Abstract
Importance
Studies have shown variation in the use of red blood cell transfusion among patients with acute coronary syndromes. There are no definitive data for its efficacy in improving outcomes and concerning data about possible association with harm. Current transfusion practices in patients undergoing percutaneous coronary intervention (PCI) are not well understood.
Objectives
To determine the current patterns of blood transfusion among patients undergoing PCI and the association of transfusion with adverse cardiac outcomes across hospitals in the U.S.
Design, Setting, Participants
Retrospective cohort study. The study population included all patient visits from the CathPCI Registry® from July 2009 to March 2013 which included PCI, excluding those with missing data on bleeding complications or underwent in-hospital CABG(N=2258711).
Main Outcomes and Measure
Transfusion rates in the overall population and by hospitals (N=1431) were the primary outcomes. The association of transfusion with myocardial infarction, stroke, and death after accounting for a patient’s propensity for transfusion was also measured.
Results
The overall rate of transfusion was 2.14%(95% CI: 2.13% to 2.16%) and transfusion rates slightly declined from 2009Q3 to 2013Q (2.11% (95% CI: 2.03% to 2.19%) to 2.04%(95% CI: 1.97% to 2.12%); P<0.001). Patients who received transfusion were more often older(70.5 vs. 64.6 years of age), female(56.3% vs. 32.0%), and had hypertension(86.4% vs. 82.02%), diabetes(44.8% vs. 34.61%), advanced renal dysfunction(8.7% vs. 2.28%), prior myocardial infarction(33.0% vs. 30.15%), or prior heart failure(27.0% vs. 11.76%). Over 90% of sites transfused <5% of patients, while ~6% of sites transfused ≥5% of patients. Variation in hospital risk-standardized rates of transfusion persisted after adjustment and hospitals showed variability in their transfusion thresholds. Receipt of transfusion was associated with MI(42803 events; 4.5% vs. 1.8%; OR 2.60; 95%CI 2.57–2.63), stroke(5011 events; 2.0% vs. 0.2%; OR 7.72; 95% CI 7.47–7.98), and in-hospital death(31885 events; 12.5% vs. 1.2%; OR 4.63; 95% CI 4.57–4.69), irrespective of bleeding complications.
Conclusions
Among patients undergoing PCI at US hospitals, there was considerable variation in blood transfusion practices, and receipt of transfusion was associated with increased risk of in-hospital adverse cardiac events. These observational findings may warrant a randomized trial of transfusion strategies for patients undergoing PCI.
Introduction
Red blood cell transfusion among patients with coronary artery disease is controversial. There is a growing body of evidence that transfusion in the setting of acute coronary syndromes1–8 and in hospitalized patients with a history of coronary artery disease (CAD) may be associated with an increase in the risk of myocardial infarction and death. 9 This is in addition to the other risks described with transfusion of allogeneic blood such as infection and circulatory overload. On the other hand, anemia is a well-known risk factor for exacerbation of myocardial ischemia10, 11 and increasing hemoglobin through red blood cell transfusion should increase oxygen delivery and mitigate ischemic outcomes. This paradox between the pathophysiological rationale for transfusion and observational studies demonstrating worse clinical outcomes has led to uncertainty surrounding transfusion practice in these patients. Indeed, current guideline statements make cautious recommendations for restricted transfusion strategies in hospitalized patients with a history of CAD, and make no recommendation on transfusion in the setting of ACS citing an absence of definitive evidence12.
Given the lack of evidence-based guidelines for transfusion in patients with CAD, a registry-based analysis showed that there is marked variation in the use of red blood cell transfusion among ACS patients 13. Similar to ACS, patients undergoing percutaneous coronary intervention (PCI) receive potent antithrombotic therapies and undergo arteriotomy, placing this subset of patients at particularly high risk for bleeding and transfusion. A single center study showed that a large proportion of patients undergoing PCI received transfusion for indications outside of published guidelines14; however, as mentioned above, the transfusion guidelines have been updated to reflect uncertainty regarding transfusion recommendations in patients with CAD. Moreover, the practice of PCI has evolved to include “bleeding avoidance strategies.15 Therefore, the use of red cell transfusion may have undergone significant change over time. Using data from the CathPCI Registry we sought to describe transfusion practice patterns in a broadly representative population of patients undergoing PCI across the United States. We also sought to evaluate how patient factors are associated with red blood cell transfusion, and to determine the association between transfusion and outcomes in the PCI population.
Methods
Study sample
The CathPCI Registry is an initiative of the American College of Cardiology Foundation and the Society for Cardiovascular Angiography and Interventions, and is the largest ongoing registry of PCI in the United States. Descriptions of the registry have been published previously16. Briefly, the registry collects data on patient and hospital characteristics, clinical presentation, procedural characteristics, and in-hospital outcomes for PCI procedures from over 1400 sites across the United States (approximately 85% of all cardiac catheterization labs). Data are entered into NCDR®-certified software at participating institutions, and exported in a standard format to the American College of Cardiology (ACC). The registry has a comprehensive data quality program, including both data quality report specifications for data capture and transmission, as well as an auditing program. An NCDR committee prospectively defines the variables, which are available at: http://www.ncdr.com.
All patients who underwent cardiac catheterization or PCI from July 2009 to March 2013 were included in the study sample with the following exceptions: patient visits in which the patient subsequently underwent in-hospital coronary artery bypass grafting (CABG), patient visits in which a PCI was not performed or did not represent the first PCI visit during a hospital stay, and procedures with missing data on bleeding events, procedural complications, or discharge status. The study was approved by the Institutional Review Board of Yale University Medical Center and was determined to meet the definition of research not requiring informed consent given that patient information is collected anonymously without unique patient identifiers and only aggregate data are reported.
