Abstract
Objectives:
To examine the efficacy of an integrative cognitive training program (REHACOP) to improve cognition, clinical symptoms, and functional disability of patients with Parkinson disease (PD).
Methods:
Forty-two patients diagnosed with PD in Hoehn & Yahr stages 1 to 3 were randomly assigned to either the cognitive training group (REHACOP) or the control group (occupational activities) for 3 months (3 sessions, 60 min/wk). Primary outcomes were change on processing speed, verbal memory, visual memory, executive functioning, and theory of mind. Secondary outcomes included changes on neuropsychiatric symptoms, depression, apathy, and functional disability. The trial was registered with clinicaltrials.gov (NCT02118480).
Results:
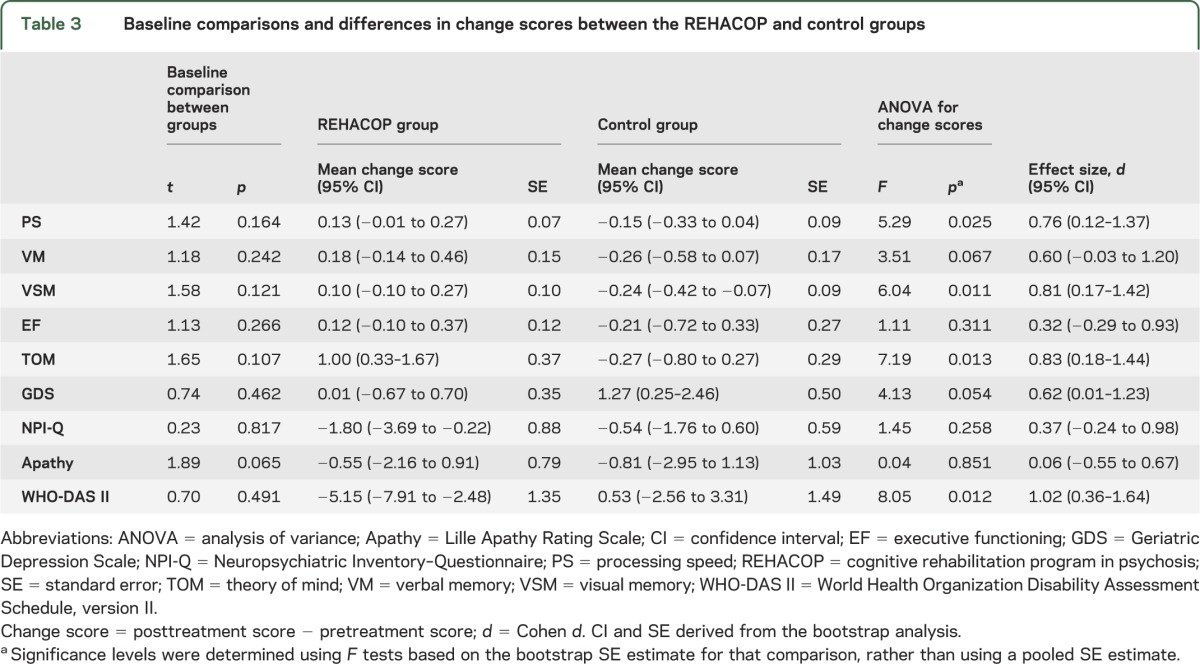
No baseline group differences were found. Bootstrapped analysis of variance results showed significant differences in the mean change scores between the REHACOP group and control group in processing speed (0.13 [SE = 0.07] vs −0.15 [SE = 0.09], p = 0.025), visual memory (0.10 [SE = 0.10] vs −0.24 [SE = 0.09], p = 0.011), theory of mind (1.00 [SE = 0.37] vs −0.27 [SE = 0.29], p = 0.013), and functional disability (−5.15 [SE = 1.35] vs 0.53 [SE = 1.49], p = 0.012).
Conclusions:
Patients with PD receiving cognitive training with REHACOP demonstrated statistically significant and clinically meaningful changes in processing speed, visual memory, theory of mind, and functional disability. Future studies should consider the long-term effect of this type of intervention. These findings support the integration of cognitive training into the standard of care for patients with PD.
Classification of evidence:
This study provides Class II evidence that for patients with PD, an integrative cognitive training program improves processing speed, visual memory, theory of mind, and functional disability.
The presence of cognitive impairment, including processing speed, visual and verbal memory, and executive function in Parkinson disease (PD)1 is currently widely known. Moreover, these deficits have been associated with impairments on the activities of daily living.2 Because of this association, there have been an increasing number of studies supporting nonpharmacologic interventions for PD. A recent systematic review3 concluded that the research in this area is very limited and urged for additional controlled studies. Social cognition has been less studied in PD than other cognitive domains. Previous studies nonetheless indicate that patients with PD exhibit impaired theory of mind (TOM).4
The efficacy of cognitive training has been previously demonstrated in other pathologies, including traumatic brain injury,5 dementia,6 and schizophrenia.7 The REHACOP was developed in this context, incorporating recent suggestions regarding the strategy of learning and transfer techniques.8 Originally created for schizophrenia and after having demonstrated its efficacy in improving core symptoms refractory to pharmacologic treatment9 and functional disability, the authors adapted it for the elderly population.
