop false beliefs that they prevented these negative outcomes39.
According to tenets of emotional processing theory and exposure therapy26, effective psychological interventions for fear and anxiety disorders require activation of an individual’s “fear structure,” which opens the door for new information about safety to compete with existing beliefs or memories of fear. In this way, exposure-based therapy is functionally and neurally similar to laboratory extinction training, and directly targets avoidant behavior. SAD, PTSD, and OCD are marked by behavioral avoidance of situations associated with potential threat or harm. Patients with PD develop beliefs that they can engage in safety-seeking thoughts or actions that prevent panic attacks, while in actuality such activities protect the CS+ from being extinguished30,118, consistent with animal models of avoidance learning119. By engaging in worry, individuals with GAD and other anxiety disorders avoid intense negative emotions about potential feared outcomes, but also miss out on the opportunity to correct inaccurate beliefs about the likelihood and consequences of such events. Pushing patients to overcome their avoidant tendencies – whether that entails challenging thoughts about threat in cognitive behavioral therapy24 (CBT) or exposure to feared scenarios in exposure therapy26 – is a crucial first step in reducing elevated expectancies of threat in the face of uncertainty.
Active avoidance learning paradigms in animal models have demonstrated the importance of circuitry involving the striatum and basal amygdala in acquiring learned avoidance behavior120–122, and have shown that inhibition of the CeA by infralimbic cortex is required to inhibit freezing responses to a CS+ and allow adaptive avoidance of the US123. Initial human imaging studies indicate a key role in active avoidance for the amygdala and interconnected regions involved in decision-making and subsequent action, including the OFC and lateral PFC, ventral and dorsal striatum, and aMCC124–126 (Figure 1D). Additionally, heightened expectancies about the emotional impact of potential feared outcomes resulting from anterior insula dysfunction lead to avoidance of situations involving threat uncertainty28,125. Successful treatment of avoidance behavior in spider phobics led to reductions in activity in the anterior insula and aMCC127,128 and to increased dorsolateral PFC activity128. As research on active avoidance in anxiety disorders evolves, the simultaneous investigation of deficient fear extinction will be highly informative for understanding interactions between avoidance and impaired safety learning.
Heightened reactivity to threat uncertainty
Because anticipating the future almost always involves some uncertainty, neural processes that influence reactivity and attitudes toward uncertainty are crucial for determining adaptive responses to this state. Across species, physiological responding to threat is enhanced when there is uncertainty about its nature, probability, or timing15,16,129–134. Humans show larger startle responses for cues that can precede either low or high intensity shocks than for cues that always precede high intensity shocks129, for cues preceding shock on 20% or 60% of trials than for cues that predict shock with 100% certainty130, and under conditions of temporal unpredictability131. Furthermore, aversive events that are not fully predictable have a greater negative impact on mood, state anxiety, and physiological indices of reactivity than those that are fully predictable132–134. Exposure to unpredictably timed, neutral tones also elicits more amygdala activity and anxious behavior in both mice and humans than predictably timed tones135, underscoring the notion that uncertainty itself – without aversive outcomes – can increase anxiety.
Relative to healthy controls, individuals with PD90 and PTSD92 showed elevated startle responses during a temporally unpredictable interstimulus interval (ISI), but not for a predictable threat condition. Distinct extended amygdala regions mediate behavioral, autonomic, and endocrine responses to predictable and unpredictable threat through descending projections to hypothalamic, midbrain, and brainstem regions6,10,13. Whereas the medial CeA coordinates rapid, phasic fear responses to stressors that are imminent and relatively certain, the BNST is activated by conditions of sustained, unpredictable threat4,13. This functional dissociation is mirrored by differential responses to benzodiazepines, which reduce behavioral expressions of fear for sustained but not phasic threat in rodents4, due at least in part to decreased BNST activity136. In humans, benzodiazepines also reduced fear-potentiated startle to unpredictable137 but not predictable threats138. Spider phobics showed greater BNST activity than controls during temporally unpredictable anticipation of spider pictures139. BNST activation has also been reported in healthy populations during sustained, temporally unpredictable threat140–142, with particularly elevated activity in individuals with high trait anxiety143.
Individual differences in reactivity to threat uncertainty are also reflected in subjective reports. Intolerance of uncertainty (IU) is defined as the inability to accept the possibility that a negative event may occur in the future, irrespective of the probability of its occurrence144. For individuals high in self-reported IU, uncertainty results in depleted attentional resources and disruptions to cognitive, behavioral, and emotional functioning144. IU scores are elevated in GAD144,145, SAD146, OCD146, and depression146.
Many imaging studies have shown the importance of the anterior insula (Figure 1E) in responding to uncertainty48,133,147,148. For example, anterior insula activity tracked levels of risk and risk prediction errors during decision-making tasks48 and was associated with less risky decisions under uncertain conditions147. Patients with anterior insula lesions were insensitive to the favorability of betting odds149, suggesting that this region biases decision-making by signaling the consequences of unfavorable bets. Increased anterior insula activation was seen during the anticipation of negative events in the absence of decision-making133,142,150, with some studies reporting particularly heightened anticipatory insula responses under conditions of threat uncertainty141,151. Integrating data on anticipation and uncertainty with this region’s established role in interoception and subjective emotional awareness152,153 (Box 5), we posit that the anterior insula generates anticipatory emotional responses for hypothetical future events154 that answer the question “How is it going to feel?”. This process contributes to subjective predictions about the probability and cost of future threat45 (Box 4). This role becomes particularly important when future events are less predictable, as anticipated feeling states contribute to adaptive decision-making and preparatory cognitive or behavioral actions under such conditions148.
Box 5. The anterior insula and subjective emotional awareness.
The insula is a band of cortex, tucked within the folds of the Sylvian fissure, that stretches from prefrontal to posterior parietotemporal regions of the brain (see the figure, part a). Its anatomical position allows extensive connections with cortical and subcortical regions, including the lateral PFC and OFC, vmPFC, cingulate, amygdala, BNST, and ventral striatum177,178 (Figure 1A, D–F). Superimposed on the insula is a posterior-anterior functional gradient, in which increasingly rich and complex representations of one’s bodily state arise153. The posterior insula is primary somatosensory cortex that receives interoceptive and exteroceptive information regarding pain, temperature, touch, itch, taste, and visceral changes153. As this basic sensory information is transmitted to middle and anterior regions of the insula, it is integrated with homeostatic, motivational, emotional, and cognitive information from an array of cortical and subcortical regions. At the top of this ascending hierarchy, the anterior insula is involved in the perception of subjective interoceptive states, and might be involved more broadly in supporting subjective emotional awareness or a “global feeling state” across time153. Hyperactivation of the anterior and mid-insula is one of the most common neuroimaging findings across different anxiety disorders and during fear conditioning77 (see the figure, part b: hyperactivation is depicted in red (with anterior and mid-insula circled), and hypoactivation in blue). NTS=nucleus tractus solitarius. Part b is adapted, with permission, from77.
Elevated activity and altered connectivity of the anterior insula help account for the negative emotional states associated with uncertainty in highly anxious individuals, as well as heightened subjective estimates or feelings about potential future threat. This region showed hyperactivity in anticipation of negative pictures in individuals with PTSD155, GAD and SAD156, and high trait anxiety157. Spider phobics showed elevated anterior insula activity while anticipating spider pictures that appeared in a temporally unpredictable manner139. In addition, elevated IU was associated with increased anterior and mid-insula responses to affectively ambiguous faces158.
