Abstract
The polyphenol content and antioxidant activity of Nonpareil, Carmel, Butte, Sonora, Fritz, Mission, and Monterey almond cultivars harvested over three seasons in California were examined. LC–MS was employed to quantify 16 flavonoids and two phenolic acids in acidified methanol extracts of almond skins. The 3-year mean polyphenol content of cultivars ranged from 4.0 to 10.7 mg/100 g almonds. Isorhamnetin-3-O-rutinoside was the most abundant flavonoid, present at 28–49% of total polyphenols among cultivars. Almonds from 2006 and 2007 had 13% fewer polyphenols than 2005, but FRAP and total phenols were comparable. Cultivar, but not season, had a differential impact on individual polyphenol synthesis. Using the results of polyphenol, total phenol, and FRAP, multivariate analysis distinguished harvest years and most cultivars with 80% confidence. Flavonoid content and antioxidant activity of almonds may be more dependent on cultivar than on seasonal differences.
Keywords: Almonds, Flavonoid, Polyphenols, Antioxidant, Year, Folin, FRAP, LC–MS, Skin
1. Introduction
For more than 100 years, tree nuts have been advocated as a nutritious component of diet (Reiling, 2008). Almonds are the most popularly consumed tree nut, and increasing experimental evidence suggests that almonds improve serum lipid profiles and cholesterol status (Chen, Milbury, Lapsley, & Blumberg, 2005; Jenkins et al., 2008; King, Blumberg, Ingwersen, Jenab, & Tucker, 2008), changes associated with reduced risk of cardiovascular disease. Almonds are a good dietary source of vitamin E, sterols, and flavonoids, each of which has been suggested to play a role in the promotion of health. In particular, increased consumption of flavonoids has been associated with an anti-obesity effect in women and reduced risk of stroke, cardiovascular disease, and some forms of cancer (Geleijnse, Launer, Hofman, Pols, & Witteman, 1999; Hertog, Hollman, Katan, & Kromhout, 1993; Hughes et al., 2008; Keli, Hertog, Feskens, & Kromhout, 1996; Yochum, Kushi, Meyer, & Folsom, 1999). Previous efforts by our laboratory and others have focused on the identification and quantification of almond polyphenols to ascertain their contribution to flavonoid in-take and health outcomes (Alasalvar & Shahidi, 2009; Garrido, Monagas, Gomez-Cordoves, & Bartolome, 2008; Harnly et al., 2006; Milbury, Chen, Dolnikowski, & Blumberg, 2006).
Almond polyphenols are concentrated in the skins, which can be removed by blanching. Almonds are often blanched for commercial reasons even though this lowers their polyphenol content. Flavonoids and other polyphenols comprise 0.2–0.8% of the dry weight of almond skins (Garrido et al., 2008). Flavonoid-containing almond skin extracts can inhibit LDL-oxidation and DNA damage in vitro (Wijeratne, Abou-Zaid, & Shahidi, 2006). Polyphenols identified from almonds also have strong 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity (Sang et al., 2002). We previously reported that flavonoid content varied at least 2-fold among eight California almond cultivars harvested in 2004 (Milbury et al., 2006). Barreira, Ferreira, Oliveira, and Pereira (2008) found that flavonoid content varied 4-fold in 10 Portuguese almond cultivars. Besides cultivar differences, climate and geography may also affect almond flavonoid concentration. These parameters also affect the content of fibre, total phenols, minerals, and tocopherols in almonds and other tree nuts (Amaral, Valentao, Andrade, Martins, & Seabra, 2008; Laverdrine, Ravel, Villet, Ducros, & Alaray, 2000; Parcerisa et al., 1995; Sanchez-Bel, Egea, Martinez-Madrid, Flores, & Romojaro, 2008). However, data regarding the seasonal variability of almond flavonoids are lacking. This information may contribute to better estimates of flavonoids intakes, broaden the understanding of cultivar-specific flavonoid and phenolic syntheses in almonds, and inform agricultural practices to increase the flavonoid content of almonds or improve their resistance to pests.
California is the largest almond growing region in the world. In 2008, 1.38 billion pounds of almonds were harvested, accounting for 80% of global production (Almond Board of California, 2008). Nonpareil, Carmel, and Butte are the main almond cultivars and represented 60% of the almond harvested in 2007 (Bolling, Dolnikowski, Blumberg, & Chen, 2009). The objective of this study was to quantify the major flavonoids and phenolic acids, total phenols, and antioxidant activity from Nonpareil, Carmel, Butte, Monterey, Fritz, Mission, and Sonora almonds harvested over a 3-year period.
2. Materials and methods
2.1. Chemicals and reagents
Quercetin-4-O-glucoside, quercetin-3-O-galactoside, isorhamnetin, kaempferol-3-O-rutinoside, isorhamnetin-3-O-glucoside, isorhamnetin-3-O-rutinoside, naringenin-7-O-glucoside, and rutin were obtained from Extrasynthese (Genay, France). Naringenin, quercetin, and eriodictyol were acquired from Indofine (Belle Mead, NJ). Methanol was HPLC grade from Fischer (Fair Lawn, NJ). Water was ultrapure grade. All other chemicals and reagents were acquired from Sigma–Aldrich (St. Louis, MO).
2.2. Almond samples
Raw almonds harvested in 2005, 2006, and 2007 from California were generously provided by the Almond Board of California. Monterey, Fritz, Mission, and Sonora almond samples had one sample from one orchard each in the central California growing region. Nonpareil, Carmel, and Butte almonds had one sample from three different orchards. In total, 13 samples of seven cultivars were collected from the same orchards in three consecutive years across the counties of Yolo, Sacramento, Stanislaus, Fresno, and Kern. A total of 39 almond samples were included in the study. For each cultivar, the mass of skin in 100 g almonds was determined by blanching almonds in hot water for 3 min, removing skins by hand, and air-drying them at room temperature. The fractional percentage of almond skin was used to calculate the polyphenol content and antioxidant activity per 100 g almonds to facilitate comparison to the USDA flavonoid database.
