Abstract
Aim. To investigate the relationship between hepatitis B e antigen seroconversion and the function of dendritic cells (DC) in patients with hepatitis B virus. Methods. The peripheral blood mononuclear cells (PBMC) from 21 chronic HBV patients in immune tolerance state, 23 patients in inactive HBsAg carrier state, and 10 healthy HBV-naive blood donors were incubated and induced into DC in presence of granulocyte-macrophage colony stimulating factor (GM-CSF) and interleukin-4 (IL-4), respectively. The expressions of surface markers on DC were detected by flow cytometry, and the stimulatory capacity of DC in allogenic mixed leukocyte reaction (MLR) was tested by CCK-8, and the level of cytokines released by DC was analyzed by enzyme-linked immunosorbent assay (ELISA). Results. DC from patients in immune tolerance showed a remarkably lower surface expression of CD80, CD86, and HLA-DR and exhibited an impaired stimulatory capacity in MLR and reduced secretion of IL-12, as compared to the patients in inactive HBsAg carrier state. There was no significant difference between the indicators from the patients in inactive HBsAg carrier state and healthy subjects. There was a significant difference of HBV DNA level between immune tolerance and inactive HBsAg carrier group (P < 0.01) and a negative correlation between HBV DNA level and the expressions of dendritic cells in both groups, respectively (P = 0.01). Conclusion. DC from patients in inactive HBsAg carrier state shows stronger function in comparison with patients in immune tolerance, the expressions of dendritic cells correlate with HBV DNA level, and the function stage of DC may play an important role in HBeAg seroconversion.
1. Background
According to statistics, chronic infection with hepatitis B virus (HBV), which poses serious risks to human, currently affects about 350 million people [1–3], and China alone has over 120 million people. Hepatitis B infection is a leading cause of chronic hepatitis, cirrhosis, and hepatocellular carcinoma [4], accounting for 1 million deaths annually. The natural course of chronic HBV infection can be divided into four phases: immune tolerance, immune clearance, low or nonreplication, and reactivation [5, 6]. Inphase 1 patients are HBeAg positive with high HBV replication but minimal liver disease, and phase 3 is the inactive phase during which HBeAg is absent, viral concentrations are low, and there is minimal inflammatory activity in the liver. Although liver disease activities are low in phase 1, 25% of them tend to have liver disease progression through frequency, extent, and severity of hepatitis flares or acute exacerbations in the second immune clearance; in general, early termination of immune tolerance and subsequent HBeAg seroconversion usually confer a favorable outcome, whereas delayed HBeAg seroconversion may accelerate the progression of liver disease.
In the pathogenesis of persistent HBV infection, both the virus and the immune response of the host play a major role. Recently, accumulating reports have shown that the number and the function of dendritic cells are impaired to a certain extent in patients infected with hepatitis B virus [7, 8]. An impaired function of DC may be suggested to account for HBV antigen-presenting and T cells stimulation defect, which may be the cause of chronic hepatitis B virus infection.
Thus, in this study, we investigated the phenotype and function of dendritic cells generated from peripheral blood mononuclear cells (PBMCs) of patients in immune tolerance and low or nonreplication to determine whether there is any difference between them. We hope that these data will be useful in explaining the difference of immune status and level of virus between the patients from two phases.
2. Objectives
The objectives are to detect the functional state of dendritic cells from different patients and relate the function and the state of HBV replication.
3. Study Design
3.1. Subjects
In total, 44 patients with chronic HBV infection were enrolled from the First Affiliated Hospital of Wenzhou Medical College. All the cases were identified according to chronic hepatitis B diagnosis standard (Conference in Xi'an, China, September 2000); patients were positive for hepatitis B surface antigen for at least 6 months; we excluded the patients with other viral hepatitis infections, and anti-HIV were negative. No patient studied had received antiviral treatment before or had any complication such as hepatic cirrhosis and hepatocellular carcinoma (HCC). The 44 patients were divided into two groups: 21 patients were HBeAg positive in immune tolerance (IT) with high serum levels of HBV-DNA, also seropositivity for HBsAg and anti-HBcAbs and normal alanine aminotransferase (ALT) levels; the level of ALT in serum was measured by autoanalyzer (HITACHI 7600-110ISE); the cutoff for the upper limit of normal (ULN) was ALT 50 U/L; 23 patients were anti-HBeAb positive in inactive HBsAg carrier state (ISC) with undetectable or low levels of HBV-DNA and also HBsAg and anti-HBcAb positivity and normal ALT levels. All the patients in group IT and group ISC were analysed for the HBV genotype. The baseline clinical data were shown in Table 1. As controls, 10 healthy HBV-naive blood donors (NC) were assessed. The study protocol was approved by the ethics committee of the author's unit, and written informed consent was obtained from all individuals.