The primary aim was to evaluate the efficacy of cognitive training (REHACOP) in patients with PD for improving processing speed, visual learning and memory, verbal learning and memory, executive functioning, and TOM. The secondary aim was to analyze whether this program would improve clinical symptoms (depression, apathy, and neuropsychiatric symptoms) and functional disability, as previously noted for patients with schizophrenia.9
METHODS
Participants.
Forty-two outpatients with PD were recruited from the Department of Neurology at Galdakao Hospital and the Parkinson's Disease Association (ASPARBI), both in Biscay, for a collaborative study coordinated by the Department of Methods and Experimental Psychology at the University of Deusto.
A neurologist specialized in movement disorders reached the diagnosis of PD based on the UK PD Society Brain Bank diagnostic criteria for PD.10 Other inclusion criteria were as follows: (1) age 45 to 75 years; (2) either male or female; and (3) Hoehn & Yahr disease stage 1 to 3 as evaluated by the neurologist. Exclusion criteria were as follows: (1) the presence of dementia as defined by the DSM-IV-TR11 and the Movement Disorders Society specific clinical criteria for PD dementia12,13; (2) the presence of other neurologic illness or injury (traumatic brain injury, multiple sclerosis); (3) unstable psychiatric disorders such as schizophrenia or major depression; and (4) the presence of visual hallucinations as assessed by the Neuropsychiatric Inventory–Questionnaire (NPI-Q).14 All patients were symptomatically stable at the time of recruitment. All patients were provided with pharmacologic treatment and were tested while on their medication.
Standard protocol approvals, registrations, and patient consents.
The study protocol was approved by the Ethics Committee at the Health Department of the Basque Mental Health System in Spain. This study was registered at clinicaltrials.gov (registration number NCT02118480). All patients were volunteers who provided written informed consent to participate in the study.
Measures.
Cognitive evaluation.
The cognitive battery included assessments to evaluate processing speed, verbal learning and memory, visual learning and memory, and executive functioning. All cognitive measures were converted into z scores based on the pooled PD group, and the sign of some measures was adjusted so that higher scores indicated better cognitive performance. All composite cognitive domains maintained satisfactory internal consistency. Processing speed (Cronbach α = 0.85) was quantified based on the Trail Making Test–A15 and Salthouse Letter Comparison Test.16 For verbal learning and memory (α = 0.88), learning and long-term recall performance on the Hopkins Verbal Learning Test17 (version 2 at baseline and 4 at posttreatment) were utilized. For visual learning and memory (α = 0.96), learning and long-term recall performance on the Brief Visual Memory Test18 (version 1 at baseline and 3 at posttreatment) were used (α = 0.96). Executive functioning (α = 0.78) was determined based on the Stroop test,19 using the word-color and interference scores.
Theory of mind.
The Happé test20 was administered to evaluate TOM. Four different stories (concerning double bluff, mistakes, persuasion, and white lies) were administered at baseline and follow-up, and they were summed into a total TOM score with a possible range of 0 to 8. Higher scores indicate better TOM.
Premorbid IQ and cognitive reserve.
The Accentuation Reading Test (TAP),21 the Spanish version of the National Adult Reading Test, was included to estimate patients' premorbid IQ. The scale ranged from 0 to 30. Cognitive reserve was estimated using the Cognitive Reserve Questionnaire.22 This 15-item multiple-choice questionnaire includes questions about education/culture, working activity, leisure and hobbies, physical activities, and social activities. Higher scores indicate a better cognitive reserve, and the scale ranged from 0 to 26.
Global cognitive status.
The Mini-Mental State Examination23 was administered to obtain a general mental status score.
Medication use.
Medications, dosages, and dose frequencies were used to calculate the levodopa equivalent daily dose (mg/d).24
PD assessment.
The Unified Parkinson's Disease Rating Scale25 and the Hoehn & Yahr scale were used for the assessment of the course and stage of the disease.
Depressive symptoms.
Geriatric Depression Scale (GDS)26 includes 15 items. Higher scores represent a higher degree of depression (range from 0 to 15).
Neuropsychiatric symptoms.
The NPI-Q14 was administered to evaluate neuropsychiatric symptoms. The test includes 10 items (delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, euphoria, apathy, disinhibition, irritability, and aberrant motor behavior). These subscales were summed into a total score with a possible range of 0 to 120. Higher scores indicate more frequent and severe neuropsychiatric symptoms.
Apathy.
The Lille Apathy Rating Scale27 consists of 33 items, including 9 subscales (everyday productivity, interests, taking initiative, novelty seeking/motivation, emotional responses, concern, social life, and self-awareness). These subscales were summed into a total apathy score with a possible range of −36 to 36. Lower scores indicate less apathy.
Functional disability.
