Abstract
Since the discovery of C60 fullerene in 1985, scientists have been searching for biomedical applications of this most fascinating of molecules. The unique photophysical and photochemical properties of C60 suggested that the molecule would function well as a photosensitizer in photodynamic therapy (PDT). PDT uses the combination of non-toxic dyes and harmless visible light to produce reactive oxygen species that kill unwanted cells. However the extreme insolubility and hydrophobicity of pristine C60, mandated that the cage be functionalized with chemical groups that provided water solubility and biological targeting ability. It has been found that cationic quaternary ammonium groups provide both these features, and this review covers work on the use of cationic fullerenes to mediate destruction of cancer cells and pathogenic microorganisms in vitro and describes the treatment of tumors and microbial infections in mouse models. The design, synthesis, and use of simple pyrrolidinium salts, more complex decacationic chains, and light-harvesting antennae that can be attached to C60, C70 and C84 cages are covered. In the case of bacterial wound infections mice can be saved from certain death by fullerene-mediated PDT.
Keywords: Fullerene, Photodynamic Therapy, Reactive Oxygen Species, Cancer, Infections, Electron Transfer, Singlet Oxygen, Cationic Charge
INTRODUCTION
Photodynamic therapy (PDT) is based on the administration of nontoxic light absorbing dyes called photosensitizers (PS), either systemically, locally or topically, followed by irradiation of harmless visible to near infrared (NIR) light, in the presents of oxygen, leading to the generation of cytotoxic reactive oxygen species (ROS).1 Dual selectivity of PDT can be obtained by the therapeutic gradient of photosensitizer concentration between tumor and normal tissues and precise delivery of light exposure within the tumor.2 However, the major limitations of PDT are non-specific cellular uptake of PS by normal cells and short light penetation depth. PDT is a widely recognized valuable treatment option for neoplastic and non-malignant diseases. Recently with the development of fiber-optic systems, light can be delivered accurately into many parts of the body for the treatment of tumors. Therefore, PDT applications have been expended to many endoscopically accessible tumors, including lung cancer, superficial gastric cancer, head and neck cancer, cervical cancer and bladder cancer. In general, there are a number of advantages for PDT over chemotherapy and radiotherapy: PDT shows no long-term side effects when an effective PS is employed; it is a minimally-invasive procedure with little or no scarring after the site heals as compared to surgery; it can deliver highly targeted precision at the disease site; there can be repeatable treatments at the same site if needed; PDT can be less costly than other cancer treatments; it may take only a short period of time for each session allowing treatment as an outpatient. The main limitation of PDT is its action occurs only at areas where light can reach. This implies that the major sites of tumors treated by PDT are the lining of organs or just under the skin that can be reached by the light source.
TRADITIONAL PHOTOSENSITIZERS
Photosensitizers can be categorized by their chemical structures as porphyrins (tertrapyrroles) and nonporphyrins. 3 First, second or third generation PS are terms further used to label tetrapyrrole-derived PS. Porphyrins are derived from the tetrapyrrole aromatic macrocycle which is a major component of many naturally occuring pigments such as heme, chlorophyll and bacteriochlorophyll. Porphyrins contain a structure with a 22 π-electron system which gives rise to their long wavelenth absorption of light.4 Hematoporphyrin (Hp), hematoporphyrin devivative (HpD) and Photofrin are referred to as first generation PS. HpD and Photofrin have been widely used in clinical for cancer.5 However, the side effects associated with 1st generation PS, such as prolonged skin photosensitiztion and suboptimal tissue penetation of the 630 nm light have stimulated interst in development of new PS. Second generation of PS are chemically purified or synthetic tetrapyrrole derivatives, which absorb longer wavelength light and cause less skin photosensitization. Some promising second generation photosensitizers have been approved or tested in clinical trials.6 These include, but are not limited to, palladium-bacteriopheophorbide (TOOKAD),7 meso-tetra-hydroxyphenylchlorin (Foscan®, Temoporfin),8 tin-ethyletiopurpurin (SnET2, Purlytin), Visudyne® (verteporfin, benzoporphyrin derivative monoacid ring A, BPD-MA; Novartis Pharmaceuticals), NPe6 (mono-L-aspartyl chlorin e6, taporfin sodium, talaporfin, LS11; Light Science Corporation), Levulan® (5-aminolevulinic acid, a precursor of protoporphyrin IX),9 and phthalocyanines (Pc4).10 The term “third generation PS” refers to the 2nd generation PS bound to carriers such as antibody conjugates11 liposomes and other targeted structures for increasing the selectively for tumor tissues. Although the majority of PS are porphyrin derivatives, non-porphyrin PS are being employed to improve PDT efficacy and minimal side effects. Synthetic, non-naturally-ocurring, conjugated or expanded pyrrolic ring systems are another class of non-porphyrin PS, including texaphyrins, porphycenes, phthalocyanines and naphthalocyanines. Other compounds that have been studied as PS are not derived from the tetrapyrrole backbone, but can be classed as miscellaneous dyes including chalcogenopyrylium dyes,12 phenothiazinium dyes including methylene blue and toluidine blue,13 Nile blue derivatives,14 hydroxylated perylenequinones such as hypericin,15 BODIPY derivatives,16 squaraines17 etc. These compounds have provided a new focus to the field of PDT.
It is generally accepted that the characteristics that the ideal PS used against cancer should possess are:
single compound with known composition and good stability,
preferential uptake and retain in the target tumour tissue,
minimal toxicity in the absence of light to prevent harmful side-effect to the surrounding normal tissue,
high quantum yield of triplet state and ROS generation and
high molar extinction coefficient to minimize the dose of PS needed to achieve the desired PDT effect,
intrinsic fluorescence to permit their detection by optical imaging (microscopy) techniques, and
high absorbance, particularly in the red part of the spectrum, which leads to a deeper light penetration into the tissues; for instance, the light depth penetration at 500 nm is about 4 mm, whereas at the 600–800 nm range it is about 8 mm2.
Photophysics and Photochemistry of PDT
Most PS have 2 electrons with opposite spins located in an energetically lower energy orbital, the so-called highest occupied molecular orbital (HOMO), in their ground (usually single) state. Absorption of light (photons) leads to an excited singlet state by the elevation of one electron with unchanged spin to a higher energy obital, called the lowest unoccupied molecular obital (LUMO).18 This excited singlet PS is short-lived (nanoseconds) and emits excess energy as fluorescence and/or heat. Alternatively, an excited PS may undergo an intersystem crossing (ISC) by inverting spin of one electron to form a relatively long-lived (microseconds to milliseconds) triplet state.
The excited triplet PS can either decay radiationlessly to the ground state or can survive long enough to transfer its energy to molecular oxygen O2 (ground triplet state) and produce excited state singlet oxygen (1O2). This reaction is referred to as a Type II process. A Type I process can also occur whereby the PS reacts directly with neighboring molecules and gains or donates an electron to form a radical. Subsequent electron donation from the PS radical anion to oxygen produces the superoxide anion radical ( ). Therefore, the efficient ISC process giving a high quantum yield of triplet state is essential for the generation of ROS. Strong absorbtion of light, high triplet state quantum yield (effective ISC) and a long-lived triplet excited state are required to be an ideal PS. However, there has not yet been established a clear relationship between the efficiency of ISC and the chemical structure.