Outcomes and definitions
The primary outcome was transfusion in the overall population. This outcome was examined with calculation of transfusion rates in the overall population and by hospital sites (N=1431), as well as by the occurrence of a bleeding event. These were also calculated quarterly from Q3-2009 to Q1-2013. Secondary outcomes included inhospital myocardial infarction, congestive heart failure, cardiogenic shock, stroke, and death. The definitions used for transfusion, bleeding events, myocardial infarction, congestive heart failure, cardiogenic shock, stroke, and death are taken from the CathPCI data collection form version 4.417 and can be found in the appendix.
Statistical Analysis
Rates of transfusion were examined in the overall cohort, by hospital sites, and in groups of patients with or without documentation of a procedural bleeding complication. We also examined the change in rates over time by quarters from Q3-2009 to Q1-2013 using the Cochrane-Armitage trend test. Hierarchical logistic regression modeling was used to calculate risk-standardized, site-based rates of transfusion (RSTR). The variables included in the model were age, gender, body mass index (BMI), ACS presentation, PCI status, cardiogenic shock, NYHA Class IV CHF, history of CHF, peripheral vascular disease, chronic lung disease, diabetes, dialysis, previous PCI, coronary lesion ≥ 50%, and glomerular filtration rate (GFR). All of these variables have been previously validated in the CathPCI Registry mortality and bleeding risk models18, 19. For the hospital-level analysis, hospitals were divided into low-, middle-, and high-transfusing groups based on the tertiles of their risk-standardized transfusion rate (RSTR). Hospitals with a RSTR at or below the 33th percentile were considered low transfusing (transfused <1.78% of patients), hospitals between the 33th and 66th percentiles were considered middle transfusing (transfused 1.78 to <2.79% of patients), and hospitals above the 66th percentile (transfused ≥ 2.79% of patients) were considered high transfusing. Transfusion frequencies were then plotted by post-procedure hemoglobin values for each group to determine whether hospital level transfusion practices were different, specifically by transfusion threshold. Hospital site characteristics were also reported, again by division into low, middle, and high transfusing hospitals according to their RSTR. Characteristics such as bed number, region, ownership etc. are reported per number of hospital sites. Procedural characteristics such as anticoagulant use and discharge medications are reported per patient visit. The median odds ratio for transfusion among hospital sites to quantify the variation of transfusion use among different hospitals and the between-hospital variance were also calculated.
The patient population was divided into cohorts according to whether they had received transfusion and baseline characteristics and in-hospital outcomes were compared between these two groups. Differences were evaluated using chi-square test for categorical variables and using t-test for continuous variables. Mean and standard deviation for continuous variables and frequency rate for categorical variables were presented.
To account for potential confounding in the use of transfusion, inverse probability weighting based on the propensity modeling for transfusion was utilized in the logistic regression models to determine the association between transfusion and myocardial infarction, congestive heart failure, stroke, and death. Variables included in the propensity model for transfusion were age, gender, race, BMI, prior MI, prior CABG/valvular surgery, cardiogenic shock, cardiac arrest, use of intra-aortic balloon pump (IABP), prior CHF, peripheral vascular disease, cerebrovascular disease, tobacco use, chronic lung disease, diabetes, hyperlipidemia, family history, dialysis, GFR, NYHA class IV, coronary lesion ≥ 50%, location of lesion, PCI indication, PCI status, and hospital characteristics such as public vs. private ownership, core based statistical area, number of beds, PCI volume, teaching facility status and region. Many of these variables have been previously validated in the NCDR mortality risk model18. The model showed excellent discrimination with a C-statistic = 0.839. Odds ratios for patient outcomes comparing the use of transfusion to no receipt of transfusion were reported with 95% confidence intervals. To account for the possibility that bleeding events could drive transfusion as well as outcomes, a secondary analysis was performed to determine the association between transfusion and outcomes among patients who did or did not have reported post-procedure bleeding events. Finally, to determine the relationship between pre-procedure hemoglobin, transfusion, and outcomes, the study sample was stratified by pre-procedure hemoglobin levels and the modeling was repeated. A two-sided p value of <0.05 was considered significant for all tests. All the statistical calculations were performed at the Yale Center for Outcomes Research and Evaluation (CORE) with SAS version 9.2.0 (SAS Institute Inc. Cary, NC).