Functional disability was self-administered using the World Health Organization Disability Assessment Schedule II (WHO-DAS II), short version (12 items).28
Study outcome measures.
The primary outcome measures were the change in mean processing speed, verbal memory, visual memory, executive functioning, and TOM total scores from baseline to the end of treatment.
The secondary outcome measures included change in mean NPI-Q, GDS, apathy, and WHO-DAS II scores from baseline to the end of treatment.
Procedure.
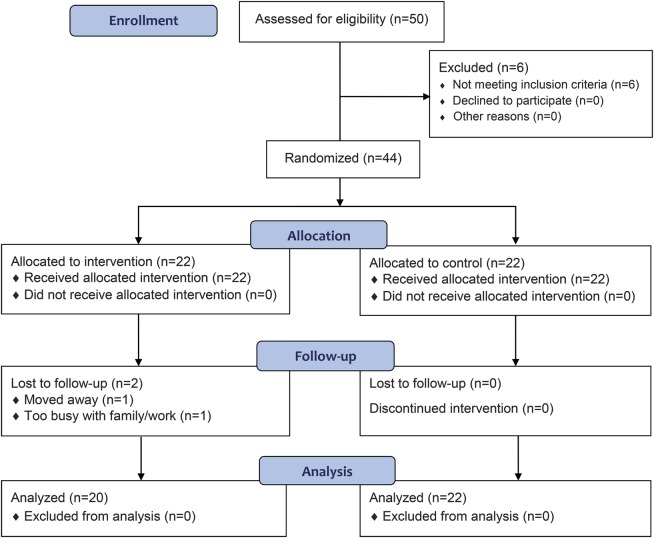
A priori power analyses were conducted to determine the sample size based on a previous study that used REHACOP.9 A sample size of 44 subjects, 22 in each group, was sufficient to attain an effect size of 0.88 to detect a difference in neurocognitive deficits between groups, with 80% power and a 5% level of significance. The study design was a parallel-group randomized trial with equal randomization. Recruitment and enrollment were conducted between June 2012 and January 2013. Patients were offered participation in the study via the neurologist and ASPARBI. Afterward, the participants were randomly allocated to either the REHACOP group or the control group (see figure 1). Assignment to the program was conducted on the basis of a computer-generated randomization of the list of participants. Posttreatment assessment (finished by June 2013) was performed within the first week after completing the intervention. All raters were blinded to treatment condition and had no other role in the project that would undermine the blinding. There was an absence of prior relationship between psychologists and participants. Following ethical aspects, the control group was offered participation in a remediation program when the posttreatment assessment was finished.
Figure 1. CONSORT flow diagram.
CONSORT = Consolidated Standards of Reporting Trials.
Intervention.
REHACOP is a structured program using paper-pencil tasks29 (based on restoration, compensation, and optimization strategies of rehabilitation) with a gradual level of cognitive effort and demand. REHACOP trains different cognitive domains, such as attention, memory, processing speed, language, executive functioning, and social cognition. The program also includes one module for functional outcome: activities of daily living.
REHACOP includes up to 300 different tasks hierarchically organized into at least 3 levels of complexity and subtypes of abilities. Several tasks are timed, so processing speed is trained throughout various modules. The therapist moves forward to the next level of difficulty after a basic cognitive strategy has been well acquired. The program format allows for either individual or group sessions (between 5 and 8 patients per group), although for the purpose of this study, group sessions were chosen.
In this study, 2 psychologists conducted the REHACOP group attending 60-minute-long sessions 3 days per week at ASPARBI (2 groups) or the Hospital of Galdakao (1 group). Both psychologists prepared the sessions together, used the same materials and instructions, and received the same training on REHACOP. Specifically, REHACOP group remediation with patients with PD consisted of the following: attention unit (4 weeks) training sustained, selective, alternant, and divided attention; memory unit (3 weeks) focusing on visual and verbal learning, recall, and recognizing memory; language unit (3 weeks) including grammar, syntax, vocabulary, verbal fluency, verbal comprehension, and abstract language; executive functions unit (2 weeks) training cognitive planning, proverbs, and analogies; and social cognition unit (1 week) exercising TOM, social reasoning, and moral dilemmas.
The control group consisted of occupational group activities conducted by a psychologist at ASPARBI. The activities included drawing, reading the daily news, and constructing using different materials (such as paper or wood). These activities were accomplished in a group format and with the same frequency as the implementation of REHACOP in the experimental group.
Analyses.
Normality of data was tested using the Kolmogorov-Smirnov test. All variables appeared as normal distributions, with the exception of GDS, which was log-transformed for further analyses. Categorical data were analyzed with the χ2 test or Fisher exact test, as indicated. Sociodemographic variables, clinical variables, cognition, and functional disability at baseline were compared using 2-tailed t tests.
Change scores (posttreatment − baseline) were compared between REHACOP and control group on each of the cognitive, clinical, and functional disability variables with an analysis of variance. To obtain adjusted mean differences in change scores, we used bootstrapping,30 a resampling technique in which random subsamples are generated from the observed sample. We generated 1,000 subsamples from within each group (with replacement).