FULLERENES AS PHOTOSENSITIZERS
Besides occupying an important place in biomedicine, nanoparticles have also been shown to have potential to act as a photosensitizing drug in PDT. Fullerenes are considered to have advantages as potential PS on the basis of certain favorable PDT characteristics when compared to conventional PS with the tetrapyrrole structure.19 The fullerenes are known for their photostability, a prerequisite to behave as an effective PS, and they undergo relatively less photobleaching than the tetrapyrroles. They can also be modified to get the desired the degree lipophilicity by chemical functionalization.20 There is the option of attaching the light-harvesting antennae to the fullerenes to increase the quantum yield of production of ROS. Fullerenes can self-assemble into vesicles called fullerosomes that can act as multivalent drug delivery vehicles with the possibility of different targeting properties.21
Fullerenes have been said to offer “a wide open playing field to chemists”22 by providing synthetic opportunities to attach a wide variety of hydrophilic or amphiphilic side chains or fused-ring structures to the spherical C60 core.22 Furthermore, fullerenes have hollow interiors, where other atoms, ions or small clusters can be entrapped and form endohedral fullerenes. Those fullerenes that encapsulate metal atoms are called endohedral metallofullerenes.23 Hydroxylation (attaching OH-groups) is the most common functionalization, which renders the molecule more hydrophilic,24 but other polar adducts will also have the same effect.25
There are also several unfavorable characteristics of fullerenes for PDT, but by applying different strategies they can be overcome. The main absorption of fullerenes occurs in the UV, blue and green regions of the spectrum while the absorption of tetrapyrrole PS (such as chlorins, bacteriochlorins, and phthalocyanines) shows substantial peaks in the red or far-red regions where the penetration of light into tissue is much deeper. This major disadvantage of fullerenes however can be overcome in several ways, for example by chemical attachment of one or more red-wavelength absorbing antennae onto a C60 cage.26 The absorption of many light photons simultaneously can be achieved by the attachment of multiple light-harvesting antennae on one C60 cage. The unfavorable absorption spectrum of fullerenes can also be overcome by using optical clearing agents to the tissue27–30 or by using two-photon excitation where two NIR photons are simultaneously delivered in a very short pulse to be equivalent to one photon of twice the energy and shorter wavelength.31–35
Photophysics and Photochemistry of Fullerenes and Derivatives
Fullerenes with attached side chains, called “functionalized fullerenes,” are known to demonstrate high efficiency in the formation of singlet oxygen, hydroxyl radicals and superoxide anions, which are considered as effective mediators of PDT. The photophysics of fullerenes is quite favorable for PDT. The absorption spectra of a typical set of mono-substituted, bis-substituted and tris-substituted fullerenes show almost monotonic decay between 300 and 700 nm.20 When C60 is irradiated with visible light, it is excited from the S0 ground state to a short-lived (<1.3 ns) S1 excited state. The S1 state quickly decays to triplet state. The triplet yield is 1 and the lifetime is as long as 50–100 μs. By energy transfer mechanism (Type II) there is the generation of singlet oxygen (1O2) by quenching of fullerenyl T1 state in the presence of dissolved molecular oxygen. At 532 nm excitation the singlet oxygen quantum yield (ΦΔ) for this process is close to theoretical maximum, 1.0.36 Calculations in 1986 by Haddon et al. had indicated that the lowest unoccupied molecular orbital (LUMO) of C60 would be low-lying, triply degenerate, and, hence, capable of accepting up to 6 electrons.37 The pristine C60 itself alone is not a good PS due to the very weak absorption of visible light and water insolubility. However, C60 is an ideal spin converter due to its efficient inherent ISC and a low S1 state energy level (about 1.72 eV). When a visible-light-harvesting antenna is attached onto C60 to produce C60-dyads, it turns out to be a potentially ideal PS.38 Figure 1 shows a schematic outline centered upon a Jablonski diagram of PDT mediated by fullerene and its derivatives.
Figure 1.
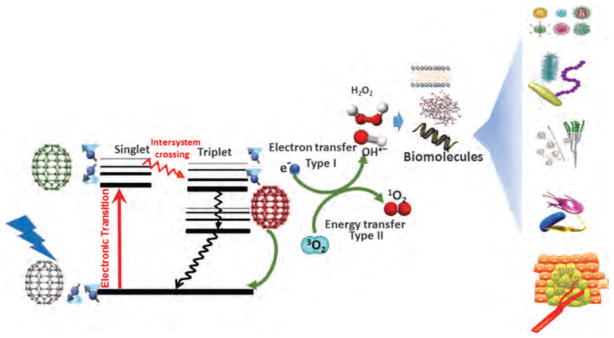
Schematic of PDT mediated by fullerenes.
Both pristine and functionalized fullerenes have the potential to produce ROS after illumination.39 The ROS produced by fullerene derivatives during illumination are inclined towards Type I photochemical products (superoxide radical, hydroxyl radical, lipid hydroperoxides, and hydrogen peroxide), compared to the standard Type II ROS (singlet oxygen) for most PS. During this process they are able accept electrons very efficiently as many as six to each C60 cage.40,41 It is thought that the reduced fullerene triplet or radical anion can transfer an electron to molecular oxygen, forming the superoxide anion radical.42 One apparent incongruity that arises in this area needs to be addressed. It is a well-known fact that fullerenes can act as antioxidants, and that C60 and derivatives can act as scavengers of ROS in the absence of light. One clue that might explain this inconsistency was proposed in 2009, by Andrievsky et al.43 who showed that the major mechanism by which hydrated C60 can inactivate the highly reactive ROS, hydroxyl radical, not by covalently scavenging the radicals but rather by action of the coat of “ordered water” that was linked with the fullerene nanoparticle.44 One of the explanations is that hydroxyl radicals can be slowed down or trapped for a sufficient time allowing the two radicals to react with each other, which produce the comparatively less-reactive ROS, hydrogen peroxide. However, the mechanism may be significantly different with more water-compatible C60 derivatives.45
DESIGN OF FULLERENE DERIVATIVES
One of the concerns that have been raised about the use of fullerenes concerns their biodegradability, as nanostructures have the possibility of accumulation in the body during blood circulation or in the environment after use.46 Though it has been shown that pristine C60 is nontoxic its insolubility and its pronounced tendency to aggregate decreases its potential to be useful in biomedicine. Many strategies have been demonstrated or applied to either solubilize or modify fullerenes for improving their utility in drug-delivery and medical applications. The following approaches: liposomes;47–49 micelles;50,51 dendrimers;52,53 PEGylation;53–56 self-nanoemulsifying systems (SNES);57–60 encapsulation in cyclodextrins.47, 61, 62 have all been explored by various groups throughout the world to overcome this problem with fullerenes.
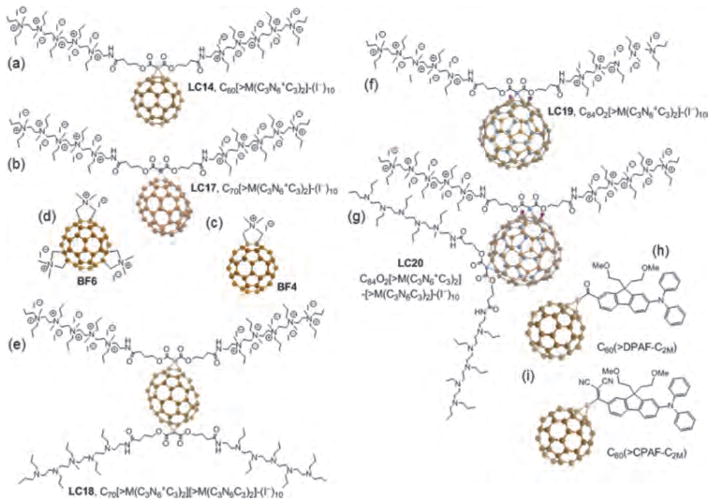
It is known that cationic functional groups provide good solubility for molecules of diverse structures and also have the potential to bind to the anionic residues present on cancer cells and also on the bacterial cell wall via static charge interactions. For these reasons cationic groups can be considered a good choice for attachment on the fullerene cage. A number of chemical functionalization techniques for derivatizing fullerenes have been evaluated.63, 64 Among them, general suitable methods for the preparation of cationic fullerene derivatives include cyclopropanation65 e.g., (LC14, Fig. 2(a))66, 67 and (LC17, Fig. 2(b))68 and pyrrolidination e.g., quaternized dimethylpyrrolidinium fullerenyl monoadduct (BF4,20 Fig. 2(c)) and trisadduct (BF6,69 Fig. 2(d)) and structures such as LC22, , and LC24, .70, 71
Figure 2.
The chemical structure of fullerene derivatives applied in PDT studies.
