Abstract
Based on the phenolic profiles obtained by high performance liquid chromatography-electrospray ionization mass spectrometry (HPLC-DAD-ESI/MS), 24 common bean samples, representing 17 varieties and 7 generic off-the-shelf items, belonging to ten US commercial market classes can be organized into six different groups. All of them contained the same hydroxycinnaminic acids, but the flavonoid components showed distinct differences. Black beans contained primarily the 3-O-glucosides of delphinidin, petunidin and malvidin, while pinto beans contained kaempferol and its 3-O-glycosides. Light red kidney bean contained traces of quercetin 3-O-glucoside and its malonates, but pink and dark red kidney beans contained the diglycosides of quercetin and kaempferol. Small red beans contained kaempferol 3-O-glucoside and pelargonidin 3-O-glucoside, while no flavonoids were detected in alubia, cranberry, great northern, and navy beans. This is the first report of the tentative identification of quercetin 3-O-pentosylhexoside and flavonoid glucoside malonates, and the first detailed detection of hydroxycinnamates, in common beans.
Keywords: 24 Common beans, Phaseolus vulgaris L.: Market classes, Polyphenol profile, Hydroxycinnamates, Glycosylated flavonols, Anthocyanin, HPLC-DAD-ESI/MS
1. Introduction
The common bean (Phaseolus vulgaris L.) is by far the most important pulse crop (i.e., annual leguminous food crops, such as chickpea, cowpea, lentils, pea and others that are harvested for dry seeds) in the world (Singh, 1999). Among all the major food legumes, the common bean is the world’s third most important bean after soybeans (Glycine max (L.) Merr.) and peanut (Arachis hypogea L.). The common bean is an important source of protein, dietary fiber, iron, complex carbohydrates, minerals, and vitamins for millions of people in developing and developed nations and is one of the basic foods of the indigenous populations of South America and Eastern and Southern Africa.
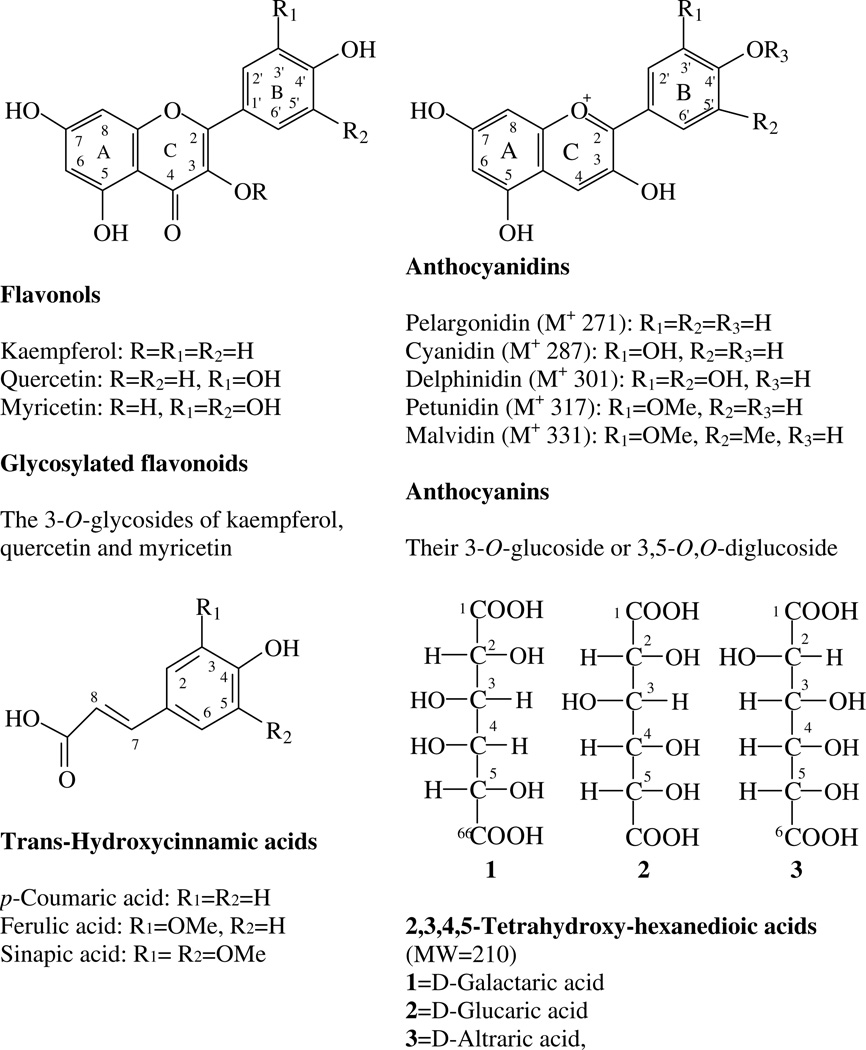
The common bean is primarily consumed as dry seeds (dry beans) but also as green pods (snap beans) and green shelled seeds. There are 10 major US commercial market classes of dry beans: black beans (Aparicio-Fernades et al., 2006; Aparicio-Fernandez, Yousef, Loarca-Pina, Gonzales de Mejia, & Lila, 2005; Takeoka et al., 1997), pinto beans (Beninger et al., 2005; Beninger & Hosfield, 2003), light and dark red kidney beans (Beninger & Hosfield, 1999; Choung, Choi, An, Chu, & Cho, 2003), pink beans, navy beans, great northern beans (Kelly et al., 2003; Luthria & Pastor-Corrales, 2006), alubia beans (Kelly & Copeland, 1998; Kelly, Hosfield, Varner, Uebersax, & Taylor, 1999) cranberry beans (Beaver, 1999), and small red beans, also known as redMexican beans (Hosfield, Varner, Uebersax,& Kelly, 2004). Common dry beans contain a wide range of flavonoids, including flavonols, their glycosides, anthocyanins, proanthocyanidins, and isoflavones, as well as some phenolic acids (Aparicio-Fernades & Garica-Gasca et al., 2006; Aparicio-Fernades & Yousef et al., 2005; Beninger & Hosfield, 1999; Beninger & Hosfield, 2003; Choung et al., 2003; Diaz-Batalla, Widholm, Fahey, Casano-Tostado, & Paredes-López, 2006; Macz-Pop, González-Parama´s, Pérez-Alonso, & Rivas-Gonzalo, 2006; Romani et al., 2004; Takeoka et al., 1997) (Fig. 1).
Fig. 1.
Structures of flavonols, anthocyanins, and hexanedioic acids and hydroxycinnamic acids.
A previous study from our laboratory characterized the phenolic acids obtained following alkaline hydrolysis of fifteen dry edible bean varieties from ten bean classes (Luthria & Pastor-Corrales, 2006). However, there is no systematic study of the polyphenolic profiles of common beans. As a part of a project to determine naturally occurring flavonoids and other polyphenolic compounds in food plants, we examined seeds of 24 dry bean samples, representing 10 US commercial market classes (17 were identified varieties, and 7 generic off-the-shelf items from the local grocery store). Using high performance liquid chromatography with diode array and electrospray ionization mass spectrometric detection (HPLC-DAD-ESI/MS) (Lin & Harnly, 2007), retention times and UV/Vis (ultraviolet/visible) and mass spectra were acquired for each of the 24 samples. In general, all of the tested beans contained the same hydroxycinnamic acid derivatives, but the flavonoid components were significantly different allowing the beans to be classified into six groups. Acid and base hydrolysis of the dry bean samples was carried out to isolate and identify the aglycones. This study reports the first detection of quercetin 3-O-pentosylhexoside and flavonoid glucoside malonates and the first detailed detection of hydroxycinnamates in common beans.
