Abstract
Large-scale lentiviral vector (LV) concentration can be inefficient and time consuming, often involving multiple rounds of filtration and centrifugation. This report describes a simpler method using two tangential flow filtration (TFF) steps to concentrate liter-scale volumes of LV supernatant, achieving in excess of 2000-fold concentration in less than 3 hours with very high recovery (>97%). Large volumes of LV supernatant can be produced easily through the use of multi-layer flasks, each having 1720 cm2 surface area and producing ~560 mL of supernatant per flask. Combining the use of such flasks and TFF greatly simplifies large-scale production of LV. As a demonstration, the method is used to produce a very high titer LV (>1010 TU/mL) and transduce primary human CD34+ hematopoietic stem/progenitor cells at high final vector concentrations with no overt toxicity. A complex LV (STEMCCA) for induced pluripotent stem cell generation is also concentrated from low initial titer and used to transduce and reprogram primary human fibroblasts with no overt toxicity. Additionally, a generalized and simple multiplexed real- time PCR assay is described for lentiviral vector titer and copy number determination.
Keywords: Lentiviral vector concentration, Tangential flow filtration, Large-scale, iPS cells, Genomic DNA normalization
1. TYPE OF RESEARCH
Since their development nearly fifteen years ago, VSV-G-pseudotyped self-inactivating (SIN) lentiviral vectors (LV) have become an indispensible part of the experimental biologist’s toolbox and have met with success in clinical gene therapy trials (Naldini et al. 1996, Cartier et al. 2009, Cavazzana-Calvo et al. 2010). Unlike the γ-retroviral vectors that preceded them, LV are capable of transducing non-dividing cells, can carry more complex transgene cassettes, more frequently maintain long-term transgene expression, and generally yield higher titers in producer cells (Zufferey et al. 1997). LV are also less genotoxic than γ-retroviral vectors, although this difference has become less significant since the advent of SIN γ-retroviral vectors (Modlich et al. 2006, Modlich et al. 2009, Arumugam et al. 2009).
Titers in the supernatant of producer cells are generally more than sufficient for transducing cell lines, but primary cells are more difficult to transduce and require a vector that is far more concentrated. Additionally, some vector designs incorporate genetic elements that severely reduce titers, effectively rendering the viral supernatant useless without concentration. Many cell types also cannot tolerate either the growth medium or secreted proteins from vector producer cells, and post-production concentration and cleanup is necessary.
Various methods have been employed to concentrate viral particles. Ultracentrifugation is a well-established strategy, but each spin yields only about 100-fold concentration and multiple spins risk diminished viral particle recovery. Furthermore, processing large volumes via ultracentrifugation is cumbersome and time-consuming, as typical research centrifuges are limited to ~230 mL of raw LV per rotor. Ultrafiltration via centrifuged filtration units enables LV to be more easily concentrated, but these units trap a significant amount of LV input. Tangential flow filtration (TFF), on the other hand, does not appreciably trap LV and allows for easier processing as well as for a diafiltration step to reduce metabolites and small secreted proteins from producer cells. TFF is therefore an attractive alternative to centrifugation for concentrating large volumes of vector supernatant, and this is evidenced by its recent use to produce a clinical-grade LV (Cavazzana-Calvo et al. 2010). The sole disadvantage of TFF is that one-step TFF only yields 50- to 100-fold concentration of LV (Geraerts et al. 2005)
The present study describes a rapid method using two tandem TFF steps to concentrate up to 5.5 L of raw LV-containing supernatant down to ~1 mL final volume with a reliably high recovery rate (>97%). The final product is demonstrated to be of a quality sufficient to transduce, with no overt toxicity, both primary human CD34+ hematopoietic stem/progenitor cells (HSPCs) and primary human fibroblasts for iPS generation.
2.1 MATERIALS
2.1.1 Special Equipment
KrosFlo Research II TFF System (Spectrum labs, Rancho Dominguez, CA, Cat No. SYR2-U20- 01N)
-
Flow Path 1 [FPI] (Spectrum labs, Rancho Dominguez, CA, Cat No. EZ-M1-500S-260-01N-I).
■ The hollow fiber filter in FP1 contains 320 fibers (0.5 mm internal diameter) with a total surface area of 615 cm2 and a 500 kDa cut-off.
-
Flow Path 2 [FPII] (Spectrum labs, Rancho Dominguez, CA, Cat No. EZ-CHIL07-01-I)
■ The hollow fiber filter in FP2 contains 12 fibers (0.5 mm internal diameter) with a total surface area of 40 cm2 and a 500 kDa cut-off.