The cyclopropanation reaction of C60 functionalization was recently applied to attach a highly complex decacationic moiety to the fullerene cage leading to the formation of fullerenes bearing ten positive charges. The decacationic functional moieties of C60, C70, and C84O2 fullerenes were designed to increase both the water-solubility and provide surface binding interactions with −D-Ala-D-Ala residues of the bacteria cell wall by incorporating multiple H-bonding interactions and positive quaternary ammonium charge to bind to anionic lipopolysaccharides and lipoteichoic acids.70 The structure included two esters and two amide moieties to give a sufficient number of carbonyl and −NH groups in a short length of ~20 Å to provide effective multi-binding sites with the presence of a well-defined water-soluble pentacationic moiety at each side of the arm. A similar reaction sequence with a malonate precursor arm M(C3N6C3)2 was also employed in the preparation of (LC18, Fig. 2(e)), (LC19, Fig. 2(f)), (LC20, Fig. 2(g)).67
To circumvent the shortcoming of weak absorption of light at visible wavelengths, a variety of highly fluorescent donor chromophore antennae have been covalently attached to the fullerene, such as porphyrins.72 Fullerene-porphyrin hybrids are more efficient in terms of singlet oxygen generation and also have improved cell penetration. Dialkyldiphenylaminofluorene (DPAF-C2M, shown in Fig. 2(h)) is also a light-harvesting donor chromophore antenna that can be attached to the C60 cage to facilitate ultrafast intramolecular energy- and electron-transfer from the donor antenna to C60 and can therefore be used to enhance PDT efficacy.73 DPAF-C2M was constructed to have increased optical absorption at 400 nm and also possessed good two-photon absorption (2PA) cross-sections in the NIR wavelengths. Later another set derivatives C60(>CPAF-C2M) (Fig. 2(i)) was formed by structural modification via chemical conversion of the keto group in C60(>DPAF-C2M) to a stronger electron-withdrawing 1,1-dicyanoethylenyl (DCE) unit. This structural modification induced a large bathochromic shift of ground-state absorption of CPAF-C2M moieties beyond 450–550 nm and an increased electronic polarization of the molecule. The modification also led to a large bathochromic shift of the major band in visible spectrum giving measureable absorption up to 600 nm and extended the photoresponsive capability of C60–DCE–DPAF nanostructures to longer red wavelengths than C60(>DPAF-C2M).
It was reported that the majority of the HOMO electron density was delocalized over the dialkyldiphenylaminofluorene (DPAF-Cn) moiety, whereas the LUMO electron density was located on the C60 spheroid.74 Therefore charge-separated states may be generated by intramolecular electron-transfer between the diphenylaminofluorene donor and C60 > acceptor moieties during the photoexcitation process.
Intramolecular formation of transient charge-separated states is crucial for generation of radical ROS, initially with and subsequently HO•. is the most stable charge-separated state in polar solvents, including H2O.74 In nonpolar solvents, the intramolecular energy-transfer event that produces transient state dominates with nearly 6-fold higher generation of singlet O2 compared to .
Examples of the Synthesis of Monocationic and Polycationic Fullerene Derivative
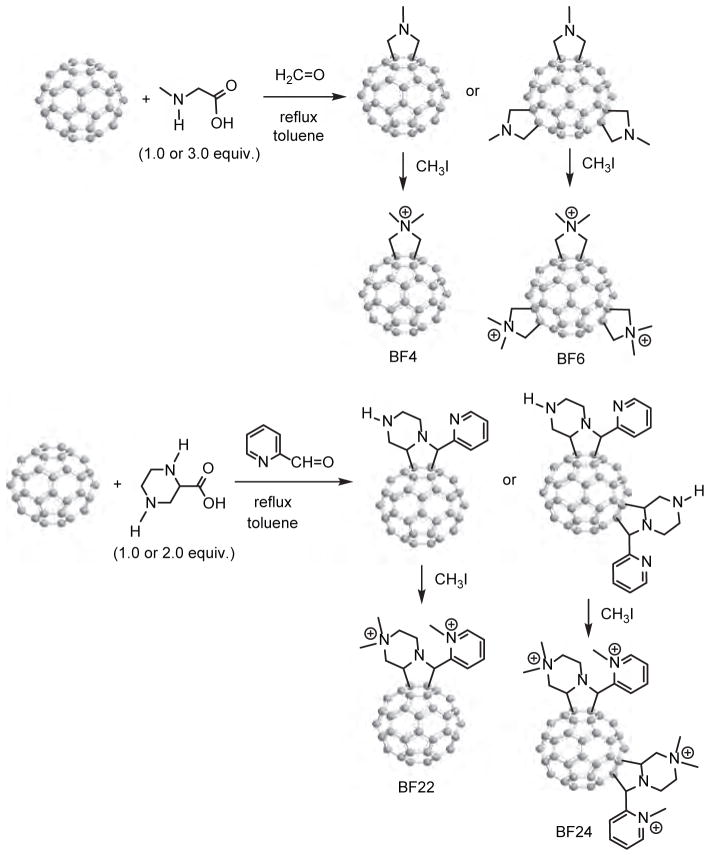
As mentioned above cationic functional groups are generally considered as the addend of choice for attachment on the fullerene cage due to their potential surface binding contact with anionic residues of the bacteria cell wall via static charge interactions. A systematic trend to increase the number of positive charges per fullerene cage was described in a recent report75 to maximize such interactions and use them as the approach for targeting bacteria having a significant density of anionic residues at the cell wall surface. A number of chemical functionalization methods of fullerenes have been reviewed.64, 76–78 Among them, common convenient methods for the preparation of cationic fullerene derivatives include cyclopropanation79 and pyrrolidination80 reactions due to their high consistency allowing product reproducibility. Examples of the latter were given in the preparation of quaternized dimethylpyrrolidinium [60] fullerenyl monoadduct (BF4) and trisadduct (BF6).81, 82 In a typical reaction condition, C60 was treated with 1.0 or 3.0 equivalent of N-methylglycine (sarcosine) and paraformalaldehyde in toluene at the reflexing temperature to afford either mono-N-methylpyrrolidino[60] fullerene (BF4) or a large number of regioisomers of tris(N-methylpyrrolidino)[60] fullerene (BF6) derivatives, as shown in Figure 3. Upon quaternization of these intermediates using methyl iodide as the methylation agent, corresponding monocationic and tricationic products as BF4 and BF6 were obtained, respectively.
Figure 3.
Synthesis of monocationic and tricationic dimethylpyrrolidinium [60]fullerenes BF4 and BF6, respectively, and mono- and bis(piperazinopyrrolidinium) [60]fullerenes BF22 and BF24, respectively.
Similarly, the reaction of C60 in toluene with either 1.0 or 2.0 equivalent of azomethine ylide produced by piperazine-2-carboxylic acid dihydrochloride dissolved in methanol and triethylamine in the presence of 4-pyridinecarboxaldehyde at the refluxing temperature gave the corresponding mono-piperazinopyrrolidino[60] fullerene or a number of regioisomers of bis(piperazinopyrrolidino)[60] fullerene derivatives. Quaternization of both intermediates with methyl iodide led to the corresponding monocationic and dicationic products as BF22 and BF24 (Fig. 3), respectively.