Table 1.
Clinical characteristics of patients and normal controls (mean ± SD).
| Parameters | IT patients (HBV-DNA >104 copies/mL) |
ISC patients (HBV-DNA <104 copies/mL) |
Normal controls |
|---|---|---|---|
| n | 21 | 23 | 10 |
| Age (years) | 30.36 ± 7.79 | 33.15 ± 6.91 | 29.10 ± 4.33 |
| Sex (male/female) | 10/1 | 11/2 | 6/4 |
| ALT (IU/L) | 36.45 ± 16.94 | 29.62 ± 13.91 | 30.10 ± 11.82 |
| AST (IU/L) | 30.64 ± 11.84 | 29.23 ± 10.03 | 26.90 ± 12.13 |
| HBV-DNA (lg copies/mL) | 6.42 ± 1.07* | 3.32 ± 3.93 | — |
| Genotype (type B/C) | 4/17 | 9/14 | — |
Note: * P < 0.01 versus ISC patients.
3.2. Dendritic Cell Isolation and Culture
Monocyte-derived DCs (MoDCs) were prepared from peripheral blood mononuclear cells (PBMCs) mainly according to previously established protocols [9, 10]. PBMCs were isolated from freshly drawn heparinized whole blood by Ficoll-Hypaque density gradient centrifugation, washed two times, and resuspended at 2 × 106/mL in RPMI 1640 (RPMI 1640 medium was purchased from GIBCO BRL, Gaithersburg, MD, USA). After 3 h incubation at 37°C in 5% CO2 in 6-well plates, supernatant was discarded and adherent cells were incubated in RPMI 1640 plus 10% fetal bovine serum overnight. The nonadherent cells were gently removed, RPMI 1640 with 10% fetal bovine serum supplemented with 100 ng/mL of rhGM-CSF (Peprotech, England). And 50 ng/mL of rhIL-4 (Peprotech, England) was added to the wells. Half of the medium was refreshed and cytokine was added at the middle concentration every 2 days. On the 7th day of incubation, 25 ng/mL of rhTNF-α (Peprotech, England) was added to the medium, and then the cells were collected on the 9th day.
3.3. Morphology of Dendritic Cells
The morphology of dendritic cells was monitored by light microscope.
3.4. Flow Cytometry of Surface Markers
On day 9, dendritic cells were harvested and stained with conjugated monoclonal mouse-anti-human antibodies, FITC-anti-CD80, PE-anti-CD86, and PE-anti-HLA-DR (all purchased from Ebioscience, San Diego, USA), for 20 min at room temperature in darkness. Isotype-matched antibodies were used as controls. After washing once with PBS, dendritic cells were fixed in 1% paraformaldehyde and analyzed by flow cytometry.
3.5. T Cell Stimulation
After being treated with 25 μg/mL of mitomycin at 37°C for 30 min, dendritic cells were plated at concentrations of 2 × 104, 1 × 104, and 5 × 103 cells per well separately, then mixed, and incubated with nonadherent PBMC from the same healthy person at concentrations of 1 × 105 cells per well in triplicate. The total volume was adjusted to 200 μL/well, and the cells were incubated in RPMI 1640 with 10% FBS for additional 96 h at 37°C in 5% CO2, then 20 μL/well tetrazolium salt (CCK-8) (purchased from Tongren, Japan) was added to the medium for 4 h before the end of culture, while T lymphocyte group (without dendritic cells incubated together) and only RPMI 1640 medium group were, respectively, established as negative control group and background group. Absorbance (A) was measured by ELX800G (Biotech, USA) at a detection wavelength of 450 nm and a reference wavelength of 630 nm. The results were expressed as stimulation index (SI) calculated by the following formula: stimulation index = (values of the sample − the background values)/(values of the negative control − the background values).