DELTRAN I disposable pressure transducer (Utah Medical Products, Midvale, UT, Cat No. DPT 100)
150 cm2 Tissue Culture Flasks (Corning, Corning, NY, Cat No. 430825)
TripleFlask (NUNC, Rochester, NY, Cat No. 132867)
HYPERflask (Corning, Corning, NY, Cat No. 10010)
Syringe filters (Millipore, Cat No. SLGV033RS)
5 mL syringe (BD Medical, Franklin Lakes, NJ, Cat No. 309603)
0.8 μm Filter Units (Nalgene, Rochester, NY, Cat No. 127-0080)
0.22 μm Filter Units (Millipore, Billerica, MA, Cat No. SCGPU05RE)
2 mL Screw Cap Tubes (VWR, Radnor, PA, Cat No. 80078-428)
0.5 mL Screw Cap Tubes (VWR, Radnor, PA, Cat No. 89004-318)
2 mL Cryovials (Nalgene, Rochester, NY, Cat No. 5011-0020)
PureLink Genomic DNA Mini Kit (Invitrogen, Carlsbad, CA, Cat No. K1820-01)
MicroAmp Optical 96-Well Reaction Plate (Applied Biosystems, Cat No. N8010560)
MicroAmp Optical Adhesive Film (Applied Biosystems, Carlsbad, CA, Cat No. 4311971)
7500 Real-Time PCR System (Applied Biosystems, Carlsbad, CA, Cat No. 4362143)
2.1.2 Chemicals and Reagents
293T cells (ATCC, Manassas, VA, Cat No. CRL-1268)
HT-29 cells (ATCC, Manassas, VA, Cat No. CCL-218)
TransIT-293 (Mirus, Madison, WI, Cat. No. MIR2706)
Opti-MEM (Invitrogen, Carlsbad, CA, Cat. No. 31985-062)
Dulbecco’s modified Eagle’s medium [DMEM] (Mediatech, Herndon, VA, Cat. No. 15-013- CV)
Fetal Bovine Serum (Omega, Tarzana, CA, Cat. No. FB-01)
L-Glutamine/Penicillin/Streptomycin (Gemini Bioproducts, Woodland, CA, Cat. No. 400110 100ML)
Sodium Butyrate (Sigma-Aldrich, St. Louis, MO, Cat. No. B5887-5G)
1M HEPES (Invitrogen, Carlsbad, CA, Cat No. 15630-130)
Dulbecco’s Phosphate Buffered Saline [DPBS] (Mediatech, Herndon, VA, Cat. No. 21-031- CV)
UltraCULTURE (LONZA, Basel, Switzerland, Cat. No. 12-725F)
0.05% Trypsin EDTA 1X (Mediatech, Herndon, VA, Cat. No. 25-052-CI)
0.25% Trypsin-EDTA (Invitrogen, Carlsbad, CA, Cat No. 15050065)
Genomic DNA Prepared Using NucleoBond Xtra Maxi EF Kit (Clontech, Cat No. 740424.10)
Primers and Probes Ordered From Integrated DNA Technologies (IDT) (Coralville, IA)
TaqMan Universal PCR Master Mix (Applied Biosystems, Carlsbad, CA, Cat No. 4304437)
2.2 DETAILED PROCEDURE
2.2.1 Time Required
Day 1 Cell Seeding and Transfection [2 hours]
Day 2 Sodium Butyrate Induction [1 hour]
Day 4 First Harvest [1 hour]
Day 5 Second Harvest and TFF [3 hours]
Total [7-8 hours]
2.2.2 Cell Seeding and Transfection
Note: all of the amounts below are per HYPERFlask of packaging cells. Two HYPERFlasks are routinely processed in parallel to make use of the cost of the flow paths. As many as five have been processed at one time, but more than five would exceed the recommended capacity of Flow Path 1.
1) Low passage (<12) 293T cells were maintained below confluence in 500 cm2 TripleFlasks in 150 mL D10 medium, consisting of DMEM with 10% fetal bovine serum, 50 U/mL penicillin, 50 μg/mL streptomycin, and 2 mM L-glutamine. This requires 1:4-1:5 passaging if performed every two days, or 1:8-1:10 passaging if performed every three days.
2) Before cell harvesting, the transfection mix was prepared. First, 1 mL of TransIT- 293 was added to 50 mL of Opti-MEM, which was then vortexed to mix thoroughly and left to incubate for 30 minutes at room temperature. Then, 150 μg of a pCCL- based (Dull et al., 1998) vector plasmid, 150 μg of gag/pol expressing plasmid (pCMVΔR8.91, Zufferey et al., 1997) and 30 μg of the envelope expression plasmid pMD.G (VSV-G) (Naldini et al., 1996) were added to the TransIT/Opti-MEM mixture, which was then incubated for a further 20 minutes at room temperature.
3) Cells were washed with 15 mL 0.05% trypsin EDTA in HBSS to bind and remove trypsin inhibitors, and then harvested with another 15 mL of 0.05% trypsin and 3-5 minute incubation at 37°C. 15 mL of D10 was then added to inactivate the trypsin, cells were decanted into a sterile polystyrene bottle, and then a further 30 mL of D10 was used to rinse the TripleFlask and was then decanted into the bottle.
4) The transfection mixture was added to 7 × 108 cells (usually obtained from three TripleFlasks) in ~150 mL D10. This well-combined cell/transfection mix was poured into a HYPERFlask and the HYPERFlask was placed on its side to allow even distribution among the layers. The HYPERFlask was then filled with D10, the whole contents were mixed well by inverting the flask several times, the flasks were vigorously tapped while held vertically to dislodge air bubbles from the layers, and the flask was then placed in a horizontal position at 37°C overnight.
2.2.3 Sodium Butyrate Induction
Note: sodium butyrate induction does not always increase titer and can severely reduce titer if changing of medium is not done carefully and disturbs cells as a result. However, past experiments show that induction may increase titer by one half to one full log when done properly, so it is retained as a routine step in the procedure.
5) Approximately 18-20 hours post-transfection, the medium on the transfected cells was changed to D10 containing 10 mM sodium butyrate and 20 mM HEPES.
6) After 6–8 hours, the cells were rinsed once with 500 mL DPBS and then fresh harvesting medium, consisting of UltraCULTURE with 20 mM HEPES, 50 U/mL penicillin, 50 μg/mL streptomycin, and 2 mM L-glutamine, was added to fill the HYPERFlask. The user must be aware of the balance between air bubbles being left in the layers of the flask and cells being disrupted by over exuberant tapping of the flask to dislodge bubbles. It is our experience that using one finger to gently tap the side of the flask, while held in a vertical position, is sufficient to dislodge enough air without appreciably disrupting the cell monolayers.
2.2.4 Vector Harvest
7) About 40 hours after addition of harvesting medium, LV-containing medium was decanted from the HYPERFlasks into 0.8 μm bottle-top filters and the filtrate was collected in sterile polystyrene bottles. Fresh harvesting medium was then added to refill the HYPERFlask, which was then incubated again at 37°C. The harvested LV- containing medium was stored overnight at 4°C.
8) 24 hours later, LV-containing medium was again decanted from the HYPERFlasks into 0.8 μm bottle-top filters and the filtrate was collected in sterile polystyrene bottles.