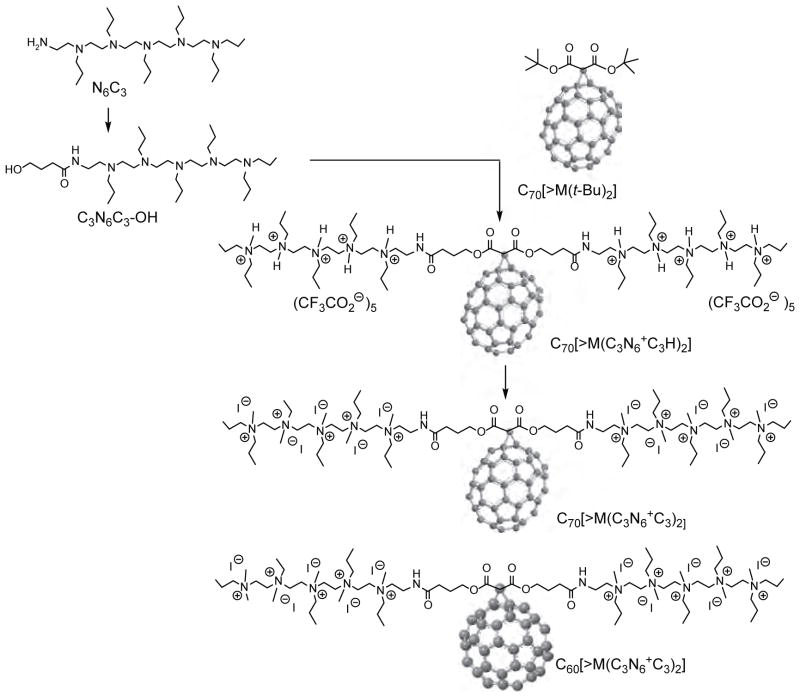
In the case of cyclopropanation reaction of C60 as the functionalization method, it was applied recently for the attachment of a highly complex decacationic moiety to the fullerene cage leading to the formation of and .83 In this reaction, a malonate precursor arm was applied to include two esters and two amide moieties, for a sufficient number of carbonyl and −NH groups in a short length of ~20 Å, with a well-defined water-soluble pentacationic moiety at each side of the arm for making effective multi-binding sites to the cell wall. The precursor was reported to be a common synthon for the structural modification of PDT nanomedicines. It was derived from the quaternization of N,N′,N,N,N,N-hexapropyl-hexa(aminoethyl)amine precursor N6C3. The best method for the preparation of and was depicted in Figure 4 to begin with a well-defined fullerene monoadduct derivatives, such as di(tert-butyl)fullerenyl malonates C60[>M(t-Bu)2] and C70[>M(t-Bu)2], respectively, followed by facile transesterification reaction with the well-characterized tertiary-amine precursor arm moiety, 4-hydroxy-[N,N′,N,N,N,N-hexapropyl-hexa(aminoethyl) butanamide (C3N6C3-OH) using trifluoroacetic acid as the catalytic reagent to afford protonated quaternary ammonium trifluoroacetate salt . Conversion of this salt to , was accompanied by neutralization of trifluoroacetic acid by sodium carbonate and subsequent quaternization by methyl iodide to give decacationic quaternary ammonium iodide salts. A similar conversion procedure was applied for the case of . This synthesis represented the first examples of decacationic fullerene monoadducts to incorporate a well-defined high number of cation without the use of multiple addend attachments to preserve the intrinsic photophysical properties of fullerene cages.
Figure 4.
Synthetic scheme for the preparation of and .
Synthesis of Hexa-Anionic Fullerene Derivatives
Fullerene molecules are highly hydrophobic. Pristine C60 can be dispersed into aqueous medium in a micelle form with the application of surfactants. However, the micelle structure may not be stable enough in biological environment. In a recent report, a strategy of creating a micelle formed from surfactants covalently bonded directly onto the fullerene cage was illustrated by the synthesis of hexa(sulfo-n-butyl)-C60 (FC4S) leading to structurally stable molecular micelles in H2O.84 The synthesis involved the use of hexaanionic chemistry85 for attaching six sulfo-n-butyl arms on C60 in one-pot reaction, as shown in Figure 5.
Figure 5.
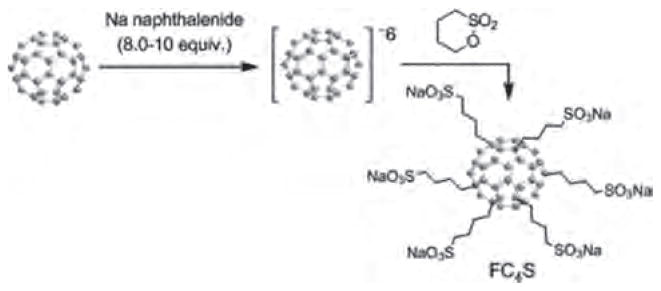
Synthesis of hexaanionic hexa(sulfo-n-butyl)-C60 (FC4S).
It was found that molecular self-assemblies of FC4S resulted in the formation of nearly monodisperse spheroidal nanospheres with the sphere radius of gyration Rg ≈19 Å, where the major axe ≈ 29 Å and the minor axe ≈21 Å for the ellipsoid-like aggregates, or an estimated long sphere diameter of 60 Å [the radius = (5/3)1/2Rg] for the aggregates, as determined by small angle neutron scattering (SANS) in D2O and small angle X-ray scattering (SAXS) in H2O.86 This radius of gyration was found to remain relatively constant over a concentration range from 0.35 to 26 mM in H2O, revealed strong hydrophobic interaction between core fullerene cages overcoming loose charge repulsion at the surface of the molecular micelle. It allowed the nanosphere formation at a low concentration despite of steric hindrance and high hydrophilicity arising from of 6 sulfo-n-butyl arms surrounding C60. Based on the SANS data, the mean number of FC4S molecules for the nanosphere was determined to be 6.5± 0.7 that led to the elucidation of its nanocluster structure with each FC4S molecule located at the vertex of an octahedron shaped nanosphere shown in Figure 6.85
Figure 6.
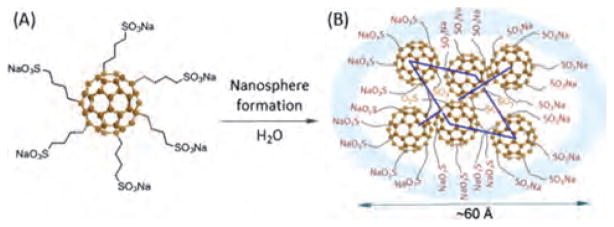
(A) Structure of hexa(sulfo-n-butyl) [60]fullerene (FC4S) and (B) a characterized FC4S-derived nanosphere formed in H2O discussed by Yu.85
Synthesis of Chromophore-Linked Fullerene Derivatives
Optical absorption of C60 is strong in UVA and weak in most of visible range. To circumvent this shortcoming, a reported approach was described to use the light-harvesting donor chromophore antenna attachment at a very close vicinity of C60 cage, within a contact distance of 2.6–3.5 Å, to facilitate ultrafast intramolecular energy- and electron-transfer from the donor antenna to C60 for enhancing PDT efficacy.73 A specific donor antenna, namely, dialkyldiphenylaminofluorene DPAF-Cn was first introduced to give an increased optical absorption at 400 nm and later being modified by replacing the keto moiety of DPAF-Cn via a highly electron-withdrawing 1,1-dicyanoethylenyl (DCE) bridging group that resulted in dark burgundy-red C60(>CPAF-Cn) derivatives. This structural modification was found to induce a large bathochromic shift of ground-state absorption of CPAF-Cn moieties beyond 450–550 nm.
Preparation of C60(>CPAF-C2M), as an example, was made by Friedel-Craft acylation of 9,9-dimethoxyethyl-2-diphenylaminofluorene with bromoacetyl bromide in the presence of AlCl3 to yield 7-bromoactyl-9,9-dimethoxyethyl-2-diphenylaminofluorene, followed by cyclopropanation reaction with C60, as shown in Figure 7. The resulting product C60(>DPAF-C2M) was then further treated with malononitrile and pyridine in the presence of titanium tetrachloride in dry toluene to yield C60(>CPAF-C2M) after chromatographic purification.
Figure 7.
Synthetic method of C60(>CPAF-C2M) discussed by Chiang.73
Photochemistry and Photophysics of Fullerenyl Molecular Micelles and Chromophore-Fullerene Conjugates
Photoexcitation of C60 and fullerene derivatives induces a singlet fullerenyl excited state that is transformed to the corresponding triplet excited state, via intersystem energy crossing, with nearly quantitative efficiency.41 Subsequent energy transfer from the triplet fullerene derivatives to molecular oxygen produces singlet molecular oxygen in aerobic media. This photocatalytic effect becomes one of key mechanisms in photodynamic treatments using fullerene derivatives as photosensitizers. However, a high degree of functionalizaton on C60 for the enhancement of solubility and compatibility in biomedia resulted in a progressive decrease of the singlet oxygen production quantum yield [Φ(1O2)]. Examples were given by Bingel-type malonic acid, C60[C(COOH)2]n, and malonic ester, C60[C(COOEt)2]n, [60] fullerene adducts,87 showing a decreasing trend of Φ(1O2) as the number of addends (n) increases. When the number n reached 6 for a hexaadduct, its Φ (1O2) value declined to only 13% or less of that for C60.88 However, it was not the case for molecular micellar FC4S, a relatively high singlet oxygen production quantum yield for FC4S may indicate its unique electronic features in difference with Bingel-type malonic hexaadducts of C60.85 The efficiency was substantiated by direct detection of 1O2 emission at 1270 nm upon photoirradiation of self-assembled FC4S nanospheres at 500–600 nm.
