Abstract
Neuroglia are a diverse non-neuronal population of cells in the central and peripheral nervous system. These cells have a variety of functions that can all be summed up as the maintenance of homeostasis of the nervous system. It is the loss of homeostasis that represents the culprit of all disorders. Thus, neuroglia can be envisioned as the pivotal element in all neural disorders, be that neurological or psychiatric. In this review, we discuss the role of glia in homeostasis and defence of the nervous system as well as changes in the morpho-functional characteristics of these cells in various disorders.
Keywords: neuroglia, astrocyte, oligodendrocyte, microglia, reactive gliosis, NG2 glia, neurological diseases, neurodegeneration, psychiatric diseases
Prelude: neurological and psychiatric disorders as a homeostatic failure
Last century witnessed a remarkable progress in medicine that made most of the somatic diseases cureable; antibiotics conquered infections, advances in immunology and surgery allowed organ transplantation, while oncology developed treatments for many types of cancer. These successes, however, are in stark contrast with the status of medicinal options in neurology, and especially in disorders of the central nervous system (CNS). Indeed, mechanical trauma of the spinal cord invariably results in paralysis, the best cure for stroke is represented by cooling of the brain (known already to ancient Egyptians), and neurodegenerative diseases inexorably proceed to dementia (Alzheimer’s disease), or trigger rapid death (motor neurone disease, also referred to as amyotrophic lateral sclerosis). Similarly hopeless is the realm of psychiatric and neurodevelopmental diseases, as neither cure nor preventive care exists for major psychiatric disorders, such as schizophrenia and major depression, or for neurodevelopmental diseases represented, for example, by heterogeneous autistic spectrum disorders. Modern drugs acting on the CNS are generally agonists or antagonists of major types of neurotransmitter receptors or neurotransmitter metabolic pathways that try to modify (by inhibition or activation) the chemical transmission that underlies synaptic connectivity within neuronal networks. These agents have little spatial specificity, being indiscriminate to the receptors of its relevant kind throughout the nervous system and peripheral organs, and their action is rather generic, being manifested either in stimulation or slackening of nervous activity. When it comes to specific brain and chronic CNS disorders, the therapeutic options are simply non-existing.
The limited cure reflects a fundamental problem: the cellular pathobiology of neurological disorders is ill defined and cell-based therapy has been developed on the widespread assumption of neurones being the central element in both physiology and pathophysiology, with synapses and neurotransmitter receptors being the chief regulatory pathways in neuronal networks. This neurone-centric dogma is almost universal, being central for the philosophy of experimental and clinical neurology.
This assumption of the dominant role of neurones and neuronal networks in the initiation and progression of neurological disorders, however, is at odds with the general logic of disease nature. Indeed, every disease can be defined as a homeostatic failure in which various exo- or endogenous factors (physical, chemical or genetic) interfere with living tissues and infringe their ability to maintain homeostasis, which is the fundamental requirement of life. In other words, disease can be defined as a homeostatic failure and the depth of the failure determines the compatibility with life. According to this logic, the mechanisms of neurological and psychiatric diseases should be sought in homeostatic systems of the nervous system, which are represented by neuroglia, the long-time neglected pariah of neurobiology.
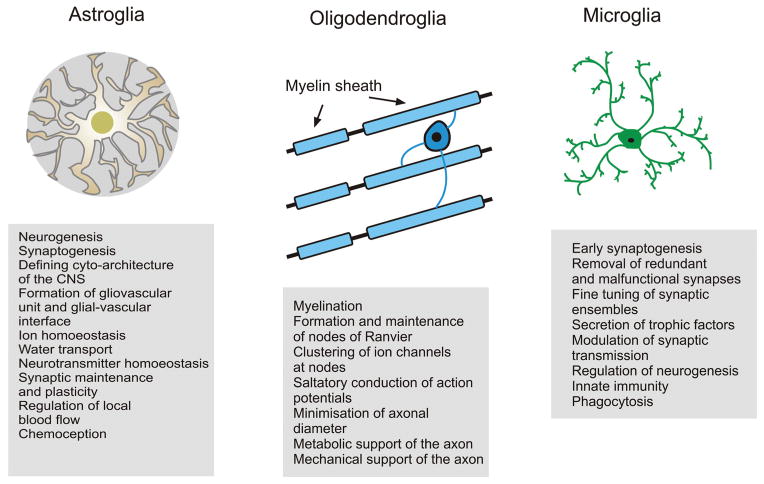
Neuroglia: the general overview
The term Neuroglia (or Nerevenkitt; the closest translation from Greek and German is “the neural putty”; the concept and the name were introduced by Rudolf Virchow in 1856–58 (1–3)) defines a highly heterogeneous population of cells responsible for the homeostasis and defence of the nervous system. The homeostatic and defensive roles are the systemic and most fundamental functions of neuroglial cells. The neuroglia comprise cells of ectodermal (i.e., neural) and mesodermal (myeloid) origins (4); generally, neuroglia are sub-classified into peripheral glia and CNS (the brain and the spinal cord) glia (Fig. 1). The glia of the peripheral nervous system incorporate satellite glial cells that localise in sensory and sympathetic ganglia, the numerous and highly heterogeneous enteric glia, the olfactory ensheathing cells and Schwann cells that support and myelinate peripheral axons, and cover neuromuscular junctions. The neuroglia of the CNS are subdivided into macroglia and microglia. The macroglia comprises the astrocytes and cells of oligodendroglial lineage that are further subdivided into oligodendrocytes and NG2 glia. The astrocytes or astroglia (αστρoν κυτoς; astron, star and kytos, a hollow vessel, later cell or the star-shaped cell, the term introduced by Michael von Lehnossek, (5)), encompass protoplasmic and fibrous astrocytes of grey and white matter respectively, the radial glia of the developing CNS, the close relatives of which in the adult CNS are represented by the retinal Müller glia and cerebellar Bergmann glia, velate astrocytes of the cerebellum, interlaminar and polarised astrocytes of the primate cortex, tanycytes (found in the periventricular organs, the hypophysis/pituitary gland, and the raphe part of the spinal cord), pituicytes in the neuro-hypophysis, and perivascular and marginal astrocytes. Astroglia also include several types of cells that line the ventricles or the subretinal space, namely ependymocytes, choroid plexus cells and retinal pigment epithelial cells. Oligodendrocytes (identified and named so by Pío del Río-Hortega (6)) are myelinating cells in the white and grey matter of the CNS, whereas NG2 cells (discovered by William Stallcup and colleagues (7)) belong to the oligodendrocyte precursor lineage and may also contribute to the homeostasis of the CNS.
Figure 1.
Classification and main functions of neuroglia (see text for further explanations).