2. Materials and methods
2.1. Plant materials
Common dry beans from 10 different US commercial market classes were analyzed: alubia, black, cranberry, dark red kidney, great northern, light red kidney, navy, pink, pinto, and small red. Eight varieties, belonging to four market classes, pinto (Buster and Othello), black (Jaguar and T-39), navy (Seahawk and Vista) and great northern (Matterhorn and Weihing) were obtained from experimental plots grown under field conditions in the ARS-USDA South Farm in Beltsville, MD. Nine other varieties belonging to nine market classes, pinto (Maverick), black (Eclipse), navy (Norstar), dark red kidney (Red Hawk), light red kidney (California Early), small red also known as Red Mexican (Red Merlot and UI-239), Alubia (Beluga), cranberry (Taylor Hort), and pink (UI 535) beans were obtained from Greg Varner, Research Director, Michigan Dry Bean Research Board. In addition, generic pinto, black, navy, great northern, light red kidney, and pink beans (Goya products) (see Table 1) were bought from a local food market in Maryland. All the beans were finely powdered and passed through a 20 mesh sieve prior to extraction.
Table 1.
Identification of flavonoids and phenolic acids
| Market | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class | Variety | F- 1 |
F- 2 |
F- 3 |
F- 4 |
F- 5 |
F- 6 |
F- 7 |
F- 8 |
F- 9 |
F- 10 |
F- 11 |
F- 12 |
A- 1 |
A- 2 |
A- 3 |
A- 4 |
A- 5 |
A- 6 |
A- 7 |
A- 8 |
Ag- 1 |
Ag- 2 |
Ag- 3 |
Ag- 4 |
Ag- 5 |
P- 1 |
P- 2 |
P- 3 |
P- 4 |
P- 5 |
P- 6 |
P- 7 |
P- 8 |
P- 9 |
P- 10 |
P- 11 |
P- 12 |
P- 13 |
P- 14 |
P- 15 |
P- 16 |
| Alubia | Beluga | E | E | E | E | E | E | E | E | E | E | E | E | |||||||||||||||||||||||||||||
| Black | Generic | E | E | E | E | E/H | E | H | H | E | E | E | H | H | H | E | E | E | E | E | E | E | E | H | H | H | E | E | E | |||||||||||||
| T-39 | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | ||||||||||||||||||||||
| Eclipse | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | ||||||||||||||||||||||
| Jaguar | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | ||||||||||||||||||||||
| Cranberry | Taylor | E | E | E | E | E | E | E | E | E | E | E | E | E | ||||||||||||||||||||||||||||
| cranberry | ||||||||||||||||||||||||||||||||||||||||||
| Great Northern | Generic | E | E | E | E | E | E | E | E | H | H | H | E | E | E | |||||||||||||||||||||||||||
| Matterhorn | E | E | E | E | E | E | E | E | E | E | E | |||||||||||||||||||||||||||||||
| Norstar | E | E | E | E | E | E | E | E | E | E | E | |||||||||||||||||||||||||||||||
| Weihing | E | E | E | E | E | E | E | E | E | E | E | |||||||||||||||||||||||||||||||
| Kidney, Light Red | Generic | E | E | E | E | E/H | H | E | E | E | E | E | E | E | E | E | E | H | H | H | E | E | E | |||||||||||||||||||
| Cal early | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | |||||||||||||||||||||||||
| Kidney, Dark Red | Red hawk | E | E | E | E | H | H | E | E | E | E | E | E | E | E | H | H | H | E | E | E | |||||||||||||||||||||
| Navy | Generic | E | E | E | E | E | E | E | E | H | H | E/H | E | E | E | |||||||||||||||||||||||||||
| Sea hawk | E | E | E | E | E | E | E | E | E | E | E | |||||||||||||||||||||||||||||||
| Vista | E | E | E | E | E | E | E | E | E | E | E | |||||||||||||||||||||||||||||||
| Pink | Bgeneric | E | E | H | H | E | E | E | E | E | E | E | E | H | H | H | E | E | E | |||||||||||||||||||||||
| UI-537 | E | E | E | E | E | E | E | E | E | E | E | E | E | |||||||||||||||||||||||||||||
| Pinto | Generic | E | E | E | E/H | E | E | E | E | E | E | E | E | H | H | H | E | E | E | |||||||||||||||||||||||
| Buster | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | |||||||||||||||||||||||||||
| Maverick | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | |||||||||||||||||||||||||||
| Othello | E | E | E | E | E | E | E | E | E | E | E | E | E | E | ||||||||||||||||||||||||||||
| Red Mexican | Red merlot | E | E | E | E | E/H | E | E | E | E | E | H | H | E | E | E | E | E | E | E | E | H | H | H | E | E | E | |||||||||||||||
| UI-239 | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | E | |||||||||||||||||||||
E – Extract.
H – Hydrolyzed extract.
3. Flavonoid standards and chemicals
Myricetin (85% purity), quercetin dihydrate (98% purity), kaempferol (minimum 90% purity), rutin trihydrate, p-coumaric acid, sinapic acid, ferulic acid and isofeluric acid were purchased from Sigma Chemical Co. (Saint Louis, MO, USA). HPLC grade quercetin 3-O-gluctoside and kaempferol 3-O-gluctoside were purchased from Extrasynthese (Genay, Cedex, France). Cyanidin chloride, pelargonidin chloride, petunidin chloride, delphinidin chloride, malvidin chloride, pelargonidin 3-O-glucoside chloride, pelargonidin 3,5-O,O-diglucoside chloride, cyanidin 3-O-glucoside chloride and malvidin 3-O-glucoside chloride were purchased from Indofine Chemical Co. (Somerville, NJ, USA). Formic acid, hydrochloric acid (37%), sodium hydroxide, HPLC grade acetonitrile, and methanol were purchased from VWR Scientific (Seattle, WA, USA). HPLC grade water was prepared from distilled water using a Milli-Q system (Millipore Lab., Bedford, MA, USA).
3.1. LC-DAD and ESI-MSD conditions
The LC-DAD-ESI/MS instrument has been described previously (Lin & Harnly, 2007). Briefly, it consisted of an Agilent 1100 HPLC (Agilent, Palo Alto, CA, USA) coupled with a diode array detector (DAD) and mass spectrometer (MSD, SL mode). A Waters (Waters Corp., Milford, MA, USA) Symmetry column (C18, 5 (µm, 250× 4.6 mm) with a sentry guard column (C18, 5 (µm, 3.9 × 20 mm) was used at flow rate of 1.0 ml/min. The column oven temperature was set at 25 °C. The mobile phase consisted of a combination of A (0.1% formic acid in water) and B (0.1% formic acid in acetonitrile). The gradient was varied linearly from 10–26% B (v/v) in 40 min, to 65% B at 70 min, and finally to 100% B at 71 min and held at 100% B to 75 min. The DAD was set at 270, 310, 350 and 520 nm for real-time read-out and UV/VIS spectra, from 190 to 650 nm, were continuously collected. Mass spectra were simultaneously acquired using electrospray ionization in the positive and negative ionization (PI and NI) modes at low and high fragmentation voltages (100 V and 250 V, respectively) for the mass range of 100–2000 amu. A drying gas flow of 13 l/min, a drying gas temperature of 350 °C, a nebulizer pressure of 50 psi, and capillary voltages of 4000 V for PI and 3500 V for NI were used. The LC system was directly coupled to the MSD without stream splitting.
The hydrolyzed bean extracts were used to detect the flavonoid aglycones and some hydroxycinnamic acids. PI/NI selective ion monitoring (SIM) detection was used to confirm the existence of trace amount of some aglycones in the hydrolyzed extracts. MS2 and MS3, using an LCQ ion trap MS (ThermoQuest Corp., San Jose, CA, USA), were used to determine two minor diglycosylated flavonols in pink bean.