2.3. Method of extraction
Skins from raw almonds were removed and extracted according to Bolling et al. (2009). Briefly, almonds were blanched three times in liquid nitrogen, hand peeled, and then the skins were pulverised under liquid nitrogen to a powder. Almond powder was steeped twice in 3.5% acetic acid, 50% methanol in water at 4 °C over 20 h. Aliquots of extract were dried under nitrogen gas and stored at −20 °C in darkness until analysis.
2.4. Analysis of polyphenols
For LC–MS analysis of polyphenols, dried almond skin extract was first suspended in 497 μL of 50% methanol with 3 μL of 1 mM daidzein as an internal standard, diluted 10-fold with 1% formic acid, and centrifuged at 12,000×g for 10 min prior to injection. The concentration of 16 flavonoids and two phenolic acids were quantified by LC–MS as described by Bolling et al. (2009). Briefly, an Agilent 1100 MSD quadrupole with electrospray ionisation (ESI) was equipped with a 250 × 4.60 mm Synergi 4μ MAX-RP 80A column (Phenomenex, Torrance, CA) and set to a constant temperature of 25 °C. The polyphenols were eluted by an increasing gradient of 1% formic acid and 100% methanol at a flow rate of 0.2 mL/min. MSD signals were acquired in selected ion monitoring mode in three groups as negative ions: m/z 137, 153, 289 from retention time (Rt) of 0–22.6 min; m/z 137, 287, 433, 447, 463, 477, 593, 609, and 623 from Rt 22.6–28.2 min; and m/z 253, 271, 285, 287, 301, 315 from Rt 28.2 to 50 min.
Prior to calibration curve runs, all standards were mixed in a stock solution of methanol, aliquoted, and stored at −80 °C in amber screw-capped glass vials. On each day of analysis, different concentrations of standard solution, consisting of (+)-catechin (CA), (−)-epicatechin (EC), daidzein, eriodictyol (E), p-hydroxybenzoic acid (pHBA), isorhamnetin (Iso), isorhamnetin-3-O-glucoside (Iso3Glu), isorhamnetin-3-O-rutinoside (Iso3R), kaempferol (K), kaempferol-3-O-glucoside (K3Glu), kaempferol-3-O-rutinoside (K3R), naringenin (N), naringenin-7-O-glucoside (N7Glu), protocatechuic acid (PA), quercetin (Q), quercetin-3-galactoside (Q3Gal), and rutin (R), were serially diluted with 1% aqueous formic acid. Dihydrokaempferol was quantified on the basis of eridictyol equivalents and kaempfreol-3-O-glactoside on the basis of kaempferol-3-O-glucoside equivalents. Quercetin-3-O-galactoside coeluted with quercetin-3-O-glucoside. Routine intra- and inter-day assay coefficients of variation (CV) were 2.4% and 6.8%, respectively.
2.5. Total phenols and ferric reducing antioxidant power (FRAP)
The total phenol content of almond skin extract was determined according to Singleton, Orthofer, and Lamuela-Raventos (1999) with results expressed as mg gallic acid equivalents (GAE) per 100 g raw almonds. The FRAP assay assesses total antioxidant capacity via a redox-linked reduction of Fe3+-2,4,6-tri-pyridyl-S-triazine to a blue-coloured Fe2+ complex at pH 3.5 (Benzie & Strain, 1996). Following reconstitution in methanol, almond skin extracts were incubated at ambient temperature with the FRAP reagent and the absorbance measured at 593 nm after 1 h (Chen & Blumberg, 2008). FRAP reducing power is expressed as μmol TE/100 g almonds. Routine intra- and inter-day assay CV were 0.7% and 4.2%, respectively.
2.6. Statistics and data analysis
For all experiments, samples were analysed in duplicate. Statistical significance was determined by a mixed model ANOVA of cultivar and seasonal variation, followed by Tukey's Honestly Significant Difference as post hoc analysis by JMPIN v 3.2.6 software (SAS Institute Inc., Cary, NC). Pearson's correlation analysis was performed using GraphPad Prism v 5.01 (GraphPad Software Inc., La Jolla, CA). Differences were considered significant at P ≤ 0.05. Canonical discriminant analysis was performed by SAS v 9.1.3 program (SAS Institute Inc., Cary, NC) using standard pooled variance and 80% confidence ellipses. Cultivar and season were independently modelled using polyphenol, FRAP, and total phenol results.
3. Results
3.1. Cultivar
Over three seasons, the mean flavonoid and phenolic acid content in seven almond cultivars ranged from 3.96 to 10.7 mg/100 g almonds (Table 1). This represents a 2.7-fold difference between Fritz and Sonora cultivars, which had the lowest and highest polyphenolic content, respectively. Carmel had the second highest polyphenol content at 8.0 mg/100 g almonds, 25% less than that of Sonora. Nonpareil, which represented 40% of California almond acreage in 2007, had midrange polyphenol content of 6.2 mg/ 100 g almonds. Butte, Carmel, and Nonpareil had similar levels of polyphenols ranging from 6.2 to 8.0 mg/100 g.
Table 1.