The non-neural subpopulation of neuroglia known as microglia are the cells of myeloid origin that represent the main defensive and innate immune system of the CNS. The microglial cells were discovered and characterised by Pío del Río-Hortega in the early 20th century (8, 9). Microglial cells originate from progenitors that derive from primitive c-kit+ erythromyeloid precursors, which come from the extra-embryonic yolk sac (10). These progenitors migrate into developing CNS early in embryogenesis (about embryonic day 10 in mice (11)). After entering the nervous tissue, microglial precursors undergo a substantial transformation and acquire an idiosyncratic morphology, characterised by small cell bodies and several thin and motile processes, and physiology, characterised by the expression of numerous receptors to neurotransmitters and neurohormones concomitant with an expression of “immuno-competent” receptors (e.g., Toll-like receptors and receptors for chemokines and cytokines (12). Besides being the principle elements of CNS defence, microglial cells play an important role in the development of the nervous system being, for example, pivotal for synaptic pruning, phagocytosis of redundant neurones and shaping synaptically connected neuronal networks (13).
Neuroglia: the central element of CNS homeostasis and defence
The preservation of homeostasis of the nervous system is the main function of neuroglia, which functions include the housekeeping of the neural tissue by astrocytes, maintenance of interneuronal “connectome” by oligodendroglia-dependent axonal myelination and providing defensive homeostasis. Astrocytes perform virtually every conceivable homeostatic function (for recent reviews and extensive references lists see (4, 14, 15)). For example, astroglia are fundamental for structural integrity of the CNS, dividing the grey matter into individual gliovascular units that couple brain parenchyma to the local circulation. Astrocytes control the emergence of the blood-brain barrier (by regulating the expression of tight junctions between endothelial cells) and represent its neural side; similarly, astroglia is central for the formation of the cerebrospinal fluid (CSF)-brain barrier. Through astroglial endfeet covering 99% of CNS capillary walls astrocytes participate in regulated transport of various matter through these barriers and contribute to the regulation of local blood flow. Astrocytes, by virtue of multiple plasmalemmal transporters and channels, as well as by numerous astroglia-specific enzymes, control CNS homeostasis of ions and neurotransmitters, most notably glutamate, γ-aminobutyric acid (GABA) and ATP/adenosine, or their precursors, in particular supplying neurones with glutamine, which is a precursor for both glutamate and GABA. It is important to emphasise that glutamine supply is critical for neurotransmission, because neurones are devoid of enzymes for de novo synthesis of glutamate (and hence GABA for which glutamate is a precursor). Astroglia provide for water transport, metabolism, synaptogenesis and the removal of reactive oxygen species. Astrocytes also contribute to systemic homeostasis, being involved in central chemoception, circadian rhythm and regulation of sleep (16). Oligodendrocytes provide CNS myelination; they are involved in a complex bidirectional communication with axons and contribute to periaxonal ion and transmitter homeostasis, to axonal metabolic support, and are able to dynamically influence the action potential propagation (17–19).
Another fundamental function of neuroglia is the formation of brain defence system. First, neuroglial cells protect nervous tissue through their homeostatic mechanisms, which are, for example, fundamental for containing excitotoxic damage (by removing excess of glutamate and buffering extracellular K+), supporting brain metabolism in conditions of ischemia through mobilising glycogen, and supplying neurones with energy substrates as well as secreting numerous trophic and neuroprotective factors (20, 21). Furthermore, insults to the brain trigger evolutionarily conserved glial response, generally defined as reactive gliosis, which includes reactive astrogliosis, proliferative response of NG2 cells and the activation of microglia (12, 22–25). The gliotic response is, fundamentally, a defensive reaction responsible for neuroprotection, isolating injured area through the formation of glial scar, removing pathogens, dying cells and cellular debris, and remodelling the nervous tissue after the resolution of pathology.
Reactive astrogliosis, which is activated in most of the pathological processes in the CNS is manifested by a complex biochemical remodelling of astrocytes, their hypertrophy and proliferation and up-regulation of intermediate filaments, i.e., cytoskeletal proteins glial fibrillary acidic protein (GFAP), vimentin and nestin (23, 26). A substantial increase in GFAP immunoreactivity is regarded as a specific marker for astrogliotic response. Importantly, reactive astrogliosis is a controlled, multi-stage and diverse process, which may involve heterogeneous cell populations with distinct neuroprotective capabilities. Furthermore, the manifestation of astroglial reactivity is context-specific and different pathologies are associated with distinct reactive astroglial phenotypes (27, 28). Inhibition of reactive astrogliosis generally reduces neuronal viability and worsens the outcome of neurological pathology (24). Finally, reactive astrocytes are instrumental for post-pathology neural tissue regeneration and repair, contributing, for example, to the rewiring of neuronal networks, lesion-induced neurogenesis and reconstitution of blood-brain barrier (26, 29).
Broadly, reactive astrogliosis is classified into isomorphic (i.e. preserving morphology) or anisomorphic (i.e., changing the morphology). In isomorphic gliosis, astroglial hypertrophy, physiological and biochemical changes proceed without altering normal astroglial morphological domain organisation, which is endowed by minute overlap between individual cells at their very periphery (30). Isomorphic astrogliosis is neuroprotective, fully reversible and it facilitates neurite outgrowth and synaptogenesis. In anisomorphic gliosis, astrocytes became hypertrophic and start to proliferate; glial territorial domains are disrupted, astrocytic processes intermingle and finally a permanent glial scar is formed. The glial scar effectively seals the damaged area and prevents axonal growth, because of chondroitin and keratin secreted by reactive astrocytes (24, 31).
The NG2 glia also respond to various types of CNS pathology by increased proliferation and morphological changes. The processes of NG2 cells in the affected regions become shorter and thicker; this is also accompanied by a substantial increase in NG2 (i.e., chondroitin sulphate proteoglycan 4) expression. The reactive NG2 cells can also proliferate and, at least in spinal cord, the NG2 cells can generate oligodendrocytes that may remyelinate pathologically affected axons In the spinal cord, activated NG2 cells generate new oligodendrocytes that remyelinate the demyelinated lesions (32, 33). Arguably, NG2 cells may also contribute to scar formation by secreting chondroitin sulphate proteoglycan 4.
Another important component of neuroglial defence is represented by microglia. Insults to the nervous tissue initiate the activation of microglia, which is a multi-stage controlled process progressing through different stages and phenotypes with a variety of morphological, biochemical, functional and immunological changes and producing a variety of phenotypically distinct types of activated microglia. Responses of microglia to pathology are multi-faceted. For example, localised insults trigger rapid converging of microglial processes to the site of injury (34, 35). Stronger and more persistent lesions induce morphological remodelling; microglial somata enlarge and processes become fewer and thicker. Microglial cells alter their expression of various enzymes and receptors, and begin to secrete immune response molecules. At even stronger insults, microglial cells enter proliferative stage, become motile, acquire macrophage-like morphology, migrate and accumulate around the sites of damage and finally transform into phagocytes (12, 25, 36).
Neuroglial reactivity is a central element of CNS response to damage and chronic pathologies. Contributions of activated neuroglia can, however, be not only neuroprotective, but also deleterious. This reflects an intrinsic dichotomy of every homeostatic system. The very same molecular cascades that underlie neuroprotection can also contribute to neurotoxicity. Overstimulation of astrocytes can induce the excessive release of glutamate through various release pathways and this release can add to the glutamate toxicity and eventually neuronal death. Abnormal water transport through astroglial aquaporins is a leading mechanism of cellular oedema, whereas deficient astroglial K+ buffering contributes to spreading depression. Similarly, over-activation of microglia results in the release of neurotoxic factors and phagocytosis that can exacerbate neuronal damage.