3.2. Sample preparation
3.2.1. Plant extracts
Approximately 50 g of dried beans were ground in a coffee grinder and the powdered beans were sieved through a standard sieve (number 20 corresponding to a sieve opening size of 0.85 mm, respectively). Powdered dried bean (250 mg, particle size ≤0.85 mm) was extracted with methanol–water (5.0 ml, 60:40, v/v) using sonication (FS30 Ultrasonic sonicator, 40 KHz, 100 W, Fisher Scientific, Pittsburgh, PA, USA) for 60 min at room temperature (from 22 to <40 °C at the end). The extract was separated from the solid by centrifuging at 420 g for 15 min (IEC Clinical Centrifuge, Damon/IEC Division, Needham, MA, USA). The solvent was removed and filtered through a 0.45 (µm 13 mm PVDF syringe filter (VWR Scientific, Seattle, WA, USA). Twenty-five microlitre samples were injected onto the column for LC-DAD-ESI/MS analysis.
3.2.2. Acidic hydrolysis of the glycosylated flavonoids in bean extracts
Bean extract (0.5 ml, each) was mixed with 0.1 ml of concentrated HCl (12 N), and heated in a covered tube at 85 °C for 2 h. After cooling to room temperature, 0.4 ml of methanol was added and the mixture was sonicated for 10 min. The solution was re-filtered prior to HPLC injection, and 50 µl of the extract was injected into the column for the analysis of aglycones (Lin & Harnly, 2007).
3.2.3. Alkaline hydrolysis of the hydroxycinnamic acid derivatives in bean extracts
A 1 ml aliquot of bean extract was taken to dryness. Three hundred microlitres of 4 N NaOH was added to the dried residue and kept at room temperature under N2 atmosphere for 18 h. Then, 0.15 ml of HCl (12 N) was added to the reaction mixture to make it pH 1. Then, 0.55 ml of MeOH was added, the solution filtered, and 25 (µl injected into the column (Lin & Harnly, 2007).
4. Results and discussion
4.1. Identification of bean flavonoids
A qualitative overview for the 24 bean samples analyzed in this study is presented in Table 1. In general, the flavonoid profiles tend to follow market classes. Grouping according to flavonoid content will be discussed in the last section. In this section, the identification of specific flavonoids will be discussed on a market class basis. The results can be assumed to be similar regardless of whether a generic sample or a specific variety was used as an example.
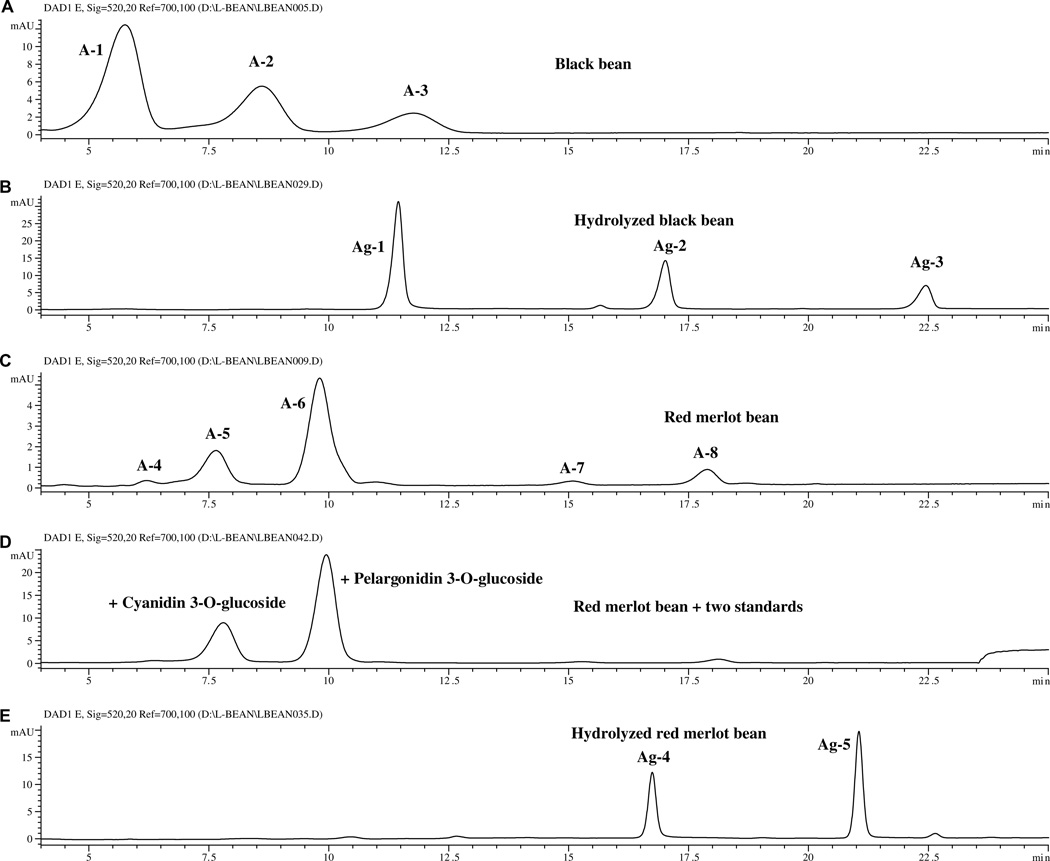
Only two dry bean market classes [the black and small red (red Mexican) beans] contained anthocyanins. The HPLC chromatograms (520 nm) for extracts of black and small red beans and their hydrolyzed extracts are shown in Fig. 2. The retention times (tR), UV/Vis absorption maxima (λmax), and PI/NI molecular and aglycone ions for each flavonoid peak are listed in Table 2. Based on this data, peaks A-1 to A-3 of the black bean and peaks A5 and A6 of the Red Merlot bean (Fig. 2) were identified as anthocyanins. From the molecular ions (m/z 465, 479, 493, 449 and 433) and aglycone ions (m/z 303, 317, 331, 287 and 271) of the unhydrolyzed and hydrolyzed extracts, peaks A-1 to A-6 were tentatively identified as hexosides of delphinidin, petunidin, malvidin, cyanidin and pelargonidin (Table 3).
Fig. 2.
Chromatograms (520 nm) of the anthocyanins in (A) black bean extract, (B) hydrolyzed black bean extract, (C) small Red Merlot bean extract, (D) small Red Merlot bean extract with added standards, and (E) hydrolyzed hydrolyzed black bean extract.
Table 2.