Results
Study sample characteristics
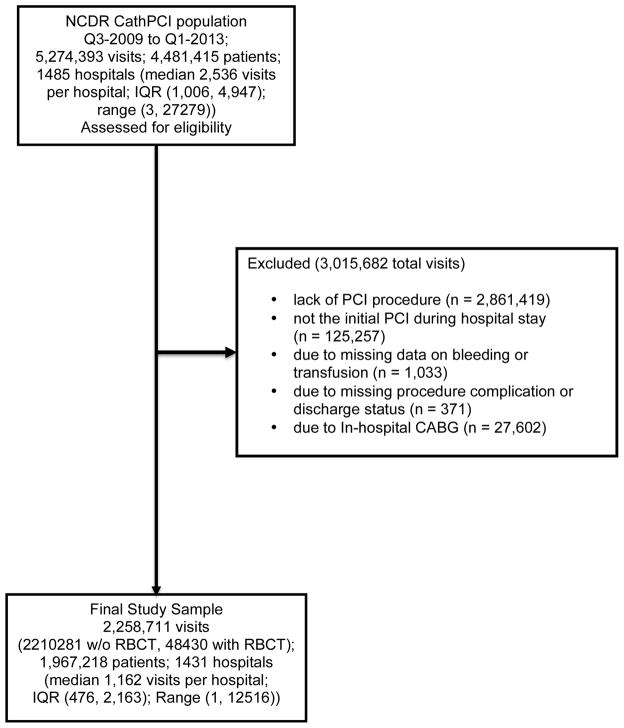
For the purpose of this study, the original sample consisted of 5,274,393 patient visits to the cardiac catheterization laboratory from 1485 sites. PCI occurred during 2,412,974 patient visits. After applying the aforementioned exclusion criteria, 2,258,711 patient visits remained in the study sample (Figure 1) from 1431 sites (96% of original sites). There were 48,430 patient visits during which a patient received a transfusion post-procedure. Baseline demographic and procedural characteristics are shown in Table 1. Patients who received transfusion were older, more often female, and more often had comorbidities such as hypertension, diabetes, advanced renal dysfunction, prior MI, and prior CHF. These patients also had a lower estimated glomerular filtration rate (eGFR), more often presented with ST-segment elevation myocardial infarction, and less often underwent PCI for elective as opposed to urgent or emergent indications.
Figure 1.
Study Sample Flow Diagram -- Displays the selection of patient visits for analysis from the NCDR CathPCI database
Table 1.
Clinical Characteristics stratified by transfusion status, % (No.)
| Overall | Inverse Probability Weighted Cohort | |||
|---|---|---|---|---|
| Patient Characteristics | Without RBCT N=2210281 |
With RBCT N=48430 |
Without RBCT N=2210281 |
With RBCT N=48401 |
| Age (mean, SD) | 64.6 (12.0) | 70.5 (12.1) | 64.8 (12.2) | 66.1 (12.0) |
| BMI (mean, SD) | 30.0 (6.4) | 28.8 (7.2) | 30.0 (6.5) | 29.7 (49.0) |
| Gender (% Female) | 32.5 (707309) | 56.3 (27275) | 32.6 (707309) | 39.8 (27266) |
| Race (% Caucasian) | 88.0 (1944032) | 84.5 (40927) | 87.9 (1944032) | 86.5 (40902) |
| Hypertension | 82.0 (1812770) | 86.4 (41864) | 82.1 (1812770) | 82.3 (41845) |
| Hyperlipidemia | 79.6 (1758531) | 74.7 (36180) | 79.4 (1758531) | 76.2 (36161) |
| Diabetes | 34.6 (765050) | 44.8 (21693) | 34.9 (765050) | 36.1 (21686) |
| Periph. Vascular Disease | 12.3 (272079) | 23.3 (11268) | 12.6 (272079) | 15.1 (11265) |
| Cerebrovascular Disease | 12.2 (269404) | 21.7 (10519) | 12.4 (269404) | 14.3 (10517) |
| Chronic Lung Disease | 15.0 (332160) | 25.1 (12139) | 15.3 (332160) | 18.2 (12135) |
| GFR (mL/min/1.73 m2) (mean, SD) | 76.3 (30.5) | 56.2 (33.2) | 75.9 (31.0) | 79 (25.7) |
| ESRD on dialysis | 2.3 (50433) | 8.7 (4207) | 2.4 (50433) | 3.6 (4207) |
| Prior MI | 30.2 (666457) | 33.0 (15970) | 30.2 (666457) | 30.0 (15962) |
| Prior CHF | 11.8 (259934) | 27.0 (13090) | 12.1 (259934) | 15.0 (13089) |
| Prior CABG/Valve Surgery | 20.1 (443168) | 22.0 (10650) | 20.1 (443165) | 20.6 (10649) |
| Family History of CAD | 24.9 (549619) | 16.2 (7829) | 24.7 (549619) | 23.5 (7820) |
| Current Tob. Use | 27.6 (609145) | 22.4 (10854) | 27.4 (609145) | 28.0 (10842) |
| CAD Presentation | ||||
| STEMI | 15.3 (337686) | 31.7 (15370) | 15.7 (337686) | 17.9 (15365) |
| NSTEMI/UA | 57.3 (1267282) | 54.3 (26286) | 57.2 (1267282) | 59.7 (26271) |
| Elective | 27.4 (604761) | 14.0 (6768) | 27.1 (604761) | 22.4 (6759) |
| NYHA Class IV CHF | 2.2 (47976) | 13.5 (6518) | 2.5 (47976) | 3.7 (6517) |
| Cardiogenic Shock | 1.6 (35700) | 15.3 (7394) | 2.0 (35700) | 3.4 (7392) |
| Cardiac Arrest | 1.8 (39202) | 9.9 (4800) | 2.0 (39202) | 3.2 (4799) |
| Use of IABP | 1.8 (40061) | 16.2 (7867) | 2.2 (40061) | 3.6 (7866) |
| Pre-procedure Hgb (g/dL) (mean, SD) | 13.6 (1.9) | 11.0 (2.1) | - | - |
GFR = glomerular filtration rate, ESRD = end stage renal disease, MI = myocardial infarction, CHF = congestive heart failure, CAD = coronary artery disease, STEMI = ST elevation myocardial infarction, NSTEMI = non-ST elevation myocardial infarction, UA = unstable angina, Hgb = Hemoglobin
