Abstract
Cellular aging programs typically rely on the asymmetric shape and growth pattern of cells. A new study shows that symmetric fission yeast cells escape classic signs of aging until they encounter environmental stress.
It will happen to the best of us. As we age, our bodies slow down and prepare for an inevitable meeting with the grim reaper. The phenomenon of aging has long fascinated biologists: what are the mechanisms that drive senescence, and how might they be controlled? A defined aging program occurs not only in complex organisms, but also at the level of single cells. Replicative life span refers to the finite number of times a single cell can generate offspring. Over 50 years ago, Mortimer and Johnston used the budding yeast Saccharomyces cerevisiae to show that a ‘mother’ cell generates about 25 newborn ‘daughter’ cells [1]. At this point, cell growth slows dramatically and is followed by death. The asymmetric growth pattern of budding yeast cells is key to this aging program because it permits the selective retention of ‘aging factors’ in the older mother cell. These factors include extra-chromosomal rDNA circles and damaged proteins, which have subsequently been shown to contribute to cellular aging in many organisms, including humans [2,3]. But not all cells exhibit asymmetric growth and division. This raises a simple question: do cells that divide symmetrically age? A new study by Coehlo et al. [4], published in this issue of Current Biology, uses the symmetrically dividing fission yeast cells to ask this question. Their answer is quite remarkable: fission yeast cells do not age when they live a stress-free life.
The growth pattern of fission yeast cells provides a strong model to test the role of symmetry in aging. These rod-shaped cells grow by linear extension at their tips, and then divide in the cell middle to generate two seemingly identical daughter cells. Despite their apparent symmetry, fission yeast cells contain at least two potential sources of asymmetry. First, the two ends of a newborn daughter cell are not equal — the ‘old’ end was present in the mother cell, while the ‘new’ end was created by division (Figure 1). Second, replication of the spindle pole body (SPB), the yeast equivalent of the centrosome, generates distinct ‘new’ and ‘old’ SPBs at opposite poles of the mitotic spindle. While some previous studies have hinted at asymmetric aging in fission yeast cells [5,6], Coehlo et al. [4] directly address the question through a simple yet elegant experiment based on long-term time-lapse microscopy. By following cells over multiple generations, they show that cells successively inheriting the old end (i.e. a really old end) show no changes in cell division time or viability (Figure 1). Similarly, inheritance of new versus old SPB has no effect on cell fate or growth rate. This suggests that fission yeast cells do not have an aging program that bears resemblance to other cell types. For a rigorous test of this possibility, the authors physically removed the new-end daughter cells for successive generations and looked for signs of aging. A cell that retains the ‘old end’ for up to 50 generations shows no signs of slowing down divisions. This contrasts a budding yeast mother cell, which slows division and dies after ~25 generations. Combined with an impressive assortment of additional experiments, the authors conclude that fission yeast cells do not age under the favorable conditions tested.
Figure 1.
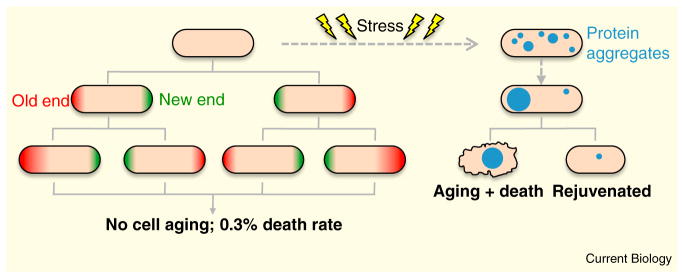
Absence of classic aging in the fission yeast S. pombe.
New work [4] now shows that rod-shaped fission yeast cells do not exhibit signs of aging over many generations, but signs of aging appear following asymmetric segregation of stress-induced protein aggregates.
Does this mean that symmetric cells have found the Holy Grail? Not exactly. Fission yeast cells exhibit a ‘death rate’ of 0.3%, far higher than the calculated age-induced death of other cell types. By retracing the steps that lead to these rare events, Coehlo et al. [4] find that slow growth and other classic signs of aging do not precede fission yeast cell death. Rather, death often occurs in one daughter cell immediately following cell division and separation, suggesting a catastrophic event during cell wall remodeling at septation. Given that protein aggregates have been linked with cell aging and death in many systems, the authors examine these aggregates (marked by the chaperone Hsp104) in fission yeast. Interestingly, protein aggregates are randomly and asymmetrically inherited during the symmetric division of fission yeast cells, and cells that receive a high amount of aggregates are likely to die [4]. This correlation raises the possibility that a threshold level of protein aggregation leads to cell death, with the underlying mechanisms unknown. Given the timing of cell death, protein aggregates might physically interfere with essential steps in cell separation. Alternatively, these aggregates might sequester vital proteins to trigger rapid cell death. The specific links between protein aggregation and cell death await identification, but the authors have found an important step in the death of these otherwise ageless cells.
All of these findings relate to the behavior of cells living a stress-free life. While we tend to pamper our cells in the laboratory, nature is not so kind. In fact, a common result of cell stress is the induction of protein aggregates. This led Coehlo et al. [4] to test the connections between cell stress, protein aggregates, and cell death. Two independent forms of stress (heat and oxidation) induced the formation of small protein aggregates that combined into one large aggregate [4]. At division, only one daughter cell inherited this large aggregate, leading to death. This largely mirrors the connection between aggregates and death in stress-free conditions, with the implication that the formation of one large protein aggregate ensures that one daughter cell is born without these toxic species. Surprisingly, the authors also found that stress induced signs of cellular aging (Figure 1). Prior to death, cells with stress-induced aggregates exhibited an increased division time. This slowing of cell division was more obvious following oxidative stress than heat stress, but raises the possibility that environmental stress triggers an otherwise ‘masked’ aging program in symmetric fission yeast cells.