Polyphenol content, total phenols, and FRAP values of seven almond cultivars over 3 years.
| Cultivar (skin%) | Polyphenols (mg/100 g) | Total phenols (mg GAE/100 g) | FRAP (μmol TE/100 g) |
|---|---|---|---|
| Sonora (4.0) | 10.7 ± 2.90a | 159 ± 21a | 891 ± 139a |
| Carmel (4.5) | 7.96 ± 1.44ab | 101 ± 30a | 888 ± 216a |
| Mission (4.4) | 6.91 ± 0.51b | 102 ± 60a | 609 ± 267ab |
| Butte (4.4) | 6.62 ± 0.79bc | 58 ± 7b | 368 ± 78b |
| Nonpareil (4.3) | 6.19 ± 0.78bc | 108 ± 25a | 645 ± 87ab |
| Monterey (4.4) | 4.88 ± 1.08c | 81 ± 12b | 530 ± 53b |
| Fritz (4.6) | 3.96 ± 2.34c | 58 ± 7b | 565 ± 274b |
Data are expressed as mean ± SD, n = 9 (Butte, Carmel, Nonpareil) and n = 3 (Sonora, Mission, Monterey, Fritz). Values within columns bearing different letters differ, tested by ANOVA and Tukey's HSD, P ≤ 0.05. Abbreviations: GAE, gallic acid equivalents; TE, Trolox equivalents.
Polyphenolic content and total phenols were slightly correlated among cultivars (R2 = 0.70, P = 0.083). However, FRAP values and polyphenol content were significantly correlated (R2 = 0.85, P = 0.016). Similar to their polyphenolic content, Sonora almonds had the highest total phenol concentration of 159 mg GAE/100 g and FRAP value of 891 μmol TE/100 g, although these values were not significantly different from the Carmel, Mission, and Nonpareil cultivars.
Of the 18 polyphenols quantified by the LC–MS analysis, cate-chin (CA), epicatechin (EC), naringenin-7-O-glucoside (N7Glu), kaempferol-3-O-rutinoside (K3R), dihydroxykaempferol (DiOH), isorhamnetin-3-O-rutinoside (Iso3R), isorhamnetin-3-O-glucoside (Iso3Glu), and naringenin (N) were the major flavonoids, each representing ≥3% of the total polyphenol content (Table 2). In Sonora almonds, CA, Iso3R, and Iso3Glu comprised 75% of the polyphenol content. Butte, Carmel, and Mission almonds were particularly high in K3R with 0.8–1.4 mg/100 g and low in Iso3G with 0.2– 0.3 mg/100 g almonds. Sonora, Nonpareil, and Monterey almonds were high in Iso3G with 2.0–2.9 mg/100 g almonds and relatively lower in K3R with 0.2–0.4 mg/100 g almonds. Iso3R was the predominant polyphenol among almond cultivars, since it comprised 30% or more of the adjusted total polyphenol content (Table 2). Relative to other cultivars, Butte almonds had greater ratios of K3R and Iso3R, and Nonpareil and Sonora had greater ratios of CA and Iso3G. Further, Mission almonds had a unique flavonoid profile, in that N7Glu and N were at least 2-fold greater than other cultivars.
Table 2.
Polyphenolic content of almond cultivars.
| Cultivar | Catechin | Epicatechin | Naringenin-7-O-glucoside | Kaemferol-3-O-rutinoside | Isorhamnetin-3-O-rutinoside | Isorhamnetin-3-O-glucoside | Naringenin |
|---|---|---|---|---|---|---|---|
| Butte | 0.536 ± 0.114c (8.2)A |
0.316 ± 0.131bc (4.7) |
0.136 ± 0.064b (2.0) | 1.38 ± 0.50a (20) | 2.98 ± 0.65a (44) | 0.290 ± 0.183d (4.7) | 0.380 ± 0.074b (5.6) |
| Carmel | 0.505 ± 0.157c (7.3) |
0.378 ± 0.167c (5.3) |
0.210 ± 0.151b (2.8) | 1.02 ± 0.24b (15) | 3.33 ± 0.50a (49) | 0.294 ± 0.090d (4.3) | 0.405 ± 0.105b (5.8) |
| Fritz | 0.569 ± 0.148bc (15) |
0.351 ± 0.209c (8.3) |
0.132 ± 0.200b (2.1) | 0.567 ± 0.228c (14) | 1.65 ± 0.98c (39) | 0.111 ± 0.088d (2.4) | 0.220 ± 0.314c (3.7) |
| Mission | 0.793 ± 0.120bc (11) |
0.408 ± 0.213a (5.6) |
0.402 ± 0.128a (5.6) | 0.772 ± 0.036bc (11) | 2.73 ± 0.14ab (38) | 0.223 ± 0.043d (3.1) | 0.801 ± 0.028a (11) |
| Monterrey | 0.568 ± 0.216bc (11) |
0.346 ± 0.168c (6.5) |
0.107 ± 0.031b (2.1) | 0.446 ± 0.221cd (9.2) | 1.98 ± 0.37bc (39) | 0.605 ± 0.386c (11) | 0.299 ± 0.050bc (5.9) |
| Nonpareil | 0.958 ± 0.167b (3.0) |
0.504 ± 0.123ab (7.9) |
0.204 ± 0.093b (3.1) | 0.218 ± 0.051d (3.4) | 2.00 ± 0.31c (31) | 1.04 ± 0.18b (16) | 0.395 ± 0.087b (6.2) |
| Sonora | 2.99 ± 1.35a (26) | 0.692 ± 0.411a (6.0) |
0.187 ± 0.043b (1.7) | 0.254 ± 0.098d (2.3) | 2.90 ± 0.33a (28) | 2.09 ± 0.30a (20) | 0.306 ± 0.106bc (2.8) |
Values in parentheses are per cent of total polyphenols measured by LC-MS. Data are expressed in mg/100 g almonds as mean ± SD, n = 9 (Butte, Carmel, Nonpareil) and n = 3 (Sonora, Mission, Monterey, Fritz). Values bearing different letters differ among columns, tested by ANOVA and Tukey's HSD, P ≤ 0.05.