9) The filtered first and second harvests were combined and samples were retained for recovery analysis.
2.2.5 Tangential Flow Filtration
10) All concentration steps were performed on custom made Spectrum flow paths (FPI and FPII shown in Figures 1 and 2 respectively) using the KrosFlo Research II TFF System.
11) Before introducing LV-containing medium, flow paths were tested for integrity. This was done by thoroughly wetting the system with DPBS, running the system until all the DPBS had been cleared as permeate, closing every valve and running the pump until the inlet pressure was around 5 PSI, and then releasing the permeate. After an initial drop in pressure to clear the PBS, an intact column exhibits a pressure drop of about 0.01 PSI/second, as the PBS-wetted filter fibers are impermeable to air.
12) Upon validation of the column’s integrity, the concentration of the LV-containing medium was commenced. Throughout the procedure, the inlet pressure was monitored and maintained below 6 PSI. FPI was used to concentrate as much as 5.5 L down to 50 mL, which is the minimal holdup volume in the flow path and represents about 100-fold concentration.
13) The concentrated vector was diafiltrated in FPI with 1000 mL of diafiltration mix, consisting of DPBS and 2.5 mL of FCS, and again concentrated down to 50 mL. This intermediate concentrate was kept in a 50 mL conical tube while FPII was tested for integrity.
14) Once FPII passed an integrity test (see step 11), it was used to further concentrate the 50 mL from FPI down to the 1 mL minimal holdup volume (50 fold concentration, up to 5000-fold concentration total). Throughout the whole procedure, the inlet pressure was monitored to keep it below 9 PSI.
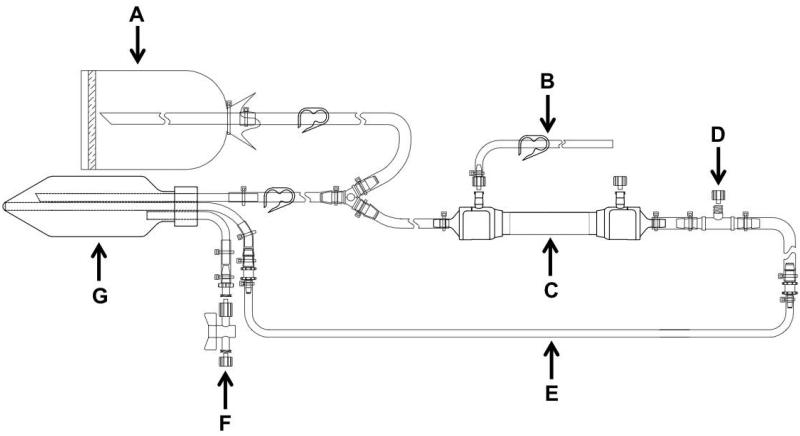
Figure 1.
Design of flow path I (FPI). A: inlet, B: permeate, C: filter column, D: pressure transducer port, E: tubing loop for peristaltic pump, F: reservoir pressure release port, G: reservoir.
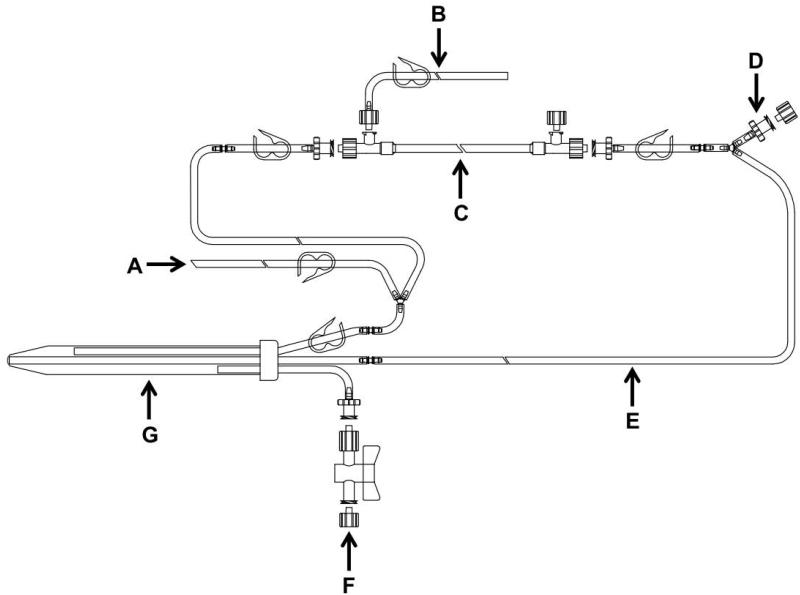
Figure 2.
Design of flow path II (FPII). A: inlet, B: permeate, C: filter column, D: pressure transducer port, E: tubing loop for peristaltic pump, F: reservoir pressure release port, G: reservoir.
2.2.6 Vector Transduction for Titer Determination
15) Six well plates were seeded with 1×105 HT-29 cells per well in 2 mL D10.
16) After 24 hours, three wells were harvested with 0.25% Trypsin EDTA and total cell count was determined. Mean cell number per well was recorded for the calculation of titer at the end of the protocol.
17) For TFF-concentrated vector, three independent 50,000-fold dilutions were prepared in D10, using three independent initial 100-fold dilutions, each followed by serial 500-fold dilutions.
18) Medium from the HT-29 cells was aspirated and then 1 mL of diluted vector was added to each well. 12-16 hours later, an additional 1 mL D10 was added to each well.
19) After another 48 hours (~60 hours post-transduction), the cells were harvested with 0.25% trypsin-EDTA.
20) Genomic DNA isolation was performed using the PureLink Genomic DNA Mini Kit.
2.2.7 Absolute Quantitation via Probe-Based Real-Time PCR
Real-time PCR amplification of the packaging signal sequence (psi) in the lentiviral provirus was used for absolute quantitation of the average number of vector DNA sequences per cell.
