Abstract
Liquid chromatography, with diode array detection and electrospray ionization mass spectrometry (LC-DAD-ESI/MS), was used to identify and quantify flavonoids in cashew apple. One anthocyanin and thirteen glycosylated flavonols were detected in a methanol–water extract. Among them, the 3-O-galactoside, 3-O-glucoside, 3-O-rhamnoside, 3-O-xylopyranoside, 3-O-arabinopyranoside and 3-O-arabinofuranoside of quercetin and myricetin, as well as kaempferol 3-O-glucoside were identified by direct comparison with standards or positively identified flavonoids in cranberry. The anthocyanin was the 3-O-hexoside of methyl-cyanidin. Trace amounts of delphinidin and rhamnetin were detected in the hydrolyzed extract, suggesting their glycosides were present, but undetectable, in the original extract. The concentrations of the 14 flavonoids in the tested sample were determined. This is the first report of these flavonoids in cashew apple.
Keywords: Cashew apple, Anacardium occidentale L. and varieties, Glycosylated flavonols, Anthocyanin, LC-DAD-ESI/MS
1. Introduction
The cashew tree, Anacardium occidentale L. (Anacardiaceae), has its origin in Brazil and is well established in many tropical regions. The edible cashew nut is an extremely important agricultural trade product for Brazil. Cashew cultivation occupies an estimated 700,000 hectares with a cashew nut production of 280,000 tons/year (Embrapa, 1992; Filgueiras, Alves, Masca, & Menezes, 1999; Leite & Paula Pessoa, 2002). In Brazil, almost 96% of the cashew cultivation is located in the northeast region. Cashew production is of high social and economical importance for many countries, including India and some African countries, due to the labour force required in the field and for processing. Therefore, identification of the apples’ health-beneficial chemical components could promote its use and have a significant economic impact (Assuncao & Mercadante, 2003; Bicalho, Pereira, Aquino Neto, Pinto, & Rezende, 2000).
Cashew apple juice has been reported to have antitumor (Cavalcante, Rübensam, Erdtmann, Brendel, & Henriques, 2005; Kubo, Ochi, Vieira, & Komatsu, 1993; Kozubek, Zarnowski, Stasiuk, & Gubernator, 2001), antimicrobial (Cavalcante et al., 2003; Kubo, Lee, & Kubo, 1999; Kubo, Nihei, & Tsujimoto, 2003; Kubo, Muroi, & Himejima, 1993), urease inhibitory (Kubo et al., 1999) and lipoxygenase activity (Ha & Kubo, 2005). Cashew apple juice and cajuina, the clarified juice, also have excellent anti-oxidant potential, as evidenced by their ability to scavenge free peroxyl radicals. The total radical-trapping antioxidant potential (TRAP) assay showed lowered oxidative damage-induced mutagenesis by co- and post-treatments with the juices (Melo-Cavalcante et al., 2003).
Chemically, the cashew apple contains volatile compounds (Bicalho et al., 2000), resorcinolic acid, anacardic acids, carotenoids (α-carotene, β-carotene and β-cryptoxanthin), vitamin C, phenols and tannin (Assuncao & Mercadante, 2003). Phenolic constituents, (anacardic acids, cardols, cardanols) were reported to be present in cashew nut shell (Paramashivappa, Phani Kumar, Vithayathil, & Srinivasa Rao, 2001). Two flavonoid aglycones have been isolated from A. occidentale (Harborne & Baxter, 1999a), but there are no reports of glycosylated flavonols or anthocyanins.
The flavonoid content of food plants has been reported to offer biological benefits, such as reduced risk of cancer and cardiovascular disease (Heim, Tagliaferro, & Bobilya, 2003; Robards, 2003; Robards, Prenzler, Tucker, Swatsitang, & Glover, 1999). Flavonoids are readily detected by liquid chromatography with diode array and/or mass spectrometric detection (Cuyckens & Cleays, 2004). As part of a project to determine flavonoids in food plants, we examined freeze-dried cashew apple, using a standardized screening method (Lin & Harnly, 2007) consisting of liquid chromatography with diode array and electrospray ionization mass spectrometry detection (LC-DAD-ESI/MS). One anthocyanin and 13 glycosylated flavonols were detected in the cashew apple for the first time. This report, describes the identification and quantification of these glycosylated flavonoids in cashew apples.