General pathology of neuroglia: Reactivity versus atrophy and asthenia
Neuroglial reactivity in neurological disorders could be considered as dedicated defensive response. At the same time, an opposite process, a loss of function, atrophy or asthenia of glial cells can be directly involved in pathological progression. Evidence for the loss of function of neuroglial cells that accompany different neurological conditions begun to accumulate in recent years. Astrocytes, for example, show signs of morphological atrophy at the early stages of several neurodegenerative conditions (37). In diverse neurotoxic impairments of the CNS, astrocytes lose their ability to control extracellular glutamate, which may be a leading mechanism for ensuing excitotoxicity and neuronal death. Similarly, atrophy or death of astroglia is observed in a variety of neuropsychiatric disorders. In demyelinating conditions, oligodendrocytes fail in remyelination, whereas a loss of function of microglia is involved in neurodevelopmental diseases and is observed in neurodegeneration and in tumorous growth in the nervous system (see (21, 38) and references therein).
Specific gliopathology in neurological and psychiatric diseases
Genetic astrogliopathology: Alexander disease
The inherited gliopathology, associated with sporadic mutations in the GFAP encoding gene, was described in 1949 by Stewart Alexander (39). Here, the impaired function of astroglia affects brain development and results in severe deficit of white matter manifested by profound leukodystrophy. Histopathologically, Alexander disease is associated with an appearance of cytoplasmic inclusions in astroglial cells known as Rosenthal fibres; these corkscrew-shaped inclusions are formed by GFAP and stress proteins. The pathogenesis of Alexander disease remains unknown and the prognosis is pessimistic with most of the patients dying in early childhood or in adolescence (40).
Neurodevelopmental disorders
The glial impairment in neurodevelopmental disorders such as autistic spectrum disorders (ASD) begun to be considered only very recently (see (41) for details and references). Many forms of ASD reflect abnormal formation of neuronal networks and disbalanced neurotransmission. These could result from environmental factors (e.g., exposure to heavy metals or other toxins), oxidative stress, hormonal impairments or early neuroinflammation in combination with genetic predisposition. Astrocytes are the main source for reactive oxygen species scavengers such as glutathione or ascorbic acid, and hence astroglial weakness can lie at the core of oxidative damage to nervous tissue. Astrocytes are also involved in the regulation of neurogenesis and neuronal migration in early postnatal period and hence astroglial weakness can contribute to the malformation of neuronal networks. Astroglia are critical for synaptogenesis (42), and hence for shaping the synaptically connected neuronal networks. Astroglia-derived cholesterol is one of the critical elements of the synaptogenesis and abnormalities of cholesterol metabolism have been detected in ASD (43); these abnormalities may reflect impaired astroglial function and could be linked to oxytocin-mediated signalling pathways acting through oxytocin receptors expressed in astroglia. Finally, ASD is associated with neuro-immune alterations such as an increase in the levels of numerous cytokines (44), which are mainly secreted my microglia. Microglial cells and astrocytes are also implicated in the pathogenesis of Rett syndrome (45, 46) and trichotillomania (47).
Toxic encephalopathies
Astrocytes play a primary role in neurotoxic damage to the brain. Astroglial cells are primary targets for heavy metal induced brain damage in Minamata disease (poisoning with methylmercury), and in manganese, lead or aluminium toxic encephalopathies (21, 48–50). In all these toxicities, astroglial cells accumulate heavy metals through astroglial-specific transporters, which in turn affect the plasmalemmal glutamate transporters. Decrease in the activity of the latter results in chronic elevation of extracellular glutamate with ensuing glutamate neurotoxicity and neuronal death. Similarly, astrocytes appear as a primary target in hepatic encephalopathy, which accompanies liver failure. Here, the brain is being poisoned by ammonia, concentration of which markedly increases in the blood and in the CNS following liver insufficiency; the symptoms of ammonia toxicity include confusion, memory deficits, lethargy, somnolence and, in the terminal stages, coma. Astrocytes are chiefly responsible for ammonia detoxification; ammonia is metabolised by glutamine synthetase, astroglia-specific enzyme catalising the condensation of glutamate and ammonia to form glutamine (51). Increased activity of glutamine synthetase in response to elevated ammonia concentration overloads astrocytes with glutamine, impairs K+ buffering and inhibits glutamate transporters. All these result in osmotic shock and cell swelling, brain oedema and glutamate excitotoxicity (52, 53).
Ischaemia and stroke
In ischaemic damages to the CNS, neuroglial cells are intimately involved into pathological progression, contributing to both neuroprotection and neurotoxicity (54–56). Normal astrocytes are substantially more resistant to hypoxia than neurones and oligodendrocytes, and hence they can survive in conditions of limited oxygen supply that is characteristic for ischaemic penumbra. Here, astroglial performance is critical for neuroprotection, through removal of glutamate, K+ buffering, release of reactive oxygen species scavengers and supplying stressed neurones with lactate. Removal of astrocytes greatly increases neuronal vulnerability in experiments in vitro (57). Such astroglia-dependent neuroprotection is critical for containing the spread of neuronal death through penumbra, which in turn defines post-ischaemic neurological deficit. Astrocytes, however, could exert fundamentally different effects, mediating neurotoxicity, especially in conditions of severe and prolonged ischeamia. The astroglia-mediated neurotoxicity can be mediated through the release (instead of removal) of glutamate via, e.g., the reversal of glutamate transporters or glutamate diffusion through astroglial hemichannels. Astroglial cells can increase extracellular acidosis as a by-product of anaerobic glycolysis; this could be seen in experimental conditions whereby an increase in glucose levels exacerbated the ischemic neuronal damage. Finally, astrocytes can mediate neuronal death through propagating aberrant astroglial Ca2+ waves causing distal (to the infarction core) release of glutamate and other neurotoxic factors (58).
Neuropsychiatric diseases
The causes, nature and pathogenesis of neuropsychiatric diseases remain generally enigmatic, albeit there is a recent shift towards the role for disbalance of neurotransmission and in particular deficient glutamatergic mechanisms that include altered glutamate homeostasis and possible endogenous inhibition of N-methyl-D-aspartate (NMDA) glutamate receptors (59, 60). These alterations may certainly be centered on neuroglia which is indispensable for glutamate turnover, catabolism and synthesis. Morphological studies have confirmed neuroglial alterations such as reduced density and atrophic changes in astroglia and oligodendroglia to be prominent in all three major psychiatric disorders, that is in schizophrenia, bipolar disorder and major depressive disorder; incidentally, no signs of apparent neuroglial reactivity were identified (61). The pathological remodelling of astroglial biochemistry may also be relevant for the progression of schizophrenia. Astrocytes are the main producers of kynurenic acid (through astroglial-specific enzyme kynurenine aminotransferase II, or KAT II (62)); kynurenic acid acts as an endogenous inhibitor of NMDA receptors, and the levels of kynurenic acid are increased in the cortex and in the CSF of patients with schizophrenia (63). Finally, astroglia and kynurenic acid may be a critical link between Toxoplasma gondii infection and an increased risk for schizophrenia. It appeared that T. gondii selectively infects astrocytes, which results in an increased production of kynurenic acid; this may account for the increased risk of schizophrenia (64).