3.6. Cytokine Secretion by Dendritic Cell
Supernatants from mixed lymphocyte reaction (MLR) were collected on day 4 and IL-12p70 was detected using an enzyme-linked immunosorbent assay (ELISA), which was purchased from R&D Systems (Minneapolis, MN, USA) and following the manufacturer's instruction.
3.7. Data Analysis
Data were expressed as mean ± SD. All data were analyzed utilizing SPSS-XII software. Parameters collected with homogeneity of variance between groups were determined by least significant difference test (LSD), while data with heterogeneity of variance were determined by Dennett T3 test. Correlations between HBV DNA level and the expressions of dendritic cells were evaluated using Spearman's correlation coefficient.
4. Results
4.1. Morphological Characteristics of Dendritic Cells
On the third day, many cells grew branched projections and small adherent aggregates could be observed. On the 7th day, more and more cells were induced and began to suspend. In contrast to another two groups, dendritic cells of patients in immune tolerance have more dead or apoptosis cells with particles in cytoplasm, which are shown in Figure 1.
Figure 1.
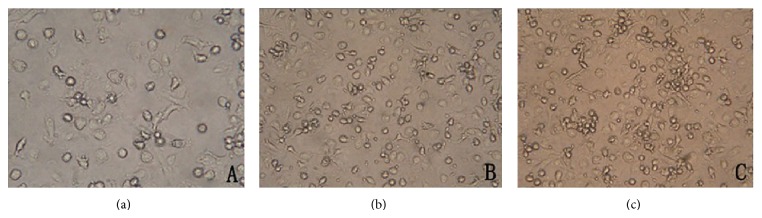
Morphological characteristics of dendritic cells in light microscope that were cultured for 7 days from two kinds of chronic HBV carriers and healthy controls ×200. (a) Dendritic cells cultured from patients in immune tolerance; (b) dendritic cells cultured from patients in inactive HBsAg carrier state; (c) dendritic cells cultured from normal controls.
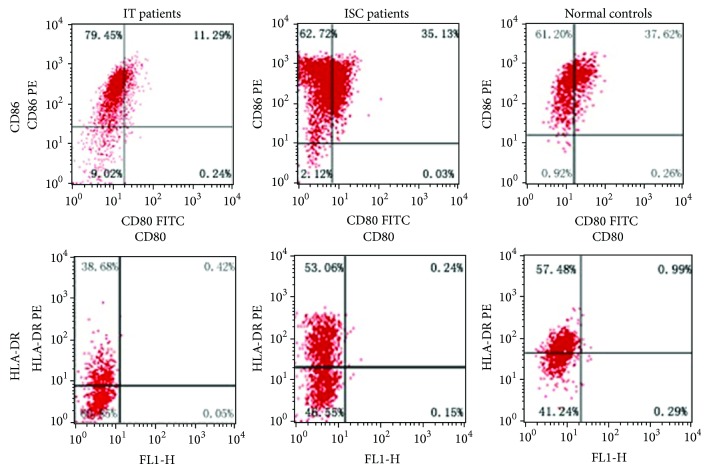
4.2. Phenotype of Dendritic Cells
On day 9 of culture, phenotypic analysis showed that expression levels of CD80, CD86, and HLA-DR were lower in patients in immune tolerance than that in another two groups. Isolated dendritic cells from patients in inactive HBsAg carrier state exhibited similar expression of surface molecules as dendritic cells from healthy controls. The results were shown in Table 2 and Figure 2.
Table 2.
Detection of dendritic cells' phenotypes in patients and normal controls (%, mean ± SD).
| Group | Case | CD80 (%) | CD86 (%) | HLA-DR (%) |
|---|---|---|---|---|
| IT patients | 21 | 11.98 ± 6.69∗# | 90.03 ± 3.01∗# | 38.40 ± 8.52∗# |
| ISC patients | 23 | 34.83 ± 9.62 | 95.99 ± 4.59 | 54.38 ± 12.45 |
| Normal controls | 10 | 37.62 ± 9.88 | 96.15 ± 3.33 | 58.32 ± 7.68 |
Note: * P < 0.01 versus ISC patients and # P < 0.01 versus normal controls.