Rates of transfusion
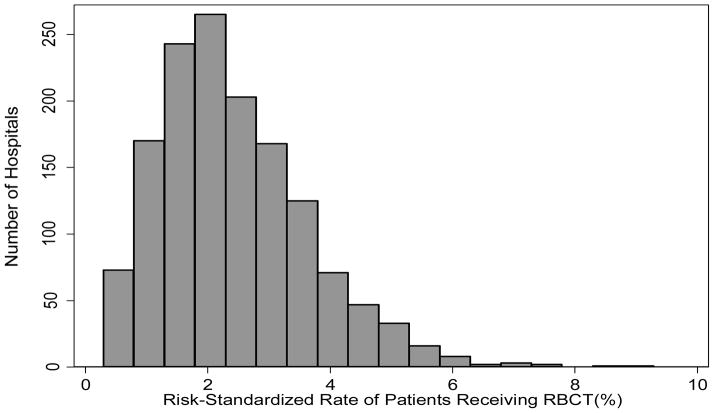
Transfusion rates quarterly from July 2009 to March 2013 slightly declined from 2.11% (95% CI: 2.03% to 2.19%) to 2.04% (95% CI: 1.97% to 2.12%) of patients receiving blood (P-value for trend <0.0001). Unadjusted transfusion rates by hospital sites varied between 0 and 13% (Figure S1). The majority of sites transfused less then 5% of their patients, with over 17% of sites transfusing less than 1% of patients. However, nearly 3% of hospitals in the population transfused more than 5% of patients. After adjustment, there was still a broad variation in patterns of transfusion across hospitals. As shown in Figure 2, the risk-standardized rates of transfusion across hospital sites ranged from 0.3% to 9.3% with a median of 2.5%.
Figure 2.
Distribution of hospital risk-standardized rates of transfusion -- Displays the variations in frequency of receipt of transfusion by hospital site, after adjustment for patient risk factors such as age, gender, body mass index (BMI), ACS presentation, PCI status, cardiogenic shock, NYHA Class IV CHF, history of CHF, peripheral vascular disease (PVD), chronic lung disease, diabetes, dialysis, previous PCI, coronary lesion ≥ 50%, and glomerular filtration rate (GFR)
When stratified by the occurrence of a bleeding event, more patients who experienced a bleeding event received transfusion at all post-procedure hemoglobin values compared with patients who did not experience a bleeding event (Figure S2). Among patients who did not bleed, the rates of transfusion increased once the post-procedure hemoglobin value was ≤ 8 g/dl.
Hospital characteristics by transfusion rate
When hospital sites were divided into low (<1.78%), middle (1.78 to <2.79%), and high (≥2.79%) transfusing sites by the tertiles of risk-standardized transfusion rates (RSTR), transfusion was more frequent at all post-procedure hemoglobin (≤7g/dL to ≥12 g/dL) values at high transfusing centers compared with the middle and low transfusing centers (Figure 3). High transfusing sites seemed to have a transfusion threshold between 9gm/dL and 10gm/dL, while low-transfusing sites seemed to have a transfusion threshold between 8 gm/dL and 9 gm/dL.
Figure 3.
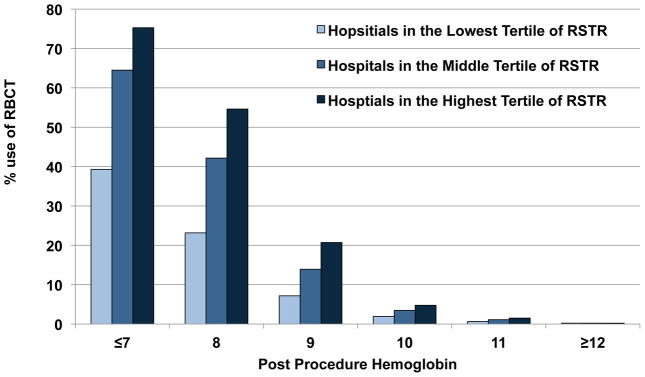
Use of RBC transfusion by post-procedure Hemoglobin at High, Middle, and Low Transfusing sites. Displays the frequency of RBC transfusion by post procedure hemoglobin value when the study sample of hospital sites is divided into High, Middle, and Low transfusing sites. Rates of transfusion are higher at High transfusing sites across all hemoglobin values.
High-transfusing hospital sites were larger with respect to number of beds, and had higher PCI volume when compared with the other sites (Table S1). They also were less likely to be privately owned, and less likely to be in rural areas but more likely to be teaching hospitals. High-transfusing sites were more likely to be in the New England and Pacific regions, though there was significant variation by region. They were also less likely to use bivalirudin during procedures, and more likely to use GIIbIIIa medications, but with similar use of radial access site between groups. Finally, high-transfusing hospital sites prescribed evidenced based medications on discharge at a similar high frequency when compared with lower-transfusing hospital sites. The median odds ratio for likelihood of transfusion by hospital site was 1.85 (95% CI: 1.79 to 1.90), and the between-hospital variation was 0.42 (95% CI: 0.38 to 0.46), indicating that hospital site was responsible for a significant amount of the variation seen in transfusion rates.