In summary, exaggerated physiological and subjective emotional responses to uncertainty in anxiety are proposed to reflect alterations to the BNST and anterior insula. Anterior insula dysfunction leads to negatively biased predictions about the emotional consequences of uncertain future events and a failure to learn from errors in these predictions28, resulting in a dissociation between heightened subjective feelings of threat and objectively accurate “cognitive” threat calculations39 (Box 4). These biased threat expectancies contribute to persistently elevated BNST activity under conditions of uncertainty, resulting in behavioral and physiological manifestations of anxiety. The resulting negative anticipatory emotions make uncertainty particularly “intolerable” for anxious individuals144.
Translating uncertainty into action
Unlike conditions of relative certainty, in which automatic or habitual processes allow navigation of the environment and goal attainment, uncertainty introduces potential conflict between competing options or motivating factors. Gray proposed that the septo-hippocampal system responds to such conflict by increasing vigilance and inhibiting motor function to allow for risk assessment, which in turn results in behavioral avoidance6. Another candidate region for mitigating the conflict introduced by uncertainty is the aMCC. The recently proposed “adaptive control hypothesis”126 posits that the aMCC integrates motivational, affective, and interoceptive information to provide an instructive signal that influences subsequent action under conditions of uncertainty. The aMCC is anatomically well-positioned to serve such a role, with widespread efferent and afferent connections to the regions featured for the five UAMA processes (Figure 1F).
Supporting this central role for the aMCC in responding to uncertainty are reciprocal connections with the anterior insula159,160 that allow information regarding interoceptive and subjective emotional states to be re-represented in the aMCC153. Projections from the spinothalamic column, basal nucleus of the amygdala, and midbrain dopaminergic regions provide the aMCC with information about pain and other negative reinforcers126. Through its projections to motor centers, the amygdala, and midbrain nuclei including the periaqueductal gray, the aMCC modulates autonomic activity161,162 and directs appropriate defensive responses163. Afferent projections from multiple medial and lateral prefrontal regions converge on the aMCC, which could act as a relay between those regions and the amygdala164. Finally, projections to dorsolateral PFC and parietal regions facilitate response selection or signal the need for increased attentional resources126. Taken together, disturbed function of the aMCC or its connections would have deleterious consequences for optimal responding in situations involving uncertainty: exaggerated autonomic responses and behavioral reactivity, compromised associative learning about fear and safety, heightened avoidance, altered allocation of attentional resources, and hypervigilance.
There is extensive evidence that the structure, function, and connectivity of the aMCC are altered in clinical anxiety. Individuals with PTSD showed reduced aMCC volume165 as well as increased aMCC activity to an extinguished CS+102, to a context in which electric shocks had previously been administered166, and during cognitive interference tasks167. Elevated baseline aMCC metabolism in veterans with PTSD and their monozygotic twins73 suggests that aMCC hyperactivity represents a genetically influenced risk factor for developing PTSD. Individuals with SAD showed reductions in functional connectivity between the aMCC and anterior insula while viewing fearful faces168, and specific phobics exposed to conditions of sustained, temporally unpredictable threat showed aMCC hyperactivity139. Structural imaging showed reductions in aMCC volume in PD169, and two cases of aMCC surgical resection were associated with subsequent panic symptoms170. Cingulotomies (targeting the aMCC) resulted in significant symptom reduction in patients with OCD171, and the aMCC showed the most consistent reductions in gray matter volume in a meta-analysis of structural MRI studies of OCD172. Trait anxiety has been associated with abnormal functional coupling of the aMCC and amygdala163,173. Finally, anxious adolescents with elevated IU scores had increased aMCC activation during decision-making under conditions of uncertainty174.
Despite extensive evidence for aMCC abnormalities in clinical anxiety, additional research is needed to test the hypothesis that maladaptive behavioral, cognitive, or emotional control is directly linked to aMCC dysfunction. Investigation of functional activation, functional connectivity, and structural connectivity in the same subjects will help to clarify the precise role of the aMCC and its many connections in maladaptive anticipatory responses to threat uncertainty in anxiety.
Uncertainty and Anticipation Model of Anxiety (UAMA)
The evidence reviewed here provides strong support for the central and disruptive role of uncertainty about potential threat in subclinical and clinical anxiety. An interconnected set of neurobiological and psychological processes are involved in adaptive anticipatory responding under conditions of uncertainty, and deficits in one or more of these processes underlie maladaptive responses to future uncertainty in anxious individuals.
As depicted in Figure 2, at the core of UAMA are elevated expectancies of threat under conditions of uncertainty, which can take the form of either disrupted “cognitive” estimates of probability and cost, or heightened subjective feelings about negative future events. These elevated threat expectancies reflect alterations to the ventral striatum and OFC, which are involved in expected value calculations and reinforcement learning. Heightened subjective feelings about threat under conditions of uncertainty suggest dysfunction of the anterior insula and vmPFC. Amygdala hyperactivity results in increased vigilance, biased attention toward threat, and deficient associative learning, all of which contribute to heightened threat expectancies. These biased expectancies result in a feedback loop in which anxious individuals are increasingly vigilant and ever more attentive toward perceived threat. Also contributing to elevated threat expectancies are impaired safety learning and an inability to inhibit fearful responding under conditions of safety, the result of deficient inhibitory vmPFC-amygdala communication.
Figure 2. Altered anticipatory processes in response to threat uncertainty in anxiety.
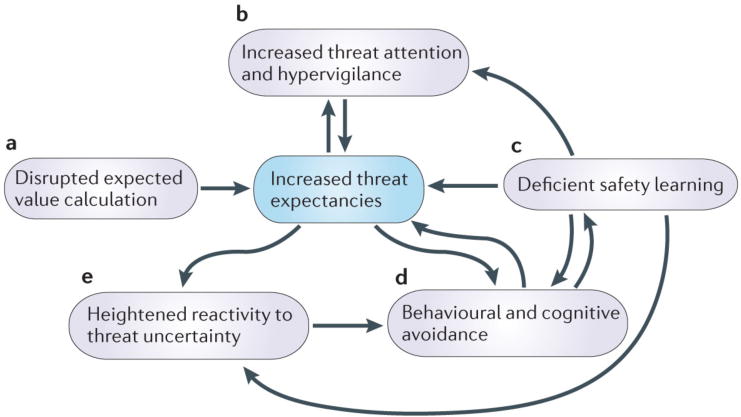
Dynamic interactions among five key psychological processes (in purple) allow for anticipatory responses to uncertainty about future threat. The UAMA posits that alterations to these processes and associated core brain circuitry (see Figure 1) are responsible for maladaptive cognitive, behavioral, and affective responses to uncertainty in highly anxious individuals. At the core of UAMA are heightened expectancies about the probability and cost of future threat (in blue). These elevated expectancies are the result of alterations in the calculation of expected value and aversive prediction error signaling (A), increased threat attention and hypervigilance (B), and deficient safety learning or an inability to inhibit anxious responding in the presence of safety (C). These heightened expectancies and an inability to identify safety in situations of uncertainty contribute to elevated cognitive and behavioral avoidance (D), which leads to further difficulties in identifying safety and reducing threat expectancies. Heightened threat expectancies and an inability to identify safety signals contribute to exaggerated physiological and behavioral reactivity under conditions of uncertainty (E), and this heightened reactivity to uncertainty leads to further avoidance of such conditions.