Retention time and UV/Vis and mass spectra of the flavonoids in common bean extract and hydrolyzed extractsa
| Peak | Flavonoid | tR (min) | [M+H]+ /[M–H]−(m/z) | PI/NI (m/z) Aglycone and other ions | λmax (nm) |
|---|---|---|---|---|---|
| Flavonols | |||||
| F-1 | Myricetin 3-O-glucoside | 21.28 | 481/479 | 319/317 | 260, 358 |
| F-2 | Quercetin 3-O – xylosylglucoside | 22.02 | 597/595 | 465, 303/301 | 256, 356 |
| F-3 | Rutin (Quercetin 3-O-rutinoside)b | 24.62 | 611/609 | 465, 303/301 | 258, 266sh, 356 |
| F-4 | Kaempferol 3-O – xylosylglucoside | 26.84 | 581/579 | 449, 287/285 | 266, 348 |
| F-5 | Quercetin 3-O-glucosideb | 27.06 | 465/463 | 303/301 | 258, 266sh, 356 |
| F-6 | Quercetin 3-O-(6″-O-malonyl)glucoside | 31.14 | 551/549 | 303/301 | 258, 266sh, 356 |
| F-7 | Kaempferol 3-O-glucosideb | 31.97 | 449/447 | 287/285 | 266, 348 |
| F-8 | Myricetinb | 36.65 | 319/317 | – | 256, 274 |
| F-9 | Kaempferol 3-O-(6″-O-malonyl)glucoside | 37.11 | 535/533 | 287/285 | 266, 348 |
| F-10 | Kaempferol 3-O-(malonyl)glucoside | 38.76 | 535/533 | 287/285 | 266, 348 |
| F-11 | Quercetinb | 48.44 | 303/301 | – | ndc |
| F-12 | Kaempferolb | 54.46 | 287/275 | – | 266, 366 |
| Anthocyanins | |||||
| A-1 | Delphinidin 3-O-glucoside | 5.68 | 465 | 303 | 278, 524 |
| A-2 | Pelargonidin 3,5-O-diglucoside | 6.18 | 595 | 271 | ndc |
| A-3 | Petunidin 3-O-glucoside | 8.52 | 479 | 317 | 280, 526 |
| A-4 | Malvidin 3-O-glucosideb | 11.68 | 493 | 331 | 270, 516 |
| A-5 | Cyanidin 3-O-glucosideb | 7.65 | 449 | 287 | 274, 516 |
| A-6 | Pelargonidin 3-O-glucosideb | 9.81 | 433 | 271 | 272, 502 |
| A-7 | Cyanidin 3-O-(6″-malonyl)glucoside | 15.11 | 535 | 287 | ndc |
| A-8 | Pelargonidin 3-O-(6″-malonyl)glucoside | 17.89 | 519 | 271 | ndc |
| Anthocyanidins in Hydrolyzed extracts | |||||
| Ag-1 | Delphinidinb | 11.46 | – | 303 | 274, 532 |
| Ag-2 | Petunidinb | 16.93 | – | 317 | 276, 532 |
| Ag-3 | Malvidinb | 20.91 | – | 331 | 270, 308, 534 |
| Ag-4 | Cyanidinb | 16.76 | – | 287 | 276, 522 |
| Ag-5 | Pelargonidinb | 21.15 | – | 271 | 268, 512 |
This determination is able to detect the flavonoid content in bean equal to 0.0008 % (w/w) and larger. The tR, mass and UV data were taken from one of the tested sample.
Directly compared with standard.
Undetectable.
Table 3.
Retention time and UV/Vis and mass spectra of the hydroxycinnamaic acid and derivatives in common bean extracts and their hydrolyzed extractsa
| Peak | tR (min) | [M–H]−(m/z) | λmax (nm) | Identification |
|---|---|---|---|---|
| Hydroxycinnamic acid derivatives | ||||
| P-1 | 7.04 | 355 | 232, 312 | P-coumaric acid derivative |
| P-2 | 8.43 | 385 | 240, 326 | Ferulic acid derivativesb |
| P-3 | 9.05 | 355 | 232, 314 | P-coumaric acid derivative |
| P-4 | 9.31 | 355 | 232, 314 | P-coumaric acid derivative |
| P-5 | 10.64 | 355 | 232, 314 | P-coumaric acid derivative |
| P-6 | 10.95 | 385 | 240, 328 | Ferulic acid derivativesb |
| P-7 | 12.32 | 385 | 240, 328 | Ferulic acid derivativesb |
| P-8 | 13.19 | 355 | 232, 314 | P-coumaric acid derivative |
| P-9 | 13.79 | 355 | 232, 314 | P-coumaric acid derivative |
| P-10 | 15.24 | 385 | 240, 328 | Ferulic acid derivativesb |
| P-13 | 25.22 | 193 | 220, 234sh, 324 | Ferulic acidd |
| P-14 | 60.05 | ndc | 226, 314 | – |
| P-15 | 61.86 | ndc | 224, 296 | – |
| P-16 | 62.68 | ndc | 224, 296 | – |
| Hydroxycinnamic acids | ||||
| P-11 | 22.30 | 163 | 224, 310 | P-coumaric acidd |
| P-12 | 24.82 | 223 | 238, 324 | Sinapic acidd |
| P-13 | 25.22 | 193 | 220, 234sh,324 | Ferulic acidd |
The tR, mass and UV data were taken from one of the tested navy bean and red kidney bean samples.
Contained around 10% of sinapic acid derivatives.
nd: un-detectable.
By direct comparison with standard.
Specific identification of these compounds was made by comparison of the retention time and UV/Vis and mass spectral data of standards for 3-O-glucosides of cyanidin, pelargonidin and malvidin (separated using the same conditions) with that of the sample peaks. All the peaks (with the exception of peak A-4) were confirmed to be 3-O-glucosides (Table 2). Of these, 3-O-glucosides of delphinidin, petunidin and malvidin had been previously isolated from black beans (Aparicio-Fernades & Garica-Gasca et al., 2006; Aparicio-Fernades & Yousef et al., 2005; Romani et al., 2004; Takeoka et al., 1997). Pelargonidin 3-O-glucoside and cyanidin 3-O-glucoside had been previously reported to exist in red kidney beans (Choung et al., 2003). Based on the data presented in Table 2, the trace peaks A-4, A-8 and A-7 of small red beans were tentatively identified as pelargonodin 3,5-O,O-diglucoside, pelargonidin 3-O-(6″-malonyl)glucoside and cyandin 3-O-(6″ -malonyl) glucoside, respectively. The later two anthocyanins had molecular ions that were 248 amu larger than their aglycones and 86 amu larger than that of their glucosides; a typical pattern for flavonoid malonylglucosides (Cuyckems & Cleays, 2004; Lin et al., 2000).
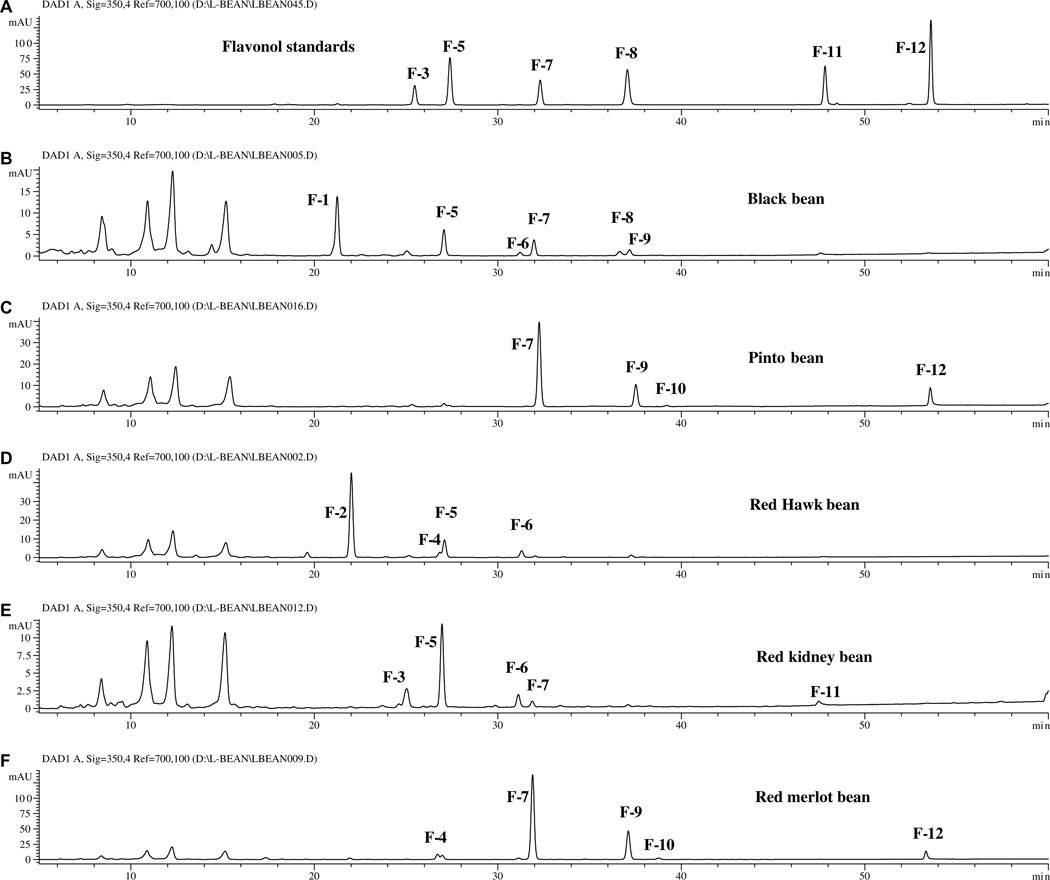
Five of the market classes (black, pinto, dark red kidney, light red kidney, and small red beans) contained detectable levels of flavonoids. The HPLC chromatograms (350 nm) for the extracts of black, pinto, red hawk, light red kidney beans, and Red Merlot bean and a mixture of 6 flavonol standards are shown in Fig. 3. The retention times (tR), UV/Vis absorption maxima (λmax), and PI/NI molecular and aglycone ions for each flavonoid peak are listed in Table 2.