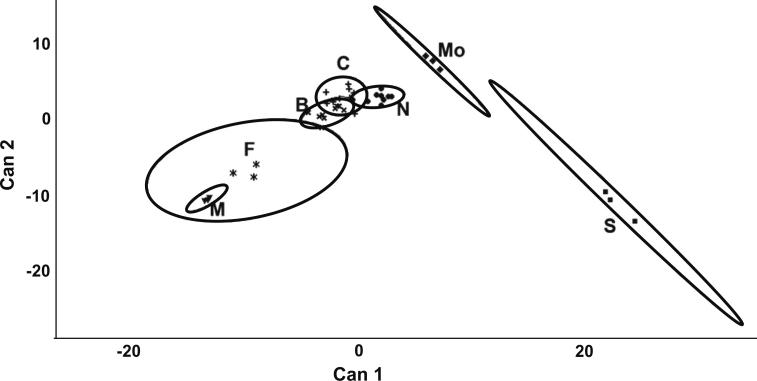
Canonical discriminant analysis of polyphenolic content and antioxidant activity of seven almond cultivars resulted in six variables with P-values less than 0.05 (Table 3). Reduced-space plots of the first two canonical variables distinguished cultivars on the basis of 80% confidence ellipses (Fig. 1). The first and second canonical variables accounted for 59% and 18% of the variation between cultivars, respectively. The flavonoids Iso, Iso3Glu, K, Q3Gal, CA, K3Glu, K3Gal, and Q provided the best discrimination between cultivars, having absolute CV1 values greater than 0.5 for these factors (Table 4). Butte, Carmel and Nonpareil almonds were the least distinguishable cultivars, having reduced space distances ranging from 2.5 to 4.9. Fritz and Mission were also less distinct, separated by a distance of 5.1. Monterey was closest to Sonora, with a distance of 6.7. All other distances ranged from 9.2 to 36.
Table 3.
Canonical discriminant analysis parameters for California almond cultivars and harvest season models.
| Model | Variable number | Canonical correlation | F-value | DF | P-valuea | Eigen value | Proportion of variance |
|---|---|---|---|---|---|---|---|
| Cultivar | 1 | 0.9772 | 13.5061 | 48 | <0.0001 | 21.1585 | 0.5941 |
| 2 | 0.9334 | 9.4895 | 35 | <0.0001 | 6.7794 | 0.1904 | |
| 3 | 0.9029 | 7.6686 | 24 | <0.0001 | 4.4097 | 0.1238 | |
| 4 | 0.8049 | 5.7487 | 15 | <0.0001 | 1.8399 | 0.0517 | |
| 5 | 0.7270 | 4.8069 | 8 | 0.0001 | 1.1207 | 0.0315 | |
| 6 | 0.4829 | 3.0410 | 3 | 0.0441 | 0.3041 | 0.0085 | |
| Season | 1 | 0.9155 | 3.1476 | 38 | 0.0004 | 5.1773 | 0.7189 |
| 2 | 0.8182 | 2.1371 | 18 | 0.0546 | 2.0246 | 0.2811 |
Probability that canonical correlation and all smaller ones are zero in the population (SAS Inc.).
Fig. 1.
Canonical discriminant analysis of almond cultivars based on polyphenol content and antioxidant activity. Labels: M, Mission; F, Fritz; B, Butte; C, Carmel; N, Nonpareil; Mo, Monterey; S, Sonora; Can1, first canonical variable; Can2, second canonical variable. Data represents the first two canonical variables of almond samples by cultivar with 80% confidence ellipses.
Table 4.
Canonical discriminant analysis factors for the first and second canonical variables (Can1, Can2) for cultivar and season models.
| Cultivar |
Season |
||||
|---|---|---|---|---|---|
| Factor | Can1 | Can2 | Factor | Can1 | Can2 |
| Iso | 0.8629 | –0.4245 | EC | 0.5565 | –0.2427 |
| Iso3Glu | 0.8383 | –0.2245 | PA | 0.4348 | 0.403 |
| K | 0.8156 | –0.372 | R | 0.4024 | –0.5197 |
| Q3Gal | 0.7536 | –0.4402 | pHBA | 0.4007 | 0.2846 |
| CA | 0.7135 | –0.5134 | E | 0.3926 | –0.1597 |
| K3Glu | 0.6835 | –0.1091 | DiOHK | 0.2199 | –0.2429 |
| K3Gal | 0.5292 | –0.1619 | N7Glu | 0.1942 | –0.1788 |
| Q | 0.5279 | 0.233 | Q3Gal | 0.1862 | –0.2296 |
| Total Phenols | 0.4243 | –0.2972 | N | 0.1679 | –0.1766 |
| EC | 0.3879 | –0.2102 | Q | 0.1557 | –0.424 |
| FRAP | 0.3424 | –0.1496 | K3Glu | 0.1398 | –0.1788 |
| E | 0.1191 | –0.3778 | CA | 0.1346 | –0.0433 |
| PA | 0.0166 | –0.1056 | K3Gal | 0.129 | –0.1451 |
| Iso3R | 0.014 | –0.0108 | K | 0.1223 | –0.1039 |
| pHBA | –0.0205 | 0.0174 | Iso3R | 0.12 | –0.0602 |
| DiOHK | –0.1117 | –0.0785 | K3R | 0.0697 | 0.1583 |
| R | –0.1687 | –0.234 | Iso3Glu | 0.0635 | –0.1113 |
| N7Glu | –0.186 | –0.2591 | Iso | 0.0323 | –0.1007 |
| N | –0.3595 | –0.2375 | Total Phenols | 0.0202 | –0.3386 |
| K3R | –0.3774 | 0.0986 | FRAP | –0.0461 | –0.2351 |
3.2. Season
The impact of season on polyphenol content and antioxidant activity was examined only on Butte, Carmel, and Nonpareil cultivars. Butte, Carmel, and Nonpareil almonds (n = 9) had significant differences in flavonoid and phenolic acid content, but not total phenols or FRAP values between seasons (Table 5). Polyphenols measured by LC–MS were the highest at 7.0 mg/100 g almonds in the 2005 harvest year, 13% greater than 2007. Among years, EC was the only major flavonoid that had a significant seasonal difference. Almonds from 2005 and 2006 had 0.479 ± 0.174 and 0.434 ± 0.100 mg EC/100 g, respectively, while 2007 had 0.284 ± 0.132 mg EC/100 g, 1.7-fold less than 2005 (P < 0.05). The polyphenolic profile (ratio of individual flavonoid or phenolic acid to total polyphenols) was unchanged between harvest years.