Elevated threat expectancies naturally lead to avoidance of situations involving uncertainty about threat. By avoiding situations in which negative outcomes are expected, however, the anxious individual cannot accumulate disconfirmatory evidence or learn about safety cues, and therefore consolidates biased expectancies. Greater threat expectancies exacerbate BNST-dependent physiological and behavioral reactivity under conditions of uncertainty, whereas anterior insula dysfunction contributes to heightened anticipatory emotional responses and subjective feelings of negative events’ probability and cost. Finally, looming over all of these disrupted processes outlined in UAMA are abnormal function and connectivity of the aMCC (Figure 1F), which prevents the anxious individual from identifying and executing adaptive anticipatory responses in the face of uncertainty.
Directions for future research
The UAMA is based primarily on three lines of evidence: neural responses to uncertainty in healthy individuals; behavioral, self-reported, or peripheral physiological responses to uncertainty in anxiety disorders; and neurobiological disruptions not directly related to uncertainty in anxiety disorders. There is little data on the convergence of these three areas. Functional imaging research in anxiety has largely assessed neural responses to symptom provocation stimuli or negative emotional stimuli, which we argue fail to engage those processes most central to clinical anxiety. Anxiety is a future-oriented emotion, and anticipating or “pre-viewing” the future induces anxiety largely because the future is intrinsically uncertain. Studies in healthy individuals have used paradigms that elicit anticipatory anxiety through exposure to sustained, unpredictable threat100,140–143. These paradigms engage brain regions implicated in pathological responses to uncertainty, including the amygdala, anterior insula, BNST, rostral cingulate, and vmPFC. We propose that these and other paradigms be extended to specifically target hypothesized disruptions to the five processes highlighted above, as framed in several questions below.
Does heightened anxiety in response to sustained, unpredictable threat reflect abnormally elevated BNST activation4? A combination of high-resolution imaging, differential temporal response profiles141,142, probabilistic fiber tracking techniques175, and pharmacological fMRI would allow for improved localization of extended amygdala subdivisions.
Are biased threat expectancies in anxiety directly related to increased amygdala activity and resulting hypervigilance? For a paradigm with different cue/outcome contingencies, anxious individuals would be expected to show biased threat expectancies in proportion to elevated amygdala responses for unpredictable contexts135. Functional connectivity analyses could be used to address whether elevated amygdala responses under conditions of uncertainty are related to deficient vmPFC inhibition, altered communication with the aMCC163 or both.
Do anxious individuals show deficits in reinforcement learning? If so, are such deficits specific to aversive outcomes? Disrupted negative prediction error signaling (i.e., the non-occurrence of expected aversive events) would result in a prolonged state of uncertainty despite the absence of predicted aversive events. Reversal learning paradigms could identify abnormalities in brain regions involved in aversive prediction error signaling (ventral striatum, anterior insula, and rostral cingulate)57–59.
Is there evidence in anxiety for deficits in “somatic” aversive prediction error signaling28? This could help explain heightened anticipatory “feelings” about threat likelihood, despite accurate “cognitive” probabilistic estimates (Box 4). Does such dysfunction result from inaccurate interoceptive feedback to the insula regarding errors in predicting somatic states, or from a failure to update predictions based on accurate interoceptive feedback?
Does heightened responding to objectively safe cues reflect impaired safety learning, or deficits in fear inhibition? Conditional discrimination tasks95 and functional imaging could differentiate between these possibilities. Modified safety learning or fear extinction paradigms that provide the option to avoid the CS117 could be used to investigate relationships among avoidance, safety learning, and fear extinction.
Speaking more generally, causality needs to be assessed using longitudinal designs in high-risk populations. Do elevated threat expectancies, deficient identification of safety, and heightened responses to uncertainty increase one’s risk of developing an anxiety disorder? Or are these disruptions consequences of living with anxiety? Is successful treatment associated with normalization of behavioral and neural responses to threat uncertainty?
An assessment of which UAMA processes are intact vs. disrupted could provide insight into the nosology of affective pathology and advance biologically informed, individualized diagnosis and treatment176. Adaptive responses to uncertainty require flexible coordination among these different processes, and alterations to any region would have consequences for functions of additional regions, particularly given the heavy reciprocal structural connections and functional co-activation of many of the regions described here4,87,88,126,177,178. Assessment of the functional and structural integrity of these networks is likely to provide a more informative picture of anxious pathology than the measurement of any one region in isolation179–181.
Implications for treatment
The UAMA supports two avenues for treatment. First, to the extent that deleterious consequences of uncertainty result from elevated threat expectancies, patients might benefit from interventions that emphasize accurate prediction of future events and learning from inaccurate predictions. Bias modification182 could be used to target elevated threat estimates. Individuals deficient in identifying safety signals might benefit from therapy emphasizing attention to contexts, cues, and coping strategies to reduce threat uncertainty27. For individuals with objectively accurate predictions but subjectively exaggerated threat expectancies39,45, the therapist can highlight this inconsistency and bring subjective feelings about threat in line with objectively accurate predictions. Pharmacological agents designed to enhance neuroplasticity and emotional learning processes could further promote the efficacy of these therapies. For example, D-cycloserine (DCS) has been used to enhance the effects of exposure therapy in specific phobia183,184, SAD185, OCD186, and PTSD187, behavioral therapy in OCD188, CBT in PD189, and attentional bias modification in highly trait-anxious individuals190.
Second, treatment efforts must also encourage individuals to become more tolerant of uncertainty27. Real-time fMRI, which allows participants to monitor and alter activity in specific brain regions during fMRI scanning191, could help anxious individuals learn to down-regulate anterior insula activity in response to uncertainty, and thereby reduce negative anticipatory emotions192. Similarly, modulation of aMCC activity could encourage adaptive control over cognitive, affective, and behavioral responses to uncertainty.
A simpler strategy involves encouraging patients to spend less time worrying about what might come, and instead to focus on life in the present193. Complete absorption in the present moment obviates anxiety about the future. One path toward the reduction of anxiety might involve transitioning “from inaccurate expectations to more accurate expectations to no expectations at all”193. The incorporation of mindfulness traditions into CBT – namely, emphasizing awareness of moment-to-moment internal and external events, and non-judgmental acceptance (rather than avoidance) of negative emotional states – allow one to tolerate unavoidable uncertainties194, and help those suffering from anxiety to understand that uncertainty about the future need not rule their lives.
Acknowledgments
The authors wish to thank Lyn Abramson, Richie Davidson, Ned Kalin, John Curtin, and members of the Curtin lab for feedback on previous versions of this manuscript. This work was supported by the National Science Foundation (Graduate Research Fellowship to DWG) and the National Institute of Mental Health (R01-MH74847, K02-MH082130 to JBN).