2. Materials and methods
2.1. Plant materials
Two cashew apple cultivars were harvested at the Embrapa Tropical Agroindustry Experimental Station, located at Paraipaba, Ceará, Brazil. The fresh cashew apples were freeze-dried (humidity of 84.77%), finely powdered, and passed through a 20 mesh sieve prior to extraction.
2.2. Flavonoid standards and chemicals
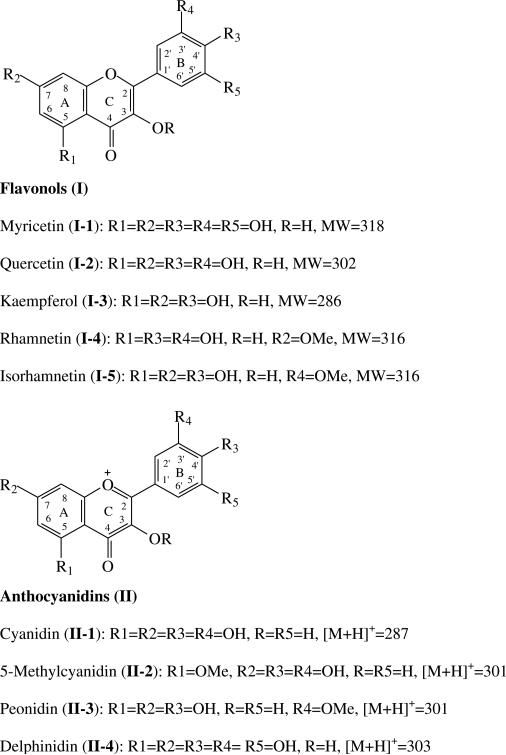
Myricetin (I-1, Fig. 1, approx. 85%), quercetin dihydrate (I-2, 98%), and kaempferol (I-3, minimum 90%, HPLC) (Fig. 1) were purchased from Sigma Chemical Co. (Saint Louis, MO). HPLC grade myricetin 3-O-rhamnoside, quercetin 3-O-galactoside, quercetin 3-O-glucoside, quercetin 3-O-rhamnoside, kaempferol 3-O-glucoside, rhamnetin (I-4) and isorhamnetin (I-5) were purchased from Extrasynthese (Genay, Cedex, France). Peonidin chloride (II-2), cyanidin chloride (II-1), delphinidin chloride (II-4), and cyanidin 3-O-glucoside were purchased from Indofine Chemical Co. (Somerville, NJ). Formic acid, hydrochloric acid (37%), HPLC grade acetonitrile and methanol, were purchased from VWR Scientific (Seattle, WA). HPLC grade water was prepared from distilled water using a Milli-Q system (Millipore Lab., Bedford, MA).
Fig. 1.
The structures of the flavonoids.
2.3. LC-DAD and ESI-MSD conditions
The LC-DAD-ESI/MS instrument consisted of an Agilent 1100 HPLC (Agilent, Palo Alto, CA) coupled with a diode array detector (DAD) and mass spectrometer (MSD, SL mode). A waters (Waters Corp., Milford, MA) symmetry column (C18, 5 μm, 250 × 4.6 mm) with a sentry guard column (Symmetry 5 μm, 3.9 × 20 mm) was used at a flow rate of 1.0 ml/min. The column oven temperature was set at 25 °C. The mobile phase consisted of a combination of A (0.1% formic acid in water) and B (0.1% formic acid in acetonitrile). The gradient was varied linearly from 10% to 26% B (v/v) in 40 min, to 65% B at 70 min, and finally to 100% B at 71 min and held at 100% B to 75 min. The DAD was set at 350, 310, 270 and 520 nm for real-time read-out and UV/VIS spectra, from 190 to 650 nm, were continuously collected for plant component identification. Mass spectra were simultaneously acquired using electrospray ionization in the positive and negative ionization (PI and NI) modes at low and high fragmentation voltages (100 V and 250 V, respectively) for the mass range of 100–2000 amu (Lin & Harnly, 2007). A drying gas flow of 13 l/min, a drying gas temperature of 350 °C, a nebulizer pressure of 50 psi, and capillary voltages of 4000 V for PI and 3500 V for NI were used. The LC system was directly coupled to the MSD without stream splitting (Lin & Harnly, 2007).
