Abstract
The role of electrical synapses in synchronizing neuronal assemblies in the adult mammalian brain is well documented. However, their role in learning and memory processes remains unclear. By combining Pavlovian fear conditioning, activity-dependent immediate early gene expression, and in vivo electrophysiology, we discovered that blocking neuronal gap junctions within the dorsal hippocampus impaired context-dependent fear learning, memory, and extinction. Theta rhythms in freely moving rats were also disrupted. Our results show that gap junction–mediated neuronal transmission is a prominent feature underlying emotional memories.
Unlike chemical synapses, the role of electrical synapses in fear learning and memory remains largely unknown (1–3). In the adult mammalian brain, gap junctions formed by connexin 36 (Cx36) couple γ-aminobutyric acid–releasing (GABAergic) interneurons that participate in the generation of synchronized oscillations (2–4). Cx36 expression has been localized within the amygdala-hippocampus-cortical axis (4, 5), and disrupted hippocampal and cortical oscillations have been reported in Cx36 knockout mice (6, 7). Electrical synapses undergo posttranslational modifications and activity-dependent plasticity similar to chemical synapses (8, 9). Thus, we hypothesized that electrical synapses may be important for the formation and maintenance of fear behaviors and memories.
Rats received intraperitoneal injections of the general gap junction blocker carbenoxolone (Cbx) (10, 11) or the selective Cx36 blocker mefloquine (Meflo) (12) and were fear-conditioned using three pairings of a neutral tone (conditional stimulus, CS) with an aversive footshock (unconditional stimulus, US) (Fig. 1, A and B). All animals exhibited equal levels of freezing when tested 24 hours later for their tone fear memories (Fig. 1C). However, both drugs significantly reduced context fear (F2,34 = 31.1, P < 0.0001; Bonferroni corrected post hoc tests at P < 0.05 indicated that both drugs were different from vehicle but not from each other) (Fig. 1D). During training, all rats froze similarly during tone presentations and the intertrial interval, indicating that the drugs did not interfere with short-term memory or the ability to freeze [3 (drug) × 3 (trial) analysis of variance (ANOVA) for freezing during tone: trial, F2,82 = 133.2, P < 0.0001; drug, F2,82 = 1.82, n.s.; interaction, F4,82 = 1.19, n.s.] (Fig. 1B; see fig. S1 for intertrial interval). Locomotor activity and shock reactivity were identical in all groups, ruling out indirect effects on sensorimotor processes (figs. S2 and S3). To determine whether the drugs affected acquisition, consolidation, or expression of context fear, we injected Cbx and Meflo posttraining, pretest, or both pretraining and pretest (Fig. 1E). Posttraining injections of Cbx and Meflo attenuated later context fear expression (one-way ANOVA, F2,25 = 9.78, P < 0.001) (Fig. 1G). Pretest manipulations did not affect fear expression (Fig. 1G), indicating that once context memories were consolidated, they became resistant to disruption of electrical communication. The deficit seen in the pretraining group was maintained in the pretraining and pretest group, ruling out a state-dependent effect (Fig. 1G).
Fig. 1.
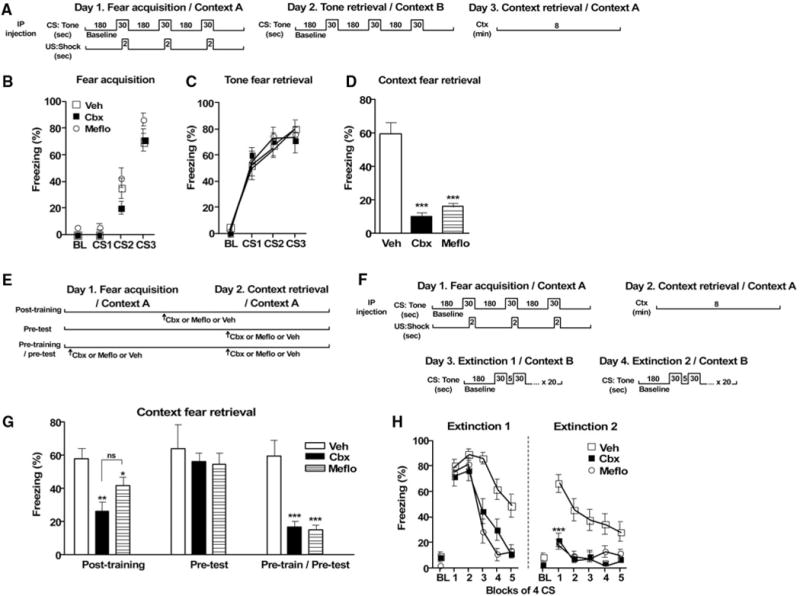