Glossary Terms
- fear-potentiated startle
The enhanced response to a startling stimulus observed under negative arousing states such as fear or anxiety
- anxiety
The suite of anticipatory affective, cognitive, and behavioral changes in response to uncertainty about potential future threat
- orbitofrontal cortex (OFC)
Medial and lateral aspects of the orbital surface of the prefrontal cortex, including Brodmann areas 11, 13, 14, and ventral portions of 10 and 47/12
- prediction error
The difference between predicted and actual outcomes, which results in a neural signal that leads to increasingly accurate future predictions
- fear conditioning
Process by which a neutral conditioned stimulus (CS+) becomes associated with an aversive, unconditioned stimulus (US) through repeated contingent presentations of the CS+ and US, resulting in fear expression following presentation of CS+ alone
- rostral cingulate cortex
Encompasses ACC and aMCC, including Brodmann areas 24, 25, 32, and 33
- hypervigilance
State of increased attention to perceived threat in one’s environment
- associability
The propensity of a stimulus to form associations with other stimuli in the environment; associability increases following surprising or unpredicted outcomes
- conditional discrimination task
Variant of fear conditioning paradigms that allows for the independent investigation of safety learning and the inhibition of fear responses in the presence of learned safe cues
- fear extinction
An active learning process in which a CS+ is repeatedly presented in the absence of a contingent US, leading to a new association between the CS+ and safety that competes with the original association between the CS+ and US
- ventromedial prefrontal cortex (vmPFC)
Encompasses medial OFC, posterior frontopolar cortex, subgenual ACC, and inferior pregenual ACC, including Brodmann areas 11, 14, 25, and portions of 10, 24, and 32
- diffusion tensor imaging (DTI)
MRI technique that assays the diffusion properties of water molecules, allowing for inferences regarding microstructural properties of white matter
- uncinate fasciculus
The primary white matter tract connecting ventral portions of the PFC and ACC with medial temporal lobe structures including the amygdala
- exposure therapy
Therapeutic technique in which individuals are presented with feared objects, situations, or memories in a safe setting, thus allowing for the reduction of fearful associations
- cognitive behavioral therapy
Diverse collection of therapies that emphasize the correction or restructuring of maladaptive behaviors and inaccurate beliefs
- benzodiazepines
A widely used class of GABA agonists for the treatment of anxiety disorders
- interoception
The perception of sensory events occurring within one’s body
- D-cycloserine (DCS)
Partial agonist of the NMDA glutamate receptor that has been shown to enhance learning
Contributor Information
Dan W. Grupe, Email: grupe@wisc.edu.
Jack B. Nitschke, Email: jnitschke@wisc.edu.
References
- 1.Gilbert DT. Stumbling on Happiness. Random House; New York: 2006. [Google Scholar]
- 2.Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychol Rev. 1998;105:325–350. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- 3.Borkovec TD. The role of cognitive and somatic cues in anxiety and anxiety disorders: Worry and relaxation-induced anxiety. Anxiety and the anxiety disorders. 1985:463–478. [Google Scholar]
- 4.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barlow DH. Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. Am Psychol. 2000;55:1247–1263. doi: 10.1037//0003-066x.55.11.1247. [DOI] [PubMed] [Google Scholar]
- 6.Gray JA, McNaughton N. The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. Oxford University Press; Oxford: 2000. [Google Scholar]
- 7.Grillon C. Startle reactivity and anxiety disorders: Aversive conditioning, context, and neurobiology. Biol Psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- 8.Lissek S, Pine DS, Grillon C. The strong situation: A potential impediment to studying the psychobiology and pharmacology of anxiety disorders. Biol Psychol. 2006;72:265–270. doi: 10.1016/j.biopsycho.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Blanchard DC, Blanchard RJ. Ethoexperimental approaches to the biology of emotion. Annu Rev Psychol. 1988;39:43–68. doi: 10.1146/annurev.ps.39.020188.000355. [DOI] [PubMed] [Google Scholar]
- 10.Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychon Bull Rev. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- 11.Seligman MEP. Helplessness: On depression, development, and death. W.H. Freeman and Company; San Francisco: 1975. [Google Scholar]
- 12.Graeff FG. Neuroanatomy and neurotransmitter regulation of defensive behaviors and related emotions in mammals. Braz J Med Biol Res. 1994;27:811–829. [PubMed] [Google Scholar]
- 13.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 14.Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV-TR) American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 15.Mineka S, Kihlstrom JF. Unpredictable and uncontrollable events: A new perspective on experimental neurosis. J Abnorm Psychol. 1978;87:256–271. doi: 10.1037//0021-843x.87.2.256. [DOI] [PubMed] [Google Scholar]
- 16.Mineka S, Hendersen RW. Controllability and predictability in acquired motivation. Annu Rev Psychol. 1985;36:495–529. doi: 10.1146/annurev.ps.36.020185.002431. [DOI] [PubMed] [Google Scholar]
- 17.Fanselow MS. Signaled shock-free periods and preference for signaled shock. J Exp Psychol Anim Behav Process. 1980;6:65–80. [Google Scholar]
- 18.Seligman MEP, Maier SF, Solomon RL. Unpredictable and uncontrollable aversive events. Aversive Conditioning and Learning. 1971:347–400. [Google Scholar]
- 19.Bach DR, Dolan RJ. Knowing how much you don’t know: A neural organization of uncertainty estimates. Nat Rev Neurosci. 2012;13:572–586. doi: 10.1038/nrn3289. [DOI] [PubMed] [Google Scholar]
- 20.Thompson SC. Will it hurt less if I can control it? A complex answer to a simple question. Psychol Bull. 1981;90:89–101. [PubMed] [Google Scholar]
- 21.Lazarus RS, Averill JR. Emotion and cognition: With special reference in anxiety. Stress and anxiety. 1972:121–128. [Google Scholar]
- 22.LeDoux JE. The emotional brain. Simon and Schuster; New York: 1996. [Google Scholar]
- 23.Mowrer OH, Lamoreaux RR. Fear as an intervening variable in avoidance conditioning. Journal of Comparative Psychology. 1946;39:29–50. doi: 10.1037/h0060150. [DOI] [PubMed] [Google Scholar]
- 24.Beck AT. Cognitive therapy and the emotional disorders. International Universities Press; Oxford: 1976. [Google Scholar]
- 25.Borkovec TD, Alcaine OM, Behar E. Avoidance theory of worry and generalized anxiety disorder. Generalized anxiety disorder: Advances in research and practice. 2004:77–108. [Google Scholar]
- 26.Foa EB, Kozak MJ. Emotional processing of fear: Exposure to corrective information. Psychol Bull. 1986;99:20–35. [PubMed] [Google Scholar]
- 27.Lohr JM, Olatunji BO, Sawchuk CN. A functional analysis of danger and safety signals in anxiety disorders. Clin Psychol Rev. 2007;27:114–126. doi: 10.1016/j.cpr.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 29.Whalen PJ. Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Curr Dir Psychol Sci. 1998;7:177–188. [Google Scholar]
- 30.Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychol Rev. 2001;108:4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- 31.Foa EB, Zinbarg R, Rothbaum BO. Uncontrollability and unpredictability in post-traumatic stress disorder: An animal model. Psychol Bull. 1992;112:218–238. doi: 10.1037/0033-2909.112.2.218. [DOI] [PubMed] [Google Scholar]
- 32.Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders: It’s not what you thought it was. Am Psychol. 2006;61:10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- 33.Öhman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychol Rev. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- 34.Grillon C. Associative learning deficits increase symptoms of anxiety in humans. Biol Psychiatry. 2002;51:851–858. doi: 10.1016/s0006-3223(01)01370-1. [DOI] [PubMed] [Google Scholar]