21) A standard curve was prepared from samples of HT29 DNA mixed with DNA from a HT29 clone that has 2 copies/cell of a lentiviral vector.
22) To obviate DNA concentration determination and normalization, a multiplex probebased real-time PCR reaction was used combining primers and probe to detect a conserved LV sequence (HIV-1 Psi region) with primers and probe targeted to the autosomal gene syndecan 4 (SDC4, De Preter et al. 2002) for normalization (Table 1). The SDC4 internal control allows the cycles to threshold (Ct) value of psi to be normalized to that of the endogenous control, which is reflective of the number of cell equivalents of DNA present in the reaction. Therefore, the same volume of DNA can be added to each reaction even though the concentrations will be somewhat different. Substitution of the SDC4 primers and probe with those directed to the ultra-conserved region uc483 (200 nM each primer, 100 nM probe) allows normalization of genomic DNA from mouse as well as human cells and many other vertebrate cells, if there is a need to determine titer in cells of another species (Bejerano et al. 2004). However, the uc483 primers and probe must be used in parallel reactions rather than in multiplex.
23) Real-time PCR was performed using ABI TaqMan Universal PCR Master Mix and an ABI 7500 Fast Real-Time PCR System. A total volume of 25 μL was used for reactions with 400 nM each for SMPU primers, 50 nM each for SDC4 primers, 50 nM each probe, and 1 μL of genomic DNA template (50-300 ng). Cycling conditions were as recommended by the manufacturer, and the ‘Fast’ option of the system was not used.
24) In order to interpret the data, the ΔCt was determined for each well (ΔCt = Ctpsi - CtSDC4). A standard curve was plotted as a log2(copy number) vs. ΔCt. The standard curve DNA had copy numbers of 2, 0.2, 0.02, 0.002, and 0.0002, corresponding to 10° through 10−4 dilutions. A linear equation was obtained for a best-fit line of the standard curve. The ΔCt values for each experimental sample were put into the equation to obtain the log2(copy number).
- 25) The copy number for each sample was calculated as:
and titer was determined with the following equation:
TABLE 1.
Oligonucleotide sequences
| SMPU F | ACCTGAAAGCGAAAGGGAAAC |
| SMPU R | CGCACCCATCTCTCTCCTTCT |
| SMPU-FAM | 6-FAM-AGCTCTCTC-ZEN-GACGCAGGACTCGGC-Iowa Black FQ |
| SDC4 F | CAGGGTCTGGGAGCCAAGT |
| SDC4 R | GCACAGTGCTGGACATTGACA |
| SDC4-HEX | HEX-CCCACCGAACCCAAGAAACTAGAGGAGAAT-Iowa Black FQ |
| uc483 F | GCATGCTTCATTAACAGTGACC |
| uc483 R | TTTAAAATCTGAATGCATGATAAGAATGG |
| uc483-HEX | HEX-AGATCCCCAGCTCATCCGTGATTG-Iowa Black FQ |
2.3 ADDITIONAL MATERIALS AND METHODS
2.3.1 CD34+ Culture and Transduction
CD34+ cells were isolated from human bone marrow obtained from The National Disease Research Interchange (NDRI, Philadelphia, PA) using Ficoll-Hypaque (Amersham Pharmacia Biotech, Piscataway, NJ) density gradient centrifugation followed by Milteyni MidiMACS separation columns (Milteyni Biotech, Sunnyvale, CA). CD34+ cells were frozen after collection and thawed prior to transduction. CD34+ cells (1 × 10^5/well) were pre-stimulated overnight on fibronectin fragment CH-296 (Takara Shuzo Co., Otsu, Shiga, Japan)-coated 6 well plates in serum-free X-Vivo-15 medium (Lonza, Basel, Swutzerland) containing 50 ng/mL FLT-3 ligand (R&D Systems, Minneapolis, MN), 50 ng/mL c-kit ligand (Biosource International, Camarillo, CA) and 50 ng/mL thrombopoietin (R&D Systems). The next day, the cells were exposed to 1×106 to 1×109 TU/mL of CCL-c-MNDU3-EGFP vector (Haas et al. 2003) in 1 mL final volume of the X-Vivo medium with cytokines described above. 24 hours following transduction, the medium was exchanged for basal bone marrow medium (BBMM: IMDM, 20 % FCS, 0.5 % BSA) with 5 ng/mL human IL-3, 10 ng/mL IL-6 and 25 ng/mL c-kit ligand (Biosource International). Seven days after transduction, cells were analyzed for EGFP expression by flow cytometry performed on a FACSCalibur (Beckton-Dickinson Immunocytometry Systems, San Jose, CA) using CellQuest software.
2.3.2 iPS Culture and Transduction
NHDF 17622 female fibroblasts were obtained from Lonza (Basel, Switzerland). All cells were grown and procedures performed under a protocol approved by the Chancellor’s Animal Research Committee (ARC) and Embryonic Stem Cell Research Oversight (ESCRO) committee at UCLA. 100,000 fibroblasts (passage 3) were exposed overnight to 1.75×106 to 3×107 TU/mL of both concentrated HAGE-EF1α-STEMCCA LV (Sommer et al. 2009) as well as CCL-c-MNDU3-EGFP LV in 1 mL of standard fibroblast medium (DMEM supplemented with 10% FBS, L-glutamine, nonessential amino acids, and penicillin-streptomycin) with 5 ug/mL polybrene. Cells were trypsinized and re-plated onto 10 cm dishes and parallel wells on 6-well plates (for NANOG staining) containing gamma irradiated male CF1 mouse embryonic fibroblasts on day 5 post-transduction. Medium was replaced with standard hESC medium (DMEM/F12 supplemented with 20% knockout serum replacement, L- glutamine, nonessential amino acids, penicillin-streptomycin, 2-mercaptoethanol, and 20 ng/mL bFGF) the next day and changed every day thereafter. hESC-like colonies were seen at day 20 post-transduction and alkaline phosphatase positive as well as NANOG positive colonies were scored at day 30 post-transduction.