Table 5.
The effect of harvest season on polyphenol content and antioxidant activity of Butte, Carmel, and Nonpareil almonds.
| Season | Polyphenols (mg/100 g) | Total Phenols (mg GAE/100 g) | FRAP (μmol TE/100 g) |
|---|---|---|---|
| 2005 | 7.019 ± 0.103a | 87 ± 26a | 584 ± 207a |
| 2006 | 6.284 ± 0.124b | 96 ± 39a | 616 ± 251a |
| 2007 | 6.117 ± 0.959b | 87 ± 38a | 630 ± 200a |
Data are expressed as mean ± SD, n = 9. Values within the same column bearing different letters differ, tested by ANOVA and Tukey's HSD, P ≤ 0.05. Abbreviations: GAE, gallic acid equivalents; TE, Trolox equivalents.
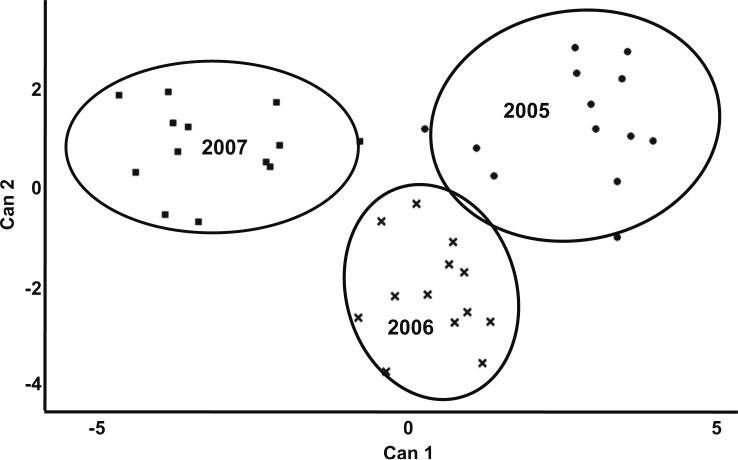
Canonical discriminant analysis of seasonal differences in Butte, Carmel, and Nonpareil cultivars resulted in two canonical variables that accounted for 72% and 28% of variation, respectively (Fig. 2). EC was the strongest factor for the first canonical variable, which best distinguished almonds from 2005 and 2007, with a distance of 5.9 (Table 4). For the second canonical variable, rutin (R) and procatechuic acid (PA) best separated 2006 from the 2005 and 2007 seasons.
Fig. 2.
Canonical discriminant analysis of almond harvest seasons based on polyphenol content and antioxidant activity. Data represents the first two canonical variables of Butte, Carmel, and Nonpareil almond samples by harvest year with 80% confidence ellipses. Labels: Can1, first canonical variable; Can2, second canonical variable.
4. Discussion
Polyphenols and other antioxidant constituents may contribute to the health promoting effect of fruits, vegetables, whole grains, and nuts. Revealing an association between the dietary intake of polyphenols and their potential health benefits requires an accurate assessment of the content of these constituents in plant foods. The polyphenol content of many plant foods have been reported in the flavonoid, isoflavone, and proanthocyanidin databases published by the USDA. However, the scope of this information is limited, because of a number of unaccounted factors such as differences in cultivars, growing environments, seasons, processing, and storage. These factors are known to affect polyphenol content and profiles of plant foods. In our previous study with one almond sample each for eight cultivars, we found that almond flavonoid content might be dependent on cultivar (Milbury et al., 2006). Almond polyphenols include simple phenols, flavonoids, tannins, condensed or polymerised flavonoids or phenols, and proanthocyanidins. We have further characterised here the impact of cultivar and harvest year on almond polyphenol profile and antioxidant activity.
4.1. Cultivar
The cultivars reported here represented 82% of almond commercial acreage in 2007 (Almond Board of California, 2008). The 3-year mean phenolic acid and flavonoid content of seven California almond cultivars varied by 2.7-fold. This is comparable to our previous study where total phenols and polyphenols varied by 2.7-fold (60.2–175.1 mg GAE/100 g) and 1.9-fold (14.6–27.2 mg/ 100 g almonds), respectively, in a single year across eight almond cultivars; similarly, Fritz and Monterey were low polyphenol-producing cultivars (Milbury et al., 2006). Hughey et al. (2008) reported that Carmel almonds had 47% more flavonoids than Nonpareil almonds harvested in 2006. This degree of variation is similar to the 29% difference of the same cultivars over a 3-year period reported here. In contrast to our finding that Carmel almonds contain more polyphenols than Nonpareil, we previously found that Nonpareil almonds harvested in 2004 had 14% more polyphenols than Carmel (Milbury et al., 2006). Season and growing region may account for this discrepancy.
The major California almond cultivars had less variability in flavonoid content, total phenols, and antioxidant activity than almonds harvested in Portugal and Iran. A study of 10 Portuguese almond cultivars found four and 18-fold differences in flavonoids and total phenols between cultivars, respectively (Barreira et al., 2008). Analysis of 18 Iranian almond genotypes showed a 4.6-fold variation in the phenolic content of hulls and a similar variability of the antioxidant activity of extracts (Sfahlan, Mahmoodzadeh, Hasanzadeh, Heidari, & Jamei, 2009).