Effect size (Cohen d and 95% confidence interval [CI]) was calculated based on change score differences between groups. The χ2 test was used to compare the percentage of patients in the 2 groups who disclosed a score improvement after the training.
Number needed to treat (NNT) was calculated on the basis of the number of patients who needed treatment in order to show improvement at various levels of change (10%–30%) compared with a patient who did not receive treatment. Absolute risk reduction (ARR) was calculated as the difference between the control group's and REHACOP group's rate of improvement at various levels of change (10%–30%). The significance level was set at 0.05. All tests were 2-tailed.
Classification of evidence.
The primary research question was whether REHACOP could improve performance in processing speed, verbal memory, visual memory, executive functioning, and TOM among patients with early to moderate stages of PD. The secondary research question was whether REHACOP could also alleviate the patients' clinical symptoms and functional disabilities. This study provides Class II evidence that for patients with PD, an integrative cognitive training program improves processing speed, visual memory, social cognition, and functional disability.
RESULTS
Forty-two patients completed the posttest assessment, which reflects an attrition rate of 4.54% (figure 1). The sociodemographic and clinical characteristics of the REHACOP and control groups are provided in table 1. Differences between the groups were analyzed to confirm the success of the randomization. There were no significant differences between the groups in age, sex distribution, years of education, Mini-Mental State Examination score, premorbid IQ, cognitive reserve, illness duration, levodopa equivalent daily dose, Unified Parkinson's Disease Rating Scale score, Hoehn & Yahr stage (see table 1). There were no significant differences at baseline in other clinical characteristics (such as apathy, GDS, or NPI-Q), or functional disability (see table 2) or cognitive performance (see table 3). Based on a matched healthy control group (not reported here), 27.3% of patients in REHACOP showed impairment in processing speed at baseline (vs 27.3% in control group, p = 1), 68.2% in verbal memory (vs 54.5% in control group, p = 0.353), 50% in visual memory (vs 31.8% in control group, p = 0.220), 45.5% in TOM (vs 31.8% in control group, p = 0.354), and 22.7% in executive functioning (vs 13.6% in control group, p = 0.434). Although not significant, the cognitive performance in the control group was consistently better than in the REHACOP group at baseline. Raw scores are shown in table e-1 on the Neurology® Web site at Neurology.org. However, 15.4% of patients in REHACOP and 16.4% of patients in the control group showed depressive symptoms.
Table 1.
Participant characteristics at baseline
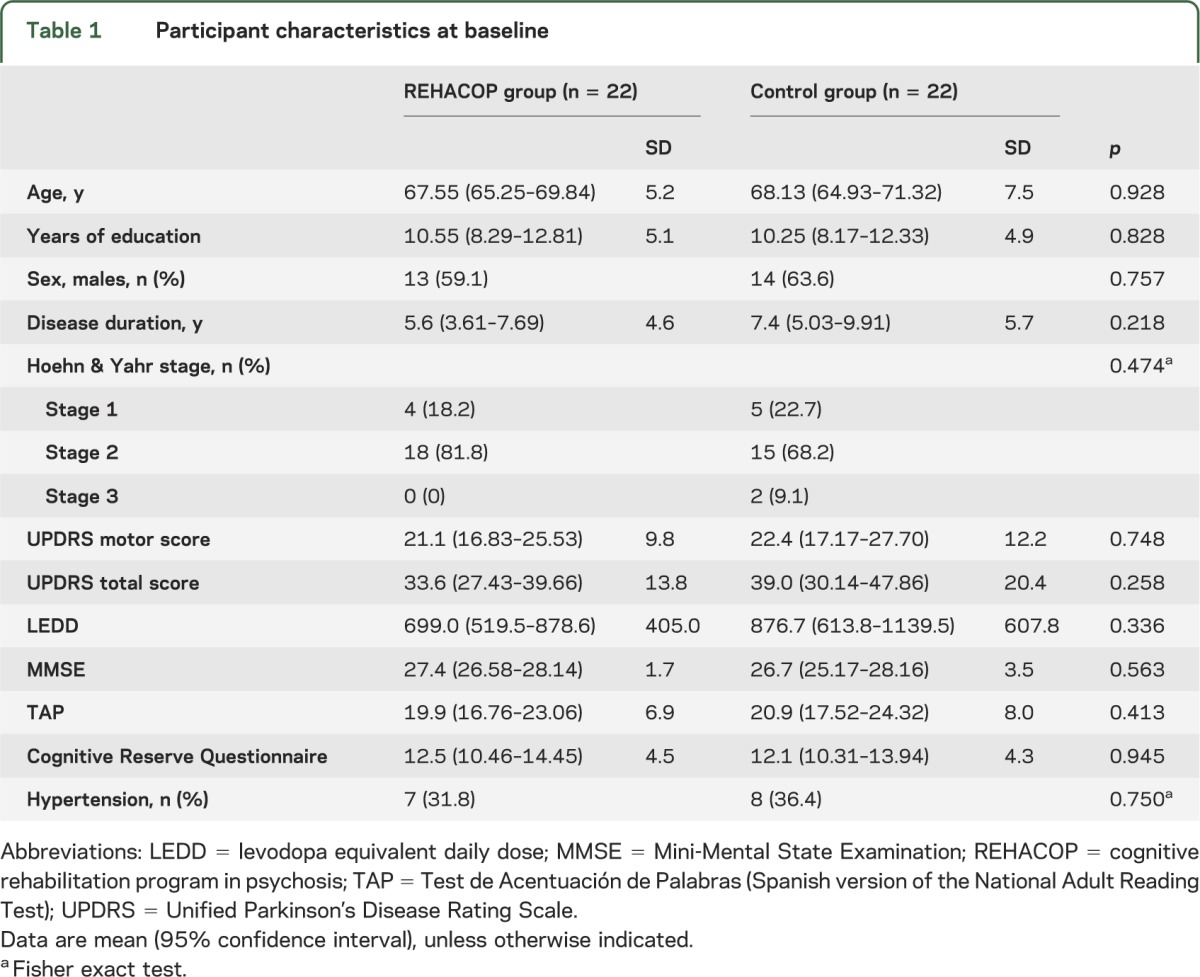
Table 2.