Epilepsy
The pathological cellular substrate of epilepsy is represented by a synchronous slow depolarisation of neurones within an epileptic focus, known as a paroxysmal depolarization shift, which in turn is mediated by the activation of ionotropic glutamate receptors. Epilepsy, in its various forms, is usually associated with prominent reactive astrogliosis, which often underlies the formation of glial scar. Astroglial reactivity develops at the early stages of the epileptic disorders (which has been observed in both human post-mortem tissues and in animal models) and proceeds in anisomorphic fashion so that reactive astrocytes in epileptic tissue lose their domain organisation (65). Specific feature of astroglial reactivity in epilepsy is represented by (i) an increased expression of ionotropic and metabotropic glutamate receptors, (ii) aberrant calcium signalling; (iii) a decreased presence of inward rectifier K+ channels and aquaporins and (iv) a decreased expression and activity of glutamate transporters and glutamine synthetase. All these changes result in aberrant K+ buffering and deregulated glutamate/GABA homeostasis, which may affect neuronal excitability and contribute to the generation of seizures (66–69).
Neurodegenerative diseases
Neuroglia play much more important role in neurodegeneration than has been previously thought, and likely it does play the leading role in some (if not in all) forms of neurodegenerative diseases. Sporadic neurodegenerative process (in contrast to acute neurodegeneration that is a consequence of trauma or ischemic attack), occurs almost exclusively in the CNS of humans; Alzheimer’s disease (AD), Huntington disease (HD), Parkinson disease, motor neurone disease (MND)/ amyotrophic lateral sclerosis (ALS) or other forms of dementia do not affect animals. This specificity to humans remains an unsolved conundrum that represents a substantial obstacle to experimental studies of these diseases. In the recent decade, numerous animal models of neurodegenerative diseases, that transgenically insert disease-associated human genes into mice, have been developed (70–73). It has to be remembered, however, that all these models, although reproducing certain parts of human pathologies and often showing disease-specific histopathology, remain imperfect replicas of the naturally occurring diseases.
In MND/ALS (also known as Lou Gehrig’s disease in the US in memory of a baseball player who suffered and died from this pathology) astrocytes are the first cells to undergo pathological remodelling. In a mouse model of MND/ALS (which expresses a human mutant superoxide dismutase 1, or SOD1, associated with a familial form of the disease) astroglial cells in the spinal cord undergo degeneration and become atrophic; these cells have deficient plasmalemmal glutamate transporters and hence cannot contain the excitotoxic build-up of extracellular glutamate (74) that is arguably the leading cause for consequent neuronal death. Furthermore, the MND/ALS progression could be mimicked by astroglia-specific genetic deletion of glutamate transporter 1 in mice (of which excitatory amino acid transporter 2, EAAT2, is a human analogue) (75), whereas selective silencing of the SOD1 mutant gene in astrocytes markedly delayed the progression of MND/ALS (76, 77).
Impairment of the astrocytic ability to clear extracellular glutamate appears as a key pathogenetic mechanism for Wernicke’s encephalopathy that represents an organic substrate for Korsakoff’s psychosis (78, 79). In this disorder, the expression of astroglial-specific glutamate transporters EAAT1/EAAT2 in humans is decreased by 60 – 70% of the physiological norm. A similar decrease in glutamate transporters was observed in astrocytes from the beriberi (thiamine deficiency) rat model (80, 81). Here, the failure of astroglial glutamate uptake causes profound neurotoxicity, rapid neuronal death with consequent psychotic abnormalities, cognitive deficiency and death.
In AD, which is arguably one of the most common cases of dementia in the developed world, all types of neuroglia are affected and are most likely, linked to pathological progression. AD is characterised by conspicuous atrophy of brain tissue and histopathological hallmarks in the form of the extracellular deposits of β-amyloid protein, known as senile plaques, and intra-neuronal accumulation of abnormal tau-protein filaments, known as neuronal tangles (82, 83). Astrocytes in AD show two types of apparently opposing changes: the relatively early and region-specific atrophy and, at the later stages of the disease characterised by the formation of senile plaques, region-specific reactivity (37, 84, 85). Morphological atrophy, detected as a decrease in astroglial profiles positive to astroglia-specific proteins GFAP, S100β and glutamine synthetase, has been observed in entorhinal and prefrontal cortices, and the hippocampus of several AD animal models (86–91); it also seems to exist in post-mortem human tissues (Rodriguez & Verkhratsky own observations). The early dystrophy of astroglial cells can be pathologically relevant because reduced astroglial synaptic coverage could impair the synaptic strength and synaptic maintenance. Moreover, this reduced astroglial coverage may also influence β-amyloidogenesis. The latter is apparently regulated by glutamatergic transmission; in particular, the activation of synaptic NMDA receptors favours non-amyloidogenic processing of amyloid precursor protein, whereas the stimulation of extra synaptic NMDA receptors stimulates β-amyloid production (92). Reduced astroglial perisynaptic coverage facilitates glutamate spillover from the synaptic cleft and hence may increase activation status of extra-synaptic NMDA receptors and thus favours β-amyloid production.
At the later stages of the AD, the appearance of senile plaques presents a signal for reactive astrogliosis, and, indeed, an accumulation of reactive hypertrophic astrocytes around β-amyloid deposits have been detected in post-mortem human tissues as well as in AD animal models (85, 87). The reactive astrocytes show an increased expression of GFAP and S100β, along with a reduced expression of glutamine synthetase, which indicates an impairment of glutamate homeostatic function (93). In addition, reactive astrocytes localised in senile plaques display aberrant Ca2+ signalling (94). Astroglial reactivity is region-specific, and it is absent in entorhinal and prefrontal cortices of AD mouse model (89, 90), which may contribute to a higher vulnerability of these brain portions to AD-like pathology.
Progression of AD also affects oligodendroglia and myelination; oligodendrocytes show atrophic changes (95) and reduced densities (96) in AD-affected tissues, which may result in a decreased myelination in the CNS. AD pathology also affects microglia; in the early, preplaque stages, a substantial increase in the microglial densities was observed in AD mice; at the later stages, activated microglia is associated with senile plaques (97, 98). Importantly, however, the activated microglia in AD brains show a loss of function, manifested in the impairment of phagocytosis (99).