Systemic blockade of gap junctions impairs context-dependent memories and accelerates extinction. (A) Experimental design. (B) Fear acquisition [vehicle (Veh), n = 20; Cbx, n = 13; Meflo, n = 11]. (C) Tone fear memory was intact in all groups (Veh, n = 14; Cbx, n = 13; Meflo, n = 11). (D) Context fear retrieval was impaired in the drug groups (Veh, n = 14; Cbx, n = 13; Meflo, n = 11). (E) Experimental design for posttraining, pretraining, and dual pretraining and pretest injections. (F) Posttraining injections of Cbx and Meflo impaired consolidation of context fear memories (Veh, n = 10; Cbx, n = 10; Meflo, n = 8). Pretesting injections did not affect context fear retrieval (Veh, n = 12; Cbx, n = 8; Meflo, n = 6). Dual pretraining and pretesting injections showed no drug state dependency (Veh, n = 10; Cbx, n = 6; Meflo, n = 6). (G) Experimental design for extinction experiment. (H) Cbx and Meflo groups exhibited rapid reduction in freezing on days 1 and 2 (Veh, n = 15; Cbx, n = 13; Meflo, n = 10). Between-group differences: *P < 0.05, **P < 0.01, ***P < 0.001; n.s., not significant. Results are presented as means ± SEM. BL, baseline; CS, conditional stimulus; Ctx, context; US, unconditional stimulus. Contexts A and B refer to two different conditioning chambers.
Because the drugs prevented context fear learning, tone fear may have been acquired in a context-independent manner and thus might be more susceptible to extinction (13). Rats were fear-conditioned as previously described and were given 2 days of extinction training in a novel context (Fig. 1F). On extinction day 1, all groups initially exhibited similar tone fear responses (Fig. 1H). However, 40 tones across two extinction days were required for the vehicle group to fully extinguish, whereas in the drug groups, one session was sufficient to induce an accelerated decrease in freezing [3 (drug) × 4 (trial block) ANOVA; drug, F2,140 = 10.86, P = 0.0002; block, F4,140 = 75.15, P < 0.0001; interaction, F8,140 = 6.291, P < 0.0001] (Fig. 1H). When tested for extinction memory on extinction day 2, freezing in the Cbx and Meflo groups had already reached baseline levels (percent time spent freezing, average of the first four tone presentations: Cbx, 21.6 ± 6.1; Meflo, 18.5 ± 4.9; vehicle, 66.7 ± 7.4; P < 0.0001) (Fig. 1H).
Given that extinction is context-dependent, it is possible that the accelerated loss of tone fear resulted from impaired contextual learning and that the drugs’ effects were mediated by the dorsal hippocampus (DH) (14). Thus, we blocked gap junctions specifically in the DH with Cbx, Meflo, and the mimetic connexin peptides GAP27 and GAP36 (15) (Fig. 2), which were injected at both pretraining and posttraining to ensure blockade during acquisition and consolidation. Similar to systemic injections, DH microinfusions reduced context fear memories (F4,51 = 17.14, P < 0.0001) (Fig. 2, A and B) and accelerated extinction [5 (drug) × 4 (trial block) ANOVA; drug, F4,172 = 10.74, P = 0.0001; block, F4,172 = 121.7, P < 0.0001; interaction, F16,172 = 4.35, P < 0.0001] (Fig. 2C). Post hoc tests revealed a significant reduction in context fear and facilitated extinction in all drug groups, with no differences between peptides and blockers (Fig. 2, B and C). When animals were reconditioned to a white noise in a novel context and tested for context fear the next day, all groups exhibited similar amounts of context freezing (fig. S5), ruling out permanent DH damage from the infusion.
Fig. 2.