Patient outcomes
With regard to patient outcomes by transfusion status, patients who underwent transfusion were more likely to have in-hospital MI, Stroke, CHF, cardiogenic shock, or death (Table S2). After adjustment, receipt of transfusion remained associated with an increased risk for in-hospital MI, stroke, or death individually, and also the composite outcome (Table 2). The analysis was repeated after stratifying patients by whether or not they experienced a bleeding event. Regardless of the occurrence of bleeding, transfusion was independently associated with increased risk for in-hospital MI, stroke, or death (Table 2). In an analysis of the relationship between hemoglobin (pre-procedure) and clinical outcomes, transfusion was consistently associated with an increased risk of in-hospital MI, stroke, or death regardless of hemoglobin value, except in patients with bleeding and pre-procedure Hbg values < 10g/dL (Table 3). In this group of patients, transfusion was associated with a significantly decreased risk of the composite outcome. The risk in all other groups increased with higher hemoglobin levels.
Table 2.
Association of transfusion and outcomes: Adjusted Odds Ratios from Inverse Probability Weighted Analysis
| Patient Outcomes | With RBCT N=48430 # (%,95% CI) |
Without RBCT N=2210281 # (%, 95% CI) |
Total Population OR (95% CI) |
Patient with Bleeding OR (95% CI) |
Patients without bleeding OR (95% CI) |
|---|---|---|---|---|---|
| MI, Stroke, or In-hospital Death | 8418 (17.4, 17.0 – 17.7) | 67907 (3.07, 3,05 – 3.10) | 3.62 (3.59–3.66) | 1.16 (1.11–1.22) | 3.66 (3.63–3.69) |
| MI | 2202 (4.54, 4.36 – 4.73) | 40601 (1.84, 1.82 – 1,85) | 2.60 (2.57–2.63) | 1.35 (1.24–1.46) | 2.38 (2.35–2.41) |
| Stroke | 988 (2.04, 1.91 – 2.17) | 4023 (0.182, 0.176–0.188) | 7.72 (7.47–7.98) | 1.54 (1.34–1.77) | 8.49 (8.21–8.78) |
| In-hospital Death | 6052 (12.5, 12.2 – 12,8) | 25833 (1.17, 1.15 – 1.18) | 4.63 (4.57–4.69) | 1.07 (1.01–1.13) | 4.96 (4.89–5.03) |
MI = myocardial infarction; IPW model included the following variables: age, gender, race, BMI, prior MI, prior CABG/valvular surgery, cardiogenic shock, cardiac arrest, use of intra-aortic balloon pump (IABP), prior CHF, peripheral vascular disease, cerebrovascular disease, tobacco use, chronic lung disease, diabetes, hyperlipidemia, family history, dialysis, GFR, NYHA class IV, coronary lesion ≥ 50%, location of lesion, PCI indication, PCI status, and hospital characteristics such as public vs. private ownership, core based statistical area, number of beds, PCI volume, teaching facility status and region
Table 3.
Association of transfusion and outcomes by pre-procedure hemoglobin level: Adjusted Odds Ratios from Inverse Probability Weighted Analysis
| Pre-Procedure Hgb Value (g/dL) | ||||
|---|---|---|---|---|
| MI, Stroke, or In-hospital Death | Hgb ≤ 10 | 10< Hgb ≤ 13 | 13< Hgb ≤ 15 | Hgb > 15 |
| Patients with RBCT No. (%,95% CI) | 2384 (13.3, 12.8 – 13.8) | 3588 (18.1, 17.5 – 18.6) | 1508 (23.0, 22.0 – 24.1) | 504 (25.9, 24.0 – 27.9) |
| Patients without RBCT No. (%,95% CI) | 4425 (5.7, 5.5 – 5,9) | 22329 (3.38, 3.34 – 3,42) | 24377 (2.71, 2.68 – 2.74) | 12519 (2.81, 2.76 – 2.86) |
| Total Population OR (95% CI) | 1.56 (1.51–1.62) | 3.62 (3.57–3.68) | 5.86 (5.78–5.95) | 8.12 (7.96–8.29) |
| With Bleeding OR (95% CI) | 0.74 (0.66–0.83) | 1.01 (0.93–1.08) | 1.51 (1.38–1.65) | 2.24 (1.99–2.53) |
| Without Bleeding OR (95% CI) | 1.54 (1.48–1.60) | 3.83 (3.77–3.89) | 8.84 (8.71–8.98) | 10.06 (9.84–10.29) |
MI = myocardial Infarction; IPW model included the following variables: age, gender, race, BMI, prior MI, prior CABG/valvular surgery, cardiogenic shock, cardiac arrest, use of intra-aortic balloon pump (IABP), prior CHF, peripheral vascular disease, cerebrovascular disease, tobacco use, chronic lung disease, diabetes, hyperlipidemia, family history, dialysis, GFR, NYHA class IV, coronary lesion ≥ 50%, location of lesion, PCI indication, PCI status, and hospital characteristics such as public vs. private ownership, core based statistical area, number of beds, PCI volume, teaching facility status and region
Discussion
There was marked variation in transfusion practice patterns across the United States among patients undergoing PCI. Within this variation there appeared to be patients who were transfused in the absence of clinical bleeding events and patients who were transfused with nearly normal post-procedure hemoglobin values. These patient-level data, as well as our finding that transfusions were more common across all hemoglobin values at some hospitals, suggest that thresholds to transfuse may have been driven more by local practice patterns than by clinical necessity. We also found that transfusion was associated with an increased risk for in-hospital adverse outcomes. In the context of prior observational studies that have shown a similar association1–8, 13, or small randomized trials that have shown no benefit of liberal transfusion20–22, the present analysis suggests that further research is needed to clearly delineate the appropriate use of transfusion in patients undergoing PCI.