Relative to other reports of polyphenol content variability, the 3-year variation of California almond polyphenols appears to be equivalent or less than tree fruits. Over a 1-year period, plum, peach, and nectarine skin total phenols varied 2.0, 2.7, and 4.8-fold, respectively, between cultivars (Gil, Tomas-Barberan, Hess-Pierce, & Kader, 2002). Apple polyphenols varied at least 5.2-fold between 67 cultivars (Wojdylo, Oszmainski, & Laskowski, 2008).
In addition to the variation in polyphenol content between cultivars, the seven California almond cultivars had unique polyphenolic profiles (Table 2). Flavonoids are products of the shikimate pathway from phenylalanine and acetate in plants. The genetic variation in the shikimate pathway of almond cultivars is likely responsible for the different flavonoid profiles between cultivars. Since polyphenols are phytoalexins, overlaying cultivar-specific polyphenol composition could yield insight into genetic traits favourable for stress adaptation and disease resistance. Therefore, more rigorous investigations into the extent of cultivar-environment interactions that affect almond polyphenol content are warranted.
Polyphenol and antioxidant content could differentiate almond cultivars and harvest years. Using multivariate analysis, studies of the chemical composition of wines (Sivertsen, Holen, Nicolaysen, & Risvik, 1999), olives (Ocakoglu, Tokatli, Ozen, & Korel, 2009), and cabbage (Sousa et al., 2008) have also distinguished cultivar and fertilisation methods. We found that canonical discriminant analysis of polyphenol content and antioxidant activity could distinguish almonds harvested in different seasons and from some cultivars with 80% confidence. Similarly, fatty acid composition (Carratala, Garcia-Lopez, Berenguer-Navarro, & Grane-Teruel, 1998), free amino acid profile (Seron et al., 1998), and triglycerides (Martin-Carratala, Llorens-Jorda, Berenguer-Navarro, & Grane-Teruel, 1999) also have distinguished almond cultivars. Thus, compiling cultivar nutrient composition data may help inform selection of cultivars, agricultural practices, and growth environments that improve almond nutrient content.
Using a canonical discriminant analysis, we found that polyphenol content and antioxidant activity could corroborate known almond cultivar inheritance. Fritz is an offspring of the Mission cultivar, Butte is a cross of Mission and Nonpareil cultivars, and Carmel, Monterey, and Sonora are offspring of Nonpareil (Bartolozzi, Warburton, Arulsekar, & Gradziel, 1998; Hauagge, Kester, Arulsekar, Parfitt, & Liu, 1987). In a reduced-space plot (Fig. 1) of almond cultivars, offspring are the nearest neighbours to parents. It is notable that although Monterey and Sonora are both offspring of Nonpareil, Sonora is a high polyphenol producer, while Monterey is a low polyphenol producer. Sonora, the offspring of Nonpareil and Eureka cultivars, also shares a different origin than Padre, another high polyphenol-producing cultivar (Bartolozzi et al., 1998; Milbury et al., 2006). Further monitoring of polyphenol content in almonds of different cultivars may lead to more information about gene–environment interactions.
Region could also affect concentration of polyphenols in almond skin among different cultivars. Preliminary analysis of compositional data pooled by geographic region indicated changes to polyphenols and total phenol concentrations. Because agronomic conditions and orchard locations were not controlled by this study, a more strictly-controlled study controlling for agronomic practice is warranted to characterise factors responsible for regional differences in flavonoid content.
4.2. Season
Climate variability, agricultural practice, or other factors may lead to the seasonal variation in almond polyphenol content. We found 13% greater polyphenol content in 2005 compared to 2007 among Butte, Carmel, and Nonpareil cultivars. The degree of this variation was less than that reported by Garrido et al. (2008) of unidentified American almond cultivars which reported differences of 54% for polyphenols and 36% for ORAC values between 2004 and 2006. Interestingly, results from this study and ours demonstrate that the seasonal differences in total phenols and antioxidant activities were less than polyphenol content. This implicates antioxidant constituents in almonds besides the 18 polyphenols measured in our study in maintaining total antioxidant activity between years. However, unlike the differential effect of cultivar on polyphenol synthetic pathways, season may affect pathways similarly.
4.3. Methods of analysis
Quantitative LC–MS analysis of polyphenols limits potential interference from co-eluting compounds. Differences in the absolute amount of polyphenols recovered from almond skins between studies may arise from methods of extraction and analysis (Bolling et al., 2009). Previously, we found hot water blanching followed by acidified methanol extraction recovers nearly 50% more polyphenols from almond skins (Bolling et al., 2009). The range of 4.0– 10.7 mg polyphenols/100 g almonds in the present study is less than the mean value of 15.24 mg/100 g almonds reported in the USDA flavonoid database. The flavonoid database values for almonds are based on our prior study (Milbury et al., 2006), and data from the Food Composition Nutrient Data Laboratories of the USDA (Harnly et al., 2006). These data utilise extracts from whole almonds, whereas the present study has only analysed skin, which accounted for 78–98% of the flavonoid content from whole almonds (Milbury et al., 2006). The flavonoid database also reports 2.46 mg cyanidin and 2.59 mg (−)-epigallocatechin/100 g almonds. These flavonoids were not determined in the present study and contribute to the polyphenol content of almond skins. Therefore, interpretation of polyphenol concentrations between studies should consider the effect of analytical methods.
5. Conclusion
Over three seasons, California almond cultivars had unique polyphenol profiles. The flavonoid content almond cultivars varied between seasons. This information provides evidence for variation of almond polyphenols and antioxidant activity due to genes and environment and could inform cultivation practices to enhance the polyphenol and antioxidant quality of almonds. Knowledge of cultivar-specific polyphenol profiles may also allow optimisation of agronomic and post-harvest handling to maximise health benefits of polyphenols. However, more information is needed regarding the bioavailability of individual phenolic and flavonoid constituents from almond skins to determine if these changes are nutritionally relevant. Further work is also required to determine the effect of processing and storage on the polyphenolic content of different almond cultivars.