In the cases of light-harvesting electron-donor chromophore assisted fullerene conjugate systems, such as C60(>CPAF-Cn) derivatives, their photophysical properties involve the primary photoexcitation events of either the fullerene moiety at UV wavelengths or the DPAF-Cn moiety at both UV and visible wavelengths up to 600 nm.73 Much higher optical absorption capability of DPAF-Cn than the C60> cage in visible wavelengths enables the former moiety to serve as a light-harvesting antenna. Accordingly, formation of the photoexcited 1(DPAF)*-Cn moiety should be considered as the early event in the photophysical process. Alteration of the keto group of C60(>DPAF-Cn) to the 1,1-dicyanoethylenyl group of C60(>CPAF-Cn) effectively extended its photoresponsive region to longer red wavelengths. Photoexcitation processes of C60(>DPAF-Cn) and C60(>CPAF-Cn) pump an electron from their highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO). By the molecular orbital calculation and energy minimization, the majority of the HOMO electron density was reported to be delocalized over the dialkyldiphenylaminofluorene (DPAF-Cn) moiety, whereas the LUMO electron density was located on the C60 spheroid, and therefore was suggested as the most stable charge-separated (CS) state in polar solvents, including H2O.74 These charge-separated states may be generated by photoinduced intramolecular electron-transfer between the diphenylaminofluorene donor and C60> acceptor moieties. The process effectively quenches fluorenyl fluorescence that can be observed in the most of C60(>DPAF-Cn) and C60(>CPAF-Cn) monoadducts. Even during energy-transfer events of C60(>CPAF-Cn), normally favorable in non-polar solvents, observed short fluorescence lifetime of the model compound 1CPAF*-C9 (241 ps) as compared with that of the keto analogous Br-1DPAF*-C9 (2125 ps) may be indicative of a facile photoinduced intramolecular charge polarization process forming the corresponding [C==C(CN)2]−•–DPAF+•-C9 charge-separated state that will facilitate the formation of in the subsequent electron-transfer event.
ANTICANCER EFFECT OF FULLERENE-PDT
There have been various studies demonstrating fullerene induced in-vitro phototoxicity in cells. It is considered that one condition for any PS to produce cell killing after illumination, is that the PS should really be taken up inside the cell, as the production of ROS outside the cell will not be enough to produce cell death unless it is produced in extremely large amounts. One of the limitations for the study of the uptake of fullerenes into cells is their non-fluorescent nature that limits the use of fluorescence microscopy to study the localization in cells. Some strategies though have been adopted to overcome this limitation, such as the use of radiolabeled fullerene that has been prepared to study the uptake. Indirect immunofluorescence staining with antibodies has been used to show the localization of fullerene in mitochondria and other intracellular membranes.89 Recently energy-filtered transmission electron microscopy and electron tomography was used to visualize the cellular uptake of pristine C60 nanoparticulate clusters in the plasma membrane, lysosomes and in the nucleus of cells.90
In Vitro Anti-Cancer PDT with Fullerenes
The first report of phototoxicity in cancer cells mediated by fullerenes was in the year 1993. In this study Tokuyama et al.91 used carboxylic acid functionalized fullerenes at 6.0 μM and white light to produce growth inhibition in cancer cells. Burlaka et al.92 used pristine C60 at 10 μM with visible light from a mercury lamp to produce some phototoxicity in Ehrlich carcinoma cells or in rat thymocytes. The cytotoxic and photocytotoxic effects of two water-soluble fullerene derivatives, a dendritic C60 monoadduct and the malonic acid C60 trisadduct were tested on Jurkat cells when irradiated with UVA or UVB light.93 The cell death was mainly caused by membrane damage and it was UV dose-dependent.
New approaches have been tested to overcome the requirement to utilize UV or short-wavelength visible light to activate fullerenes. In one study where two new fullerene-bis-pyropheophorbide-a derivatives were prepared: a mono-(FP1) and a hexaadduct (FHP1). The C60 hexaadduct FHP1 had a significant phototoxic activity (58% cell death, after a dose of 400 mJ/cm2 of 688 nm light) but the monoadduct FP1 had a very low phototoxicity and only at higher light doses.94 Nevertheless the activity of both adducts was less than that of pure pyropheophorbide-a, possibly due to the lower cellular uptake of the adducts.95
The hypothesis that fullerenes have the potential to destroy cancer cells by PDT was tested in our group. We have shown that the C60 molecule mono-substituted with a single pyrrolidinium group (BF4 shown in Fig. 2(c)) is an efficient PS and can mediate killing of a panel of mouse cancer cells.20 The cells lines used were lung cancer (LLC) and colon cancer (CT26) adenocarcinoma and reticulum cell sarcoma (J774) and the latter showed much higher susceptibility to fullerene mediated phototoxicity possibly due to having an increased uptake because J774 cells behave like macrophages. Besides the exceptionally active BF4, the next group of compounds has only moderate activity (BF2, BF5, and BF6 shown in Fig. 2(d)) against J774 cells showing only some killing even at high fluences, while last two compounds (BF1 and BF3) had no detectable PDT killing up to 80 J/cm2. The indirect measurement of fullerenes uptake was demonstrated by increase in fluorescence of an intracellular probe (H2DCFDA) which is specific for the formation of ROS. We also showed the initiation of apoptosis by PDT mediated by BF4 and BF6 in CT26 cells at 4–6 h after illumination. The induction of apoptosis was rapid after illumination which may perhaps suggest that fullerenes are localized in mitochondria, as it has been previously shown with benzoporphyrin derivative.96–98 The explanation for the mono-pyrrolidinium substituted fullerene as most effective PS most likely linked to its relative hydrophobicity as established by its log P value of over 2. Besides this a single cationic charge on BF4 is in addition expected to play a significant role in determining its relative phototoxicity.
Elisa Milanesio et al.97 used tetrapyrrole-fullerene conjugates and evaluated PDT effect with a porphyrin-C60 dyad (P-C60) and its metal complex with Zn(II) (ZnP-C60) and compared with 5-(4-acetamidophenyl)-10,15,20-tris(4-methoxyphenyl)porphyrin (P) on Hep-2 human larynx carcinoma cell line. The phototoxicity was dependent on light exposure level with visible light. 80% phototoxicity was observed for P-C60 after 15 min of light irradiation which was higher as compared to ZnP-C60. In case of argon atmosphere also a high photoactivity was observed with both the dyads. In another paper,99 the cell death was confirmed to occur by apoptotic mode.
As fullerenes show a relatively slower uptake we incubated the cells for 24h with (LC14) and (LC17) (shown in Figs. 2(a) and (b)). killed cells more effectively than , On the contrary, the fullerene drug LC14 killed less than 1 log at all fluencies. LC17 that was short of the decatertiary amine chain was less phototoxic than LC18 which possessed an extra deca-tertiary ethyleneamine chain. This exciting result prompted us to carry out studies with new PDT compounds (LC19) and (LC20)67 (shown in Figs. 2(f) and (g)). Different wavelengths were used for irradiation. UVA and blue light caused more killing with LC20 than with LC19. This difference can be attributed to better chance of electron transfer process occurring with shorter wavelengths and also the presence of the electron donating tertiary-polyethyleneamine chain. While when white light was employed the variation between LC20 and LC19 was smaller but LC20 still gave extra killing, while green light gave equivalent killing for the two fullerenes. The situation was upturned and LC19 gave considerably more killing than LC20 when red light was used. It is important to state that the compounds used here induced a very low dark toxicity.