- 35.Lissek S, et al. Classical fear conditioning in the anxiety disorders: A meta-analysis. Behav Res Ther. 2005;43:1391–1424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Butler G, Mathews A. Anticipatory anxiety and risk perception. Cognit Ther Res. 1987;11:551–565. [Google Scholar]
- 37.Stöber J. Trait anxiety and pessimistic appraisal of risk and chance. Pers Individ Dif. 1997;22:465–476. [Google Scholar]
- 38.Mitte K. Anxiety and risky decision-making: The role of subjective probability and subjective costs of negative events. Pers Individ Dif. 2007;43:243–253. [Google Scholar]
- 39.Borkovec TD, Hazlett-Stevens H, Diaz ML. The role of positive beliefs about worry in generalized anxiety disorder and its treatment. Clin Psychol Psychother. 1999;6:126–138. [Google Scholar]
- 40.Butler G, Mathews A. Cognitive processes in anxiety. Adv Behav Res There. 1983;5:51–62. [Google Scholar]
- 41.Foa EB, Franklin ME, Perry KJ, Herbert JD. Cognitive biases in generalized social phobia. J Abnorm Psychol. 1996;105:433–439. [PubMed] [Google Scholar]
- 42.Gilboa-Schechtman E, Franklin ME, Foa EB. Anticipated reactions to social events: Differences among individuals with generalized social phobia, obsessive compulsive disorder, and nonanxious controls. Cognit Ther Res. 2000;24:731–746. [Google Scholar]
- 43.Warda G, Bryant RA. Cognitive bias in acute stress disorder. Behav Res Ther. 1998;36:1177–1183. doi: 10.1016/s0005-7967(98)00071-0. [DOI] [PubMed] [Google Scholar]
- 44.Tversky A, Kahneman D. Judgment under uncertainty: Heuristics and biases. Science. 1974;185:1124–1131. doi: 10.1126/science.185.4157.1124. [DOI] [PubMed] [Google Scholar]
- 45.Loewenstein GF, Weber EU, Hsee CK, Welch N. Risk as feelings. Psychol Bull. 2001;127:267–286. doi: 10.1037/0033-2909.127.2.267. [DOI] [PubMed] [Google Scholar]
- 46.Volz KG, Schubotz RI, Von Cramon DY. Predicting events of varying probability: Uncertainty investigated by fMRI. Neuroimage. 2003;19:271–280. doi: 10.1016/s1053-8119(03)00122-8. [DOI] [PubMed] [Google Scholar]
- 47.Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25:4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Preuschoff K, Bossaerts P, Quartz SR. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28:2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peters J, Büchel C. Neural representations of subjective reward value. Behav Brain Res. 2010;213:135–141. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 51.Plassmann H, O’Doherty JP, Rangel A. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. J Neurosci. 2010;30:10799–10808. doi: 10.1523/JNEUROSCI.0788-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rangel A, Hare T. Neural computations associated with goal-directed choice. Curr Opin Neurobiol. 2010;20:262–270. doi: 10.1016/j.conb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 53.Schoenbaum G, Takahashi Y, Liu TL, McDannald MA. Does the orbitofrontal cortex signal value? Ann N Y Acad Sci. 2011;1239:87–99. doi: 10.1111/j.1749-6632.2011.06210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wallis JD. Cross-species studies of orbitofrontal cortex and value-based decision-making. Nat Neurosci. 2012;15:13–19. doi: 10.1038/nn.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 56.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seymour B, et al. Temporal difference models describe higher-order learning in humans. Nature. 2004;429:664–667. doi: 10.1038/nature02581. [DOI] [PubMed] [Google Scholar]
- 58.Schiller D, Levy I, LeDoux JE, Niv Y, Phelps EA. From fear to safety and back: Reversal of fear in the human brain. J Neurosci. 2008;28:11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li J, Schiller D, Schoenbaum G, Phelps EA, Daw ND. Differential roles of human striatum and amygdala in associative learning. Nat Neurosci. 2011;14:1250–1252. doi: 10.1038/nn.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paulus MP, Yu AJ. Emotion and decision-making: Affect-driven belief systems in anxiety and depression. Trends Cogn Sci. 2012;16:476–483. doi: 10.1016/j.tics.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, Van Ijzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychol Bull. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- 62.Beck AT, Emery G. Anxiety disorders and phobias: A cognitive perspective. Basic Books; New York: 1985. [Google Scholar]
- 63.Mathews A, Richards A, Eysenck M. Interpretation of homophones related to threat in anxiety states. J Abnorm Psychol. 1989;98:31–34. doi: 10.1037//0021-843x.98.1.31. [DOI] [PubMed] [Google Scholar]
- 64.Hazlett-Stevens H, Borkovec TD. Interpretive cues and ambiguity in generalized anxiety disorder. Behav Res Ther. 2004;42:881–892. doi: 10.1016/S0005-7967(03)00204-3. [DOI] [PubMed] [Google Scholar]
- 65.Stopa L, Clark DM. Social phobia and interpretation of social events. Behav Res Ther. 2000;38:273–283. doi: 10.1016/s0005-7967(99)00043-1. [DOI] [PubMed] [Google Scholar]
- 66.Yoon KL, Zinbarg RE. Interpreting neutral faces as threatening is a default mode for socially anxious individuals. J Abnorm Psychol. 2008;117:680–685. doi: 10.1037/0021-843X.117.3.680. [DOI] [PubMed] [Google Scholar]
- 67.Richards JC, Austin DW, Alvarenga ME. Interpretation of ambiguous interoceptive stimuli in panic disorder and nonclinical panic. Cognit Ther Res. 2001;25:235–246. [Google Scholar]
- 68.Kimble MO, et al. Sentence completion test in combat veterans with and without PTSD: Preliminary findings. Psychiatry Res. 2002;113:303–307. doi: 10.1016/s0165-1781(02)00229-9. [DOI] [PubMed] [Google Scholar]
- 69.Sakai Y, et al. Cerebral glucose metabolism associated with a fear network in panic disorder. Neuroreport. 2005;16:927–931. doi: 10.1097/00001756-200506210-00010. [DOI] [PubMed] [Google Scholar]
- 70.Semple WE, et al. Higher brain blood flow at amygdala and lower frontal cortex blood flow in PTSD patients with comorbid cocaine and alcohol abuse compared with normals. Psychiatry. 2000;63:65–74. doi: 10.1080/00332747.2000.11024895. [DOI] [PubMed] [Google Scholar]
- 71.Chung YA, et al. Alterations in cerebral perfusion in posttraumatic stress disorder patients without re-exposure to accident-related stimuli. Clin Neurophysiol. 2006;117:637–642. doi: 10.1016/j.clinph.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 72.Bremner JD, et al. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biol Psychiatry. 2003;53:879–889. doi: 10.1016/s0006-3223(02)01891-7. [DOI] [PubMed] [Google Scholar]
- 73.Shin LM, et al. Resting metabolic activity in the cingulate cortex and vulnerability to posttraumatic stress disorder. Arch Gen Psychiatry. 2009;66:1099–1107. doi: 10.1001/archgenpsychiatry.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Furmark T, et al. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Arch Gen Psychiatry. 2002;59:425–433. doi: 10.1001/archpsyc.59.5.425. [DOI] [PubMed] [Google Scholar]
- 75.Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS ONE. 2008;3:e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oler JA, et al. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466:864–868. doi: 10.1038/nature09282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Etkin A, Wager TD. Functional neuroimaging of anxiety: A meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nitschke JB, et al. Anticipatory activation in the amygdala and anterior cingulate in generalized anxiety disorder and prediction of treatment response. Am J Psychiatry. 2009;166:302–310. doi: 10.1176/appi.ajp.2008.07101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167:545–554. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lorberbaum JP, et al. Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport. 2004;15:2701–2705. [PubMed] [Google Scholar]