Fig. 3.
Chromatograms (350 nm) of (A) 6 flavonol standards and extracts from (B) black, (C) pinto, (D) red hawk, (E) red kidney, and (F) red merlot beans.
A comparison of the data in Table 2 with that obtained for standards revealed that peaks F-3, F-5, F-7, F-8, F-11 were rutin (quercetin 3-O-rutinoside), quercetin 3-O-glucoside, kaempferol 3-O-glucoside, myricetin, quercetin, and kaempferol, respectively. F1 has UV absorption maxima at 260 and 358 nm, PI/NI molecular ions of m/z 481/479, and aglycone ions of m/z 319/317, suggesting that it is a myricetin 3-O-hexoside. Positive identification as myricetin 3-O-glucoside was obtained by comparison of the data in Table 2 with that previously obtained for myricetin 3-O-glucoside in cranberries (Lin & Harnly, 2007).
Peak F-6 had the same UV spectra and aglycone ion as peak F-5 (quercetin 3-O-glucoside), but its molecular ion is 86 amu larger than that of F5 and or 248 amu larger than that of its aglycone. Thus, F-6 was tentatively identified as quercetin 3-O-(6″-malonyl) glucoside (Cuyckems & Cleays, 2004; Lin et al., 2000). Similarly, peaks F-9 and F-10 were identified as kaempferol 3-O-(6″-malonyl)glucoside and kaempferol 3-O-(malonyl)glucoside. F-9 was identified as kaempferol 3-O-(6″-malonyl)glucoside because linkage of the malonyl at the 6″-position of the sugar is far more common (Lin et al., 2000; Svelikova et al., 2004). The position of malonyl group on the sugar moiety was not determined for peak F-10.
Peak F-2 of Red Hawk bean, of the dark red kidney market class (Fig. 3) showed PI/NI molecular ions at m/z 597/595, aglycone ions at m/z 303/301, and fragment ions at m/z 465/463 (i.e., M+-132, 132 for a pentosyl) from loss of a secondary sugar. The mass spectra of the hydrolyzed extract indicated that aglycone was quercetin. The data suggest that this quercetin glycoside contains one pentose more than that of peak F-5 (quercetin 3-O-glucoside). Since the UV absorption spectrum is the same as that for peak F-5, peak F-2 is most likely quercetin 3-O-pentosylhexoside.
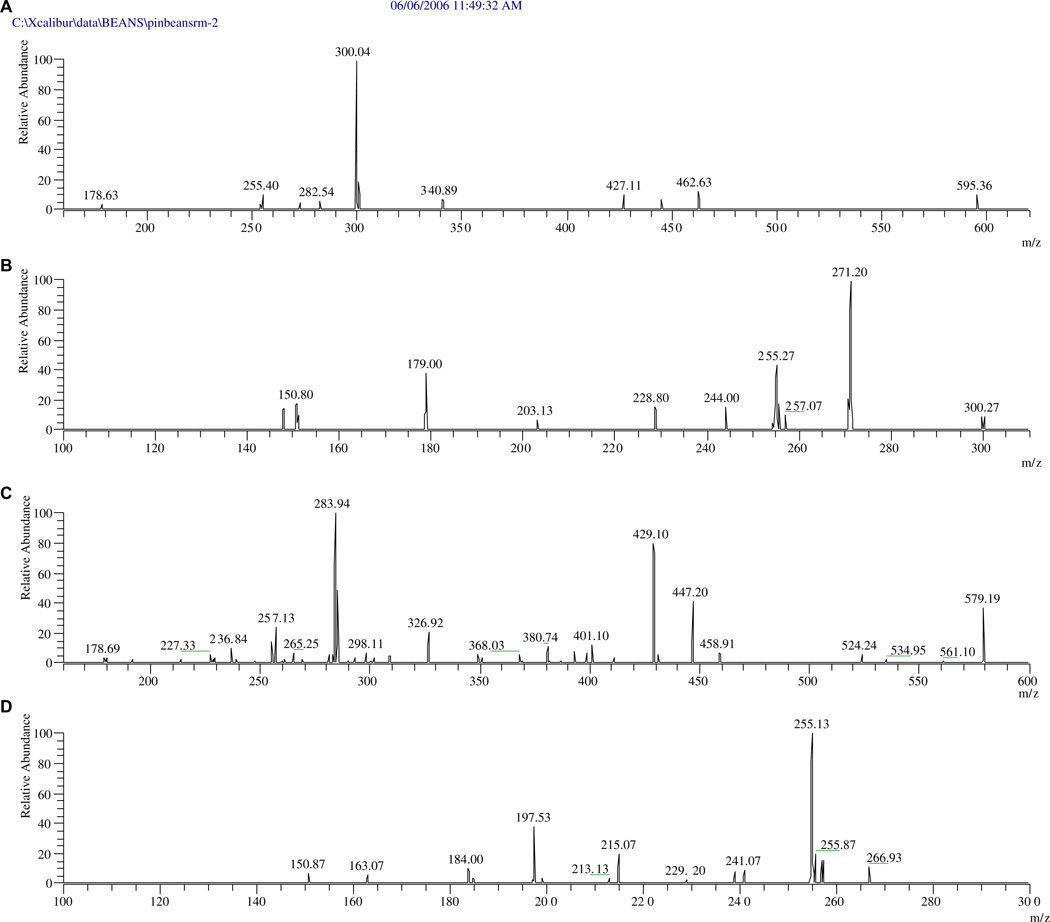
The minor peak, F-4 of Red Hawk beans, with PI/NI molecular ions of m/z 581/579 and aglycone ions of m/z 287/285, should be kaempferol 3-O-pentosylhexoside or kaempferol 3-O-hexosylpentoside. The order of the sugars cannot be determined by LC-DAD-ESI/MS, since the mass spectra did not offer any fragments to indicate the mass of the secondary sugar. Both sugar orders, kaempferol 3-O-glucosylxylose in Montcalm dark kidney beans seed coats (Beninger & Hosfield, 2003; Beninger & Hosfield, 1999) and keampferol 3-O-xylosylglucoside in the Zolfino common bean landraces (P. vulgaris L., cv. Zolfino) (Romani et al., 2004), have been reported. MS2 experiments support the existence of kaempferol 3-O-xylosylglucoside in the tested dark red kidney bean extract based on observation of an ion with m/z 447 formed by the loss of pentosyl (132 amu) from the diglycosylated flavonoid (Fig. 4). Otherwise, the existence of kaempferol 3-O-glucosylxoloside would have required an ion at m/z 417 formed by the loss of glucosyl from the diglycoside. This ion cannot be seen in Fig. 4.