This work adds to the growing connection between protein aggregates and cellular aging. In the context of symmetric fission yeast cells, aggregates are linked to cell death in both stressed and unstressed conditions, raising a host of questions regarding the mechanisms that position, sense, and respond to toxic aggregates. Asymmetrical inheritance of such toxic species ensures the generation of ‘clean’ daughter cells following stress. For yeast cells, this effectively prevents clonal senescence even after stress — the population survives by sacrificing a few daughter cells. These findings suggest that the position of the toxic aggregate is key to cell destiny, so how is this determined? The movement of protein aggregates appears random, although the old cell end is more likely to inherit large stress-induced aggregates. In asymmetric budding yeast, cell polarity ensures the retention of toxic aggregates in the old mother compartment, but the underlying mechanisms are hotly debated [7–9]. The links between environmental stress, protein aggregation, and cell aging appear to operate in a wide range of cell types and organisms [3]. Thus, uncovering the mechanisms that generate, move, and respond to protein aggregates in yeast cells might identify conserved principles in eukaryotic cell aging. Moreover, control of aggregate formation and movement may be coordinated with additional components of a larger aging system.
Cellular aging programs appear to function as dynamic systems that are modulated by the environment. Cell shape and symmetry play an important role in the makeup of an aging program. Asymmetric eukaryotic cells such as S. cerevisiae and Candida albicans display a defined aging program [2], whereas symmetric Schizosaccharomyces pombe cells escape this fate [4]. This may be reflected in prokaryotes, where asymmetric Caulobacter crescentus cells age [10] but symmetric Escherichia coli cells may not. Initial studies suggested that E. coli cells, which look like a miniaturized fission yeast cell, segregate aging with the old cell pole [11]. However, subsequent work using more optimal growth conditions found a lack of clear aging [12]. The mechanisms that allow symmetric cells to reveal hidden aging programs under stressful conditions may have implications for controlling the growth of immortalized cells such as cancer. Symmetry does not provide cells with immortality, but continued work on these systems may reveal unexpected twists and turns on the way to mortality’s final stop.
References
- 1.Mortimer RK, Johnston JR. Life span of individual yeast cells. Nature. 1959;183:1751–1752. doi: 10.1038/1831751a0. [DOI] [PubMed] [Google Scholar]
- 2.Longo VD, Shadel GS, Kaeberlein M, Kennedy B. Replicative and chronological aging in Saccharomyces cerevisiae. Cell Metab. 2012;16:18–31. doi: 10.1016/j.cmet.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coehlo M, Dereli A, Haese A, Kühn S, Malinovska L, DeSantis ME, Shorter J, Alberti S, Gross T, Tolić-Nørrelykke IM. Fission yeast does not age under favorable conditions, but does so after stress. Curr Biol. 2013;23:1844–1852. doi: 10.1016/j.cub.2013.07.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erjavec N, Cvijovic M, Klipp E, Nystrom T. Selective benefits of damage partitioning in unicellular systems and its effects on aging. Proc Natl Acad Sci USA. 2008;105:18764–18769. doi: 10.1073/pnas.0804550105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker MG, Walmsley RM. Replicative ageing in the fission yeast Schizosaccharomyces pombe. Yeast. 1999;15:1511–1518. doi: 10.1002/(sici)1097-0061(199910)15:14<1511::aid-yea482>3.3.co;2-p. [DOI] [PubMed] [Google Scholar]
- 7.Aguilaniu H, Gustafsson L, Rigoulet M, Nystrom T. Asymmetric inheritance of oxidatively damaged proteins during cytokinesis. Science. 2003;299:1751–1753. doi: 10.1126/science.1080418. [DOI] [PubMed] [Google Scholar]
- 8.Liu B, Larsson L, Caballero A, Hao X, Oling D, Grantham J, Nystrom T. The polarisome is required for segregation and retrograde transport of protein aggregates. Cell. 2010;140:257–267. doi: 10.1016/j.cell.2009.12.031. [DOI] [PubMed] [Google Scholar]
- 9.Zhou C, Slaughter BD, Unruh JR, Eldakak A, Rubinstein B, Li R. Motility and segregation of Hsp104-associated protein aggregates in budding yeast. Cell. 2011;147:1186–1196. doi: 10.1016/j.cell.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ackermann M, Stearns SC, Jenal U. Senescence in a bacterium with asymmetric division. Science. 2003;300:1920. doi: 10.1126/science.1083532. [DOI] [PubMed] [Google Scholar]
- 11.Stewart EJ, Madden R, Paul G, Taddei F. Aging and death in an organism that reproduces by morphologically symmetric division. PLoS Biol. 2005;3:e45. doi: 10.1371/journal.pbio.0030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang P, Robert L, Pelletier J, Dang WL, Taddei F, Wright A, Jun S. Robust growth of Escherichia coli. Curr Biol. 2010;20:1099–1103. doi: 10.1016/j.cub.2010.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]


