Narcolepsy type 1 (NT1) is characterized by hypersomnolence associated with early occurrence of REM sleep (i.e., sleep-onset REM periods [SOREMP]) and cataplexy (i.e., sudden loss of muscle tone evoked by emotions), or with CSF hypocretin-1 (CSF hcrt-1) low levels (below 110 pg/mL, undetectable in most cases). Compelling evidence indicates that NT1 is caused by an autoimmune disease affecting HLA-DQB1*06:02 carriers.1 Diagnostic workup requires a history of cataplexy and multiple SOREMP at sleep studies (nocturnal polysomnography and multiple sleep latency test [MSLT]) or CSF hcrt-1 deficiency.2 At diagnosis, most patients with NT1 already have very reduced CSF hcrt-1 levels. Hypersomnolence and cataplexy presumably become evident when hypocretin cell loss is above 85%–90%, according to postmortem studies.3 A rapid and dramatically severe picture has been documented often in childhood-onset NT1, but it is also commonly recalled by history taking that the disorder can also slowly progress, and sleepiness may anticipate cataplexy by years. In full-blown NT1 cases, CSF hcrt-1 tends to maintain stable values through the disease course, and in only one case has CSF hcrt-1 been documented by chance before and after development of NT1, showing an abrupt step down from normal to pathologic values when the disease occurs.4 We document in an adult patient the slow onset of CSF hcrt-1 deficiency paralleled by a slow clinical progression to clear-cut NT1.
Case history.
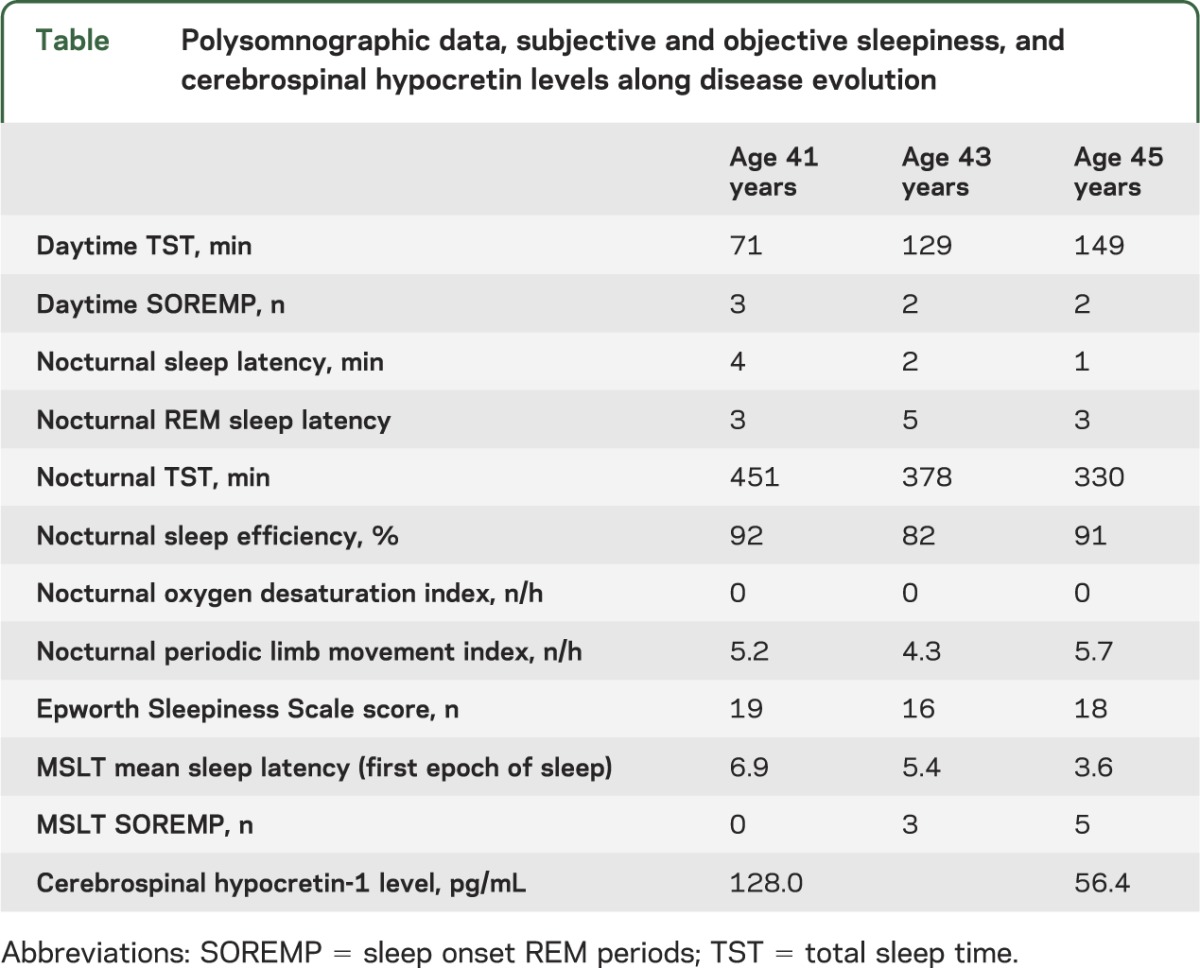
A 45-year-old woman presented with hypersomnolence, daily hypnopompic hallucinations, sleep paralysis, and frequent nightmares since age 39. Despite objective evidence of intermediary CSF hcrt-1 levels (128 pg/mL), and SOREMP at both spontaneous daytime naps and nocturnal sleep during ad libitum 24-hour recording, due to the lack of SOREMP at MSLT the case fit with the diagnosis of idiopathic hypersomnia according to current nosography (table).2 Brain MRI had normal results, and human leukocyte antigen typing was positive for the DQB1*06:02 allele. Modafinil was titrated up to 400 mg with temporary hypersomnolence improvements, despite regular, unchanged, sleep-wake habits. At age 43, hypersomnolence qualitatively changed with appearance of unpredictable daytime sleep attacks together with disruption of nocturnal sleep. Repeated MSLT (after 2 months of drug withdrawal) disclosed a shorter sleep latency with 3 SOREMP (table), pointing to a clear-cut diagnosis of narcolepsy type 2 (NT2).2 Modafinil was re-instated at doses up to 400 mg/day with only partial improvement. At age 45, the patient reported further hypersomnolence worsening and monthly episodes of leg weakness while laughing, consistent with cataplexy. The clinical change was associated with a further increase in the number of SOREMP during the MSLT (n = 5, performed after a 2-week withdrawal period) and decrease in CSF hcrt-1 to 56 pg/mL, levels now diagnostic for NT1. CSF total tau and 14-3-3 protein concentrations were within the normal ranges. Neurologic and neuropsychological examinations remained normal. The study was approved by internal review board and the patient signed a written informed consent.