For immunostaining, cells grown on coverslips were washed in PBS and then fixed for 10 min at room temperature (RT) in PBS containing 4% paraformaldehyde. Cells were then permeabilized by incubation with PBS containing 0.5% Triton X-100 for 5 minutes at RT, transferred into PBS with 0.2% Tween- 20 (PBS/Tween), and then incubated for 30 min in blocking buffer (5% goat serum, 0.2% fish skin gelatin, 0.2% Tween in PBS). Primary NANOG antibody (Abcam ab21624, Cambridge, Massachusetts) incubations were performed for 1 hour at RT in blocking solution, and cells were washed three times in PBS/Tween and incubated with Alexa 546 labeled secondary antibodies in blocking buffer for 30 minutes. Primary TRA-1-60 antibody (Millipore MAB4360, Billerica, Massachusetts) incubations were performed for 1 hour at RT in blocking solution, and cells were washed three times in PBS/Tween and incubated with Alexa 647 labeled secondary antibodies in blocking buffer for 30 minutes. Cells were then washed with PBS/Tween, stained with DAPI, and mounted in Aqua-polymount (Polysciences Inc., Warrington, Pennsylvania).
2.3.3 Statistical analysis
Statistical analyses for correlations between vector dose and cell number and viability and for the exponential decay coefficient of vector through freeze/thaw cycles were performed with GraphPad Prism software (GraphPad, La Jolla, CA).
2.3.4 Recovery Calculations
To calculate recovery, the titer of the raw LV supernatant (after one freeze/thaw cycle) was multiplied by the total volume of supernatant at the beginning of the concentration process to obtain the total number of initial transducing units (ITU). The titer of the concentrated LV was multiplied by the final volume of the product to obtain the total number of final transducing units (FTU). Recovery (in %) was calculated as 100 * (FTU/ITU).
3.1 RESULTS
3.1.1 Recovery
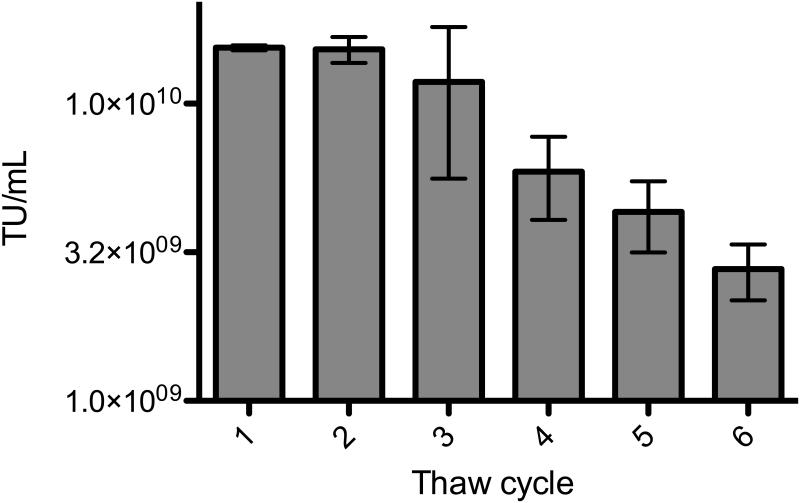
TFF very efficiently concentrated multiple LV preparations, with a mean recovery of 117% for both simple single promoter/transgene vectors and more complex vectors possessing multiple promoters/transgenes (Table 2). Two very low titer vector preparations, resulting from poor transfection efficiencies, were also efficiently concentrated. The mean recovery value of over 100% can probably be attributed to enhanced cryopreservation of the concentrated LV samples relative to the raw LV samples. The final product resembles a highly concentrated suspension of LV and cellular debris, and high protein concentrations are generally agreed to contribute to cryopreservation. In order to assess the stability of our final product through freeze/thaw cycles, an aliquot of concentrated vector was repeatedly thawed and refrozen. Each time, a sample was taken and used to transduce cells, and this was repeated to yield five samplings (Figure 3). TFF-concentrated LV exhibited higher than expected stability through multiple freeze/thaw cycles, losing on average only 15% of its TU per freeze/thaw cycle as determined by exponential decay analysis of the freeze/thaw data. The ratio of transducing units to nanograms of p24 was determined for several vector preparations, and the mean TU/ng p24 was 2.0 × 104 (Table 3). This value is within a log of values typically reported for LV preparations (Follenzi and Naldini Methods Enzymol. 2002, Kutner et al. Nat. Protoc. 2009).
TABLE 2.
Recovery data
| Vector | Raw titer (TU/mL) |
Fold concentration |
Expected titer (TU/mL) |
Actual titer (TU/mL) |
Recovery (%) |
|---|---|---|---|---|---|
| STEMCCA | 2.0×105 | 1833 | 3.6×108 | 5.0×108 | 137 |
| Single Promoter | 1.3×107 | 420 | 5.4×109 | 6.7×109 | 124 |
| Single Promoter | 5.3×106 | 1690 | 8.9×109 | 9.6×109 | 108 |
| Single Promoter | 7.×106 | 1690 | 1.3×1010 | 1.2×1010 | 97 |
| Single Promoter | 2.7×106 | 2000 | 5.4×109 | 5.8×109 | 109 |
| Single Promoter | 3.1×104 | 1100 | 3.4×107 | 4.0×107 | 116 |
| Single Promoter | 1.7×105 | 1222 | 2.1×108 | 2.0×108 | 94 |
| Single Promoter | 3.7×105 | 2200 | 8.2×108 | 1.0×109 | 127 |
| Single Promoter | 8.0×105 | 2200 | 1.8×109 | 2.5×109 | 141 |
| Dual Promoter | 4.6×106 | 730 | 3.3×109 | 3.8×109 | 113 |
| Dual Promoter | 9.8×106 | 360 | 3.6×109 | 3.6×109 | 101 |
| Failed Transfection | 3.1×104 | 1100 | 3.4×107 | 4.0×107 | 116 |
| Failed Transfection | 6.5×103 | 1467 | 9.6×106 | 1.4×107 | 142 |
Figure 3.