2.4. Sample preparation
2.4.1. Plant extracts
Powdered dried cashew apple (300 mg) was extracted with methanol–water (10.0 ml, 60:40, v/v) using sonication (FS30 Ultrasonic sonicator, Fisher Scientific, Pittsburg, PA) at 40 kHz, 100 W for 60 min at room temperature (from 22 °C to <40 °C at the end). The solvent was separated from the solid by centrifuging at 2500 rpm for 15 min (IEC Clinical Centrifuge, Damon/IEC Division, Needham, MA). Then, 8 ml of the extract were taken to dryness and the residue (around 185–190 mg) was redissolved in 1.0 ml of methanol–water (60:40, v/v) to provide a total volume of 1.1 ml. This final volume was established by dissolving 1.875 g of sugar in 10.0 ml of methanol–water (60:40, v/v). The methanol–water solution was sonicated for 10 min, filtered through a 0.45 μm 13 mm PVDF syringe filter (VWR Scientific, Seattle, WA), and 50 μl were injected onto the column for LC-DAD-ESI/MS analysis.
2.4.2. Hydrolyzed purified glycosylated flavonoid extract
The concentrated extract was purified using a polyamide-6 cartridge (Macherey-Nagel GmbH & Co, Düren, Germany). Sugars were removed by washing with water (10.0 ml). The flavonoids absorbed on the cartridge were eluted with methanol (10.0 ml). The eluent was taken to dryness and the residue redissolved in 1.0 ml of methanol–water (60:40, v/v). Then, 0.5 ml of the solution was mixed with 0.1 ml of concentrated HCl (37%) and heated in a covered tube at 85 °C for 2.0 h. Then, 0.4 ml of methanol was added to the mixture and it was sonicated for 10 min. The solution was filtered through a 0.45 μm 13 mm PVDF syringe filter (VWR Scientific, Seattle, WA), and 50 μl were injected onto the column for LC-DAD-ESI/MS analysis of the aglycones.
3. Results and discussion
3.1. Identification of cashew apple flavonoids by LC-DAD-ESI/MS
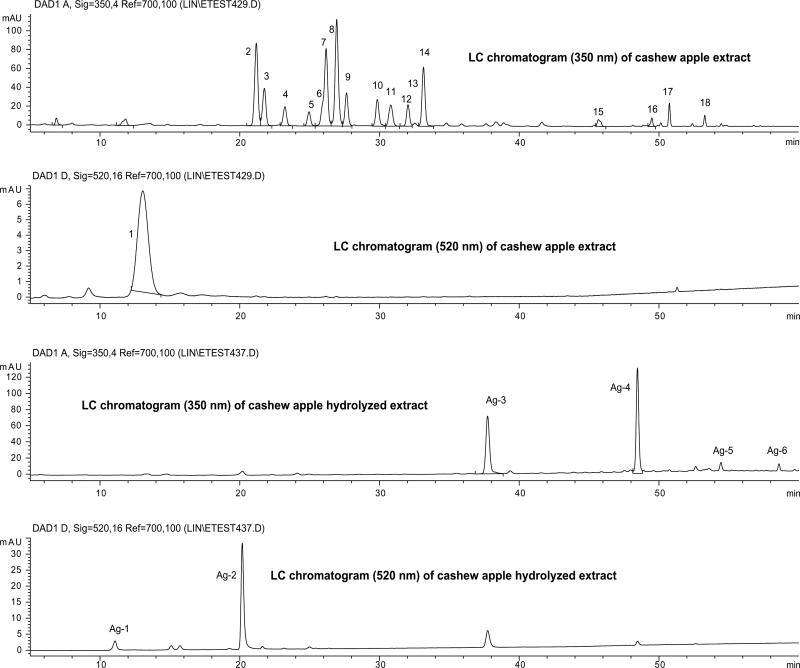
The HPLC chromatograms (350 and 520 nm) of cashew apple extract and the hydrolyzed extract are shown in Fig. 2. The retention times (tR), UV/Vis absorption maxima (λmax), and PI/NI molecular and aglycone ions for each LC peak are listed in Table 1. Fig. 1 summarized the aglycones of the glycosides found.