Fig. 4.
MS2 and MS3 spectra of peaks F-2 (quercetin 3-O-pentosylhexoside) and F-4 (kaempferol 3-O-xylosylglucoside, bottom two) of red hawk bean: (A) MS2 of F-2, (B) MS3 of F-2, (C) MS2 of F-4, and (D) MS3 of F-4.
Similar reasoning was used to identify peak F-4 for the pink bean as quercetin 3-O-pentosylhexoside. The peak at m/z 463 corresponds to the mass of the ion formed by the loss of the pentosyl (132 amu) from the diglycosylated flavonoid. Based on the logic that both 3-O-pentosylhexosides of quercetin and kaempferol in same beans might be formed through the same biogenetic pathway, peak F-2 is most likely quercetin 3-O-xylosylglucoside.
It is worth noting that this may be the first determination of quercetin 3-O-xylosylglucoside and all the malonyl esters of the 3-O-glucosylated flavonols and anthocyanidins in these beans.
4.2. Identification of hydrolysable hydroxycinnamates
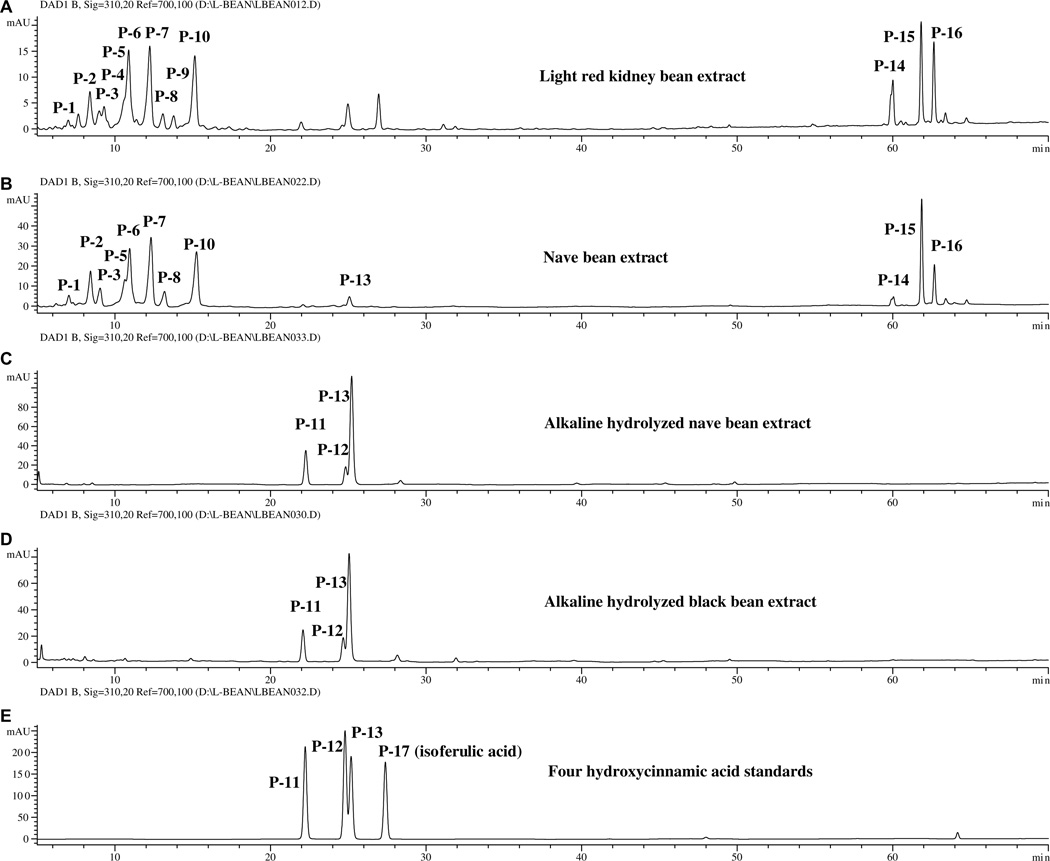
After hydrolysis, all the dry beans of the US. commercial market classes had very similar hydroxycinnamic acid profiles. Prior to hydrolysis, there were minor variations (Fig. 5). The profiles of red kidney, cal early, and cranberry beans showed slightly greater amounts of P-4, and P-9 than the profiles of the other common beans. Their total hydroxycinnamate content, however, is slightly lower than the others. Fig. 5 shows the chromatograms for the extracts of light red kidney and navy bean extracts, the NaOH hydrolyzed extracts of navy and black bean, and a mixture of hydroxycinnamic acid standards (ferulic acid, sinapic acid, p-coumaric acid, and isoferulic acid). Peaks P-1 to P-10 are hydroxycinnamic acid derivatives and P-11 to P-13 are hydroxycinnamic acids (Table 2). Peaks P-14 to P-16 were not identified.
Fig. 5.
Chromatograms (310 nm) of the extract of (A) light red kidney and (B) navy beans, alkaline hydrolyzed extracts of (C) navy and (D) black beans, and (E) four hydroxycinnamic acid standards (P-17, isoferulic acid).
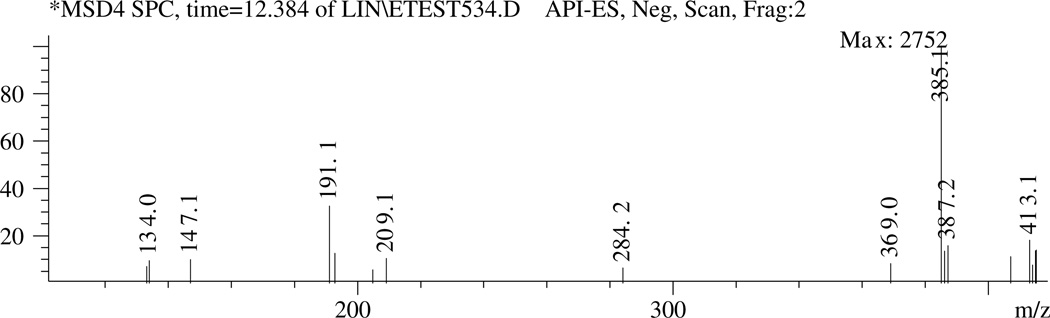
Peaks P-2, P-6, P-7, and P-10 (Fig. 5) appear to be ferulic acid derivatives. They have UV absorption maxima at 326–328 nm, similar to that of ferulic acid (324 nm), and the strongest NI ions at m/z 385 (Fig. 6). These peaks disappear upon alkaline hydrolysis of the sample (Fig. 5) and release ferulic acid (peak P-13, MW = 194). Thus, peaks P-2, P-6, P-7 and P-10 are suggested to be ferulate derivatives (MW = 386) of 2,3,4,5-tetrahydroxy-hexanedioic acids (such as galactaric acid, glucaric acid, and altraric acid) with NI ions at m/z 209 (Fig. 6). The mass spectra of these peaks also contained an ion of m/z 415 (about 10% the intensity ratio of the strongest ion of m/z 385) indicating the presence of similar derivatives for sinapic acid (MW = 224, UV: 324 nm).
Fig. 6.
The NI mass spectrum (250 V) of peak P-10, a ferulic acid derivative.
Similarly, peaks P-1, P-3, P-4, P-5, P-8 and P-9 are suggested to be p-coumaric acid (peak P9, MW = 164, UV: 310 nm) derivatives. These peaks have UV absorption maxima at 312–14 nm and their strongest NI ion at m/z 355. These peaks disappear a upon NaOH hydrolysis and give rise to peak P-9. Mass spectra (not shown) suggest that peaks P-1, P-3, P-4, and P-7 are p-coumaric acid condensed with galactaric acid, glucaric acid, or altraric acid.