Astroglia are also affected in non-AD type dementias, such as frontotemporal dementia, Pick’s disease, frontotemporal lobar degeneration, thalamic dementia, or -associated dementia in which both astroglial atrophy and reactive astrogliosis have been identified (100, 101). Primary astroglial pathology, represented by both gliotic and dystrophic changes, is observed in thalamic dementia, in which the loss of astroglial homeostatic functions induces widespread neuronal loss, hippocampal sclerosis and white matter lesions (102). Loss of function (due to mutations) of astroglia-specific protein NPC-1, which appears to function as a transporter in the endosomal-lysosomal system, contributes to the Niemann-Pick disease type C (103). In Huntington’s (HD) disease decrease in astroglial glutamate transporters and possibly in the production of ascorbic acid may contribute to neurotoxicity (104). It should be noted that HD causes preferential loss of a subset of neurones in the brain, although the huntingtin protein is expressed broadly in various neural cell types. Recently, it has been demonstrated that full-length mutant huntingtin expression perturbs astrocyte gliotransmitter release. Hence, BACHD astrocytes show augmented exocytotic glutamate release with unaltered Ca2+ dynamics. These astrocytes have a biochemical footprint that would lead to increased availability of cytosolic glutamate, i.e., augmented de novo glutamate synthesis due to an increase in the level of the astrocyte specific mitochondrial enzyme, pyruvate carboxylase. This work identified a new mechanism in astrocytes that could lead to increased levels of extracellular glutamate in HD and thus may contribute to excitotoxicity in this devastating disease (105). Similarly, a loss of astroglia-dependent neuroprotection may contribute to the demise of dopaminergic neurones in Parkinson’s disease (106).
Conclusions: Translational outlook
Neurological and psychiatric disorders have been almost entirely considered from the neurone-centric point of view, with neurons being the principal, if not the sole, cellular element of disorderly process. However, it is neuroglia, but not neuronese that control the nervous system homeostasis, the dysregulation of which is the common denominator in all diseases. Recently, it is becoming clear that neuroglial cells play an active role in pathophysiological processes and that understanding the underlying mechanisms shall provide novel targets for much needed therapeutic intervention.
Acknowledgments
We thank Manoj K. Gottipati for comments on a previous version of this manuscript. VP research is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD078678). AV was supported by the Alzheimer’s Research Trust (UK), by European Commission, by IKERBASQUE and by a research grant of Nizny Novgorod State University.
References
- 1.KETTENMANN H, VERKHRATSKY A. Neuroglia: the 150 years after. Trends Neurosci. 2008;31:653–659. doi: 10.1016/j.tins.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 2.VIRCHOW R. Gesammelte Abhandlungen zyr wissenschaftlischen Medizin. Verlag von Meidinger Sohn & Comp; Frankfurt a.M: 1856. [Google Scholar]
- 3.VIRCHOW R. Zwanzig Vorlesungen gehalten während der Monate Februar, März und April 1858 im pathologischen Institut zu Berlin. 1. Berlin: August Hirschwald; 1858. Die Cellularpathologie in ihrer Begründung auf physiologische and pathologische Gewebelehre. [Google Scholar]
- 4.VERKHRATSKY A, BUTT AM. Glial Physiology and Pathophysiology. Wiley-Blackwell; Chichester: 2013. [Google Scholar]
- 5.LENHOSSÉK MV. Der feinere Bau des Nervensystems im Lichte neuester Forschung. 2. Berlin: Fischer‘s Medicinische Buchhandlung H. Kornfield; 1895. [Google Scholar]
- 6.DEL RÍO-HORTEGA P. Estudios sobre la neuroglia. La glia de escasas radiaciones oligodendroglia. Biol Soc Esp Biol. 1921;21:64–92. [Google Scholar]
- 7.STALLCUP WB. The NG2 antigen, a putative lineage marker: immunofluorescent localization in primary cultures of rat brain. Dev Biol. 1981;83:154–165. doi: 10.1016/s0012-1606(81)80018-8. [DOI] [PubMed] [Google Scholar]
- 8.DEL RÍO-HORTEGA P. Poder fagocitario y movilidad de la microglia. Bol de la Soc esp de biol. 1919;9:154. [Google Scholar]
- 9.DEL RÍO-HORTEGA P. Microglia. In: Penfield W, editor. Cytology and cellular pathology of the nervous system. Hoeber; New York: 1932. pp. 482–534. [Google Scholar]
- 10.KIERDORF K, ERNY D, GOLDMANN T, SANDER V, SCHULZ C, PERDIGUERO EG, WIEGHOFER P, HEINRICH A, RIEMKE P, HOLSCHER C, MULLER DN, LUCKOW B, BROCKER T, DEBOWSKI K, FRITZ G, OPDENAKKER G, DIEFENBACH A, BIBER K, HEIKENWALDER M, GEISSMANN F, ROSENBAUER F, PRINZ M. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci. 2013;16:273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- 11.GINHOUX F, GRETER M, LEBOEUF M, NANDI S, SEE P, GOKHAN S, MEHLER MF, CONWAY SJ, NG LG, STANLEY ER, SAMOKHVALOV IM, MERAD M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.KETTENMANN H, HANISCH UK, NODA M, VERKHRATSKY A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 13.KETTENMANN H, KIRCHHOFF F, VERKHRATSKY A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 14.KETTENMANN H, RANSOM BR, editors. Neuroglia. Oxford University Press; Oxford: 2013. [Google Scholar]
- 15.PARPURA V, HENEKA MT, MONTANA V, OLIET SH, SCHOUSBOE A, HAYDON PG, STOUT RF, JR, SPRAY DC, REICHENBACH A, PANNICKE T, PEKNY M, PEKNA M, ZOREC R, VERKHRATSKY A. Glial cells in (patho)physiology. J Neurochem. 2012;121:4–27. doi: 10.1111/j.1471-4159.2012.07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GOURINE AV, KASPAROV S. Astrocytes as brain interoceptors. Exp Physiol. 2011;96:411–416. doi: 10.1113/expphysiol.2010.053165. [DOI] [PubMed] [Google Scholar]
- 17.YAMAZAKI Y, HOZUMI Y, KANEKO K, FUJII S, GOTO K, KATO H. Oligodendrocytes: facilitating axonal conduction by more than myelination. Neuroscientist. 2010;16:11–18. doi: 10.1177/1073858409334425. [DOI] [PubMed] [Google Scholar]
- 18.DU Y, DREYFUS CF. Oligodendrocytes as providers of growth factors. J Neurosci Res. 2002;68:647–654. doi: 10.1002/jnr.10245. [DOI] [PubMed] [Google Scholar]