Fig. 2.
HPLC chromatograms (350 and 520 nm) of the cashew apple extract and its hydrolyzed sample. The peak assignments are listed in Table 1.
Table 1.
Peak assignment of the aqueous methanol extract and its hydrolyzed extract of cashew apple sample
| Peak no. | tR (min) | [M + H]+/[M–H]– (m/z) | PI/NI aglycone ion (m/z) | λmax (nm) | Identification |
|---|---|---|---|---|---|
| 1 | 13.05 | 463 | 301 | 282, 514 | 5-Methylcyanidin 3-O-hexoside |
| 2 | 21.18 | 481/479 | 319/317 | 262, 356 | Myricetin 3-O-galactosidea |
| 3 | 21.76 | 481/479 | 319/317 | 262, 356 | Myricetin 3-O-glucosidea |
| 4 | 23.23 | 451/449 | 319/317 | 262, 356 | Myricetin 3-O-xylopyra-nosidea |
| 5 | 24.95 | 451/449 | 319/317 | 262, 356 | Myricetin 3-O-arabino-pyranosidea |
| 6 | 25.91 | 451/449 | 319/317 | 262, 356 | Myricetin 3-O-arabino-furanosidea |
| 7 | 26.18 | 465/463 | 319/317 | 262, 356 | Myricetin 3-O-rhamnosidea |
| 8 | 26.94 | 465/463 | 303/301 | 256,266sh, 354 | Quercetin 3-O-galactosidea |
| 9 | 27.64 | 465/463 | 303/301 | 256,266sh, 354 | Quercetin 3-O-glucosidea |
| 10 | 29.84 | 435/433 | 303/301 | 272, 354 | Quercetin 3-O-xylo-pyranosidea |
| 11 | 30.81 | 435/433 | 303/301 | 266,296sh, 352 | Quercetin 3-O-arabino-pyranosidea |
| 12 | 32.04 | 435/433 | 303/301 | 266,296sh, 352 | Quercetin 3-O-arabino-furanosidea |
| 13 | 32.55 | 435/433 | 287 | nd | Kaempferol 3-O-glucosidea |
| 14 | 33.15 | 449/447 | 303/301 | 258, 354 | Quercetin 3-O-rhamnoside |
| 15 | 45.67 | nd | /315 | 254, 354 | Rhamnetin O-glycoside |
| 16 | 49.49 | nd | /317 | 266, 360 | Myricetin O-glycoside |
| 17 | 50.75 | nd | /317 | nd | Myricetin O-glycoside |
| 18 | 53.28 | nd | /301 | nd | Quercetin O-glycoside |
| Aglycones detected in the hydrolyzed cash apple extract | |||||
| Ag-1 | 11.07 | 303/301 | – | nd | Delphinidin chloridea |
| Ag-2 | 20.17 | 301/299 | – | 278, 332sh, 520 | 5-Methylcyanidin chloride |
| Ag-3 | 37.73 | 319/317 | – | 254, 372 | Myricetina |
| Ag-4 | 48.46 | 303/301 | – | 258, 370 | Quercetina |
| Ag-5 | 54.43 | 287/285 | – | nd | Kaempferola |
| Ag-6 | 58.59 | 317/315 | – | nd | Rhamnetina |
nd: The data were not determined.
The identification was confirmed by direct comparison with standards or reference compounds in plant sample.