Supplementary Material
Acknowledgement
This work was supported by US Department of Agriculture (USDA)/Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707 and a grant from the Almond Board of California. Dr. Bolling was supported by award K12GM074869 from the National Institute of Medical Sciences. The authors are grateful for the excellent technical assistance of Jennifer O'Leary, Desire Kelley, and Sisca Bolling.
Abbreviations
- B
Butte
- C
Carmel
- CA
catechin
- EC
epicatechin
- DiOHK
dihydroxykaempferol
- E
eriodictyol
- ECD
electrochemical detection
- F
Fritz
- GAE
gallic acid equivalents
- FRAP
ferric reducing antioxidant power
- Iso
isorhamnetin
- Iso3Glu
isorhamnetin-3-O-glucoside
- Iso3R
isorhamnetin-3-O-rutinoside
- K
kaempferol
- K3Gal
kaempferol-3-O-galactoside
- K3Glu
kaempferol-3-O-glucoside
- K3R
kaempferol-3-O-rutinoside
- M
Mission
- Mo
Monterey
- N
naringenin
- N
Nonpareil
- N7Glu
naringenin-7-O-glucoside
- ORAC
oxygen radical absorbance capacity
- PA
procatechuic acid
- pHBA
p-hydroxybenzoic acid
- Q
quercetin
- Q3Gal
quercetin-3-O-galactoside
- R
rutin
- TE
Trolox equivalents
Footnotes
Supported by the Almond Board of California, US Department of Agriculture (USDA)/Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707, and K12GM074869 from the National Institute of General Medical Sciences. The contents of this publication do not necessarily reflect the official views or policies of the NIGMS, NIH, or USDA nor does mention of trade names, commercial products or organisations imply endorsement by the US government.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.foodchem.2010.03.068.
References
- Alasalvar C, Shahidi F. Tree nuts: Composition, phytochemicals and health effects. CRC Press; Boca Raton, FL: 2009. [Google Scholar]
- Almond Board of California . Modesto; Almond almanac. CA: 2008. [Google Scholar]
- Amaral JS, Valentao P, Andrade PB, Martins RC, Seabra RM. Do cultivar, geographical location and crop season influence phenolic profile of walnut leaves? Molecules. 2008;13:1321–1332. doi: 10.3390/molecules13061321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreira JC, Ferreira IC, Oliveira MB, Pereira JA. Antioxidant activity and bioactive compounds of 10 Portuguese regional and commercial almond cultivars. Food and Chemical Toxicology. 2008;46:2230–2235. doi: 10.1016/j.fct.2008.02.024. [DOI] [PubMed] [Google Scholar]
- Bartolozzi F, Warburton ML, Arulsekar S, Gradziel TM. Genetic characterization and relatedness among California almond cultivars and breeding lines detected by randomly amplified polymorphic DNA (RAPD) analysis. Journal of the American Society of Horticultural Science. 1998;123:381–387. [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Analytical Biochemistry. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bolling B, Dolnikowski GG, Blumberg J, Chen CY. Quantification of almond skin polyphenols by liquid chromatography–mass spectrometry. Journal of Food Science. 2009;74:C326–C332. doi: 10.1111/j.1750-3841.2009.01133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carratala MLM, Garcia-Lopez C, Berenguer-Navarro V, Grane-Teruel N. New contribution to the chemometric characterization of almond cultivars on the basis of their fatty acid profiles. Journal of Agricultural and Food Chemistry. 1998;46:963–967. [Google Scholar]
- Chen CY, Blumberg JB. In vitro activity of almond skin polyphenols for scavenging free radicals and inducing quinone reductase. Journal of Agricultural and Food Chemistry. 2008;56:4427–4434. doi: 10.1021/jf800061z. [DOI] [PubMed] [Google Scholar]
- Chen CY, Milbury PE, Lapsley K, Blumberg JB. Flavonoids from almond skins are bioavailable and act synergistically with vitamins C and E to enhance hamster and human LDL resistance to oxidation. Journal of Nutrition. 2005;135:1366–1373. doi: 10.1093/jn/135.6.1366. [DOI] [PubMed] [Google Scholar]
- Garrido I, Monagas M, Gomez-Cordoves C, Bartolome B. Polyphenols and antioxidant properties of almond skins: Influence of industrial processing. Journal of Food Science. 2008;73:C106–C115. doi: 10.1111/j.1750-3841.2007.00637.x. [DOI] [PubMed] [Google Scholar]
- Geleijnse JM, Launer LJ, Hofman A, Pols HA, Witteman JC. Tea flavonoids may protect against atherosclerosis: The Rotterdam study. Archives of Internal Medicine. 1999;159:2170–2174. doi: 10.1001/archinte.159.18.2170. [DOI] [PubMed] [Google Scholar]
- Gil MI, Tomas-Barberan AT, Hess-Pierce B, Kader AA. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. Journal of Agricultural and Food Chemistry. 2002;50:4976–4982. doi: 10.1021/jf020136b. [DOI] [PubMed] [Google Scholar]
- Harnly JM, Doherty RF, Beecher GR, Holden JM, Haytowitz DB, Bhagwat S, et al. Flavonoid content of US fruits, vegetables, and nuts. Journal of Agricultural and Food Chemistry. 2006;54:9966–9977. doi: 10.1021/jf061478a. [DOI] [PubMed] [Google Scholar]
- Hauagge R, Kester DE, Arulsekar S, Parfitt DE, Liu L. Isozyme variation among California almond cultivars: II. Cultivar characterization and origins. Journal of the American Society for Horticultural Science. 1987;112:693–698. [Google Scholar]