Figure 2.
Expression of surface markers on dendritic cells cultured for 9 days.
4.3. Allogeneic T Cell Proliferation of Dendritic Cells
In allergenic mixed leukocyte reaction, the level of T cell proliferation induced by dendritic cells increased in ratio between DC and T cell-dependent manner. Dendritic cells from ISC patients had a stronger stimulatory capacity than that from IT patients in Figure 3. There was a significant difference between them, especially when DC and T cell were at a ratio of 1 : 5 and 1 : 10. The experiment did not reveal significant differences between DC from ISC patients and healthy control in T cell proliferation.
Figure 3.
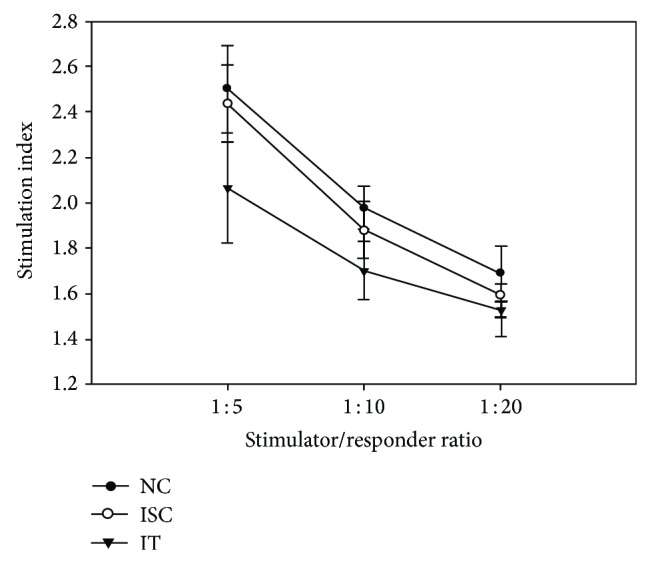
Effect of dendritic cells' stimulation on proliferation of T lymphocytes in mixed lymphocytes reaction.
4.4. Cytokine Secretion by Dendritic Cells
The levels of IL-12p70 were 28.11 ± 4.29 pg/mL in IT patients, 34.05 ± 6.11 pg/mL in ISC patients, and 35.46 ± 4.93 pg/mL in healthy controls. The results showed that IL-12p70 was reduced quantitatively in dendritic cells cultured from IT patients compared with ISC patients or healthy controls. However, there were no statistically significant differences between dendritic cells from patients in inactive HBsAg carrier state or healthy people in the secretion of IL-12p70.
4.5. HBV DNA Level and Genotype
There was a significant difference of HBV DNA level between immune tolerance and inactive HBsAg carrier group (P < 0.01) and a negative correlation between HBV DNA level and the expressions of dendritic cells in both groups, respectively (P = 0.01). All the patients were of genotype B or genotype C in these two groups. The percentage of genotype C was higher than genotype B in group IT and group ISC, respectively. Furthermore, the percentage of genotype C in group IT was higher than that in group ISC.
5. Discussion
The mechanism of how viral infections evade the immune response and lead to chronic infection has become a research hot spot. It is believed that the cytotoxic T lymphocyte (CTL) response plays a major role in controlling HBV infection [11]. Patients with acute viral hepatitis, who successfully cleared the virus, mounted a large number of HBV-specific CTL response. On the contrary, this response is absent or extremely weak in chronically infected patients who do not clear the virus. Thus, viral persistence or chronicity is associated with an inadequate CTL response [12, 13].
Dendritic cells are considered as the most powerful antigen-presenting cells (APCs) playing a strategic role in initiating and modulating the immune response [14]. DCs are uniquely well equipped in antigen-capturing, processing, and presenting function and act as key players in initiating T lymphocyte activation against viral agents [15, 16]. Beckebaum et al. [17] proposed that HBV infection compromised the antigen-presenting function of DC with concomitant impairment of T helper cell type 1 responses.
In this present study, by the means of GM-CSF and IL-4, DC isolated from IT patients showed decreased expression of CD80, CD86, and HLA-DR and lower allostimulatory capacity when DC and T cell were at a ratio of 1 : 5 and 1 : 10 compared with ISC patients. However, there was no significant difference between them when DC and T cell were at a ratio of 1 : 20. Perhaps the main reason for that is that the number of DCs is too small, leading to no obvious difference in lymphocyte proliferation.