Based on the data in Table 1, peak 1 (λmax 280, 516 nm, molecular ion [M + H]+ m/z 463, and aglycone ion [A + H]+ m/z 301), was identified as an anthocyanin, tentatively peonidin 3-O-hexoside. The only detected anthocyanidin (Ag-2, Fig. 2) in the hydrolyzed extract had a mass [M + H]+ at m/z 301. This anthocyanidin should be the aglycone of peak 1. However, the tR (20.16 min) and λmax (280, 334, 518 nm) of this anthocyanidin were different from the expected values for peonidin (tR 20.78 min; λmax 274, 430, 524 nm) (II-2, Fig. 1) and agreed well with the values found for the cyanidin standard. These data suggest that this anthocyanidin is a methyl derivative of cyanidin, most likely 5-methylcyanidin (II-2). The 5-methylcyanidin is the only naturally occurring methyl-anthocyanidin other than peonidin (Harborne & Baxter, 1999b; Kong, Chin, Goh, Chia, & Brouillard, 2003). Thus, peak 1 is suggested to be 5-methylcyanidin 3-O-hexoside. Furthermore, the anthocyanin can be suggested to be 5-methylcyanidin 3-O-galactoside, based on the fact that the concentrations of galactosides of myricetin and quercetin are much higher than those of their related glucosides in cashew apple (see Table 2).
Table 2.
The glycosylated flavonol and anthocyanin contents of the tested freeze-dried cashew apple
| Compound | Cashew apple (mg/g) ± DSe |
|---|---|
| Myricetin 3-O-galactosidea | 0.0532 ± 0.01 |
| Myricetin 3-O-glucosidea | 0.0274 ± 0.00 |
| Myricetin 3-O-xylopyranosidea | 0.0124 ± 0.00 |
| Myricetin 3-O-arabinopyrannosidea | 0.0104 ± 0.00 |
| Myricetin 3-O-arabinofuranosidea | 0.0097 ± 0.00 |
| Myricetin 3-O-rhamnosidea | 0.0400 ± 0.00 |
| Total myricetin glycosides | 0.1511 ± 0.01 |
| Quercetin 3-O-galactosideb | 0.0465 ± 0.00 |
| Quercetin 3-O-glucosideb | 0.0144 ± 0.00 |
| Quercetin 3-O-xylopyranosideb | 0.0116 ±0.00 |
| Quercetin 3-O-arabinopyrannosideb | 0.0108 ± 0.00 |
| Quercetin 3-O-arabinofuranosideb | 0.0079 ± 0.00 |
| Quercetin 3-O-rhamnosideb | 0.0227 ± 0.00 |
| Total quercetin glycosides | 0.1139 ± 0.00 |
| Kaempferol 3-O-glucosided | Trace amount |
| 5-Methylcyanidin 3-O-hexosidec | 0.0197 ±0.01 |
| Total glycosylated flavonoids | 0.2847 ± 0.01 |
All the monoglycosylated myricetins and quercetins were expressed as the molar equivalent of myricetin, 3-O-rhamnoside, and quercetin 3-O-glucoside, at 350 nm, respectively.
All the monoglycosylated myricetins and quercetins were expressed as the molar equivalent of myricetin, 3-O-rhamnoside, and quercetin 3-O-glucoside, at 350 nm, respectively.
Calculated as the molar equivalent of cyanidin 3-O-glucoside based on its vis peak absorption at 520 nm.
Kaempferol 3-O-glucoside used for the determination at 350 nm.
Average (mean) concentration ± SD of triplicate.