- 19.FIELDS RD. Oligodendrocytes changing the rules: action potentials in glia and oligodendrocytes controlling action potentials. Neuroscientist. 2008;14:540–543. doi: 10.1177/1073858408320294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.SUH SW, BERGHER JP, ANDERSON CM, TREADWAY JL, FOSGERAU K, SWANSON RA. Astrocyte glycogen sustains neuronal activity during hypoglycemia: studies with the glycogen phosphorylase inhibitor CP-316,819 ([R-R*,S*]-5-chloro-N-[2-hydroxy-3-(methoxymethylamino)-3-oxo-1-(phenylmet hyl)propyl]-1H-indole-2-carboxamide) J Pharmacol Exp Ther. 2007;321:45–50. doi: 10.1124/jpet.106.115550. [DOI] [PubMed] [Google Scholar]
- 21.VERKHRATSKY A, RODRIGUEZ JJ, PARPURA V. Astroglia in neurological diseases. Future Neurol. 2013;8:149–158. doi: 10.2217/fnl.12.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.GIAUME C, KIRCHHOFF F, MATUTE C, REICHENBACH A, VERKHRATSKY A. Glia: the fulcrum of brain diseases. Cell Death Differ. 2007;14:1324–1335. doi: 10.1038/sj.cdd.4402144. [DOI] [PubMed] [Google Scholar]
- 23.PEKNY M, NILSSON M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 24.SOFRONIEW MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.HANISCH UK, KETTENMANN H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 26.BURDA JE, SOFRONIEW MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.PEKNA M, PEKNY M. The neurobiology of brain injury. Cerebrum. 2012;2012:9. [PMC free article] [PubMed] [Google Scholar]
- 28.PEKNY M, WILHELMSSON U, PEKNA M. The dual role of astrocyte activation and reactive gliosis. Neurosci Lett. 2014 doi: 10.1016/j.neulet.2013.12.071. [DOI] [PubMed] [Google Scholar]
- 29.ROBEL S, BERNINGER B, GOTZ M. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci. 2011;12:88–104. doi: 10.1038/nrn2978. [DOI] [PubMed] [Google Scholar]
- 30.BUSHONG EA, MARTONE ME, JONES YZ, ELLISMAN MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.SOFRONIEW MV, VINTERS HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.TRIPATHI RB, RIVERS LE, YOUNG KM, JAMEN F, RICHARDSON WD. NG2 glia generate new oligodendrocytes but few astrocytes in a murine experimental autoimmune encephalomyelitis model of demyelinating disease. J Neurosci. 2010;30:16383–16390. doi: 10.1523/JNEUROSCI.3411-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ZAWADZKA M, RIVERS LE, FANCY SP, ZHAO C, TRIPATHI R, JAMEN F, YOUNG K, GONCHAREVICH A, POHL H, RIZZI M, ROWITCH DH, KESSARIS N, SUTER U, RICHARDSON WD, FRANKLIN RJ. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell. 2010;6:578–590. doi: 10.1016/j.stem.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DAVALOS D, GRUTZENDLER J, YANG G, KIM JV, ZUO Y, JUNG S, LITTMAN DR, DUSTIN ML, GAN WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 35.NIMMERJAHN A, KIRCHHOFF F, HELMCHEN F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 36.RANSOHOFF RM, PERRY VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 37.RODRIGUEZ JJ, VERKHRATSKY A. Neuroglial roots of neurodegenerative diseases? Mol Neurobiol. 2011;43:87–96. doi: 10.1007/s12035-010-8157-x. [DOI] [PubMed] [Google Scholar]
- 38.DE KEYSER J, MOSTERT JP, KOCH MW. Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J Neurol Sci. 2008;267:3–16. doi: 10.1016/j.jns.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 39.ALEXANDER WS. Progressive fibrinoid degeneration of fibrillary astrocytes associated with mental retardation in a hydrocephalic infant. Brain. 1949;72:373–381. doi: 10.1093/brain/72.3.373. [DOI] [PubMed] [Google Scholar]
- 40.MESSING A, BRENNER M, FEANY MB, NEDERGAARD M, GOLDMAN JE. Alexander disease. J Neurosci. 2012;32:5017–5023. doi: 10.1523/JNEUROSCI.5384-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.ZEIDAN-CHULIA F, SALMINA AB, MALINOVSKAYA NA, NODA M, VERKHRATSKY A, MOREIRA JC. The glial perspective of autism spectrum disorders. Neurosci Biobehav Rev. 2014;38:160–172. doi: 10.1016/j.neubiorev.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 42.PFRIEGER FW. Role of glial cells in the formation and maintenance of synapses. Brain Res Rev. 2010;63:39–46. doi: 10.1016/j.brainresrev.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 43.TIERNEY E, BUKELIS I, THOMPSON RE, AHMED K, ANEJA A, KRATZ L, KELLEY RI. Abnormalities of cholesterol metabolism in autism spectrum disorders. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:666–668. doi: 10.1002/ajmg.b.30368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.WEI H, MORI S, HUA K, LI X. Alteration of brain volume in IL-6 overexpressing mice related to autism. Int J Dev Neurosci. 2012;30:554–559. doi: 10.1016/j.ijdevneu.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 45.MAEZAWA I, JIN LW. Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. J Neurosci. 2010;30:5346–5356. doi: 10.1523/JNEUROSCI.5966-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MAEZAWA I, SWANBERG S, HARVEY D, LASALLE JM, JIN LW. Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J Neurosci. 2009;29:5051–5061. doi: 10.1523/JNEUROSCI.0324-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.CHEN SK, TVRDIK P, PEDEN E, CHO S, WU S, SPANGRUDE G, CAPECCHI MR. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141:775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.STRUYS-PONSAR C, GUILLARD O, VAN DEN BOSCH DE AGUILAR P. Effects of aluminum exposure on glutamate metabolism: a possible explanation for its toxicity. Exp Neurol. 2000;163:157–164. doi: 10.1006/exnr.2000.7355. [DOI] [PubMed] [Google Scholar]
- 49.SUAREZ-FERNANDEZ MB, SOLDADO AB, SANZ-MEDEL A, VEGA JA, NOVELLI A, FERNANDEZ-SANCHEZ MT. Aluminum-induced degeneration of astrocytes occurs via apoptosis and results in neuronal death. Brain Res. 1999;835:125–136. doi: 10.1016/s0006-8993(99)01536-x. [DOI] [PubMed] [Google Scholar]
- 50.YIN Z, MILATOVIC D, ASCHNER JL, SYVERSEN T, ROCHA JB, SOUZA DO, SIDORYK M, ALBRECHT J, ASCHNER M. Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Res. 2007;1131:1–10. doi: 10.1016/j.brainres.2006.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.ROSE CF, VERKHRATSKY A, PARPURA V. Astrocyte glutamine synthetase: pivotal in health and disease. Biochem Soc Trans. 2013;41:1518–1524. doi: 10.1042/BST20130237. [DOI] [PubMed] [Google Scholar]
- 52.BRUSILOW SW, KOEHLER RC, TRAYSTMAN RJ, COOPER AJ. Astrocyte glutamine synthetase: importance in hyperammonemic syndromes and potential target for therapy. Neurotherapeutics. 2010;7:452–470. doi: 10.1016/j.nurt.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.BUTTERWORTH RF. Hepatic encephalopathy: a central neuroinflammatory disorder? Hepatology. 2011;53:1372–1376. doi: 10.1002/hep.24228. [DOI] [PubMed] [Google Scholar]