Practice patterns
Our data showed that the majority of transfusions among patients without bleeding occurred at hemoglobin values ≤8 g/dl. In contrast, patients with bleeding events received transfusion across the spectrum of hemoglobin values. While this may have indicated brisk blood loss in some patients despite higher post-procedure hemoglobin values, the overall rate of bleeding was low in our study sample. Thus this aggressive transfusion practice may, in fact, have reflected the biases of the physicians caring for these patients. The variation seen in transfusion practice patterns throughout this study was consistent with the limited data that have been previously reported13, 23.
This variation may have stemmed from several sources, including previously held beliefs about the benefit of transfusion, and recently published data indicating the lack of benefit and potential hazard associated with transfusion1–6. However, among these studies there is little randomized clinical trial evidence for transfusion practice, and none for the broad population of patients undergoing PCI. This creates a lack of consensus that is reflected in the American Association of Blood Banks’ guidelines published in 201212, which do not make any recommendations for transfusion strategies in patients with ACS. The guidelines do present cautious recommendations for transfusion in patients hospitalized with coronary artery disease. These include a restrictive strategy, limiting transfusion to those with either symptomatic anemia or those with a hemoglobin ≤8mg/dL. The uncertainty in the guidelines may be reflected by the slight decline of transfusion rates over time seen in our population, perhaps due to observational data that raises questions about the benefit of transfusion in these patients. The current data highlight the need for further evidence, in the form of randomized clinical trials, to assess the role of transfusion as therapy in these patients.
Transfusion and outcomes
Although we found one group in whom transfusion may be associated with improved outcomes, namely patients who suffer post-PCI bleeding and have a hemoglobin value <10g/dl, our study cannot determine which transfusion “trigger”, as defined by a hemoglobin value, is appropriate for patients undergoing PCI. There have been clinical trials in the critical care population to address this issue22, 24, 25. These trials have consistently shown that there is no benefit in maintaining higher hemoglobin levels in patients who are critically ill 26; however, whether these data are applicable to patients with ischemic heart disease is controversial.27 Moreover, these studies excluded patients who were actively bleeding. Data on patients with ischemic heart disease are available only from two small clinical trials that compared transfusion thresholds of 8 g/dl and 10 g/dl in patients presenting with ACS or stable angina21. The CRIT Pilot trial showed a higher rate of death, MI, or heart failure in patients assigned to maintaining a hemoglobin of 10 g/dl. Conversely, in the MINT Trial patients assigned to maintaining a Hgb ≥10 g/dl had a significantly lower rate of 30-day mortality and numerically lower rates of MI and unscheduled revascularization28. The FOCUS trial, which was conducted in patients with a history of coronary artery disease recovering from hip arthroplasty showed no difference in clinical outcomes between a hemoglobin of 8 g/dl or 10 g/dl, but the trial did not meet its pre-specified sample size and thus may have been underpowered to detect a difference in outcomes.20
In contrast, there are data from observational studies that show an association between more aggressive transfusion in patients with either MI or ACS and adverse outcomes.1–8 While observational data examining transfusion and outcome are subject to significant bias, there may be physiological reasons to explain why transfusion may reduce oxygen delivery and thus increase ischemic risk. The so-called “storage lesion” that occurs in stored red blood cells (RBCs) may impair oxygen delivery. Moreover, stored red cells are depleted of nitric oxide which may be important for interaction with vascular endothelium and transfer of oxygen to ischemic tissues 9. Transfusion of blood products may also have a prothrombotic effect through the release of platelet activation agents, a phenomenon that would be particularly harmful in the post-PCI patient9. In the context of this equipoise, a prospective randomized trial is needed to guide transfusion practice in patients with ischemic heart disease and those undergoing PCI.
Limitations
This study has several limitations. First, the data utilized are observational, and thus have measured confounding as shown through our univariable, multivariable and propensity modeling analyses. They may also have unmeasured confounding that cannot be mitigated. Second, while the CathPCI Registry captures data from the majority of United States cardiac catheterization laboratories, it does not include all sites, and thus may not fully represent practice in the United States. Third, we analyzed transfusion patterns on the site and patient level, but did not evaluate for individual practitioner level variation. It is likely that physicians other than the interventional cardiologist who performed the procedure care for many patients undergoing PCI in the United States, and these physicians may have made post-procedure transfusion decisions. The CathPCI registry does not contain information on these other practitioners. Finally, while hemoglobin level, transfusion, and events were all defined as post procedure, the absolute temporal relationship between these elements cannot be determined. Thus the data indicate there was an association between transfusion and adverse in-hospital outcomes, but causality could not be inferred based on these data.
Conclusions
In conclusion, there was considerable variation in blood transfusion practice among patients undergoing PCI in the United States, and this variation persisted after adjustment for patient differences. Moreover, transfusion thresholds varied widely across hospitals. Transfusion was independently associated with in-hospital adverse cardiac events, and this association remained significant in patients with and without bleeding events and at nearly all hemoglobin levels. These data highlight the need for randomized trials of transfusion strategies to guide practice in patients undergoing PCI. Until these trials have been completed, operators should utilize strategies that reduce the risk for bleeding and subsequent transfusion.
Supplementary Material
Acknowledgments
Funding and Support
This was an investigator initiated study. The analytic work was performed by the Yale Center for Outcomes Research, with financial support from The American College of Cardiology. The manuscript was reviewed by the NCDR for compliance with registry description and representation but the sponsor had no role in the design and conduct of the study, analysis and interpretation of the data, preparation of the manuscript or decision to submit the manuscript for peer review.