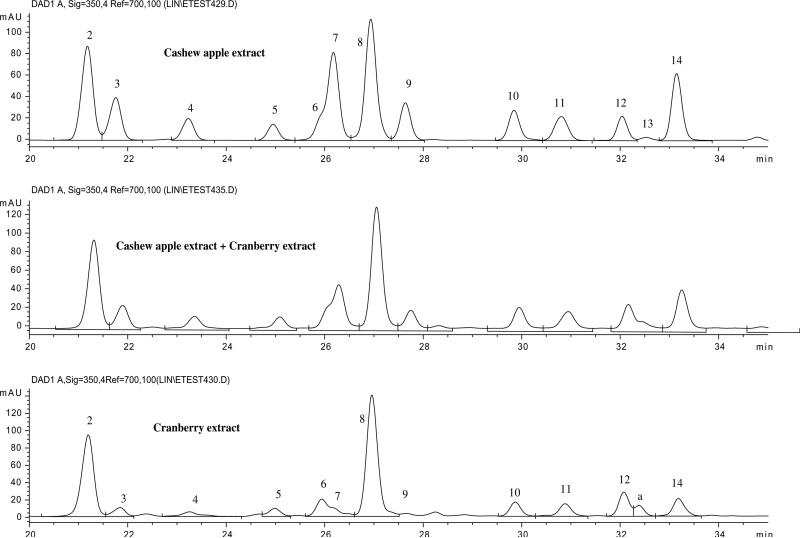
Based on the data in Table 1, peaks 8–12 and 14 were suggested to be two hexosides, three pentosides and one deoxyhexoside of a flavonol with its PI/NI aglycone ions at m/z 303/301. Since quercetin (I-2) was the only flavonol detected in the hydrolyzed extract (Ag-4 in Fig. 2), with PI/NI molecular ions of m/z 303/301, peaks 8–12 and 14 can be tentatively identified as the glycosides of quercetin. By direct comparison with collected standards of quercetin glycosides, peaks 8, 9 and 14 were positively identified as quercetin 3-O-galactoside, 3-O-glucoside and 3-O-rhamnoside, respectively. The similarity of the UV spectra for these six peaks, suggested that the three pentosides of quercetin were 3-O-pentosides. Since three quercetin 3-O-pentosides were detected in cranberry extract (100 mg of dried powdered cranberry/5.0 ml of methanol–water, 60:40, v/v), and positively identified (Vvedenskaya et al., 2004; Lin & Harnly, 2007), a direct comparison of the LC-DAD-ESI/MS data of cranberry and cashew apple led to the identification of peaks 10, 11 and 12 as quercetin 3-O-xylopyranoside, 3-O-arabinopyranoside and 3-O-arabinofuranoside, respectively. The LC chromatograms of cashew apple, cranberry and their mixture (1:1, v/v) are shown in Fig. 3. The retention times and spectral data for the quercetin 3-glycosides in the LC chromatograms of cashew apple and cranberry were consistent with each other and they appeared as a single peak in the chromatogram of the combined samples.
Fig. 3.
HPLC chromatograms (350 nm) of the extracts of cashew apple extract, cranberry extract, and their mixture. *Peak a = quercetin 3-O-6″-acetylgalactoside.
In a similar manner, based on the data in Table 1, peaks 2–7 were suggested to be two hexosides, three pentosides and one deoxyhexoside of a flavonol with PI/NI molecular ions of m/z 319/317. Since myricetin (I-1) is the only flavonol (Ag-3, Fig. 2) with PI/NI molecular ions of m/z 319/317 detected in the hydrolyzed extract, these peaks were tentatively identified as the glycosides of myricetin. Peak 7 was positively identified as myricetin 3-O-rhamnoside by direct comparison with a pure standard. Peaks 2–6 were identified as myricetin 3-O-galactoside, 3-O-glucoside, 3-O-xylopyranoside, 3-O-arabinopyranoside and 3-O-arabinofuranoside, respectively, by direct comparison with the same compounds in cranberry (Lin & Harnly, 2007; Vvedenskaya et al., 2004) (Fig. 3).
Two separate patterns suggest that these identifications are correct. First, using the same LC conditions, the same elution order was found for each group of 3-O-glycosides for myricetin and quercetin. Second, these data are consistent with the possibility of a common biosynthetic pathway for the cashew and cranberry. The same 3-O-glycosides exist for quercetin and myricetin in both plants. This suggests a common pathway with the same glycosylation mechanism for quercetin and myricetin.
Among the remaining minor peaks, peak 13 was positively identified as kaempferol 3-O-glucoside. This was supported by the identification of the aglycone of kaempferol (II-5, Fig. 1; Ag-5 in Fig. 2) in the hydrolyzed extract and by direct comparison with a pure standard. Similarly, peak 15 was tentatively identified as rhamnetin 3-O-hexoside instead of its isomer isorhamnetin (II-5), since rhamnetin (II-4, Fig. 1; Ag-6 in Fig. 2) was detected in the hydrolyzed extract as a minor peak. Peaks 16–18 are unidentified glycosides of myricetin and quercetin since their exact molecular weights could not be established. In addition, the plant might also contain trace amounts of other anthocyanins, such as delphinidin glycosides, since delphinidin (II-4 in Fig. 1; Ag-1, in Fig. 2) was detected in the hydrolyzed extract at very low concentrations.