Table.
Polysomnographic data, subjective and objective sleepiness, and cerebrospinal hypocretin levels along disease evolution
Discussion.
This case is the first prospective documentation of the slow appearance of NT1 symptoms in parallel with decreasing CSF hcrt-1 levels up to definite hcrt-1 deficiency. Slow appearance of NT1 has been reported only retrospectively, and NT2 may be an intermediate stage of partial hypocretinergic cell loss,5 with a CSF hcrt-1 threshold of 200 pg/mL a suggested diagnostic cutoff to identify cases with subsequent evolution in NT1. Postmortem studies of a patient with NT2 carrying the HLA-DQB1*0602 allele also showed a 33% hypocretin cell loss (vs 90%–95% in NT1).6 Moreover, our case confirms that spontaneous SOREMP during daytime recordings could have led to an earlier diagnosis despite negative polysomnography and MSLT studies.7
The fact that the syndrome first manifested with hypersomnolence, with mounting appearance of REM sleep abnormalities and finally cataplexy, is also consistent with the role of hypocretin in diminishing wake (as observed following hypocretin blockers such as suvorexant and almorexant), and then increasing REM sleep at higher doses.
An ambiguous electroclinical picture can herald the full-blown hypocretin deficiency syndrome (NT1), creating diagnostic challenges and contrasting with the dramatic cluster of symptoms in post-H1N1 cases, pinpointing that NT1 may also present with a primary progressive course like other autoimmune diseases. Indeed, cases with clinical features contrasting with neurophysiologic findings (e.g., frequently reported REM-related symptoms without SOREMP at the MSLT) should undergo repeated and in-depth evaluations over time. Finally, although we cannot rule out a neurodegenerative disease, suggested by a slow progression of symptoms, the normal concentration of CSF tau protein indicates a very targeted neurodegeneration, if any. Slow evolution cases, if diagnosed early, may benefit from immunomodulatory treatments to avoid destruction of the hypocretinergic system.1
Footnotes
Author contributions: F. Pizza: study design, data collection, manuscript drafting. S. Vandi: data collection, manuscript revising. R. Liguori: data collection, manuscript revising. P. Parchi: data collection, manuscript revising. P. Avoni: data collection, manuscript revising. E. Mignot: manuscript revising. G. Plazzi: study design, manuscript revising.
Study funding: No targeted funding reported.
Disclosure: F. Pizza, S. Vandi, R. Liguori, P. Parchi, P. Avoni, and E. Mignot report no disclosures relevant to the manuscript. G. Plazzi received honoraria from serving on the scientific advisory board for UCB Pharma and Jazz Pharmaceuticals. Go to Neurology.org for full disclosures.
References
- 1.Partinen M, Kornum BR, Plazzi G, Jennum P, Julkunen I, Vaarala O. Narcolepsy as an autoimmune disease: the role of H1N1 infection and vaccination. Lancet Neurol 2014;13:600–613. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 3.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron 2000;27:469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savvidou A, Knudsen S, Olsson-Engman M, Gammeltoft S, Jennum P, Palm L. Hypocretin deficiency develops during onset of human narcolepsy with cataplexy. Sleep 2013;36:147–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andlauer O, Moore H, IV, Hong SC, et al. Predictors of hypocretin (orexin) deficiency in narcolepsy without cataplexy. Sleep 2012;35:1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thannickal TC, Nienhuis R, Siegel JM. Localized loss of hypocretin (orexin) cells in narcolepsy without cataplexy. Sleep 2009;32:993–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pizza F, Moghadam KK, Vandi S, et al. Daytime continuous polysomnography predicts MSLT results in hypersomnias of central origin. J Sleep Res 2013;22:32–40. [DOI] [PubMed] [Google Scholar]