At least, two signals are necessary to activate a naive lymphocyte before it can recognize and target HBV antigen. Binding of the antigen-MHC complex and TCR on naive T lymphocyte represents the first activation signal; costimulatory signal provided by ligation of CD28 by B7 molecules on the naive T cell represents the second signal. Activated DCs have an ability to process antigens and express high levels of costimulatory molecules; thus they can provide both signals needed for T cell activation. In our study, the low expression of costimulatory molecules and reduced allostimulation by IT patients DC would indicate failure of antigen presentation, specially HBeAg presentation and T cell stimulation, inducing lack of HBV-specific immune response, which may play an important role in the difference of immune response between IT patients and ISC patients. By testing the effect of passive immunization with anti-HBe immunoglobulin free of antibody to hepatitis B surface antigen on experimental HBV infection in the chimpanzee model, Stephan et al. [18] suggested that anti-HBe might have biological activity in the modulation of HBV replication. Therefore, the reason for obvious differences in HBV replication between IT and ISC patients may be due to the lack of immune response to HBeAg caused by dysfunction of DC.
The key cytokines provided by DC are considered the third pathway to initiate an adaptive immune response. IL-12 is a main effector in the third pathway to induce helper T lymphocyte (Th) response towards Th1 cell differentiation. It is also involved in the generation of cytotoxic T lymphocyte (CTL) and activation of cytotoxicity in CD8+ T cells, especially potentiating gamma interferon (IFN-γ) production by T lymphocytes [19]. Our experimental results showed a reduced secretion of IL-12 by DC in IT patients which induced Th1/Th2 imbalance. This discrepancy may lie in HBV persistent replication.
After HBeAg seroconversion, some patients reach the fourth phase as HBeAg-negative chronic hepatitis B characterized by negative HBeAg, positive anti-HBe, detectable HBV-DNA (104–108 copies/mL), and elevated aminotransferases. There are two hypotheses explaining the reactivation of HBV replication. Precore (Pre-c) mutations abrogate HBeAg synthesis by creating a translational stop codon, while basal core promoter (BCP) mutations reduce HBeAg expression by transcriptional mechanisms [20–22]. Thus, anti-HBe in IT patients is not specific for the mutant virus. This does not seem to be true in HBeAg seroconversion sense. Therefore, no patient in HBeAg-negative chronic hepatitis B was taken into HBeAg seroconversion group.
We found isolated DC from ISC patients exhibited similar expression of surface markers, alloreactive T cell stimulation, and IL-12 secretion as control DC, corresponding to previous findings [23].
HBV genotypes were previously shown to have distinct geographic and ethnic distribution, with genotypes B and C prevailing in Southeast Asia [24], and our present study further confirmed this finding; all the patients were of genotype B or genotype C. Previous studies have shown that HBV genotypes influence disease severity and long-term clinical outcomes of HBV infection [25]. Compared to genotype B patients, genotype C patients have late or absent HBeAg seroconversion after multiple hepatitis flares that accelerate the progression of chronic hepatitis [24, 26]. Most previous studies indicated that patients with HBV genotype C infection have a higher risk of cirrhosis and HCC than those with genotype B infection [27–29]. The impact of viral load on the risk of HCC was assessed in a population-based prospective cohort of untreated CHB Taiwanese patients (REVEAL-HBV study) [30]. We found that the HBV DNA level of genotype C was higher than genotype B. The HBV DNA could influence the expression of dendritic cells concentration. But these differences need to be more strictly reconfirmed by a larger number of cases.