- 81.Guyer AE, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Arch Gen Psychiatry. 2008;65:1303–1312. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 83.Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- 84.Holland PC, Gallagher M. Different roles for amygdala central nucleus and substantia innominata in the surprise-induced enhancement of learning. J Neurosci. 2006;26:3791–3797. doi: 10.1523/JNEUROSCI.0390-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pearce JM, Hall G. A model for Pavlovian learning: Variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol Rev. 1980;87:532–552. [PubMed] [Google Scholar]
- 86.Pessoa L. Emotion and cognition and the amygdala: From “what is it?” to “what’s to be done?”. Neuropsychologia. 2010;48:3416–3429. doi: 10.1016/j.neuropsychologia.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ghashghaei HT, Barbas H. Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 88.Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Woody S, Rachman S. Generalized anxiety disorder (GAD) as an unsuccessful search for safety. Clin Psychol Rev. 1994;14:743–753. [Google Scholar]
- 90.Grillon C, et al. Increased anxiety during anticipation of unpredictable but not predictable aversive stimuli as a psychophysiologic marker of panic disorder. Am J Psychiatry. 2008;165:898–904. doi: 10.1176/appi.ajp.2007.07101581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lissek S, et al. Impaired discriminative fear-conditioning resulting from elevated fear responding to learned safety cues among individuals with panic disorder. Behav Res Ther. 2009;47:111–118. doi: 10.1016/j.brat.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grillon C, et al. Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol Psychiatry. 2009;66:47–53. doi: 10.1016/j.biopsych.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jovanovic T, et al. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 2010;27:244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Waters AM, Henry J, Neumann DL. Aversive Pavlovian conditioning in childhood anxiety disorders: Impaired response inhibition and resistance to extinction. J Abnorm Psychol. 2009;118:311–321. doi: 10.1037/a0015635. [DOI] [PubMed] [Google Scholar]
- 95.Myers KM, Davis M. AX+, BX- discrimination learning in the fear-potentiated startle paradigm: possible relevance to inhibitory fear learning in extinction. Learn Mem. 2004;11:464–475. doi: 10.1101/lm.74704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 97.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 99.Milad MR, et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 100.Mobbs D, et al. Neural activity associated with monitoring the oscillating threat value of a tarantula. Proc Natl Acad Sci USA. 2010;107:20582–20586. doi: 10.1073/pnas.1009076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research–past, present, and future. Biol Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 102.Milad MR, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shin LM, et al. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 104.Phan KL, Britton JC, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow during nontraumatic emotional processing in posttraumatic stress disorder. Arch Gen Psychiatry. 2006;63:184–192. doi: 10.1001/archpsyc.63.2.184. [DOI] [PubMed] [Google Scholar]
- 105.Greenberg T, Carlson JM, Cha J, Hajcak G, Mujica-Parodi LR. Ventromedial prefrontal cortex reactivity is altered in generalized anxiety disorder during fear generalization. Depress Anxiety. 2013;30:242–250. doi: 10.1002/da.22016. [DOI] [PubMed] [Google Scholar]
- 106.Whalen PJ, et al. A functional magnetic resonance imaging predictor of treatment response to venlafaxine in generalized anxiety disorder. Biol Psychiatry. 2008;63:858–863. doi: 10.1016/j.biopsych.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mayberg HS. Limbic-cortical dysregulation: A proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 108.Pizzagalli DA. Frontocingulate dysfunction in depression: Toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tromp DP, et al. Reduced structural connectivity of a major frontolimbic pathway in generalized anxiety disorder. Arch Gen Psychiatry. 2012;69:16–24. doi: 10.1001/archgenpsychiatry.2011.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Phan KL, et al. Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol Psychiatry. 2009;66:691–694. doi: 10.1016/j.biopsych.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci. 2009;29:11614–11618. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: Implications for mood and anxiety disorders. Mol Psychiatry. 2012;17:132–141. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Koenigs M, et al. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat Neurosci. 2008;11:232–237. doi: 10.1038/nn2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bryant RA, et al. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: An fMRI study. Hum Brain Mapp. 2008;29:517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hayes JP, Labar KS, Petty CM, McCarthy G, Morey RA. Alterations in the neural circuitry for emotion and attention associated with posttraumatic stress symptomatology. Psychiatry Res. 2009;172:7–15. doi: 10.1016/j.pscychresns.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fox AS, et al. Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J Neurosci. 2010;30:7023–7027. doi: 10.1523/JNEUROSCI.5952-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lovibond PF, Mitchell CJ, Minard E, Brady A, Menzies RG. Safety behaviours preserve threat beliefs: Protection from extinction of human fear conditioning by an avoidance response. Behav Res Ther. 2009;47:716–720. doi: 10.1016/j.brat.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 118.Salkovskis PM, Clark DM, Hackmann A, Wells A, Gelder MG. An experimental investigation of the role of safety-seeking behaviours in the maintenance of panic disorder with agoraphobia. Behav Res Ther. 1999;37:559–574. doi: 10.1016/s0005-7967(98)00153-3. [DOI] [PubMed] [Google Scholar]
- 119.Solomon RL, Kamin LJ, Wynne LC. Traumatic avoidance learning: The outcomes of several extinction procedures with dogs. The Journal of Abnormal and Social Psychology. 1953;48:291–302. doi: 10.1037/h0058943. [DOI] [PubMed] [Google Scholar]
- 120.Neill DB, Boggan WO, Grossman SP. Impairment of avoidance performance by intrastriatal administration of 6-hydroxydopamine. Pharmacol Biochem Behav. 1974;2:97–103. doi: 10.1016/0091-3057(74)90140-3. [DOI] [PubMed] [Google Scholar]
- 121.McCullough LD, Sokolowski JD, Salamone JD. A neurochemical and behavioral investigation of the involvement of nucleus accumbens dopamine in instrumental avoidance. Neuroscience. 1993;52:919–925. doi: 10.1016/0306-4522(93)90538-q. [DOI] [PubMed] [Google Scholar]
- 122.Amorapanth P, LeDoux JE, Nader K. Different lateral amygdala outputs mediate reactions and actions elicited by a fear-arousing stimulus. Nat Neurosci. 2000;3:74–79. doi: 10.1038/71145. [DOI] [PubMed] [Google Scholar]