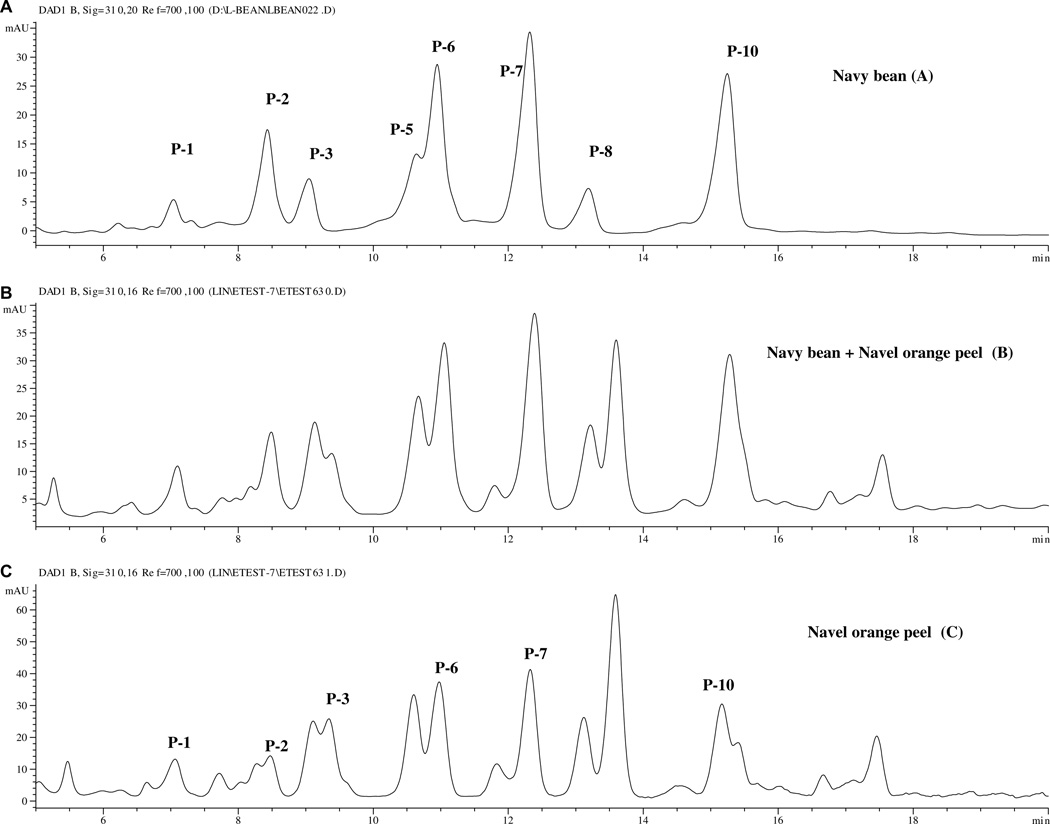
Navel orange peel [Citrus sinensis (L.) Osbeck (navel group)] have been reported to contain hydrolyzable hydroxycinnamtes which also produce p-coumaric acid, ferulic acid, and sinapic acid upon alkaline hydrolysis (Fig. 7D) (Lin & Harnly, 2007). Some of the orange hydroxycinnamates were identified as 2′-(E)-O-feruloylgalactaric acid (MW = 386), 2′-(E)-O-p-coumaroylgalactaric acid, and (E) – O – p-coumaroyl-derivatives of glucaric acid (MW = 356) (Risch, Hermann, & Wray, 1988; Risch, Hermann, Wray, & Grotjahn, 1987). Altraric acid, another isomeric acid, has also been reported to form hydroxycinnamic acid derivatives in yacon root (Takenka et al., 2003). Fig. 7 shows chromatograms for navy bean and navel orange peel extracts. Several peaks (P-1, P-2, P-3, P-6, P-7 and P-10) in both plant materials had similar retention times, UV/Vis, and mass spectral data. Spiking the navy bean extract with the navel orange peel extract (Fig. 7B) confirmed the correspondence of these peaks. This strongly suggests that both orange peel and navy bean contain hydroxycinnamic acids derivatized with 2,3,4,5-tetrahydroxy-hexanedioic acids.
Fig. 7.
Chromatorams of extracts of (A) navy bean, (B) a mixture of navy bean and navel orange peel, and (C) navel orange peel.
Each of the three tetrahydroxy-hexanedioic acids can produce some possible positional isomers with a hydroxycinnamic acid. This accounts for the complexity of the chromatograms in Fig. 7 between 5 and 18 min. The techniques used in this study, LC-MS and MSn, were insufficient to determine the exact linkage. Further studies with nuclear magnetic resonance spectroscopy are necessary to confirm their structures.
4.3. Grouping common bean according to their phenolic profiles
An examination of the phenolic profiles of all the dry bean cultivars analyzed in this study shows that all of the beans contained the same hydroxycinnamic acids in the hydrolyzed extract and very similar hydroxycinnamic acid derivatives in the extract. The hydroxycinnamic acid derivatives constituted the main phenolic component of beans. By comparison, their flavonoid components were less prominent, with the exception of small red dry bean cultivars (Red Merlot and UI 237) (Figs. 2 and 3). However, their flavonoid components of beans showed some distinct differences which allow us to separate the common beans into 6 groups. In general, these groups follow the lines of market class.
The black bean group (Jaguar, T-39, Eclipse and generic,) has chromatographic profiles (not shown) similar to that of generic black bean shown in Figs. 2 and 3. All of the black beans contained three anthocyanins (the 3-O-glucosides of delphinidin, petunidin and malvidin) as their main flavonoid components. In addition, they each contained myricetin 3-O-glucoside and trace amounts of 3-O-glucosides of quercetin and kaempfeorl and their malonyl esters.
The pinto bean group (Othello, Maverick, Buster and generic) contained kaempferol 3-O-glycosides and their free aglycone (Fig. 3). The chromatograms of other three pinto beans (not shown) were very similar, except Buster and Maverick bean had a much lower kaempferol aglycone content (much smaller absorbance peak).
The pink bean group was composed of pink bean (UI 537 and generic) and dark red kidney bean (Red Hawk). These beans contained 3-O-pentosylhexosides of quercetin and kaempferol, and quercetin 3-glucoside and its malonyl derivatives. However, the Red Hawk bean a much higher quercetin 3-O-pentosylhexoside content (the peak absorbance was approximately 17 times greater) than the other two beans.
The light red kidney bean group consisted of Cal Early and a generic light red kidney bean. Both beans contained queretin 3-O-glucoside and its malonyl derivatives. The chromatogram of Cal Early bean (not shown) is close to that of red kidney as shown in Fig. 3.
The small red bean group (Red Merlot and UI 239) contained 3-O-glucosides of pelargonidin and cyanidin and kaempferol 3-O-glucoside and its malonyl derivative. Their kaempferol 3-O-glucoside content is about 5 times higher than that of the pinto bean group and their total flavonoid content is the highest among all the beans. The chromatogram of UI 239 (not shown) is very similar to that of Red Merlot bean.
The navy bean group consists of Norstar, Vista, Seahawk and a generic bean; the great northern bean group was comprised of Matterhorn, Weihing and a generic bean; the Alubia market class was represented by the bean variety Beluga, and the cranberry market class by the Taylor Hort variety. Except for ferulic acid (present as a trace quantity in navy bean), all of the beans in this group had chromatographic profiles (not shown) very similar to that of navy beans (Fig. 5), i.e., they contained the same hydroxycinnamic acid derivatives as navy beans. However, no flavonoids were detectable for any of the beans in this group. No significant flavonoid peaks were detectable in their chromatographic profiles.