Cognitive performance, clinical characteristics, and functional disability in the REHACOP and control groups at baseline and posttreatment
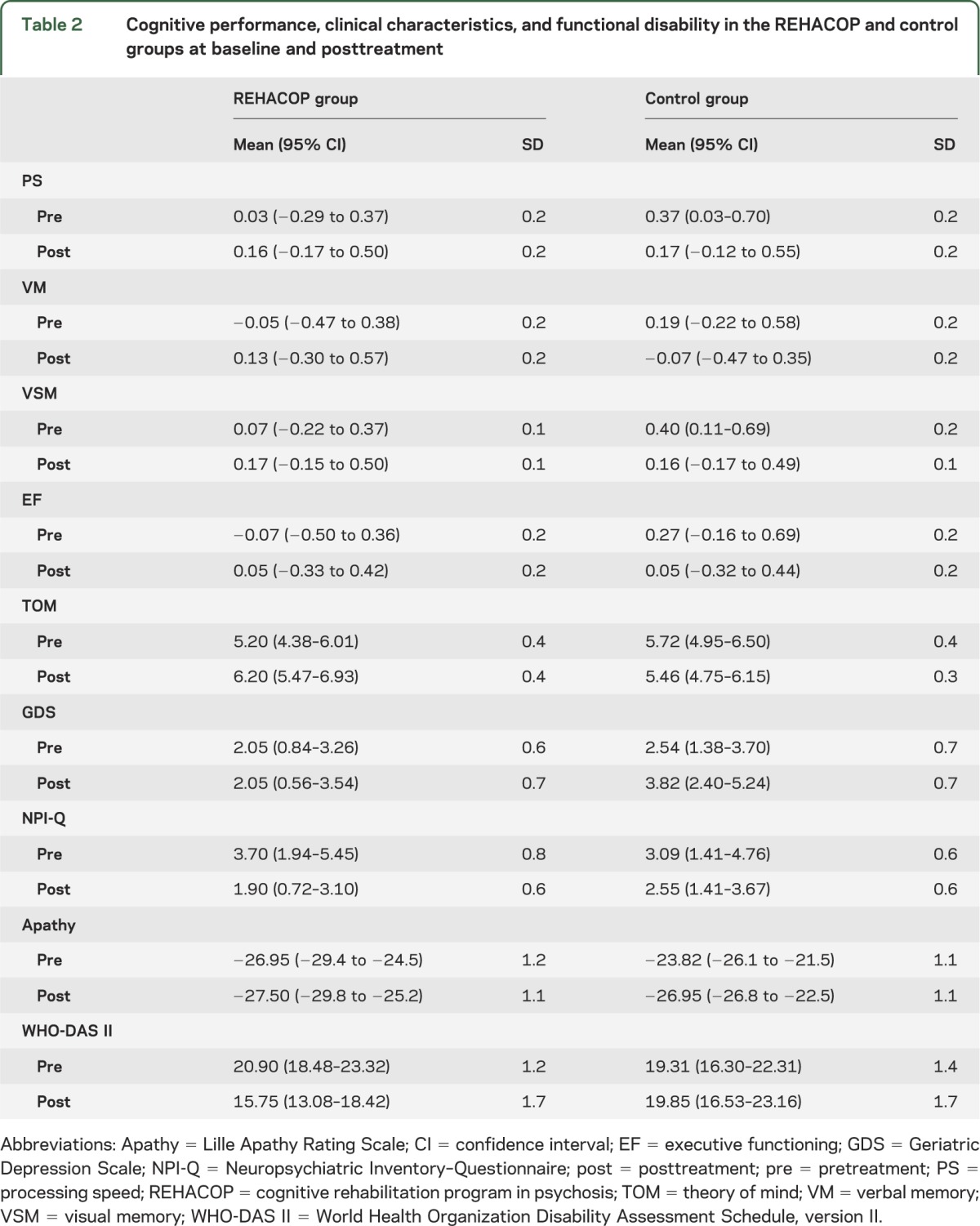
Table 3.