In conclusion, the present study demonstrates that DC shows stronger function after HBeAg seroconversion. The change of the function of DC may play an important role in the difference of immune response and replication of HBV between IT patients and ISC patients. We planned to pulse DC cells with HBeAg or HBcAg to enhance the functions of DC, especially the immune response to HBeAg in further study.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Wang F. S. Current status and prospects of studies on human genetic alleles associated with hepatitis B virus infection. World Journal of Gastroenterology. 2003;9(4):641–644. doi: 10.3748/wjg.v9.i4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee W. M. Hepatitis B virus infection. The New England Journal of Medicine. 1997;337(24):1733–1745. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 3.Kao J. H., Chen D. S. Global control of hepatitis B virus infection. The Lancet Infectious Diseases. 2002;2(7):395–403. doi: 10.1016/S1473-3099(02)00315-8. [DOI] [PubMed] [Google Scholar]
- 4.Hoofnagle J. H., di Bisceglie A. M. The treatment of chronic viral hepatitis. The New England Journal of Medicine. 1997;336(5):347. doi: 10.1056/NEJM199701303360507. [DOI] [PubMed] [Google Scholar]
- 5.Lok A. S. F., McMahon B. J. Chronic hepatitis B. Hepatology. 2007;45(2):507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 6.Yim H. J., Lok A. S.-F. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology. 2006;43(2):S173–S181. doi: 10.1002/hep.20956. [DOI] [PubMed] [Google Scholar]
- 7.van der Molen R. G., Sprengers D., Binda R. S., de Jong E. C., Niesters H. G. M., Kusters J. G., Kwekkeboom J., Janssen H. L. A. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology. 2004;40(3):738–746. doi: 10.1002/hep.20366. [DOI] [PubMed] [Google Scholar]
- 8.Duan X.-Z., Zhuang H., Wang M., Li H.-W., Liu J.-C., Wang F.-S. Decreased numbers and impaired function of circulating dendritic cell subsets in patients with chronic hepatitis B infection (R2) Journal of Gastroenterology and Hepatology (Australia) 2005;20(2):234–242. doi: 10.1111/j.1440-1746.2004.03529.x. [DOI] [PubMed] [Google Scholar]
- 9.Romani N., Gruner S., Brang D., Kämpgen E., Lenz A., Trockenbacher B., Konwalinka G., Fritsch P. O., Steinman R. M., Schuler G. Proliferating dendritic cell progenitors in human blood. Journal of Experimental Medicine. 1994;180(1):83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Voorhis W. C., Hair L. S., Steinman R. M., Kaplan G. Human dendritic cells. Enrichment and characterization from peripheral blood. The Journal of Experimental Medicine. 1982;155(4):1172–1187. doi: 10.1084/jem.155.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertoletti A., Maini M., Williams R. Role of hepatitis B virus specific cytotoxic T cells in liver damage and viral control. Antiviral Research. 2003;60(2):61–66. doi: 10.1016/j.antiviral.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Sprengers D., Molen R. G. V. D., Kusters J. G., Man R. A. D., Niesters H. G. M., Schalm S. W., Janssen H. L. A. Analysis of intrahepatic HBV-specific cytotoxic T-cells during and after acute HBV infection in humans. Journal of Hepatology. 2006;45(2):182–189. doi: 10.1016/j.jhep.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Chang J. J., Wightman F., Bartholomeusz A., Ayres A., Kent S. J., Sasadeusz J., Lewin S. R. Reduced hepatitis B virus (HBV)-specific CD4+ T-cell responses in human immunodeficiency virus type 1-HBV-coinfected individuals receiving HBV-active antiretroviral therapy. Journal of Virology. 2005;79(5):3038–3051. doi: 10.1128/JVI.79.5.3038-3051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas R., Lipsky P. E. Dendritic cells: origin and differentiation. Stem Cells. 1996;14(2):196–206. doi: 10.1002/stem.140196. [DOI] [PubMed] [Google Scholar]