- 123.Moscarello JM, LeDoux JE. Active avoidance learning requires prefrontal suppression of amygdala-mediated defensive reactions. J Neurosci. 2013;33:3815–3823. doi: 10.1523/JNEUROSCI.2596-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Delgado MR, Jou RL, LeDoux JE, Phelps EA. Avoiding negative outcomes: Tracking the mechanisms of avoidance learning in humans during fear conditioning. Front Behav Neurosci. 2009;3 doi: 10.3389/neuro.08.033.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aupperle RL, Paulus MP. Neural systems underlying approach and avoidance in anxiety disorders. Dialogues Clin Neurosci. 2010;12:517–531. doi: 10.31887/DCNS.2010.12.4/raupperle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shackman AJ, et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Straube T, Glauer M, Dilger S, Mentzel HJ, Miltner WHR. Effects of cognitive-behavioral therapy on brain activation in specific phobia. Neuroimage. 2006;29:125–135. doi: 10.1016/j.neuroimage.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 128.Hauner KK, Mineka S, Voss JL, Paller KA. Exposure therapy triggers lasting reorganization of neural fear processing. Proc Natl Acad Sci USA. 2012;109:9203–9218. doi: 10.1073/pnas.1205242109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shankman SA, Robison-Andrew EJ, Nelson BD, Altman SE, Campbell ML. Effects of predictability of shock timing and intensity on aversive responses. Int J Psychophysiol. 2011;80:112–118. doi: 10.1016/j.ijpsycho.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hefner KR, Curtin JJ. Alcohol stress response dampening: Selective reduction of anxiety in the face of uncertain threat. J Psychopharmacol. 2012;26:232–244. doi: 10.1177/0269881111416691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Grillon C, Baas JP, Lissek S, Smith K, Milstein J. Anxious responses to predictable and unpredictable aversive events. Behav Neurosci. 2004;118:916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- 132.Dunsmoor JE, Bandettini PA, Knight DC. Neural correlates of unconditioned response diminution during Pavlovian conditioning. Neuroimage. 2008;40:811–817. doi: 10.1016/j.neuroimage.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sarinopoulos I, et al. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cereb Cortex. 2010;20:929–940. doi: 10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Grupe DW, Nitschke JB. Uncertainty is associated with biased expectancies and heightened responses to aversion. Emotion. 2011;11:413–424. doi: 10.1037/a0022583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Herry C, et al. Processing of temporal unpredictability in human and animal amygdala. J Neurosci. 2007;27:5958–5966. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bechtholt AJ, Valentino RJ, Lucki I. Overlapping and distinct brain regions associated with the anxiolytic effects of chlordiazepoxide and chronic fluoxetine. Neuropsychopharmacology. 2008;33:2117–2130. doi: 10.1038/sj.npp.1301616. [DOI] [PubMed] [Google Scholar]
- 137.Grillon C, et al. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biol Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 138.Baas JMP, Grillon C, Morgan CA, Leon IIIJ. Benzodiazepines have no effect on fear-potentiated startle in humans. Psychopharmacology. 2002;161:233–247. doi: 10.1007/s00213-002-1011-8. [DOI] [PubMed] [Google Scholar]
- 139.Straube T, Mentzel HJ, Miltner WHR. Waiting for spiders: Brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37:1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 140.Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. Neuroimage. 2011;55:389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Somerville LH, et al. Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cereb Cortex. 2012;23:49–60. doi: 10.1093/cercor/bhr373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Grupe DW, Oathes DJ, Nitschke JB. Dissecting the anticipation of aversion reveals dissociable neural networks. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry. 2010;68:416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Buhr K, Dugas MJ. The intolerance of uncertainty scale: Psychometric properties of the English version. Behav Res Ther. 2002;40:931–945. doi: 10.1016/s0005-7967(01)00092-4. [DOI] [PubMed] [Google Scholar]
- 145.Dugas MJ, et al. Intolerance of uncertainty and information processing: Evidence of biased recall and interpretations. Cognit Ther Res. 2005;29:57–70. [Google Scholar]
- 146.Carleton RN, et al. Increasingly certain about uncertainty: Intolerance of uncertainty across anxiety and depression. J Anxiety Disord. 2012;26:468–479. doi: 10.1016/j.janxdis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 147.Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 148.Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–40. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 149.Clark L, et al. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131:1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nitschke JB, Sarinopoulos I, Mackiewicz KL, Davidson RJ, Schaefer HS. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 151.Dunsmoor JE, Bandettini PA, Knight DC. Impact of continuous versus intermittent CS-UCS pairing on human brain activation during Pavlovian fear conditioning. Behav Neurosci. 2007;121:635–642. doi: 10.1037/0735-7044.121.4.635. [DOI] [PubMed] [Google Scholar]
- 152.Damasio AR. Descartes’ error: Emotion, reason, and the human brain. Penguin Books; New York: 1994. [Google Scholar]
- 153.Craig AD. How do you feel — now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 154.Gilbert DT, Wilson TD. Prospection: Experiencing the future. Science. 2007;317:1351–1354. doi: 10.1126/science.1144161. [DOI] [PubMed] [Google Scholar]
- 155.Aupperle RL, et al. Dorsolateral prefrontal cortex activation during emotional anticipation and neuropsychological performance in posttraumatic stress disorder. Arch Gen Psychiatry. 2012;69:360–371. doi: 10.1001/archgenpsychiatry.2011.1539. [DOI] [PubMed] [Google Scholar]
- 156.Simmons AN, et al. Anxiety positive subjects show altered processing in the anterior insula during anticipation of negative stimuli. Hum Brain Mapp. 2011;32:1836–1846. doi: 10.1002/hbm.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Simmons AN, Strigo IA, Matthews SC, Paulus MP, Stein MB. Anticipation of aversive visual stimuli is associated with increased insula activation in anxiety-prone subjects. Biol Psychiatry. 2006;60:402–409. doi: 10.1016/j.biopsych.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 158.Simmons AN, Matthews SC, Paulus MP, Stein MB. Intolerance of uncertainty correlates with insula activation during affective ambiguity. Neurosci Lett. 2008;430:92–97. doi: 10.1016/j.neulet.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Moisset X, et al. Anatomical connections between brain areas activated during rectal distension in healthy volunteers: a visceral pain network. Eur J Pain. 2010;14:142–148. doi: 10.1016/j.ejpain.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 160.Cauda F, et al. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- 161.Milad MR, et al. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 162.Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: Awareness and response. Brain Struct Funct. 2010;214:535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Robinson OJ, Charney DR, Overstreet C, Vytal K, Grillon C. The adaptive threat bias in anxiety: Amygdala-dorsomedial prefrontal cortex coupling and aversive amplification. Neuroimage. 2012;60:523–529. doi: 10.1016/j.neuroimage.2011.11.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ray RD, Zald DH. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci Biobehav Rev. 2012;36:479–501. doi: 10.1016/j.neubiorev.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yamasue H, et al. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci USA. 2003;100:9039–9043. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rougemont-Bücking A, et al. Altered processing of contextual information during fear extinction in PTSD: An fMRI study. CNS Neurosci Ther. 2011;17:227–236. doi: 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bryant RA, et al. Neural networks of information processing in posttraumatic stress disorder: A functional magnetic resonance imaging study. Biol Psychiatry. 2005;58:111–118. doi: 10.1016/j.biopsych.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 168.Klumpp H, Angstadt M, Phan KL. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol Psychol. 2012;89:273–276. doi: 10.1016/j.biopsycho.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Asami T, et al. Anterior cingulate cortex volume reduction in patients with panic disorder. Psychiatry Clin Neurosci. 2008;62:322–330. doi: 10.1111/j.1440-1819.2008.01800.x. [DOI] [PubMed] [Google Scholar]