5. Conclusion
All of the beans examined in this study contain similar hydroxycinnaminic acid derivatives as their main phenolic component. Their flavonoid content is less prominent, but more varied. The flavonoid components allow the beans to be separated into 6 groups. These groups follow taxonomic lines except for the largest group, which is composed of beans with no detectable flavonoid component.
Acknowledgements
This research was supported by an Interagency Agreement with the Office of Dietary Supplement at the National Institutes of Health, Health and Human Services Department.
References
- Aparicio-Fernades X, Garica-Gasca T, Yousef GG, Lila MA, Gonzales de Mejia E, Loarca-Pina G. Chemopreventive activity of polyphenolics from black Jamapa bean (Phaseolus vulgaris L.) on HeLa and HaCaT cells. Journal of Agricultural Food Chemistry. 2006;54:2116–2122. doi: 10.1021/jf052974m. [DOI] [PubMed] [Google Scholar]
- Aparicio-Fernandez X, Yousef GG, Loarca-Pina G, Gonzales de Mejia E, Lila MA. of polyphenolics in the seed of black Jamapa bean (Phaseolus vulgaris L.) Journal of Agricultural Food Chemistry. 2005;53:4615–4622. doi: 10.1021/jf047802o. [DOI] [PubMed] [Google Scholar]
- Beaver JS. Improvement of large-seeded race Nueva Granada cultivars. In: Singh S, editor. Common bean improvement in the twenty-first century. Dordrecht, the Netherlands: Kluwer; 1999. pp. 275–288. [Google Scholar]
- Beninger CW, Gu L, Prior RL, Junk DC, Vandenberg A, Bett K. Changes in polyphenols of the seed coat during the after-darkening process in pinto bean (phaseolus vulgaris L.) Journal of Agricultural Food Chemistry. 2005;53:7777–7782. doi: 10.1021/jf050051l. [DOI] [PubMed] [Google Scholar]
- Beninger CW, Hosfield GL. Flavonol glycosides from Montcalm dark red kidney bean: implications for genetics of seed coat color in Phaseolus vulgaris L. Journal of Agricultural Food Chemistry. 1999;47:4079–4082. doi: 10.1021/jf990440d. [DOI] [PubMed] [Google Scholar]
- Beninger CW, Hosfield GL. Antioxidant activity of extracts, condensed tannin fractions, and pure flavonoids from Phaseolus vulgaris L. seed coat color genotypes. Journal of Agricultural Food Chemistry. 2003;51:7879–7883. doi: 10.1021/jf0304324. [DOI] [PubMed] [Google Scholar]
- Choung M-G, Choi B-R, An Y-N, Chu Y-H, Cho Y-S. Anthocyanin profile of Korean cultivated kidney bean (Phaseolus vulgaris L.) Journal of Agricultural Food Chemistry. 2003;51:7040–7043. doi: 10.1021/jf0304021. [DOI] [PubMed] [Google Scholar]
- Cuyckems F, Cleays M. Mass spectrometry in the structural analysis of flavonoids. Journal of Mass Spectrometry. 2004;39:1–15. doi: 10.1002/jms.585. [DOI] [PubMed] [Google Scholar]
- Diaz-Batalla L, Widholm JM, Fahey GC, Jr, Casano-Tostado E, Paredes-Lo´pez O. Chemical components with health implications in Wild and cultivated Mexican common bean seeds (Phaseolus vulgaris L.) Journal of Agricultural Food Chemistry. 2006;54:2045–2052. doi: 10.1021/jf051706l. [DOI] [PubMed] [Google Scholar]
- Hosfield GL, Varner GV, Uebersax MA, Kelly JD. Registration of ‘Merlot’ small red bean. Crop Science. 2004;44:351–352. [Google Scholar]
- Kelly JD, Copeland LO. Beluga. A new alubia bean for Michigan. Ext. Bull. 1998:E-2668. [Google Scholar]
- Kelly JD, Hosfield GL, Varner GV, Uebersax MA, Ender M, Taylor J. Registration of ‘Seahawk’ navy bean. Crop Science. 2003;43:2307–2308. [Google Scholar]
- Kelly JD, Hosfield GL, Varner GV, Uebersax MA, Taylor J. Registration of’Beluga’ alubia bean. Crop Science. 1999;39:293. [Google Scholar]
- Lin L-Z, Harnly JM. A screening method for the systematic identification of glycosylated flavonoids and other phenolic compounds using a standard analytical approach for all plant materials. Journal of Agricultural Food Chemistry. 2007;55:1084–1096. doi: 10.1021/jf062431s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L-Z, He X-Q, Lindenmaier M, Yang J, Cleary M, Qiu S-X, et al. Study of the flavonoid glycoside malonates of red clover (Trifolium pretense) Journal of Agricultural Food Chemistry. 2000;48:354–365. doi: 10.1021/jf991002+. [DOI] [PubMed] [Google Scholar]
- Luthria LD, Pastor-Corrales MA. Phenolic acids content of fifteen dry edible bean (Phaseolus vulgaris L.) varieties. Journal of Food Composition and Analysis. 2006;19:205–211. [Google Scholar]
- Macz-Pop GA, Gonza´lez-Parama´s AM, Pe´rez-Alonso JJ, Rivas-Gonzalo JC. New flavanol-anthocyanin condensed pigments and anthocyanin composition in Guatemalan beans (Phaseolus sp.) Journal of Agricultural Food Chemistry. 2006;54:536–542. doi: 10.1021/jf051913l. [DOI] [PubMed] [Google Scholar]
- Manthey JA. Franctionation of orange peel phenols in ultrafiltered molasses and mass balance studies of their antioxidant levels. Journal of Agricultural Food Chemistry. 2004;52:7586–7592. doi: 10.1021/jf049083j. [DOI] [PubMed] [Google Scholar]
- Risch B, Hermann K, Wray V. (E)-O-p-coumaroyl-derivatives of glucaric acid in citrus. Phytochemistry. 1988;10:3307–3309. [Google Scholar]
- Risch B, Hermann K, Wray V, Grotjahn L. 2′-(E)-O-p-coumaroylgalactaric acid and 2′ -(E)-O-feruloylgalactaric acid in citrus. Phytochemistry. 1987;10:3307–3309. [Google Scholar]
- Romani A, Vignolini P, Galardi C, Mulinacci N, Benedettelli S, Heimier D. Germplasm characterization of Zolfino landraces (Phaseolus vulgaris L.) by flavonoid content. Journal of Agricultural Food Chemistry. 2004;52:3838–3842. doi: 10.1021/jf0307402. [DOI] [PubMed] [Google Scholar]
- Singh SP. Production and utilization. In: Singh S, editor. Common bean improvement in the twenty-first century. Dordrecht, The Netherlands: Kluwer; 1999. pp. 1–24. [Google Scholar]
- Svelikova V, Bennett RN, Mellon FA, Needs PW, Piacente S, Kroon PA, et al. Isolation, Identification and stability of acylated derivatives of apigenin 7-O-glucoside from chamomile (Chamomilla recutita [L.] Rauschert) Phytochemistry. 2004;65:2323–2332. doi: 10.1016/j.phytochem.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Takenka M, Yan X, Ono H, Yoshida M, Nagaro T, Nakanishi T. Caffeic acid derivatives in the roots of Yacon (Smallanthus sonchifolius) Journal of Agricultural Food Chemistry. 2003;51:793–796. doi: 10.1021/jf020735i. and some of the cited references. [DOI] [PubMed] [Google Scholar]
- Takeoka GR, Dao LT, Full GH, Wong RY, Harden LA, Edwards RH, et al. Characterization of black bean (Phaseolus vulgaris L.) Journal of Agricultural Food Food Chemistry. 1997;45:3395–3400. [Google Scholar]