- 54.TAKANO T, OBERHEIM N, COTRINA ML, NEDERGAARD M. Astrocytes and ischemic injury. Stroke. 2009;40:S8–12. doi: 10.1161/STROKEAHA.108.533166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.VANGEISON G, REMPE DA. The Janus-faced effects of hypoxia on astrocyte function. Neuroscientist. 2009;15:579–588. doi: 10.1177/1073858409332405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.ZHAO Y, REMPE DA. Targeting astrocytes for stroke therapy. Neurotherapeutics. 2010;7:439–451. doi: 10.1016/j.nurt.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.TANAKA J, TOKU K, ZHANG B, ISHIHARA K, SAKANAKA M, MAEDA N. Astrocytes prevent neuronal death induced by reactive oxygen and nitrogen species. Glia. 1999;28:85–96. doi: 10.1002/(sici)1098-1136(199911)28:2<85::aid-glia1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 58.LIN JH, WEIGEL H, COTRINA ML, LIU S, BUENO E, HANSEN AJ, HANSEN TW, GOLDMAN S, NEDERGAARD M. Gap-junction-mediated propagation and amplification of cell injury. Nat Neurosci. 1998;1:494–500. doi: 10.1038/2210. [DOI] [PubMed] [Google Scholar]
- 59.KONDZIELLA D, BRENNER E, EYJOLFSSON EM, SONNEWALD U. How do glial-neuronal interactions fit into current neurotransmitter hypotheses of schizophrenia? Neurochem Int. 2007;50:291–301. doi: 10.1016/j.neuint.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 60.STEINER J, BOGERTS B, SARNYAI Z, WALTER M, GOS T, BERNSTEIN HG, MYINT AM. Bridging the gap between the immune and glutamate hypotheses of schizophrenia and major depression: Potential role of glial NMDA receptor modulators and impaired blood-brain barrier integrity. World J Biol Psychiatry. 2012;13:482–492. doi: 10.3109/15622975.2011.583941. [DOI] [PubMed] [Google Scholar]
- 61.VERKHRATSKY A, RODRIGUEZ JJ, STEARDO L. Astrogliopathology: A Central Element of Neuropsychiatric Diseases? Neuroscientist. 2013 doi: 10.1177/1073858413510208. [DOI] [PubMed] [Google Scholar]
- 62.GUIDETTI P, HOFFMAN GE, MELENDEZ-FERRO M, ALBUQUERQUE EX, SCHWARCZ R. Astrocytic localization of kynurenine aminotransferase II in the rat brain visualized by immunocytochemistry. Glia. 2007;55:78–92. doi: 10.1002/glia.20432. [DOI] [PubMed] [Google Scholar]
- 63.ALEXANDER KS, WU HQ, SCHWARCZ R, BRUNO JP. Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the α7 positive modulator galantamine. Psychopharmacology (Berl) 2012;220:627–637. doi: 10.1007/s00213-011-2539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.SCHWARCZ R, HUNTER CA. Toxoplasma gondii and schizophrenia: linkage through astrocyte-derived kynurenic acid? Schizophr Bull. 2007;33:652–653. doi: 10.1093/schbul/sbm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.OBERHEIM NA, TIAN GF, HAN X, PENG W, TAKANO T, RANSOM B, NEDERGAARD M. Loss of astrocytic domain organization in the epileptic brain. J Neurosci. 2008;28:3264–3276. doi: 10.1523/JNEUROSCI.4980-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.CARMIGNOTO G, HAYDON PG. Astrocyte calcium signaling and epilepsy. Glia. 2012;60:1227–1233. doi: 10.1002/glia.22318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.COULTER DA, EID T. Astrocytic regulation of glutamate homeostasis in epilepsy. Glia. 2012;60:1215–1226. doi: 10.1002/glia.22341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.SEIFERT G, CARMIGNOTO G, STEINHAUSER C. Astrocyte dysfunction in epilepsy. Brain Res Rev. 2010;63:212–221. doi: 10.1016/j.brainresrev.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 69.SEIFERT G, STEINHAUSER C. Neuron-astrocyte signaling and epilepsy. Exp Neurol. 2011 doi: 10.1016/j.expneurol.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 70.GOTZ J, STREFFER JR, DAVID D, SCHILD A, HOERNDLI F, PENNANEN L, KUROSINSKI P, CHEN F. Transgenic animal models of Alzheimer’s disease and related disorders: histopathology, behavior and therapy. Mol Psychiatry. 2004;9:664–683. doi: 10.1038/sj.mp.4001508. [DOI] [PubMed] [Google Scholar]
- 71.GOTZ J, MATAMALES M, GOTZ NN, ITTNER LM, ECKERT A. Alzheimer’s disease models and functional genomics-How many needles are there in the haystack? Front Physiol. 2012;3:320. doi: 10.3389/fphys.2012.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.ACEVEDO-AROZENA A, KALMAR B, ESSA S, RICKETTS T, JOYCE P, KENT R, ROWE C, PARKER A, GRAY A, HAFEZPARAST M, THORPE JR, GREENSMITH L, FISHER EM. A comprehensive assessment of the SOD1G93A low-copy transgenic mouse, which models human amyotrophic lateral sclerosis. Dis Model Mech. 2011;4:686–700. doi: 10.1242/dmm.007237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.SKAPER SD, GIUSTI P. Transgenic mouse models of Parkinson’s disease and Huntington’s disease. CNS Neurol Disord Drug Targets. 2010;9:455–470. doi: 10.2174/187152710791556186. [DOI] [PubMed] [Google Scholar]
- 74.ROSSI D, BRAMBILLA L, VALORI CF, RONCORONI C, CRUGNOLA A, YOKOTA T, BREDESEN DE, VOLTERRA A. Focal degeneration of astrocytes in amyotrophic lateral sclerosis. Cell Death Differ. 2008;15:1691–1700. doi: 10.1038/cdd.2008.99. [DOI] [PubMed] [Google Scholar]
- 75.STAATS KA, VAN DEN BOSCH L. Astrocytes in amyotrophic lateral sclerosis: direct effects on motor neuron survival. J Biol Phys. 2009;35:337–346. doi: 10.1007/s10867-009-9141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.WANG L, GUTMANN DH, ROOS RP. Astrocyte loss of mutant SOD1 delays ALS disease onset and progression in G85R transgenic mice. Hum Mol Genet. 2011;20:286–293. doi: 10.1093/hmg/ddq463. [DOI] [PubMed] [Google Scholar]
- 77.YAMANAKA K, CHUN SJ, BOILLEE S, FUJIMORI-TONOU N, YAMASHITA H, GUTMANN DH, TAKAHASHI R, MISAWA H, CLEVELAND DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat Neurosci. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.KORSAKOFF SS, KOPCAKOB CC. Психическое расстройство в сочетании с множественным невритом (psychosis polineuritica, s. cerebropathia psychica toxaemica). Psychic disorder in conjunction with multiple neuritis. Korsakoff SS, Victor M, Yakovlev, translators. Neurology (1955) 1889;5:394– 406. doi: 10.1212/wnl.5.6.394. [DOI] [PubMed]; Мед обозр. 32:3–18. [Google Scholar]
- 79.WERNICKE C. Lehrbuch der Gehirnkrankheiten für Aerzte und Studirende. Theodor Fischer; Kassel und Berlin: 1881–1883. [Google Scholar]