Freeze/thaw stability of concentrated vector. Each bar represents the mean of three independent dilutions of vector, each followed by a transduction of HT29 cells, genomic DNA isolation and real-time PCR measurement. Error bars represent the standard error of the mean (SEM).
TABLE 3.
Vector quality
| Titer (TU/mL) |
p24 (ng/mL) |
TU/ng p24 |
|---|---|---|
| 2.0×108 | 1.0×104 | 1.9×104 |
| 5.8×109 | 1.7×105 | 3.4×104 |
| 1.0×109 | 1.0×105 | 1.0×104 |
| 2.5×109 | 1.5×105 | 1.7×104 |
3.1.2 Transduction of primary human CD34+ HSPCs
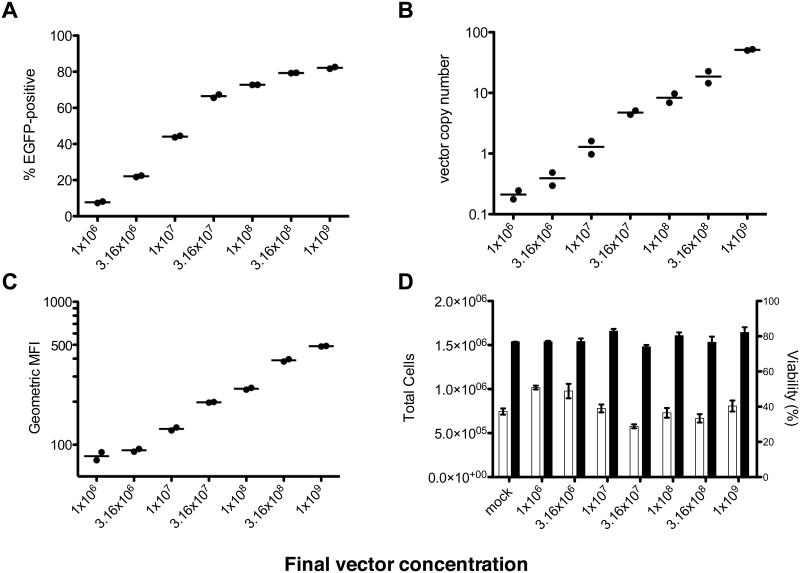
To test the quality of the concentrated LV, a vector preparation was used to transduce primary human CD34+ HSPCs isolated from bone marrow. To transduce these sensitive cells efficiently, they are typically exposed to final vector concentrations of 1×107-1×108 TU/mL (Haas et al. 2000). In this case, however, an upper concentration of 1×109 was used to see how high of a vector concentration the HSPCs would tolerate in a short-term in vitro culture assay. After an 18h prestimulation in cytokines that enhance CD34+ cell transduction, the cells were transduced overnight, cultured for seven days and then collected for flow cytometric and molecular analyses. As expected, the percentage of EGFP+ cells measured by flow cytometry increased relatively linearly at low vector doses but increased less at higher doses as cells began to incur multiple transduction events (Figure 4A). In contrast, vector copy number increased linearly across the entire range of vector doses, indicating that the increasing vector doses resulted in the expected increase in transduction events (Figure 4B). Although some of these measurements are extrapolated beyond the standard and are therefore not strictly accurate, they are taken to be reasonable estimates based on dilutions of high VCN DNA into untransduced DNA that were used previously to test the assay (data not shown). Similarly, mean fluorescence intensity of EGFP+ cells increased linearly across almost the whole range, except for the first two data points where most of the EGFP+ cells would be expected to have only one integration and thus the same EGFP expression (Figure 4C). Final cell counts were somewhat variable, but there was no significant correlation between vector dose and final cell number or viability (p=0.2357 and p=0.8397 by Spearman’s rank correlation test, respectively) (Figure 4D).
Figure 4.
Various metrics of transduction of CD34+ cells analyzed 7 days post-transduction, plotted against vector dose. (A) % EGFP+ cells by flow cytometry. (B) Vector copy number measured by real-time PCR. (C) Geometric mean fluorescence intensity of EGFP+ cells in each condition. (D) Cell count (□) and viability (■) by trypan blue dye exclusion. Bars represent mean and error bars represent range (n=2).
3.1.3 Transduction of primary human fibroblasts for iPSC generation
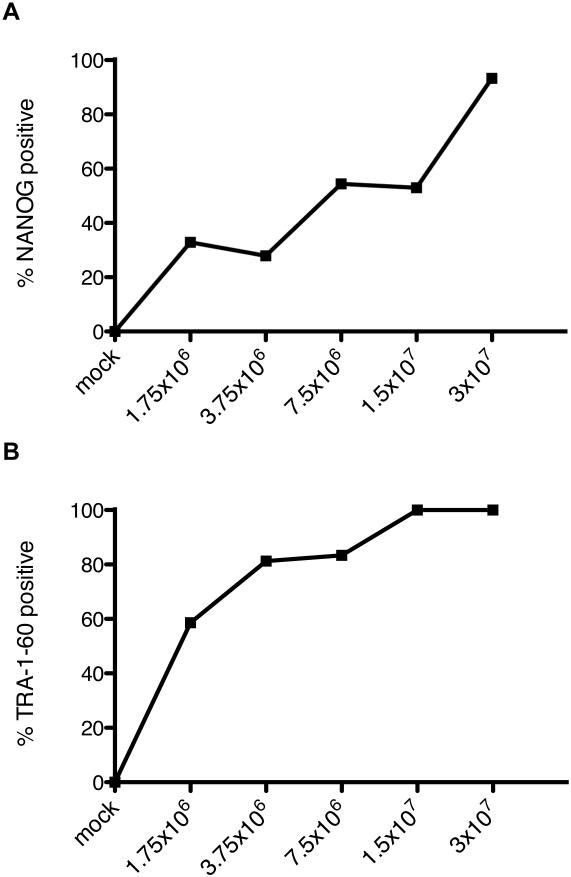
Induced pluripotent stem cells are an important new technology for biological and medical research, but vectors containing the efficient STEMCCA element for single-vector reprogramming are difficult to produce in large scale and high titer. A large 5.5L batch of HAGE-EF1α-STEMCCA was produced and concentrated down to 3 mL, representing a nearly 2000-fold concentration (Table 2). This vector was used in a dose escalation to transduce primary human dermal fibroblasts to generate iPSC colonies along with an EGFP-expressing vector as a transduction control. With increasing vector doses, the efficiency of full reprogramming as measured by the fraction of NANOG and TRA-1-61 positive colonies out of total ESC-like DAPI clusters increased continuously with vector dose (Figure 5). This suggests that the high extent of transduction by our vector preparation induced efficient reprogramming.