- Hertog MG, Hollman PC, Katan MB, Kromhout D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutrition and Cancer. 1993;20:21–29. doi: 10.1080/01635589309514267. [DOI] [PubMed] [Google Scholar]
- Hughes LA, Arts IC, Ambergen T, Brants HA, Dagnelie PC, Goldbohm RA, et al. Higher dietary flavone, flavonol, and catechin intakes are associated with less of an increase in BMI over time in women: A longitudinal analysis from the Netherlands Cohort Study. American Journal of Clinical Nutrition. 2008;88:1341–1352. doi: 10.3945/ajcn.2008.26058. [DOI] [PubMed] [Google Scholar]
- Hughey CA, Wilcox B, Minardi CS, Takehara CW, Sundararaman M, Were LM. Capillary liquid chromatography–mass spectrometry for the rapid identification and quantification of almond flavonoids. Journal of Chromatography A. 2008;1192:259–265. doi: 10.1016/j.chroma.2008.03.079. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Kendall CW, Marchie A, Josse AR, Nguyen TH, Faulkner DA, et al. Almonds reduce biomarkers of lipid peroxidation in older hyperlipidemic subjects. Journal of Nutrition. 2008;138:908–913. doi: 10.1093/jn/138.5.908. [DOI] [PubMed] [Google Scholar]
- Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: The Zutphen study. Archives of Internal Medicine. 1996;156:637–642. [PubMed] [Google Scholar]
- King JC, Blumberg J, Ingwersen L, Jenab M, Tucker KL. Tree nuts and peanuts as components of a healthy diet. Journal of Nutrition. 2008;138:1736S–1740S. doi: 10.1093/jn/138.9.1736S. [DOI] [PubMed] [Google Scholar]
- Laverdrine F, Ravel A, Villet A, Ducros V, Alaray J. Mineral composition of two walnut cultivars originating in France and California. Food Chemistry. 2000;68:347–351. [Google Scholar]
- Martin-Carratala ML, Llorens-Jorda C, Berenguer-Navarro V, Grane-Teruel N. Comparitive study on the triglyceride composition of almond kernel oil. A new basis for cultivar chemometric characterization. Journal of Agricultural and Food Chemistry. 1999;47:3688–3692. doi: 10.1021/jf981220n. [DOI] [PubMed] [Google Scholar]
- Milbury PE, Chen CY, Dolnikowski GG, Blumberg JB. Determination of flavonoids and phenolics and their distribution in almonds. Journal of Agricultural and Food Chemistry. 2006;54:5027–5033. doi: 10.1021/jf0603937. [DOI] [PubMed] [Google Scholar]
- Ocakoglu D, Tokatli F, Ozen B, Korel F. Distribution of simple phenols, phenolic acids and flavonoids in Turkish monovarietal extra virgin olive oils for two harvest years. Food Chemistry. 2009;113:401–410. [Google Scholar]
- Parcerisa J, Rafecas M, Castellote AI, Codony R, Farran A, Garcia J, et al. Influence of variety and geographical origin on the lipid fraction of hazelnuts (Corylus avellana L.) from Spain: (III) Oil stability, tocopherol content and some mineral contents (Mn, Fe, Cu). Food Chemistry. 1995;53:71–74. [Google Scholar]
- Reiling J, editor. The food value of nuts. Journal of the American Medical Association. 2008;300:2806. [Google Scholar]
- Sanchez-Bel P, Egea I, Martinez-Madrid MC, Flores B, Romojaro F. Influence of irrigation and organic/inorganic fertilization on chemical quality of almond (Prunus amygdalus cv. Guara). Journal of Agricultural and Food Chemistry. 2008;56:10056–10062. doi: 10.1021/jf8012212. [DOI] [PubMed] [Google Scholar]
- Sang S, Lapsley K, Jeong W, Lachance P, Ho C-T, Rosen R. Antioxidative phenolic compounds isolated from almond skins (Prunus amygdalus Batsch). Journal of Agricultural and Food Chemistry. 2002;50:2459–2463. doi: 10.1021/jf011533+. [DOI] [PubMed] [Google Scholar]
- Seron LH, Poveda EG, Moya MS, Carratala MLM, Berenguer-Navarro V, Grane-Teruel N. Characterisation of 19 almond cultivars on the basis of their free amino acids composition. Food Chemistry. 1998;61:455–459. [Google Scholar]
- Sfahlan AJ, Mahmoodzadeh A, Hasanzadeh A, Heidari R, Jamei R. Antioxidants and antiradicals in almond hull and shell (Amygdalus communis L.) as a function of genotype. Food Chemistry. 2009;115:529–533. [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods of Enzymology. 1999;299:152–178. [Google Scholar]
- Sivertsen HK, Holen B, Nicolaysen F, Risvik E. Classification of French red wines according to their geographical origin by the use of multivariate analyses. Journal of the Science of Food and Agriculture. 1999;79:107–115. [Google Scholar]
- Sousa C, Pereira DM, Pereira JA, Bento A, Rodrigues MA, Dopico-Garcia S, et al. Multivariate analysis of tronchuda cabbage (Brassica oleracea L. var. costata DC) phenolics: Influence of fertilizers. Journal of Agricultural and Food Chemistry. 2008;56:2231–2239. doi: 10.1021/jf073041o. [DOI] [PubMed] [Google Scholar]
- Wijeratne S, Abou-Zaid M, Shahidi F. Antioxidant polyphenols in almond and its coproducts. Journal of Agricultural and Food Chemistry. 2006;54:312–318. doi: 10.1021/jf051692j. [DOI] [PubMed] [Google Scholar]
- Wojdylo A, Oszmainski J, Laskowski P. Polyphenolic compounds and antioxidant activity of new and old apple varieties. Journal of Agricultural and Food Chemistry. 2008;56:6520–6530. doi: 10.1021/jf800510j. [DOI] [PubMed] [Google Scholar]
- Yochum L, Kushi LH, Meyer K, Folsom AR. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. American Journal of Epidemiology. 1999;149:943–949. doi: 10.1093/oxfordjournals.aje.a009738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.