Footnotes
Author Contributions
Dr. Sherwood and Dr. Rao had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclaimer
This research was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR). The views expressed in this manuscript represent those of the author(s), and do not necessarily represent the official views of the NCDR or its associated professional societies identified at www.ncdr.com.
Author Disclosure
Authors acknowledge the following conflicts of interest: Dr. Sherwood, Mr. Wang, and Dr. Rao have no conflicts of interest related to this manuscript; Dr. Curtis acknowledges research grants from NIH/NHLBI and CMS to develop measures for public reporting, salary support from the American College of Cardiology National Cardiovascular Data Registry, ownership of stock in Medtronic; Dr. Peterson acknowledges research grants to the Duke Clinical Research Institute from the American College of Cardiology, American Heart Association, Eli Lilly & Co., Janssen, Society of Thoracic Surgeons, Consulting/Honoraria from Boehringer Ingelheim, Genentech, Janssen, Merck & Co., Sanofi-Aventis.
References
- 1.Chatterjee S, Wetterslev J, Sharma A, Lichstein E, Mukherjee D. Association of blood transfusion with increased mortality in myocardial infarction: A meta-analysis and diversity-adjusted study sequential analysis. Archives of internal medicine. 2012:1–8. doi: 10.1001/2013.jamainternmed.1001. [DOI] [PubMed] [Google Scholar]
- 2.Jolicoeur EM, O’Neill WW, Hellkamp A, Hamm CW, Holmes DR, Jr, Al-Khalidi HR, Patel MR, Van de Werf FJ, Pieper K, Armstrong PW, Granger CB, Investigators A-A. Transfusion and mortality in patients with st-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. European heart journal. 2009;30:2575–2583. doi: 10.1093/eurheartj/ehp279. [DOI] [PubMed] [Google Scholar]
- 3.Nikolsky E, Mehran R, Sadeghi HM, Grines CL, Cox DA, Garcia E, Tcheng JE, Griffin JJ, Guagliumi G, Stuckey T, Turco M, Fahy M, Lansky AJ, Stone GW. Prognostic impact of blood transfusion after primary angioplasty for acute myocardial infarction: Analysis from the cadillac (controlled abciximab and device investigation to lower late angioplasty complications) trial. JACC. Cardiovascular interventions. 2009;2:624–632. doi: 10.1016/j.jcin.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Shishehbor MH, Madhwal S, Rajagopal V, Hsu A, Kelly P, Gurm HS, Kapadia SR, Lauer MS, Topol EJ. Impact of blood transfusion on short- and long-term mortality in patients with st-segment elevation myocardial infarction. JACC. Cardiovascular interventions. 2009;2:46–53. doi: 10.1016/j.jcin.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Aronson D, Dann EJ, Bonstein L, Blich M, Kapeliovich M, Beyar R, Markiewicz W, Hammerman H. Impact of red blood cell transfusion on clinical outcomes in patients with acute myocardial infarction. The American journal of cardiology. 2008;102:115–119. doi: 10.1016/j.amjcard.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Wu WC, Rathore SS, Wang Y, Radford MJ, Krumholz HM. Blood transfusion in elderly patients with acute myocardial infarction. The New England journal of medicine. 2001;345:1230–1236. doi: 10.1056/NEJMoa010615. [DOI] [PubMed] [Google Scholar]
- 7.Rao SV, Jollis JG, Harrington RA, Granger CB, Newby LK, Armstrong PW, Moliterno DJ, Lindblad L, Pieper K, Topol EJ, Stamler JS, Califf RM. Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes. JAMA: the journal of the American Medical Association. 2004;292:1555–1562. doi: 10.1001/jama.292.13.1555. [DOI] [PubMed] [Google Scholar]
- 8.Rao SV, Eikelboom JA, Granger CB, Harrington RA, Califf RM, Bassand JP. Bleeding and blood transfusion issues in patients with non-st-segment elevation acute coronary syndromes. European heart journal. 2007;28:1193–1204. doi: 10.1093/eurheartj/ehm019. [DOI] [PubMed] [Google Scholar]
- 9.Doyle BJ, Rihal CS, Gastineau DA, Holmes DR., Jr Bleeding, blood transfusion, and increased mortality after percutaneous coronary intervention: Implications for contemporary practice. Journal of the American College of Cardiology. 2009;53:2019–2027. doi: 10.1016/j.jacc.2008.12.073. [DOI] [PubMed] [Google Scholar]
- 10.Sabatine MS, Morrow DA, Giugliano RP, Burton PB, Murphy SA, McCabe CH, Gibson CM, Braunwald E. Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation. 2005;111:2042–2049. doi: 10.1161/01.CIR.0000162477.70955.5F. [DOI] [PubMed] [Google Scholar]
- 11.Pilgrim T, Vetterli F, Kalesan B, Stefanini GG, Räber L, Stortecky S, Gloekler S, Binder RK, Wenaweser P, Moschovitis A, Khattab AA, Buellesfeld L, Zwahlen M, Meier B, Jüni P, Windecker S. The impact of anemia on long-term clinical outcome in patients undergoing revascularization with the unrestricted use of drug-eluting stents. Circulation: Cardiovascular Interventions. 2012;5:202–210. doi: 10.1161/CIRCINTERVENTIONS.111.965749. [DOI] [PubMed] [Google Scholar]