- 15.Steinman R. M. The dendritic cell system and its role in immunogenicity. Annual Review of Immunology. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 16.Dubois B., Vanbervliet B., Fayette J., Massacrier C., Van Kooten C., Brière F., Banchereau J., Caux C. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. Journal of Experimental Medicine. 1997;185(5):941–951. doi: 10.1084/jem.185.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beckebaum S., Cicinnati V. R., Zhang X., Ferencik S., Frilling A., Grosse-Wilde H., Broelsch C. E., Gerken G. Hepatitis B virus-induced defect of monocyte-derived dendritic cells leads to impaired T helper type 1 response in vitro: mechanisms for viral immune escape. Immunology. 2003;109(4):487–495. doi: 10.1046/j.1365-2567.2003.01699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephan W., Prince A. M., Brotman B. Modulation of hepatitis B infection by intravenous application of an immunoglobulin preparation that contains antibodies to hepatitis B e and core antigens but not to hepatitis B surface antigen. Journal of Virology. 1984;51(2):420–424. doi: 10.1128/jvi.51.2.420-424.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong S.-Q., Lin B.-L., Gao X., Tang H., Wu C.-Y. IL-12 promotes HBV-specific central memory CD8+ T cell responses by PBMCs from chronic hepatitis B virus carriers. International Immunopharmacology. 2007;7(5):578–587. doi: 10.1016/j.intimp.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Funk M. L., Rosenberg D. M., Lok A. S. F. World-wide epidemiology of HBeAg-negative chronic hepatitis B and associated precore and core promoter variants. Journal of Viral Hepatitis. 2002;9(1):52–61. doi: 10.1046/j.1365-2893.2002.00304.x. [DOI] [PubMed] [Google Scholar]
- 21.Laras A., Koskinas J., Avgidis K., Hadziyannis S. J. Incidence and clinical significance of hepatitis B virus precore gene translation initiation mutations in e antigen-negative patient. Journal of Viral Hepatitis. 1998;5(4):241–248. doi: 10.1046/j.1365-2893.1998.00109.x. [DOI] [PubMed] [Google Scholar]
- 22.Lin C.-L., Liao L.-Y., Liu C.-J., Chen P.-J., Lai M.-Y., Kao J.-H., Chen D.-S. Hepatitis B genotypes and precore/basal core promoter mutants in HBeAg-negative chronic hepatitis B. Journal of Gastroenterology. 2002;37(4):283–287. doi: 10.1007/s005350200036. [DOI] [PubMed] [Google Scholar]
- 23.Tavakoli S., Mederacke I., Herzog-Hauff S., Glebe D., Grün S., Strand D., Urban S., Gehring A., Galle P. R., Böcher W. O. Peripheral blood dendritic cells are phenotypically and functionally intact in chronic hepatitis B virus (HBV) infection. Clinical and Experimental Immunology. 2008;151(1):61–70. doi: 10.1111/j.1365-2249.2007.03547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kao J.-H., Chen P.-J., Lai M.-Y., Chen D.-S. Hepatitis B virus genotypes and spontaneous hepatitis B e antigen seroconversion in Taiwanese hepatitis B carriers. Journal of Medical Virology. 2004;72(3):363–369. doi: 10.1002/jmv.10534. [DOI] [PubMed] [Google Scholar]
- 25.Maeshiro T., Arakaki S., Watanabe T., Aoyama H., Shiroma J., Yamashiro T., Hirata T., Hokama A., Kinjo F., Nakayoshi T., Nakayoshi T., Mizokami M., Fujita J., Sakugawa H. Different natural courses of chronic hepatitis B with genotypes B and C after the fourth decade of life. World Journal of Gastroenterology. 2007;13(34):4560–4565. doi: 10.3748/wjg.v13.i34.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kao J.-H., Chen P.-J., Lai M.-Y., Chen D.-S. Genotypes and clinical phenotypes of hepatitis B virus in patients with chronic hepatitis B virus infection. Journal of Clinical Microbiology. 2002;40(4):1207–1209. doi: 10.1128/JCM.40.4.1207-1209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu C. J., Kao J. H. Global perspective on the natural history of chronic hepatitis B: role of hepatitis B virus genotypes A to J. Seminars in Liver Disease. 2013;33(2):97–102. doi: 10.1055/s-0033-1345716. [DOI] [PubMed] [Google Scholar]
- 28.Kao J.-H., Chen P.-J., Lai M.-Y., Chen D.-S. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology. 2000;118(3):554–559. doi: 10.1016/S0016-5085(00)70261-7. [DOI] [PubMed] [Google Scholar]
- 29.Yang H.-I., Yeh S.-H., Chen P.-J., et al. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. Journal of the National Cancer Institute. 2008;100(16):1134–1143. doi: 10.1093/jnci/djn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen C.-J., Yang H.-I. Natural history of chronic hepatitis B REVEALed. Journal of Gastroenterology and Hepatology (Australia) 2011;26(4):628–638. doi: 10.1111/j.1440-1746.2011.06695.x. [DOI] [PubMed] [Google Scholar]