In Vivo Photodynamic Therapy of Cancer
The three prerequisites for the fullerene PS to have photodynamic effect on tumors are first of all it should accumulate in the tumor tissue; secondly there should be a practically efficient way to administer the compound to tumor bearing animals; and thirdly a practical way to deliver excitation light to the tumors.100 The first challenge in this direction was taken up by Tabata56 in 1997. To make the water-insoluble pristine C60 water soluble and enlarge its molecular size they chemically modified it with polyethylene glycol. This conjugate was injected intravenously into mice carrying a subcutaneous tumor on the back. C60-PEG conjugate demonstrated higher accumulation and relatively more prolonged retention in the tumor tissue than in normal tissue. On performing PDT after intravenous injection of C60-PEG conjugate or Photofrin to tumor-bearing mice, coupled with exposure of the tumor site to visible light, the volume increase of the tumor mass was suppressed and the C60_PEG conjugate exhibited a stronger suppressive effect than Photofrin. Tumor necrosis was observed without any damage to the overlying normal skin. The antitumor effect of the conjugate showed an increase with increasing fluence delivered and C60 dose, and cures were achieved by treatment with a low dose of 424 μg/kg at a fluence of 107 J/cm2. In another study Liu and others55 conjugated polyethylene glycol (PEG) to C60 (C60-PEG), and diethylenetriaminepentaacetic acid (DTPA) was subsequently introduced to the terminal group of PEG to prepare C60-PEG-DTPA that was mixed with gadolinium acetate solution to obtain Gd3+-chelated C60-PEG-DTPA-Gd. PDT induced anti-tumor effect and MRI tumor imaging was evaluated on intravenous injection of C60-PEG-DTPA-Gd into the tumor bearing mice. Equivalent generation of superoxide upon illumination was observed with or without Gd3+ chelation. Intravenous injection of C60-PEG-DTPA-Gd into tumor bearing mice plus light (400~500 nm, 53.5 J/cm2) demonstrated significant anti-tumor PDT depending on the timing of light irradiation that also correlated with tumor accumulation as detected by the enhanced MRI signal.
Yu and her coworkers101 performed a preliminary in vivo study of PDT using hydrophilic nanospheres formed from hexa(sulfo-n-butyl)-C60(FC4S, shown in Fig. 3(A)). This study was performed on ICR mice bearing sarcoma 180 subcutaneous tumors. No adverse effects were noted in the animals when the FC4S was administered orally. Water-soluble FC4S in PBS (5 mg/kg body weight) was given either intraperitoneally or intravenously with subsequent irradiation with an argon ion laser beam at a wavelength of 515 nm or an argon-pumped dye-laser at 633 nm. The beam was focused to a diameter of 7–8 mm with the total light dose of 100 J/cm2. Inhibition of tumor growth was found more effective using the low wavelength i.e., in case of better-absorbed 515nm laser than the 633 nm laser. I ·p. administration method proved to be slightly better in inhibition effectiveness than the i.v. method. Conclusively data suggest that PDT with fullerenes is not only possible in animal tumor models, but can demonstrate the potential use of these compounds as PS for PDT of cancer.
We have also recently shown102 the therapeutic effects of intraperitoneal PDT with fullerene and white light in a very challenging mouse model of disseminated abdominal cancer. In this study we prepared the monocationic BF4 (Fig. 2(c)) in micelles composed of Cremophor EL. Colon adenocarcinoma cell line (CT26) expressing firefly luciferase was used to allow monitoring of IP tumor burden by non-invasive biolumi8nescence imaging. BF4 in micelles was injected intraperitonally (5 mg/kg) followed by white-light illumination (100 J/cm2) delivered through the peritoneal wall. This produced a statistically significant reduction in bioluminescence and besides this produced a survival advantage in mice, shown in Figure 8. A drug-light interval of 24 h was more effective than a 3 h drug-light interval showing the significance of allowing enough time for the fullerene to be taken up into the cancer cells.
Figure 8.
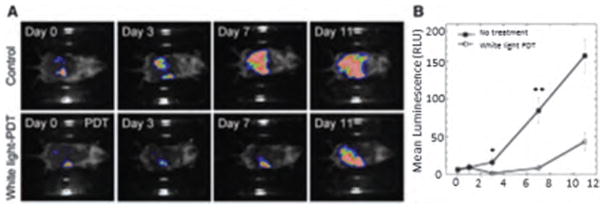
(A) Bioluminescence imaging of CT26-Luc tumors growing in a representative control mouse (upper panel) and a representative IPPDT treated mouse (lower panel). (B) Quantitative analysis of bioluminescence dynamics in control and white light treated mice (n = 10 per group). Reprinted with permission from [102], P. Mroz, et al., Intraperitoneal photodynamic therapy mediated by a fullerene in a mouse model of abdominal dissemination of colon adenocarcinoma. Nanomedicine 7, 965 (2011).
© 2011, Future Science.
As the cancer cells are known to express more glucose receptors, Otake et al. exploited this fact and synthesized group of C60-glucose conjugates which also proved to be more soluble. These conjugates demonstrated selective phototoxicity compared to fibroblast cells thus suggesting the significance of targeting glucose receptors.103 The PDT effect in vivo was investigated in human-melanoma (COLO679)-xenograft bearing mice by injecting C60-(Glc)1 (0.1 or 0.2 mg/tumor) intratumorally followed by irradiated with 10 J/cm2 UVA 1ight. The drug-light interval was 4 h. Tumor growth was suppressed better with the higher dose than the lower dose.
ANTIMICROBIAL EFFECT OF FULLERENES
Antibiotic resistance is a worldwide problem that is spreading with remarkable speed. The injudicious and over-use of antibiotics is the most important reason leading to antibiotic resistance around the world.
The “golden age” of antimicrobial therapy began with the discovery of antibiotics around the middle of the last century.104 Meanwhile many other ancient effective antibacterial treatments including photosensitizing reactions were forgotten. In the last few decades, however, the widespread use of antimicrobial agents emerges the increase of antibiotic-resistant bacteria and other infectious microorganisms and led to predictions of untreatable infections caused by “superbugs,”105 which in turn has created an ever-increasing need for new drugs.
Therefore antimicrobial PDT has become an emerging alternative strategies for destroying microorganisms especially for multi-drug resistant pathogens.106 PDT produces ROS that are toxic to the target microorganisms. PDT has a broad spectrum of action, and compared to antibiotic treatment PDT does not lead to the selection of mutant resistant strains.
Currently, topical application of a PS on infected tissues and subsequent illumination seems to be the most prominent feature of antimicrobial PDT, without damaging the surrounding tissue or disturbing the residual bacterial-flora. It was well accepted that Gram-positive bacteria are more susceptible to PDT as compared to Gram-negative bacteria. This can be explained by the different structures of their cell walls.107
There are several possible mechanisms to explain the antimicrobial activity of illuminated fullerene PS: by interfering with cell wall synthesis; plasma membrane integrity; nucleic acid synthesis; ribosomal function and folate synthesis. All of these would result in disruption of the bacterial cell function and inhibit their growth.
Martin and Logsdon hypothesized that it was possible that microorganisms were susceptible to damage by to Type I ROS when compared with Type II singlet oxygen.108 As mentioned before fullerenes can gain solubility63 and produce more hydroxyl radicals and superoxide anion, as well as singlet oxygen through functionalization109 by attaching some hydrophilic or amphiphilic functional groups.22
An ideal PS proposed for antimicrobial PDT can be judged on several criteria. These PS should have no toxicity in the dark and should selectively kill bacteria over mammalian cells. PS should be able to kill multiple classes of microorganisms at relatively low concentrations with low fluences of light. PS should ideally have high absorption around 600 nm to 800 nm and generate high triplet and singlet oxygen quantum yields.