Figure 5.
Transduction and reprogramming of primary human dermal fibroblasts. (A) Percentage of ESC-like DAPI clusters staining NANOG-positive at day 30 post-transduction by immunocytochemistry. (B) Percentage of ESC-like DAPI clusters staining TRA-1-60- positive at day 30 post-transduction by immunocytochemistry.
4. DISCUSSION AND CONCLUSIONS
This protocol using 2 tangential flow steps in tandem can be used reproducibly and reliably to concentrate up to 5.5 L of raw LV-containing supernatant down to ~1 mL final volume, with a high recovery rate (>97%). Based on our metrics of vector transduction and expression as well as total cell counts and viability determination in CD34+ cells after one week of culture, it is concluded that vectors prepared in this fashion do not intrinsically lead to overt toxicity, at least in primary human hematopoietic cells. It should be noted that vectors bearing certain transgenes can be toxic irrespective of the method of preparation. Finally, our preparation of the proven STEMCCA vector for iPSC generation and successful generation of iPSCs from primary human fibroblasts demonstrates that this production and concentration scheme is effective for producing and concentrating complicated vectors in large scale.
5. TROUBLESHOOTING
| Problem | Solution |
|---|---|
| Slow Growing 293T | This is often resulting from high passage number. Try to use 293T cells that are below passage 12. |
| DNA Precipitation | If the DNA is not pure it may cause excessive precipitation during the transfection set up. The Nucleobond Xtra Maxi EF kit is recommended for endotoxin removal, and thorough rinsing of the DNA precipitate with 70% ethanol can remove excess salt. |
| Cell Clumping | If the trypsinization step was insufficient to dissociate the cells from one another, it is recommended to rinse again with trypsin to improve recovery. |
| Uneven Cell Plating | This may occur if the incubator shelf is not level. |
| Cells Peeling Off | This can occur for several reasons: too many cells were plated, or the medium changes were performed too vigorously, or the HYPERflask was repeatedly knocked. |
| Column Integrity Fail | This is very rare but when it does occur the column must be replaced. |
| Flow Path Leak | Check all the connections throughout the flow path as this is usually caused by a single loose connection. |
| Blocked filter | If too much particulate matter or protein (serum) is in the LV-containing medium then the filter may start to block. Reduce the protein content in the LV-containing medium by using serum-free medium. If serum-free medium was used and the permeate flow seems slow then try closing the permeate to increase the internal pressure in the filter to try unblocking some of the pores in the membrane. |
| High inlet pressure | This often occurs as the filter is beginning to clog. Reduce the back pressure (if additional back pressure has been applied) and reduce the flow rate. |
| Aggregation | If too much protein is removed then there is a substantial increase in aggregation. Try to keep some protein present in the diafiltration mix and avoid diafiltrating in pure DPBS. |
| Overheating | FP2 can heat up during use, threatening the stability of the vector. As a precaution, keep the reservoir tube on ice during the second concentration stage. |
6. ALTERNATIVES
| Alternative Concentration Method |
Advantages | Disadvantages |
|---|---|---|
| Ultracentrifugation | Little co-concentration of large molecules or particulates, relatively simple in concept and execution |
Cannot achieve high-fold concentration, increased risk of contamination, cumbersome, cannot process large volumes |
| Ultrafiltration | Relatively easy to process medium-to-large volumes |
Cannot achieve high-fold concentration, increased risk of contamination, large amount of vector trapped in filters, co-concentrates negatively charged species such as phenol red |
| Chromatography | Inexpensive, no specialized equipment, little co- concentration of large molecules or particulates |
Processing medium-to- large volumes extremely cumbersome |
| Alternative protocol components |
Advantages | Disadvantages |
|---|---|---|
| Four-plasmid packaging system (Dull et al. 1998) |
Less chance of replication- competent lentivirus (RCL) formation |
Requires extra optimization and plasmid preps. No reports of RCL with three- plasmid system |
| Cell factories to replace HYPERFlasks |
Easier to change medium, available with larger culture areas |
More expensive per unit area, require more incubator space |
| Calcium phosphate transfection |
Less expensive | Less reliable, requires more plasmid, necessitates more washing during medium changes to remove precipitate |
7. QUICK PROCEDURE
7.1.1 Cell Seeding and Transfection
The transfection mixture was added to 6 × 108 cells, mixed well and poured into a HYPERFlask (placed at 37°C overnight).
7.1.2 Sodium Butyrate Induction
Approximately 18-20 hours later, the medium on the transfected cells was changed to D10 containing sodium butyrate and HEPES. After 6–8 hours, the cells were rinsed once with DPBS and then fresh harvesting medium, was added to fill the HYPERFlask.