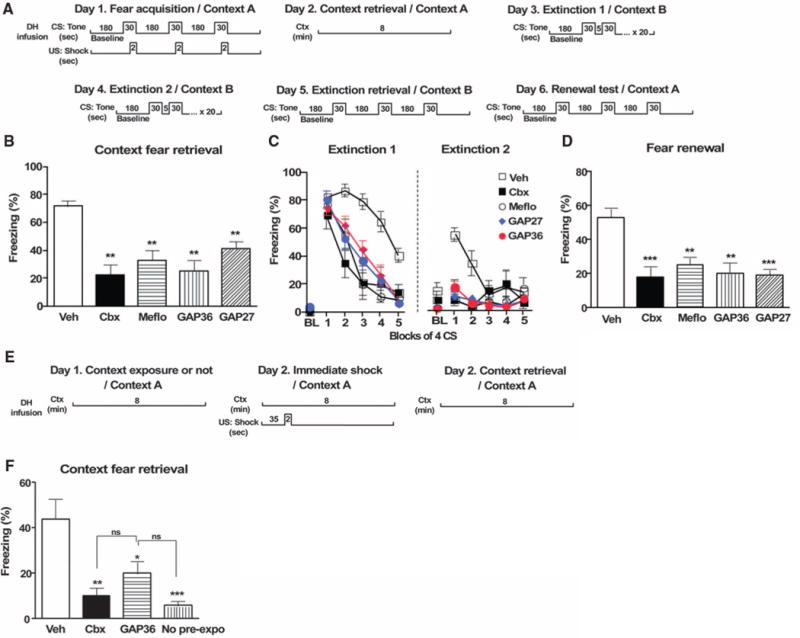
Electrical synapses within the DH control context-dependent fear memory and extinction. (A) Experimental design. (B) Context fear retrieval was impaired in Cbx, Meflo, GAP36, and GAP27 groups (Veh, n = 22; Cbx, n = 10; Meflo, n = 8; GAP36, n = 8; GAP27, n = 8). (C) Conditioned fear responses to tone extinguished faster in Cbx, Meflo, GAP36, and GAP27 groups (Veh, n = 12; Cbx, n = 8; Meflo, n = 8; GAP36, n = 10; GAP27, n = 10). (D) Fear renewal was impaired in Cbx, Meflo, GAP36, and GAP27 groups (Veh, n = 11; Cbx, n = 9; Meflo, n = 8; GAP36, n = 7; GAP27, n = 7). (E) Experimental design for immediate shock deficit paradigm and its rescue by context preexposure. (F) Microinfusions of Cbx or GAP36 in the dorsal hippocampus (DH) reduced the benefit of preexposure on the immediate shock deficit (n = 6 for all four conditions: Veh, Cbx, GAP36, and no preexposure). *P < 0.05, **P < 0.01, ***P < 0.001.
Because fear renewal is also susceptible to DH manipulations (16), we hypothesized that contextual information encoded in the presence of gap junction blockers and the subsequent enhancement of extinction might prevent renewal of fear. As predicted, fear renewal measured in the original context was compromised in the drug and peptide groups (F4,37 = 8.82, P < 0.0001) (Fig. 2, A and D). To distinguish between an effect on contextual encoding proper and encoding in the presence of an aversive experience, we used the immediate shock deficit paradigm (17, 18). Cbx or GAP36 infused before a shock-free preexposure session abolished the preexposure’s ability to rescue the immediate shock deficit (F3,20 = 9.8, P < 0.0001) (Fig. 2, E and F).
If electrical synapses in the DH are required to form and consolidate contextual representations, then rats may not recognize a context previously explored under blockade of gap junctions and treat that environment as novel. We thus examined c-fos expression as an indication of context familiarity (19, 20). Rats exposed to the testing context for the first time served as a novel environment control (“1st expo” group). In both the CA1 and CA3 regions of the hippocampus, c-fos expression was higher in the vehicle group than in the home cage controls (HC) but was lower than in animals placed in the context for the first time (1st expo, Fig. 3). However, in rats initially trained under Cbx, c-fos expression was similar to the 1st expo but higher than the vehicle and HC groups (one-way ANOVA on vehicle, CBX, 1st expo, and HC, per region: CA1, F3,12 = 65.96, P < 0.0001; CA3, F3,12 = 31.84, P < 0.0001) (Fig. 3). The 1st expo group did not receive any aversive experience in that context; this suggests that rats in the Cbx group, when reintroduced to the training context, exhibited neuronal activity similar to a first-time contextual exposure. Contrasting results were found in the basolateral amygdala (BLA), the site where context-shock associations are formed (21) (F3,13 = 38.64, P < 0.0001). Post hoc tests indicated reduced c-fos expression in both the Cbx and 1st expo groups relative to the vehicle group. There were no differences between the Cbx and 1st expo groups. However, the Cbx group showed higher c-fos expression relative to HC rats, whereas the 1st expo group did not. To distinguish the effects of blocking electrical synapses on contextual encoding versus context-shock associations, we repeated the experiment with shock-free context exposure (fig. S7). Again, c-fos expression was higher in both the Cbx and 1st expo groups in the CA1 and CA3 regions relative to the vehicle group (oneway ANOVA on Veh, CBX, and 1st expo, per region: CA1, F2,9 = 6.49, P < 0.0001; CA3, F2,9 = 5.90, P < 0.05) (fig. S7), confirming an effect on the context encoding.