Antimicrobial Effects In Vitro
The structures, especially the charges of attached groups on fullerene influence the efficacy of PS on killing microorganisms. Our lab has shown, in a series of reported experiments that cationic fullerenes fulfill many, but not all of the aforementioned criteria. At the first time we demonstrated that the soluble functionalized fullerenes were efficient antimicrobial PS and could mediate selective photodynamic inactivation (PDI) for various classes of microbial cells over mammalian cells.75 We compared the antimicrobial activity of broad-spectrum antimicrobial photodynamic activities of two series of functionalized C60; a first series with one, two, or three polar diserinol groups, and a second series with one, two, or three quarternary pyrrolidinium groups. Gram-positive bacteria (S. aureus) Gram-negative bacteria (E. coli and P. aeruginosa), and fungal yeast (C. albicans) were tested in this study. The neutral, alcohol-functionalized fullerenes had only modest activity against S. aureus, while the cationic pyrrolidinium-functionalized fullerene (BF6, Fig. 2(d)) was surprisingly effective in causing light-mediated killing of S. aureus at 1 μM, and 10 μM for E. coli, C. albicans and P. aeruginosa. However, the pyrrolidinium- functionalized fullerenes compound BF6 demonstrated high levels of dark toxicity against S. aureus, Mashino et al. showed that cationic fullerenes could inhibit the growth of E. coli and S. aureus by interfering with the respiratory chain.110, 111 This data suggests that photoactivated fullerenes may have somewhat different sites of action in bacteria compared to more traditional PS such as tetrapyrroles that generate singlet oxygen. These compounds all performed significantly better than a widely used antimicrobial photosensitizer, toluidine blue O.
In agreement with previous discussion, results from Spesia et al.112 indicated that a dicationic fullerene derivative was an interesting PS with potential applications in PDI of bacteria. They compared the PDI efficacy of fullerene derivatices with different numbers of cationic charges. The spectroscopic and photodynamic properties of a dicationic N,N-dimethyl-2-(40-N,N,N-trimethyl-aminophenyl) fulleropyrrolidinium iodide) ( ) were compared with a non-charged N-methyl-2-(4′-acetamidophenyl) fulleropyrrolidine (MAC60) and a monocationic N,N-dimethyl-2-(4′-acetamidophenyl)fulleropyrrolidinium iodide ( ) in different media and in a typical Gram-negative bacterium, E. coli. PDI of E. coli cellular suspensions by dicationic fullerene exhibits a ~3.5 log decrease of cell survival after 30 min of irradiation, which represents about 99.97% of cellular inactivation.
To determine the optimal chemical structures produced by fullerene derivatization, a QSAR relationship study employed fullerene PS with a wider range of different hydrophobicities, as well as with an increased number of cationic charges.113 The results indicated that increasing the number of cationic charges and lowering the hydrophobicity tended to increases the antimicrobial PDI efficacy of fullerene PS against both Gram-positive and Gram-negative bacteria. The charge increases the association of the PS with negatively charged pathogen membranes, whereas the hydrophobic character increases association with or penetration into the lipid components of the membrane, or both. Recently, Mizuno et al.82 from our laboratory emphasized the importance of the number of cationic charges in influencing the efficiency of the fullerenes in antimicrobial PDI when they looked at a further series of functionalized cationic fullerenes PS. They compared PDI efficacy of a new group of synthetic fullerene derivatives that possessed either basic or quaternary amino groups as antimicrobial PS against S. aureus (Gram-positive), E. coli (Gram-negative) bacteria and C. albicans (fungi). QSAR derived with Log P and hydrophilic lipophilic balance parameters showed that much better correlations were obtained when 3× the number of cationic charges were subtracted from the Log P values. The most effective ones to perform antimicrobial PDT were tetracationic compound BF21 that had more cationic charges and a lower log P. S. aureus was most susceptible; E. coli was intermediate, while C. albicans was the most resistant species tested.
Antimicrobial effect of two highly water-soluble decacationic fullerenes LC14 ( ) was and LC17 ( ) were applied for comparison in the PDT-killing of the Gram-positive S. aureus.66 The decacationic arms attached to these fullerenes affiliated the rapid binding to the anionic residues of bacterial cell walls. The large number of ionic groups dramatically enhanced water solubility of these compounds. The data showed interesting differences between the photoactivity of decacationic fullerene compounds that differ only in the number of carbon atoms in the fullerene cage. For Gram-positive bacteria was better at photokilling than , while for Gram-negative bacteria and for cancer cells the opposite was the case, in that was better at photokilling than . The results of ROS (HO• or 1O2) generation demonstrated that produced more 1O2 while produced more HO•. This finding offers an explanation of the preferential killing of Gram-positive bacteria by LC14 and the preferential killing of Gram-negative bacteria by LC17. This finding is in agreement with our previous report that Type II ROS, i.e., singlet oxygen, 1O2, are better at killing Gram-positive bacteria than Type I ROS, i.e., hydroxyl radicals, HO•, while the reverse is true for Gram-negative bacteria (HO• is better at killing than 1O2). The hypothesis is that 1O2 can diffuse more easily into porous cell walls of Gram-positive bacteria to reach sensitive sites, while the less permeable Gram-negative bacterial cell wall needs the more reactive HO• to cause real damage.114, 115
Antimicrobial Effect In Vivo
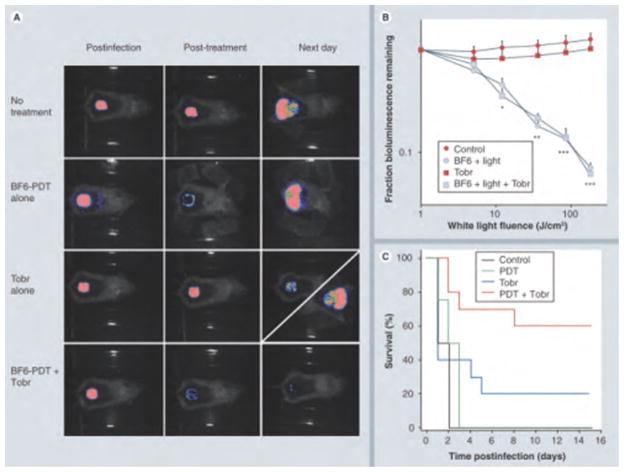
The absorption spectrum of fullerenes is, in addition to substantial UV absorption, mainly in the blue and green visible wavelengths. This property actually limits the application of fullerene in clinical disorders, once the penetration of short wavelength light into tissue is relatively poor; however, fullerenes may still be useful as antimicrobial PS for the treatment of relatively superficial infections, where the light does not need to penetrate deeper than 1 mm. A fullerene-based PS (BF6) with tricationic charges provided by quaternized dimethylpyrrolidinium groups was found to be an effective against Gram-positive bacteria, Gram-negative bacteria and fungal yeast in vitro.75 To investigate if the high degree of in vitro activity could translate into an in vivo antibacterial PDT effect, our lab81 continued to test BF6 in two potentially lethal mouse models of wounds infected with two Gram-negative bacteria (P. aeruginosa and P. mirabilis), respectively. Compared to Gram-positive bacteria, many Gram-negative bacteria are much more difficult to be photo-inactivated, and tend to produce systemic sepsis after developing infections in wounds. Higher concentrations of PS and higher fluences of light (180 J/cm2) were needed in vivo than in vitro to achieve a certain loss of bioluminescence. The fullerene-mediated PDT succeeded in saving the life of mice whose wounds were infected with P. mirabilis and could be combined with a suboptimal dose of antibiotics to save mice with P. aeruginosa wound infections. These exciting results shown in Figure 9 indicated that fullerene-mediated PDT could either treat wounds infected with virulent species of Gram-negative bacteria or be able to synergize with a suboptimal antibiotic regimen to prevent regrowth and produce significantly higher survival.81
Figure 9.
BF6-PDT and tobramycin treatment of Pseudomona aeruginosa wound-infected mice. (A) Representative bioluminescence images of P. aeruginosa-infected mice (captured immediately postinfection, immediately post-treatment and 24 h post-treatment), receiving: no treatment (top row); treated with BF6-PDT alone (180 J/cm2; second row); treated with Tobr alone (6 mg/kg for 1 day; third row, diagonal panel 24 h post-treatment shows two possible outcomes); and treated with a combination of BF6-PDT and 1 day Tobr (bottom row). (B) Quantification of luminescence values from bioluminescence images (not shown) obtained during the PDT process, or at equivalent times for non-PDT mice. *p < 0.05; **p < 0.01; ***p < 0.001; BF6 plus light (with and without Tobr) versus BF6 in dark and versus Tobr alone. (C) Kaplan–Meier survival curves for the groups of mice in Figure 4(A); no treatment control (n = 10); PDT alone (n = 12); Tobr alone (n = 2); PDT plus Tobr (n = 10). PDT: Photodynamic therapy; Tobr: Tobramycin. Reprinted with permission from [81], Z. Lu, et al., Photodynamic therapy with a cationic functionalized fullerene rescues mice from fatal wound infections. Nanomedicine (Lond.), 5, 1525 (2010).