Baseline comparisons and differences in change scores between the REHACOP and control groups
Seven patients from the REHACOP group diminished medication (vs 8 from the control group) and 2 needed to increase the medication (vs 3 from the control group). These differences were not significant (Fisher exact test = 0.39, p = 1).
The bootstrapped F test between the REHACOP and control groups for mean change scores was significant in processing speed, visual learning and memory, TOM, and functional disability, indicating that there is a reliable mean difference between these groups. These results, along with the effect size, are shown in table 3.
Figure e-1 shows the percentage of patients from each group who improved from baseline to posttreatment. There were significant differences in visual memory (χ2 = 4.62, p = 0.032), TOM (χ2 = 6.04, p = 0.014), GDS (χ2 = 6.31, p = 0.012), and WHO-DAS II (χ2 = 7.08, p = 0.006).
NNT (95% CI) and ARR (95% CI) were calculated with WHO-DAS II scores. The NNT was 2.89 (2–5) and ARR was 0.346 (2.2–4.6) for a treatment response of 10%, NNT = 4.48 (2–5) and ARR = 0.423 (0.304–0.526) for a treatment response of 20%, and NNT = 5.78 (−4 to 14) and ARR = 0.173 (0.071–0.273) for a treatment response of 30%.
DISCUSSION
This study provides evidence supporting the efficacy of REHACOP in PD. Significant differences between REHACOP and control groups in change scores were found in processing speed, visual learning and memory, TOM, and functional disability. The effect sizes were large for visual memory, TOM, and functional disability (d = 0.81, 0.83, and 1.02, respectively) and moderate for processing speed (d = 0.76). The improvement cannot be attributed to the effect of treatment duration (given that both groups received the same number of hours) nor to group vs individual interventions, because both formats were alike.
Findings in processing speed and visual memory were consistent with previous literature regarding PD.31−33 A recent study31 trained processing speed and reported significant improvements after cognitive training. In another more comprehensive cognitive training program, the authors32 detected significant improvements in processing speed, memory, visual abilities, semantic fluency, and executive functioning. A recent study33 also noted significant improvements in learning and memory. These general results, summed in a recent review,3 indicate that cognitive training is a promising tool for dealing with cognitive impairment in PD.
However, the lack of significant improvement in executive functions is not consistent with previous studies.34,35 One potential explanation for this discrepancy is that, for the purpose of this study, executive functioning was evaluated based only on the Stroop color-word and interference subtest. However, the intrinsic nature of executive functioning may be better captured by other neuropsychological tools, such as WCST (Wisconsin Card Sorting Test) or BADS (Behavioral Assessment of the Dysexecutive Syndrome).34,36
Our patients also significantly improved in TOM, measured using the Happé test. Previous literature has shown that patients with PD are impaired in social cognitive abilities.4,37,38 According to a recent review of nonpharmacologic treatment of cognitive dysfunction in PD,3 there are no previous studies that have attempted to address the treatment of TOM in PD using cognitive training. Therefore, direct comparisons with previous studies in PD are not possible. However, positive results in studies of other pathologies39,40 along with our results may be helpful to propose that TOM impairment could be improved. Nevertheless, additional studies are needed to replicate and extend our results.
Results regarding depressive symptoms showed marginally significant differences among groups, although these results must be taken with caution. REHACOP group's results were nearly identical from baseline to posttreatment assessment, whereas the control group's results decreased.
Nevertheless, the major finding of the present study was the improvement in functional disability (d = 1.02). A few studies have previously examined changes in functional status after cognitive training in PD,31,34 and none reported significant improvements. One possible explanation for the positive findings in this study may be related to the specific characteristics of REHACOP, which was developed considering factors that may boost the benefits of cognitive training.7,8 Among others, these factors include strategic learning and emphasis on transfer techniques. This finding is strengthened by the detection of similar improvements from the use of REHACOP in other populations.9
The attrition rate in this study was 4.54%. After receiving feedback (recorded in a focus group not yet published) from the patients, their positive comments about the program may explain the high adherence and low attrition rates of the experimental group. Cultural characteristics, such as broad social support, may also explain our result of a lack of attrition in the control group.
However, this study has several limitations. A longitudinal follow-up would show whether the effects of the treatments are maintained in the long term. Because the patients included in this study were in the mild to moderate stages of PD, we cannot generalize the positive results to patients in later stages of PD. The study was single-blinded. At the recruitment, participants were informed that they would receive either cognitive training or treatment with occupational activities, and they were initially blinded to this decision. However, it is possible that some of them guessed they were receiving REHACOP because of the nature of the tasks during the sessions. Moreover, some of the patients from each group were located in the same building. Future studies should also consider analyzing biomarkers of these improvements, including cerebral changes associated with cognitive rehabilitation. Combining cognitive training with other types of treatments may result in deeper and larger changes, which could also be tested in future studies.