- 80.HAZELL AS. Astrocytes are a major target in thiamine deficiency and Wernicke’s encephalopathy. Neurochem Int. 2009;55:129–135. doi: 10.1016/j.neuint.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 81.HAZELL AS, SHEEDY D, OANEA R, AGHOURIAN M, SUN S, JUNG JY, WANG D, WANG C. Loss of astrocytic glutamate transporters in Wernicke encephalopathy. Glia. 2009;58:148–156. doi: 10.1002/glia.20908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.BRAAK H, DE VOS RA, JANSEN EN, BRATZKE H, BRAAK E. Neuropathological hallmarks of Alzheimer’s and Parkinson’s diseases. Prog Brain Res. 1998;117:267–285. doi: 10.1016/s0079-6123(08)64021-2. [DOI] [PubMed] [Google Scholar]
- 83.SELKOE DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 84.RODRIGUEZ JJ, OLABARRIA M, CHVATAL A, VERKHRATSKY A. Astroglia in dementia and Alzheimer’s disease. Cell Death Differ. 2009;16:378–385. doi: 10.1038/cdd.2008.172. [DOI] [PubMed] [Google Scholar]
- 85.VERKHRATSKY A, OLABARRIA M, NORISTANI HN, YEH CY, RODRIGUEZ JJ. Astrocytes in Alzheimer’s disease. Neurotherapeutics. 2010 doi: 10.1016/j.nurt.2010.05.017. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.BEAUQUIS J, VINUESA A, POMILIO C, PAVIA P, GALVAN V, SARAVIA F. Neuronal and glial alterations, increased anxiety, and cognitive impairment before hippocampal amyloid deposition in PDAPP mice, model of Alzheimer’s disease. Hippocampus. 2014;24:257–269. doi: 10.1002/hipo.22219. [DOI] [PubMed] [Google Scholar]
- 87.BEAUQUIS J, PAVIA P, POMILIO C, VINUESA A, PODLUTSKAYA N, GALVAN V, SARAVIA F. Environmental enrichment prevents astroglial pathological changes in the hippocampus of APP transgenic mice, model of Alzheimer’s disease. Exp Neurol. 2013;239:28–37. doi: 10.1016/j.expneurol.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 88.RODRIGUEZ JJ, TERZIEVA S, OLABARRIA M, LANZA RG, VERKHRATSKY A. Enriched environment and physical activity reverse astrogliodegeneration in the hippocampus of AD transgenic mice. Cell Death Dis. 2013;4:e678. doi: 10.1038/cddis.2013.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.KULIJEWICZ-NAWROT M, VERKHRATSKY A, CHVATAL A, SYKOVA E, RODRIGUEZ JJ. Astrocytic cytoskeletal atrophy in the medial prefrontal cortex of a triple transgenic mouse model of Alzheimer’s disease. J Anat. 2012;221:252–262. doi: 10.1111/j.1469-7580.2012.01536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.YEH CY, VADHWANA B, VERKHRATSKY A, RODRIGUEZ JJ. Early astrocytic atrophy in the entorhinal cortex of a triple transgenic animal model of Alzheimer’s disease. ASN Neuro. 2011;3:271–279. doi: 10.1042/AN20110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.OLABARRIA M, NORISTANI HN, VERKHRATSKY A, RODRIGUEZ JJ. Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer’s disease. Glia. 2010;58:831–838. doi: 10.1002/glia.20967. [DOI] [PubMed] [Google Scholar]
- 92.RUSH T, BUISSON A. Reciprocal disruption of neuronal signaling and Abeta production mediated by extrasynaptic NMDA receptors: a downward spiral. Cell Tissue Res. 2014 doi: 10.1007/s00441-013-1789-1. [DOI] [PubMed] [Google Scholar]
- 93.OLABARRIA M, NORISTANI HN, VERKHRATSKY A, RODRIGUEZ JJ. Age-dependent decrease in glutamine synthetase expression in the hippocampal astroglia of the triple transgenic Alzheimer’s disease mouse model: mechanism for deficient glutamatergic transmission? Mol Neurodegener. 2011;6:55. doi: 10.1186/1750-1326-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.KUCHIBHOTLA KV, LATTARULO CR, HYMAN BT, BACSKAI BJ. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science. 2009;323:1211–1215. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.GAGYI E, KORMOS B, CASTELLANOS KJ, VALYI-NAGY K, KORNEFF D, LOPRESTI P, WOLTJER R, VALYI-NAGY T. Decreased oligodendrocyte nuclear diameter in Alzheimer’s disease and Lewy body dementia. Brain Pathol. 2012;22:803–810. doi: 10.1111/j.1750-3639.2012.00595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.BEHRENDT G, BAER K, BUFFO A, CURTIS MA, FAULL RL, REES MI, GOTZ M, DIMOU L. Dynamic changes in myelin aberrations and oligodendrocyte generation in chronic amyloidosis in mice and men. Glia. 2013;61:273–286. doi: 10.1002/glia.22432. [DOI] [PubMed] [Google Scholar]
- 97.RODRIGUEZ JJ, NORISTANI HN, VERKHRATSKY A. Microglial response to Alzheimer’s disease is differentially modulated by voluntary wheel running and enriched environments. Brain Struct Funct. 2013 doi: 10.1007/s00429-013-0693-5. [DOI] [PubMed] [Google Scholar]
- 98.RODRIGUEZ JJ, WITTON J, OLABARRIA M, NORISTANI HN, VERKHRATSKY A. Increase in the density of resting microglia precedes neuritic plaque formation and microglial activation in a transgenic model of Alzheimer’s disease. Cell Death Dis. 2010;1:e1. doi: 10.1038/cddis.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.PETERS O, SCHIPKE CG, PHILIPPS A, HAAS B, PANNASCH U, WANG LP, BENEDETTI B, KINGSTON AE, KETTENMANN H. Astrocyte function is modified by Alzheimer’s disease-like pathology in aged mice. J Alzheimers Dis. 2009;18:177–189. doi: 10.3233/JAD-2009-1140. [DOI] [PubMed] [Google Scholar]
- 100.BROE M, KRIL J, HALLIDAY GM. Astrocytic degeneration relates to the severity of disease in frontotemporal dementia. Brain. 2004;127:2214–2220. doi: 10.1093/brain/awh250. [DOI] [PubMed] [Google Scholar]
- 101.KERSAITIS C, HALLIDAY GM, KRIL JJ. Regional and cellular pathology in frontotemporal dementia: relationship to stage of disease in cases with and without Pick bodies. Acta Neuropathol. 2004;108:515–523. doi: 10.1007/s00401-004-0917-0. [DOI] [PubMed] [Google Scholar]
- 102.POTTS R, LEECH RW. Thalamic dementia: an example of primary astroglial dystrophy of Seitelberger. Clin Neuropathol. 2005;24:271–275. [PubMed] [Google Scholar]
- 103.ROSENBAUM AI, MAXFIELD FR. Niemann-Pick type C disease: molecular mechanisms and potential therapeutic approaches. J Neurochem. 2011;116:789–795. doi: 10.1111/j.1471-4159.2010.06976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.ESTRADA-SÁNCHEZ AM, REBEC GV. Corticostriatal dysfunction and glutamate transporter 1 (GLT1) in Huntington’s disease: Interactions between neurons and astrocytes. Basal Ganglia. 2012 doi: 10.1016/j.baga.2012.04.029. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.LEE W, REYES RC, GOTTIPATI MK, LEWIS K, LESORT M, PARPURA V, GRAY M. Enhanced Ca2+-dependent glutamate release from astrocytes of the BACHD Huntington’s disease mouse model. Neurobiol Dis. 2013;58:192–199. doi: 10.1016/j.nbd.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.MCGEER PL, MCGEER EG. Glial reactions in Parkinson’s disease. Mov Disord. 2008;23:474–483. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]