3.2. Quantification of flavonoids in cashew apple samples
The flavonoid concentrations were determined for triplicate extractions of a single cashew apple (Table 2). While not a valid statistical representation, these values provide an initial approximation of the levels of the flavonoids in the fruit. Myricetin 3-O-rhamnoside and quercetin 3-O-glucoside were used to quantify all the myricetin and quercetin glycosides, respectively. Since all the glycosides were located at the 3-position, the UV absorption spectrum is primarily dependent on the aglycone. Thus, absorption maxima and coefficients can be assumed to be very similar. The concentration of each glycoside was calculated as the molar equivalent of myricetin 3-O-rhamnoside or quercetin 3-O-glucoside equivalents, respectively. Similarly, cyanidin 3-O-glucoside was used for the estimation of 5-methylcyanidin 3-O-hexoside. Only kaempferol 3-O-glucoside was used for determining its concentration in the tested sample, but its content in the tested cashew apple was below the detection limit.
4. Conclusion
One uncommon anthocyanin, and 13 glycosylated flavonols were identified and quantified in cashew apple extract using LC-DAD-ESI/MS. Identification was based on direct comparison with standards and positively identified compounds in cranberry. Both cashew apple and cranberry contained the same set of 12 glycosylated flavonols, namely, 3-O-galactoside, 3-O-glucoside, 3-O-xylopyranoside, 3-O-arabinopyranoside, 3-O-arabinofuranoside and 3-O-rhamnoside of myricetin and quercetin. This is the first report of these compounds in cashew apple.
Acknowledgements
This research was supported by USDA ARS CRIS Project No. 1235-52000-04800D. To Embrapa Macroprograma 2 groups, they were supported by the cooperation agreement ARS-USDA/Embrapa Labex.
References
- Assuncao RB, Mercadante AZ. Carotenoids and ascorbic acid from cashew apple (Anacardium occidentale L.): variety and geographic effects. Food Chemistry. 2003;81:495–502. [Google Scholar]
- Bicalho B, Pereira AS, Aquino Neto FR, Pinto AC, Rezende CM. Application of high-temperature gas chromatography-mass spectrometry to the investigation of glycosidically bound compounds related to cashew apple (Anacardium occidentale L. var nanum) volatiles. Journal of Agricultural and Food Chemistry. 2000;48:1167–1174. doi: 10.1021/jf9909252. [DOI] [PubMed] [Google Scholar]
- Cavalcante AAM, Rübensam G, Erdtmann B, Brendel M, Henriques JAP. Cashew (Anacardium occidentale) apple juice lowers mutagenicity of aflatoxin B1 in S. typhimurium TA 102. Genetics and Molecular Biology. 2005;28:328–333. [Google Scholar]
- Cuyckens F, Cleays M. Mass spectrometry in the structural analysis of flavonoids. Journal of Mass Spectrometry. 2004;39:1–15. doi: 10.1002/jms.585. [DOI] [PubMed] [Google Scholar]
- Embrapa (Empresa brasileira de pesquisa agropecuária) Campanha nacional de aumento de produtividade do cajueiro e produtos derivados do cajueiro. Fortaleza. 1992:11. (Embrapa – CNPCa. Boletim de Pesquisa, 04) [Google Scholar]
- Filgueiras HAC, Alves RE, Masca JL, Menezes JB. Cashew apple for fresh consumption: research on harvest and postharvest handling technology in Brazil. Acta Horticulturae. 1999;485:155–160. [Google Scholar]
- Ha TJ, Kubo I. Lipoxygenase inhibitory activity of anacardic acids. Journal of Agricultural and Food Chemistry. 2005;53:4350–4354. doi: 10.1021/jf048184e. [DOI] [PubMed] [Google Scholar]