7.1.3 Vector Harvest
After another ~40 hours, LV-containing medium was decanted from the HYPERFlask, filtered through a 0.8 μm filter and then stored overnight at +4°C. Fresh harvesting medium was then added to refill the HYPERFlask and the cells were incubated at 37°C. After ~24 hours, LV-containing medium was filtered and combine with the first harvest.
7.1.4 Tangential Flow Filtration
Test flow paths for integrity. Concentrate down to 50 mL and diafiltrate in FPI, further concentrate to 1 mL in FPII
7.1.5 Vector Transduction for Titer Determination
Seed 6-well plates with 1×105 HT-29 cells per well in 2 mL D10. After 24 hours, count three wells and add diluted vector to the other wells. 12-16 hours later, an additional 1 mL D10 was added to each well. After 48 hours cells were harvested and their genomic DNA was isolated.
7.1.6 Absolute Quantitation via Probe-Based Real-Time PCR
A standard curve was prepared from samples of HT29 DNA mixed with DNA from a HT29 clone that has 2 copies/cell of a lentiviral vector. Run a multiplex real-time PCR reaction for PSI and SDC4. Plot a standard curve and use this to determine the copy number. The following equation was used to determine titer: Titer (TU/mL) = (cell count at transduction)(copy number)(vector dilution factor).
9. ACKNOWLEDGEMENTS
The authors acknowledge Michael Bransby and Dianne Skelton for invaluable advice and technical assistance during the establishment of the protocol.
10. REFERENCES
- Arumugam PI, Higashimoto T, Urbinati F, Modlich U, Nestheide S, Xia P, Fox C, Corsinotti A, Baum C, Malik P. Genotoxic potential of lineage-specific lentivirus vectors carrying the beta-globin locus control region. Mol. Ther. 2009;17:1929–1937. doi: 10.1038/mt.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejerano G, Pheasant M, Makunin I, Stephen S, Kent WJ, Mattick JS, Haussler D. Ultraconserved elements in the human genome. Science. 2004;304:1321–1325. doi: 10.1126/science.1098119. [DOI] [PubMed] [Google Scholar]
- Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, Vidaud M, Abel U, Dal-Cortivo L, Caccavelli L, Mahlaoui N, Kiermer V, Mittelstaedt D, Bellesme C, Lahlou N, Lefrère F, Blanche S, Audit M, Payen E, Leboulch P, l'Homme B, Bougnères P, von Kalle C, Fischer A, Cavazzana-Calvo M, Aubourg P. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, Down J, Denaro M, Brady T, Westerman K, Cavallesco R, Gillet-Legrand B, Caccavelli L, Sgarra R, Maouche-Chrétien L, Bernaudin F, Girot R, Dorazio R, Mulder GJ, Polack A, Bank A, Soulier J, Larghero J, Kabbara N, Dalle B, Gourmel B, Socie G, Chrétien S, Cartier N, Aubourg P, Fischer A, Cornetta K, Galacteros F, Beuzard Y, Gluckman E, Bushman F, Hacein-Bey-Abina S, Leboulch P. Transfusion independence and HMGA2 activation after gene therapy of human β- thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Preter K, Speleman F, Combaret V, Lunec J, Laureys G, Eussen BHJ, Francotte N, Board J, Pearson ADJ, De Paepe A, Van Roy N, Vandesompele J. Quantification of MYCN, DDX1, and NAG gene copy number in neuroblastoma using a real-time quantitative PCR assay. Modern Pathology. 2002;15:159–166. doi: 10.1038/modpathol.3880508. [DOI] [PubMed] [Google Scholar]
- Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A Third-Generation Lentivirus Vector with a Conditional Packaging System. J. Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follenzi A, Naldini L. Generation of HIV-1 derived lentiviral vectors. Methods Enzymol. 2002;346:454–465. doi: 10.1016/s0076-6879(02)46071-5. [DOI] [PubMed] [Google Scholar]
- Geraerts M, Michiels M, Baekelandt V, Debyser Z, Gijbers R. Upscaling of lentiviral vector production by tangential flow filtration. J. Gene Med. 2005;7:1299–1310. doi: 10.1002/jgm.778. [DOI] [PubMed] [Google Scholar]
- Haas DL, Case SS, Crooks GM, Kohn DB. Critical factors influencing stable transduction of human CD34(+) cells with HIV-1-derived lentiviral vectors. Mol. Ther. 2000;2:71–80. doi: 10.1006/mthe.2000.0094. [DOI] [PubMed] [Google Scholar]
- Haas DL, Lutzko C, Logan AC, Cho GJ, Skelton D, Jin Yu X, Pepper KA, Kohn DB. The Moloney murine leukemia virus repressor binding site represses expression in murine and human hematopoietic stem cells. J. Virol. 2003;77:9439–9450. doi: 10.1128/JVI.77.17.9439-9450.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutner RH, Zhang XY, Reiser J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat. Protoc. 2009;4:495–505. doi: 10.1038/nprot.2009.22. [DOI] [PubMed] [Google Scholar]
- Modlich U, Bohne J, Schmidt M, von Kalle C, Knöss S, Schambach A, Baum C. Cell-culture assays reveal the importance of retroviral vector design for insertional genotoxicity. Blood. 2006;108:2545–2553. doi: 10.1182/blood-2005-08-024976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlich U, Navarro S, Zychlinski D, Maetzig T, Knoess S, Brugman MH, Schambach A, Charrier S, Galy A, Thrasher AJ, Bueren J, Baum C. Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors. Mol. Ther. 2009;17:1919–1928. doi: 10.1038/mt.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In Vivo Gene Delivery and Stable Transduction of Nondividing Cells by a Lentiviral Vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Sommer CA, Stadtfeld M, Murphy GJ, Hochedlinger K, Kotton DN, Mostoslavsky G. Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells. 2009;27:543–549. doi: 10.1634/stemcells.2008-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufferey R, Nagy D, Mandel RJ, Naldini L, Trono D. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 1997;15:871–875. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]