- 12.Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, Holcomb JB, Illoh O, Kaplan LJ, Katz LM, Rao SV, Roback JD, Shander A, Tobian AA, Weinstein R, Swinton McLaughlin LG, Djulbegovic B Clinical Transfusion Medicine Committee of the A. Red blood cell transfusion: A clinical practice guideline from the aabb*. Annals of internal medicine. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Alexander KP, Chen AY, Roe MT, Brindis RG, Rao SV, Gibler WB, Ohman EM, Peterson ED, Investigators C. The implications of blood transfusions for patients with non-st-segment elevation acute coronary syndromes: Results from the crusade national quality improvement initiative. Journal of the American College of Cardiology. 2005;46:1490–1495. doi: 10.1016/j.jacc.2005.06.072. [DOI] [PubMed] [Google Scholar]
- 14.Moscucci M, Ricciardi M, Eagle KA, Kline E, Bates ER, Werns SW, Karavite D, Muller DW. Frequency, predictors, and appropriateness of blood transfusion after percutaneous coronary interventions. The American journal of cardiology. 1998;81:702–707. doi: 10.1016/s0002-9149(97)01018-7. [DOI] [PubMed] [Google Scholar]
- 15.Marso SP, Amin AP, House JA, Kennedy KF, Spertus JA, Rao SV, Cohen DJ, Messenger JC, Rumsfeld JS National Cardiovascular Data R. Association between use of bleeding avoidance strategies and risk of periprocedural bleeding among patients undergoing percutaneous coronary intervention. JAMA: the journal of the American Medical Association. 2010;303:2156–2164. doi: 10.1001/jama.2010.708. [DOI] [PubMed] [Google Scholar]
- 16.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The american college of cardiology-national cardiovascular data registry (acc-ncdr): Building a national clinical data repository. Journal of the American College of Cardiology. 2001;37:2240–2245. doi: 10.1016/s0735-1097(01)01372-9. [DOI] [PubMed] [Google Scholar]
- 17.Moussa I, Hermann A, Messenger JC, Dehmer GJ, Weaver WD, Rumsfeld JS, Masoudi FA. The ncdr cathpci registry: A us national perspective on care and outcomes for percutaneous coronary intervention. Heart. 2013;99:297–303. doi: 10.1136/heartjnl-2012-303379. [DOI] [PubMed] [Google Scholar]
- 18.Peterson ED, Dai D, DeLong ER, Brennan JM, Singh M, Rao SV, Shaw RE, Roe MT, Ho KK, Klein LW, Krone RJ, Weintraub WS, Brindis RG, Rumsfeld JS, Spertus JA, Participants NR. Contemporary mortality risk prediction for percutaneous coronary intervention: Results from 588,398 procedures in the national cardiovascular data registry. Journal of the American College of Cardiology. 2010;55:1923–1932. doi: 10.1016/j.jacc.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta SK, Frutkin AD, Lindsey JB, House JA, Spertus JA, Rao SV, Ou FS, Roe MT, Peterson ED, Marso SP National Cardiovascular Data R. Bleeding in patients undergoing percutaneous coronary intervention: The development of a clinical risk algorithm from the national cardiovascular data registry. Circulation. Cardiovascular interventions. 2009;2:222–229. doi: 10.1161/CIRCINTERVENTIONS.108.846741. [DOI] [PubMed] [Google Scholar]
- 20.Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, Nemo G, Dragert K, Beaupre L, Hildebrand K, Macaulay W, Lewis C, Cook DR, Dobbin G, Zakriya KJ, Apple FS, Horney RA, Magaziner J, Investigators F. Liberal or restrictive transfusion in high-risk patients after hip surgery. The New England journal of medicine. 2011;365:2453–2462. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cooper HA, Rao SV, Greenberg MD, Rumsey MP, McKenzie M, Alcorn KW, Panza JA. Conservative versus liberal red cell transfusion in acute myocardial infarction (the crit randomized pilot study) The American journal of cardiology. 2011;108:1108–1111. doi: 10.1016/j.amjcard.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion requirements in critical care investigators, canadian critical care trials group. The New England journal of medicine. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 23.Corwin HL, Gettinger A, Pearl RG, Fink MP, Levy MM, Abraham E, MacIntyre NR, Shabot MM, Duh MS, Shapiro MJ. The crit study: Anemia and blood transfusion in the critically ill--current clinical practice in the united states. Critical care medicine. 2004;32:39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- 24.Hill SR, Carless PA, Henry DA, Carson JL, Hebert PC, McClelland DB, Henderson KM. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane database of systematic reviews. 2002:CD002042. doi: 10.1002/14651858.CD002042. [DOI] [PubMed] [Google Scholar]
- 25.Freudenberger RS, Carson JL. Is there an optimal hemoglobin value in the cardiac intensive care unit? Current opinion in critical care. 2003;9:356–361. doi: 10.1097/00075198-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Hebert PC, Yetisir E, Martin C, Blajchman MA, Wells G, Marshall J, Tweeddale M, Pagliarello G, Schweitzer I Transfusion Requirements in Critical Care Investigators for the Canadian Critical Care Trials G. Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases? Critical care medicine. 2001;29:227–234. doi: 10.1097/00003246-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Rao SV, Califf RM. Is old blood bad blood? American heart journal. 2010;159:710–712. doi: 10.1016/j.ahj.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Carson JL, Brooks MM, Abbott JD, Chaitman B, Kelsey SF, Triulzi DJ, Srinivas V, Menegus MA, Marroquin OC, Rao SV, Noveck H, Passano E, Hardison RM, Smitherman T, Vagaonescu T, Wimmer NJ, Williams DO. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. American heart journal. 2013;165:964–971. e961. doi: 10.1016/j.ahj.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.