- Harborne JB, Baxter H. Occidentoside, punin 6″-p-counarate. In: Harborne JB, Baxter H, editors. The handbook of natural flavonoids. Vol. 1. Vol. 2. John Wiley & Sons, Ltd; New York: 1999a. p. 644.p. 236. [Google Scholar]
- Harborne JB, Baxter H. Anthocyanins. In: Harborne JB, Baxter H, editors. The handbook of natural flavonoids. Vol. 2. John Wiley & Sons, Ltd; New York: 1999b. pp. 1–114. [Google Scholar]
- Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. Journal of Nutritional Biochemistry. 2003;13:572–584. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- Kong J-M, Chin L-S, Goh N-K, Chia T-F, Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry. 2003;64:923–933. doi: 10.1016/s0031-9422(03)00438-2. [DOI] [PubMed] [Google Scholar]
- Kozubek A, Zarnowski R, Stasiuk M, Gubernator J. Natural amphiphilic phenols as bioactive compounds. Cellular and Molecular Biology Letters. 2001;6:351–355. [Google Scholar]
- Kubo J, Lee JR, Kubo I. Anti-helicobacter pylori agents from the cashew apple. Journal of Agricultural and Food Chemistry. 1999;47:533–537. doi: 10.1021/jf9808980. [DOI] [PubMed] [Google Scholar]
- Kubo I, Muroi H, Himejima M. Structure-antibacterial activity relations of anacardic acids. Journal of Agricultural and Food Chemistry. 1993;41:1016–1019. [Google Scholar]
- Kubo I, Nihei K, Tsujimoto K. Antibacterial action of anacardic acids against Methicillin Resistant Staphylococcus aureus (MRSA). Journal of Agricultural and Food Chemistry. 2003;51:7624–7628. doi: 10.1021/jf034674f. [DOI] [PubMed] [Google Scholar]
- Kubo I, Ochi M, Vieira PC, Komatsu S. Antitumor agents from the cashew (Anacardium occidentale) apple juice. Journal of Agricultural and Food Chemistry. 1993;41:1012–1015. [Google Scholar]
- Leite LAS, Paula Pessoa PFA. Aspectos Sócioeconômicos. In: Barros LM, editor. Caju. Producßäo: aspectos técnicos. Fortaleza: Embrapa Agroindústria Tropical. Informacßäo Tecnológica; Brasília: 2002. pp. 15–17. (Frutas do Brasil, 30) [Google Scholar]
- Lin L-Z, Harnly J. A standardized method for the identification of glycosylated flavonoids and other phenolic compound in all plant materials. Journal of Agricultural and Food Chemistry. 2007;55:1084–1096. doi: 10.1021/jf062431s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo-Cavalcante AA, Rübensam G, Picada JN, Silva EG, Moreira FJC, Henriques JAP. Mutagenic evaluation, antioxidant potential and antimutagenic activity against hydrogen peroxide of cashew (Anacardium occidentale) apple juice and cajuina. Environmental and Molecular Mutagenesis. 2003;41:360–369. doi: 10.1002/em.10158. [DOI] [PubMed] [Google Scholar]
- Paramashivappa R, Phani Kumar P, Vithayathil PJ, Srinivasa Rao A. Novel method for isolation of major phenolic constituents from cashew (Anacardium occidentale L.) nut shell liquid. Journal of Agricultural Food Chemistry. 2001;49:2548–2551. doi: 10.1021/jf001222j. [DOI] [PubMed] [Google Scholar]
- Robards K. Strategies for the determination of bioactive phenols in plants, fruits and vegetables. Journal of Chromatography A. 2003;1000:657–691. doi: 10.1016/s0021-9673(03)00058-x. [DOI] [PubMed] [Google Scholar]
- Robards K, Prenzler PD, Tucker G, Swatsitang P, Glover W. Phenolic compounds and their role in oxidative processes in fruits. Food Chemistry. 1999;66:401–436. [Google Scholar]
- Vvedenskaya IO, Rosen RT, Guido JE, Russell DJ, Mills KA, Vorsa N. Characterization of flavonols in cranberry (Vaccium macrocarpon) powder. Journal of Agricultural Food Chemistry. 2004;52:188–195. doi: 10.1021/jf034970s. [DOI] [PubMed] [Google Scholar]