- 170.Shinoura N, et al. Damage to the right dorsal anterior cingulate cortex induces panic disorder. J Affect Disord. 2011;133:569–572. doi: 10.1016/j.jad.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 171.Dougherty DD. Prospective long-term follow-up of 44 patients who received cingulotomy for treatment-refractory obsessive-compulsive disorder. Am J Psychiatry. 2002;159:269–275. doi: 10.1176/appi.ajp.159.2.269. [DOI] [PubMed] [Google Scholar]
- 172.Radua J, Van den Heuvel OA, Surguladze S, Mataix-Cols D. Meta-analytical comparison of voxel-based morphometry studies in obsessive-compulsive disorder vs other anxiety disorders. Arch Gen Psychiatry. 2010;67:701–711. doi: 10.1001/archgenpsychiatry.2010.70. [DOI] [PubMed] [Google Scholar]
- 173.Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 2011;21:1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Krain AL, et al. A functional magnetic resonance imaging investigation of uncertainty in adolescents with anxiety disorders. Biol Psychiatry. 2008;63:563–568. doi: 10.1016/j.biopsych.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 175.Saygin ZM, Osher DE, Augustinack J, Fischl B, Gabrieli JDE. Connectivity-based segmentation of human amygdala nuclei using probabilistic tractography. Neuroimage. 2011;56:1353–1361. doi: 10.1016/j.neuroimage.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Insel T, et al. Research domain criteria (RDoC): Toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- 177.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research Reviews. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 178.Reynolds SM, Zahm DS. Specificity in the projections of prefrontal and insular cortex to ventral striatopallidum and the extended amygdala. J Neurosci. 2005;25:11757–11767. doi: 10.1523/JNEUROSCI.3432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sylvester CM, et al. Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci. 2012;35:527–535. doi: 10.1016/j.tins.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Buckholtz JW, Meyer-Lindenberg A. Psychopathology and the human connectome: Toward a transdiagnostic model of risk for mental illness. Neuron. 2012;74:990–1004. doi: 10.1016/j.neuron.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 181.Suvak MK, Barrett LF. Considering PTSD from the perspective of brain processes: A psychological construction approach. J Trauma Stress. 2011;24:3–24. doi: 10.1002/jts.20618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hakamata Y, et al. Attention bias modification treatment: A meta-analysis toward the establishment of novel treatment for anxiety. Biol Psychiatry. 2010;68:982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ressler KJ, et al. Cognitive enhancers as adjuncts to psychotherapy: Use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 184.Davis M, Myers KM, Ressler KJ, Rothbaum BO. Facilitation of extinction of conditioned fear by D-Cycloserine: Implications for psychopathology. Curr Dir Psychol Sci. 2005;14:214–219. [Google Scholar]
- 185.Hofmann SG, et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- 186.Kushner MG, et al. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62:835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 187.De Kleine RA, Hendriks GJ, Kusters WJC, Broekman TG, Van Minnen A. A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biol Psychiatry. 2012;71:962–968. doi: 10.1016/j.biopsych.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 188.Wilhelm S, et al. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008;165:335–341. doi: 10.1176/appi.ajp.2007.07050776. [DOI] [PubMed] [Google Scholar]
- 189.Otto MW, et al. Efficacy of d-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol Psychiatry. 2010;67:365–370. doi: 10.1016/j.biopsych.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 190.Behar E, McHugh RK, Peckham A, Otto MW. D-cycloserine for the augmentation of an attentional training intervention for trait anxiety. J Anxiety Disord. 2010;24:440–445. doi: 10.1016/j.janxdis.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 191.DeCharms RC. Reading and controlling human brain activation using real-time functional magnetic resonance imaging. Trends Cogn Sci. 2007;11:473–481. doi: 10.1016/j.tics.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 192.Caria A, Sitaram R, Veit R, Begliomini C, Birbaumer N. Volitional control of anterior insula activity modulates the response to aversive stimuli. A real-time functional magnetic resonance imaging study. Biol Psychiatry. 2010;68:425–432. doi: 10.1016/j.biopsych.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 193.Borkovec TD. Life in the future versus life in the present. Clin Psychol Sci Pr. 2002;9:76–80. [Google Scholar]
- 194.Roemer L, Orsillo SM. Expanding our conceptulization of and treatment for generalized anxiety disorder: Integrating mindfulness/acceptance-based approaches with existing cognitive-behavioral models. Clin Psychol Sci Pr. 2002;9:54–68. [Google Scholar]
- 195.Spielberger CD, Gorsuch RL, Lushene R, Bagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- 196.Watson D, et al. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- 197.Nitschke JB, Heller W, Imig JC, McDonald RP, Miller GA. Distinguishing dimensions of anxiety and depression. Cognit Ther Res. 2001;25:1–22. [Google Scholar]
- 198.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- 199.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 200.Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behav Res Ther. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- 201.Watson D, et al. Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS) Psychol Assess. 2007;19:253–268. doi: 10.1037/1040-3590.19.3.253. [DOI] [PubMed] [Google Scholar]
- 202.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 203.Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- 204.Zinbarg RE, Barlow DH. Structure of anxiety and the anxiety disorders: A hierarchical model. J Abnorm Psychol. 1996;105:181–193. doi: 10.1037//0021-843x.105.2.181. [DOI] [PubMed] [Google Scholar]
- 205.Brown TA, Chorpita BF, Barlow DH. Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. J Abnorm Psychol. 1998;107:179–192. doi: 10.1037//0021-843x.107.2.179. [DOI] [PubMed] [Google Scholar]
- 206.Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- 207.Hettema JM, Prescott CA, Myers JM, Neale MC, Kendler KS. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Arch Gen Psychiatry. 2005;62:182–189. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- 208.Bartz JA, Hollander E. Is obsessive-compulsive disorder an anxiety disorder? Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:338–352. doi: 10.1016/j.pnpbp.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 209.Watson D. Rethinking the mood and anxiety disorders: A quantitative hierarchical model for DSM-V. J Abnorm Psychol. 2005;114:522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- 210.Brown TA, Barlow DH. A proposal for a dimensional classification system based on the shared features of the DSM–IV anxiety and mood disorders: Implications for assessment and treatment. Psychol Assess. 2009;21:256–271. doi: 10.1037/a0016608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Garber J, Miller SM, Abramson LY. On the distinction between anxiety and depression: Perceived control, certainty, and probability of goal attainment. Human helplessness: Theory and applications. 1980:131–169. [Google Scholar]
- 212.Abramson LY, Metalsky GI, Alloy LB. Hopelessness depression: A theory-based subtype of depression. Psychol Rev. 1989;96:358–372. [Google Scholar]
- 213.Alloy LB, Kelly KA, Mineka S, Clements CM. Comorbidity of anxiety and depressive disorders: A helplessness-hopelessness perspective. Comorbidity of mood and anxiety disorders. 1990:499–543. [Google Scholar]
- 214.Kishida KT, King-Casas B, Montague PR. Neuroeconomic approaches to mental disorders. Neuron. 2010;67:543–554. doi: 10.1016/j.neuron.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nesse RM, Klaas R. Risk perception by patients with anxiety disorders. J Nerv Ment Dis. 1994;182:465–470. doi: 10.1097/00005053-199408000-00008. [DOI] [PubMed] [Google Scholar]