Supplementary Material
ACKNOWLEDGMENT
The authors thank ASPARBI and all the patients involved in the study.
GLOSSARY
- ARR
absolute risk reduction
- CI
confidence interval
- DSM-IV-TR
Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition, Text Revision)
- GDS
Geriatric Depression Scale
- NNT
number needed to treat
- NPI-Q
Neuropsychiatric Inventory–Questionnaire
- PD
Parkinson disease
- REHACOP
cognitive rehabilitation program in psychosis
- TOM
theory of mind
- WHO-DAS II
World Health Organization Disability Assessment Schedule, version II
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Naroa Ibarretxe-Bilbao and Dr. Natalia Ojeda contributed to the study design and conceptualization. Statistical analyses and interpretation were performed by Dr. Javier Peña and Dr. Naroa Ibarretxe-Bilbao. Dr. Maria Angeles Gomez-Beldarrain, Dr. García-Gorostiaga, and María Díez-Cirarda contributed to data collection and management. All authors contributed to the writing and revision of the manuscript.
STUDY FUNDING
This study was supported by the Department of Health of the Basque Government (2011111117 to Dr. Naroa Ibarretxe-Bilbao) and the Spanish Ministry of Economy and Competitiveness (PSI2012-32441 to Dr. Naroa Ibarretxe-Bilbao).
DISCLOSURE
J. Peña received funding from the University of Deusto and the Department of Education and Science of the Basque Government (Equipo A), the Department of Health of the Basque Government (2011111117 to N.I.-B.), and the Spanish Ministry of Economy and Competitiveness (PSI2012-32441 to N.I.-B.). N. Ibarretxe-Bilbao received funding from the University of Deusto, the Department of Health of the Basque Government (2011111117), and the Spanish Ministry of Economy and Competitiveness (PSI2012-32441). I. García-Gorostiaga received funding from Osakidetza. M. Gomez-Beldarrain received funding from Osakidetza. M. Díez-Cirarda received a collaborative predoctoral grant from the Basque Government. N. Ojeda received funding from the University of Deusto, Department of Health of the Basque Government (2011111117, Contrato Programa), the Spanish Ministry of Economy and Competitiveness (PSI2012-32441), the Spanish Ministry of Health (PI11/01903), Diputación Foral de Bizkaia (Bizkailab program), and the Department of Education and Science of the Basque Government (Equipo A). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Emre M. Dementia associated with Parkinson's disease. Lancet Neurol 2003;2:229–237. [DOI] [PubMed] [Google Scholar]
- 2.Rosenthal E, Brennan L, Xie S, et al. Association between cognition and function in patients with Parkinson disease with and without dementia. Mov Disord 2010;25:1170–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hindle JV, Petrelli A, Clare L, Kalbe E. Nonpharmacological enhancement of cognitive function in Parkinson's disease: a systematic review. Mov Disord 2013;28:1034–1049. [DOI] [PubMed] [Google Scholar]
- 4.Ibarretxe‐Bilbao N, Junque C, Tolosa E, et al. Neuroanatomical correlates of impaired decision‐making and facial emotion recognition in early Parkinson's disease. Eur J Neurosci 2009;30:1162–1171. [DOI] [PubMed] [Google Scholar]
- 5.van Heugten C, Gregório GW, Wade D. Evidence-based cognitive rehabilitation after acquired brain injury: a systematic review of content of treatment. Neuropsychol Rehabil 2012;22:653–673. [DOI] [PubMed] [Google Scholar]
- 6.Bahar-Fuchs A, Clare L, Woods B. Cognitive training and cognitive rehabilitation for persons with mild to moderate dementia of the Alzheimer's or vascular type: a review. Alzheimers Res Ther 2013;5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wykes T. Cognitive remediation therapy needs funding. Nature 2010;468:165–166. [DOI] [PubMed] [Google Scholar]
- 8.Wykes T, Spaulding WD. Thinking about the future cognitive remediation therapy: what works and could we do better? Schizophr Bull 2011;37(suppl 2):S80–S90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sánchez P, Peña J, Bengoetxea E, et al. Improvements in negative symptoms and functional outcome after a new generation cognitive remediation program: a randomized controlled trial. Schizophr Bull 2013;40:707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniel SE, Lees AJ. Parkinson's Disease Society Brain Bank, London: overview and research. J Neural Transm Suppl 1993;39:165–172. [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 12.Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson's disease dementia: recommendations from the Movement Disorder Society Task Force. Mov Disord 2007;22:2314–2324. [DOI] [PubMed] [Google Scholar]
- 13.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord 2007;22:1689–1707. [DOI] [PubMed] [Google Scholar]