Fig. 3.
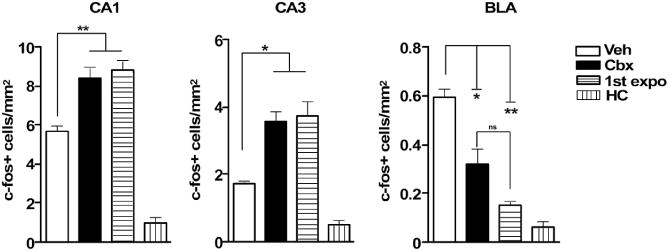
Blocking of pretraining at gap junctions affects c-fos expression within the amygdalohippocampal network, as shown by quantification of c-fos expression in CA1, CA3, and BLA. *P < 0.05, **P < 0.01.
GABAergic interneurons expressing Cx36 in the DH and medial septum drive hippocampal theta rhythms previously linked to investigatory behaviors (22–25). We recorded theta electroencephalograms (EEGs) from the CA1 of freely moving rats after Cbx intracerebroventricular (icv) infusions, which reproduced the behavioral effects on contextual memories (fig. S8). Cbx specifically disrupted theta oscillations (Fig. 4, A and B). A 3 (speed) × 2 (Cbx, pre versus post) ANOVA revealed that blocking electrical synapses attenuated the power of theta rhythms (main effect of Cbx: F1,5 = 25.6, P < 0.005) (Fig. 4C) but did not disrupt the normal positive correlation between theta power and running speed (main effect of speed: F2,10 = 5.78, P < 0.05; speed × Cbx interaction: F2,10 = 0.08, n.s.). Theta rhythm frequency was also increased after Cbx (F1,3 = 11.54, P < 0.05) (Fig. 4D), but the normal positive correlation between theta frequency and running speed was unaffected (main effect of speed: F2,6 = 5.54, P < 0.05; speed × Cbx interaction: F2,6 = 0.98, n.s.). Cbx had no effect on time spent running (F1,5 = 0.17, n.s.) or on mean running speed recorded at each frequency (Fig. 4D). Vehicle infusions did not affect theta power (F1,5 = 5.25, n.s.) or frequency (F1,5 = 0.03, n.s.).
Fig. 4.
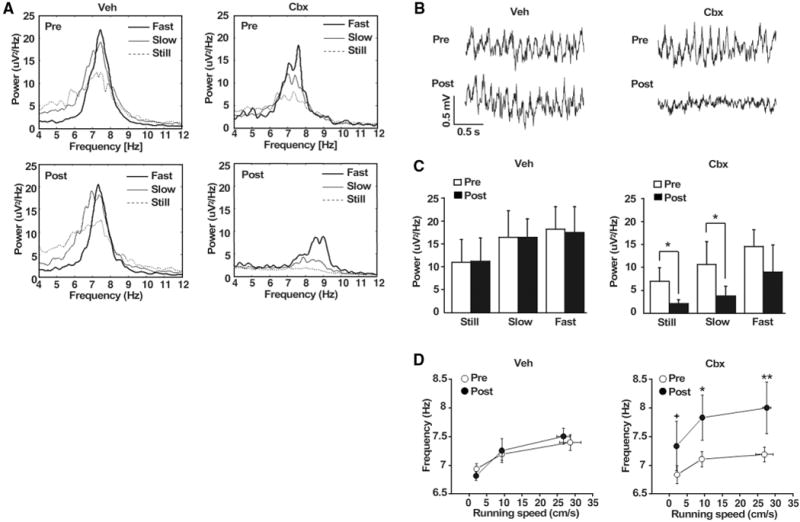
Blocking gap junctions alters hippocampal theta EEG. (A) Averaged power spectra of hippocampal EEG in the theta band (4 to 12 Hz). (B) Representative example of EEG traces. (C) Height of peak theta power was reduced by icv microinfusions. (D) Central frequency of peak theta (y axis) as a function of icv CBX or Veh infusions and running speed (n = 6). *P < 0.05, **P < 0.01, †P < 0.1 (trend).
Our c-fos analyses (Fig. 3), the immediate shock deficit experiment, and the disruption of theta rhythms during novel exploration (Fig. 4) all suggest that blocking DH gap junctions disrupts contextual encoding and thereby prevents contextual fear learning (Figs. 1 and 2). Considering that place cells fire in relation to the theta cycle (24), disrupting theta may abolish the temporal code for that location. Furthermore, blocking gap junctions in the DH before or during the consolidation of aversive experiences may disrupt theta-band synchronization within the amygdalohippocampal network required for the consolidation of fear memories (26).
Pretraining blockade of gap junctions in the DH spared tone-shock learning but rendered tone fear memories more prone to extinction and impaired renewal (Fig. 2). This suggests that extinction learning was facilitated by a lack of contextualization of the tone-shock memory. Our findings not only provide new evidence for a functional role for electrical synapses in mechanisms underlying fear learning and memory in the adult mammalian brain, but may also point toward new therapeutic avenues for the treatment of trauma and anxiety disorders.
Supplementary Material
Acknowledgments
Supported by NIMH grant RO1-MH62122 and P01NS35985 (M.S.F.), Swiss National Fund grant PA00A-115367 (S.B.), and NIMH grant RO1-MH079511 (H.T.B.).
References and Notes
- 1.Bennett MV, Zukin RS. Neuron. 2004;41:495. doi: 10.1016/s0896-6273(04)00043-1. [DOI] [PubMed] [Google Scholar]
- 2.Buzsáki G. Neuron. 2001;31:342. doi: 10.1016/s0896-6273(01)00378-6. [DOI] [PubMed] [Google Scholar]
- 3.Connors BW, Long MA. Annu Rev Neurosci. 2004;27:393. doi: 10.1146/annurev.neuro.26.041002.131128. [DOI] [PubMed] [Google Scholar]
- 4.Condorelli DF, Belluardo N, Trovato-Salinaro A, Mudò G. Brain Res Brain Res Rev. 2000;32:72. doi: 10.1016/s0165-0173(99)00068-5. [DOI] [PubMed] [Google Scholar]
- 5.Muller JF, Mascagni F, McDonald AJ. J Neurosci. 2005;25:7366. doi: 10.1523/JNEUROSCI.0899-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buhl DL, Harris KD, Hormuzdi SG, Monyer H, Buzsáki G. J Neurosci. 2003;23:1013. doi: 10.1523/JNEUROSCI.23-03-01013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frisch C, et al. Behav Brain Res. 2005;157:177. doi: 10.1016/j.bbr.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 8.Alev C, et al. Proc Natl Acad Sci USA. 2008;105:20964. doi: 10.1073/pnas.0805408105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landisman CE, et al. J Neurosci. 2002;22:1002. doi: 10.1523/JNEUROSCI.22-03-01002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Placantonakis DG, Bukovsky AA, Aicher SA, Kiem HP, Welsh JP. J Neurosci. 2006;26:5008. doi: 10.1523/JNEUROSCI.0146-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson RJ, Zhou N, MacVicar BA. Science. 2006;312:924. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- 12.Cruikshank SJ, et al. Proc Natl Acad Sci USA. 2004;101:12364. doi: 10.1073/pnas.0402044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quinn JJ, Wied HM, Ma QD, Tinsley MR, Fanselow MS. Hippocampus. 2008;18:640. doi: 10.1002/hipo.20424. [DOI] [PubMed] [Google Scholar]
- 14.Maren S, Quirk GJ. Nat Rev Neurosci. 2004;5:844. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- 15.Evans WH, Leybaert L. Cell Commun Adhes. 2007;14:265. doi: 10.1080/15419060801891034. [DOI] [PubMed] [Google Scholar]
- 16.Bouton ME, Westbrook RF, Corcoran KA, Maren S. Biol Psychiatry. 2006;60:352. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 17.Fanselow MS. Behav Brain Res. 2000;110:73. doi: 10.1016/s0166-4328(99)00186-2. [DOI] [PubMed] [Google Scholar]
- 18.Stote DL, Fanselow MS. Behav Neurosci. 2004;118:253. doi: 10.1037/0735-7044.118.1.253. [DOI] [PubMed] [Google Scholar]
- 19.Sheth A, Berretta S, Lange N, Eichenbaum H. Hippocampus. 2008;18:169. doi: 10.1002/hipo.20380. [DOI] [PubMed] [Google Scholar]
- 20.Vazdarjanova A, Guzowski JF. J Neurosci. 2004;24:6489. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fanselow MS, LeDoux JE. Neuron. 1999;23:229. doi: 10.1016/s0896-6273(00)80775-8. [DOI] [PubMed] [Google Scholar]
- 22.Buzsáki G. Neuron. 2002;33:325. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 23.Buzsáki G. Hippocampus. 2005;15:827. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- 24.Kim JJ, et al. Proc Natl Acad Sci USA. 2007;104:18297. [Google Scholar]
- 25.Freund TF, Antal M. Nature. 1988;336:170. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- 26.Seidenbecher T, Laxmi TR, Stork O, Pape HC. Science. 2003;301:846. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