- 14.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. J Neuropsychiatry Clin Neurosci 2000;12:233–239. [DOI] [PubMed] [Google Scholar]
- 15.Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Tucson: Neuropsychology Press; 1985. [Google Scholar]
- 16.Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Dev Psychol 1991;27:763. [Google Scholar]
- 17.Brandt J, Benedict RHB. Hopkins Verbal Learning Test–Revised. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 18.Benedict RH, Schretlen D, Groninger L, Dobraski M, Shpritz B. Revision of the Brief Visuospatial Memory Test: studies of normal performance, reliability, and validity. Psychol Assess 1996;8:145. [Google Scholar]
- 19.Golden CJ. STROOP: Test de Colores y Palabras. Madrid: TEA Ediciones; 2001. [Google Scholar]
- 20.Happé FG. An advanced test of theory of mind: understanding of story characters' thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. J Autism Dev Disord 1994;24:129–154. [DOI] [PubMed] [Google Scholar]
- 21.González Montalvo JI. Creación y validación de un test de lectura para el diagnóstico del deterioro mental en el anciano [dissertation]. Universidad Complutense de Madrid; 1991. [Google Scholar]
- 22.Rami L, Valls-Pedret C, Bartres-Faz D, et al. Cognitive Reserve Questionnaire: scores obtained in a healthy elderly population and in one with Alzheimer's disease [in Spanish]. Rev Neurol 2011;52:195–201. [PubMed] [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 24.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2653. [DOI] [PubMed] [Google Scholar]
- 25.Fahn S, Elton R; UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Health Care Information; 1987:153–163. [Google Scholar]
- 26.Yesavage JA, Brink T, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1983;17:37–49. [DOI] [PubMed] [Google Scholar]
- 27.Sockeel P, Dujardin K, Devos D, Deneve C, Destée A, Defebvre L. The Lille Apathy Rating Scale (LARS), a new instrument for detecting and quantifying apathy: validation in Parkinson's disease. J Neurol Neurosurg Psychiatry 2006;77:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vázquez-Barquero JL, Herrera S, Vásquez E, Gaite I. Cuestionario para la Evaluación de Discapacidad de la Organización Mundial de la Salud: WHO-DAS II (Versión Española Del World Health Organization Disability Assessment Schedule II). Madrid: Ministerio de Trabajo y Asuntos Sociales; 2006. [Google Scholar]
- 29.Ojeda N, Pena J, Bengoetxea E, et al. REHACOP: a cognitive rehabilitation programme in psychosis [in Spanish]. Rev Neurol 2012;54:337–342. [PubMed] [Google Scholar]
- 30.Efron B, Tibshirani R. An Introduction to the Bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 31.París AP, Saleta HG, de la Cruz Crespo Maraver M, et al. Blind randomized controlled study of the efficacy of cognitive training in Parkinson's disease. Mov Disord 2011;26:1251–1258. [DOI] [PubMed] [Google Scholar]
- 32.Edwards JD, Hauser RA, O'Connor ML, Valdés EG, Zesiewicz TA, Uc EY. Randomized trial of cognitive speed of processing training in Parkinson disease. Neurology 2013;81:1284–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naismith SL, Mowszowski L, Diamond K, Lewis SJ. Improving memory in Parkinson's disease: a healthy brain ageing cognitive training program. Mov Disord 2013:28;1097–1103. [DOI] [PubMed] [Google Scholar]
- 34.Sammer G, Reuter I, Hullmann K, Kaps M, Vaitl D. Training of executive functions in Parkinson's disease. J Neurol Sci 2006;248:115–119. [DOI] [PubMed] [Google Scholar]
- 35.Mohlman J, Chazin D, Georgescu B. Feasibility and acceptance of a nonpharmacological cognitive remediation intervention for patients with Parkinson disease. J Geriatr Psychiatry Neurol 2011;24:91–97. [DOI] [PubMed] [Google Scholar]
- 36.Reuter I, Mehnert S, Sammer G, Oechsner M, Engelhardt M. Efficacy of a multimodal cognitive rehabilitation including psychomotor and endurance training in Parkinson's disease. J Aging Res 2012;2012:235765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bodden ME, Mollenhauer B, Trenkwalder C, et al. Affective and cognitive theory of mind in patients with Parkinson's disease. Parkinsonism Relat Disord 2010;16:466–470. [DOI] [PubMed] [Google Scholar]
- 38.Poletti M, Enrici I, Bonuccelli U, Adenzato M. Theory of mind in Parkinson's disease. Behav Brain Res 2011;219:342–350. [DOI] [PubMed] [Google Scholar]
- 39.Horan WP, Kern RS, Shokat-Fadai K, Sergi MJ, Wynn JK, Green MF. Social cognitive skills training in schizophrenia: an initial efficacy study of stabilized outpatients. Schizophr Res 2009;107:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bölte S, Hubl D, Feineis-Matthews S, Prvulovic D, Dierks T, Poustka F. Facial affect recognition training in autism: can we animate the fusiform gyrus? Behav Neurosci 2006;120:211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.