© 2010, Future Science.
In the case of the 3rd-degree burns, they are particularly susceptible to bacterial infection as the barrier function of the skin is destroyed, the dead tissue is devoid of host-defense elements, and a systemic immune suppression is a worrying consequence of serious burns. Furthermore, the lack of perfusion of the burned tissue means that systemic antibiotics are generally ineffective.116 Although excision and skin grafting is now standard treatment for the 3rd-degree burns,117 superimposed infection is still a major problem. Patients with Gram-negative burn infections have a higher likelihood of developing sepsis than Gram-positive infections. Topical antimicrobials are the mainstay of therapy for burn infections and PDT may have a major role to play in the management of this disease.118
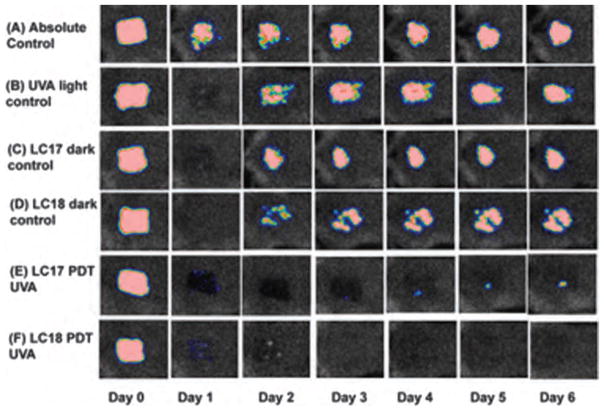
We found in previous study a decacationic fullerene LC17 ( ) was effective at mediating the photokilling of Gram-negative bacteria in vitro.66 We synthesized a new compound (LC18) with the same decacationic side chain plus an additional deca-tertiary amine groups against Gram-negative bacteria. A mouse model of the third-degree burn infection with bioluminescent Gram-negative bacteria was used to test the in vivo effectiveness of the therapeutic approach using the UVA excitation.119 The data shown in Figures 10 and 11 suggested that the attachment of an additional deca(tertiary-ethylenylamino) malonate arm to C70, producing LC18, allowed the moiety to act as a potent electron donor and increased the generation yield of hydroxyl radicals under UVA illumination. This is consistent with the reported phenomena of photoinduced intramolecular electron transfer from the tertiary amine moiety to the fullerene cage in polar solvents, including water, at the short excitation wavelength. With the availability of ten tertiary amine moieties, each capable of donating one electron to the C70 cage, LC18 may function as an electron-rich precursor for hydroxyl radical production that demonstrated a new approach in enhancing HO• radical killing of pathogenic bacteria in contrast to the more common 1O2-killing mechanism.
Figure 10.
Representative bioluminescence images from mice with Escherichia coli burn infections (day 0) and treated with successive fluences of photodynamic therapy or UVA light alone. (A) UVA control; (B) LC17 + UVA light; and (C) LC18+UVA light. There was no significant reduction in bioluminescence after application of either LC17 or LC18 without light exposure as a dark control. Reprinted with permission from [119], L. Huang, et al., Antimicrobial photodynamic therapy with decacationic monoadducts and bisadducts of [70]fullerene: In vitro and in vivo studies. Nanomedicine (Lond.) (2013).
© 2013, Future Science.
Figure 11.
Representative bioluminescence images from mice with Acinetobacter baumannii burn infections and treated with photodynamic therapy, UVA light alone or absolute control, captured day 0 (before photodynamic therapy) and then daily for 6 days. (A) Absolute control; (B) UVA control+15% DMA; (C) LC17+15% DMA; (D) LC18+15% DMA; (E) LC17+15% DMA+UVA light; and (F) LC18+15% DMA+UVA light. Reprinted with permission from [119], L. Huang, et al., Antimicrobial photodynamic therapy with decacationic monoadducts and bisadducts of [70]fullerene: In vitro and in vivo studies. Nanomedicine (Lond.) (2013).
© 2013, Future Science.
CONCLUSION
Fullerenes have been widely studied as potential PS that could mediate PDT of diverse diseases. As discussed previously fullerene-derivatives have uniquely important favorable properties and an unusual photochemical mechanism, which could make them candidates for ideal PS. As shown by us and by others, fullerene-derivatives produce a substantial amount of superoxide anion in a Type I photochemical process involving electron transfer from the excited triplet state to molecular oxygen in aqueous biomedical solutions. It is assumed that hydroxyl radicals are formed from hydrogen peroxide, and hydroxyl radicals are the most reactive and potentially the most cytotoxic of all ROS.
The chief disadvantage of fullerenes is likely to be that their absorption spectrum of fullerenes is highest in the UVA and blue regions of the spectrum, which limit the tissue penetration depth of illumination. With the correct functionalities present on the fullerene cage, these difficulties may be overcome. Since in vivo PDT usually uses red light for its improved tissue-penetrating properties it is unclear whether fullerenes would mediate effective PDT in vivo. Therefore synthesis of new fullerene derivatives will be a trend for future study, particularly those with light-harvesting antennae to broaden the absorption light, hence increasing light penetration depth into tissue. Furthermore 2-photon excitation is another promising avenue to increase penetration depth of PDT. The mechanistic study of Type I and Type II photochemistry, and the correlations between fullerene structure, photochemical mechanism and PDT efficacy will establish whether fullerenes can compete with more traditional PS in clinical applications of PDT.
Acknowledgments
Long Y. Chiang was supported by US NIH grant R01CA137108. Michael R. Hamblin was supported by US NIH grant R01AI050875.
Biographies
 Ying-Ying Huang, M.D., is a researcher in Dr. Michael Hamblin’s lab in Wellman Center for Photomedicine at Massachusetts General Hospital, an Instructor of Dermatology at Harvard Medical School. She received her M.D. from China in 2004. She earned her M.Med in Dermatology in China and she was trained as a dermatologist. She has been at MGH Wellman Center for 5 years. Her research interests lie in the areas of photodynamic therapy (PDT) for infections, cancer and mechanism of low level light therapy (LLLT) for traumatic brain injury. She has published 48 peer review articles and 15 conference proceedings and book chapters. She is the co-editor of newly released “Handbook of Photomedicine.”
Ying-Ying Huang, M.D., is a researcher in Dr. Michael Hamblin’s lab in Wellman Center for Photomedicine at Massachusetts General Hospital, an Instructor of Dermatology at Harvard Medical School. She received her M.D. from China in 2004. She earned her M.Med in Dermatology in China and she was trained as a dermatologist. She has been at MGH Wellman Center for 5 years. Her research interests lie in the areas of photodynamic therapy (PDT) for infections, cancer and mechanism of low level light therapy (LLLT) for traumatic brain injury. She has published 48 peer review articles and 15 conference proceedings and book chapters. She is the co-editor of newly released “Handbook of Photomedicine.”
 Sulbha K. Sharma, Ph.D. is a visiting fellow at Raja Ramanna centre for advanced technology, Indore, India. Earlier she was a postdoctoral fellow at Dr. Hamblin’s lab at The Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA. She completed her Ph.D. from Laser Biomedical section and instrumentation division at Raja Ramanna centre for advanced technology, Indore, India. She has published 25 peer-reviewed articles 7 conference proceedings and 4 book chapters. Her research interests are anticancer photodynamic therapy and low level light therapy.
Sulbha K. Sharma, Ph.D. is a visiting fellow at Raja Ramanna centre for advanced technology, Indore, India. Earlier she was a postdoctoral fellow at Dr. Hamblin’s lab at The Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA. She completed her Ph.D. from Laser Biomedical section and instrumentation division at Raja Ramanna centre for advanced technology, Indore, India. She has published 25 peer-reviewed articles 7 conference proceedings and 4 book chapters. Her research interests are anticancer photodynamic therapy and low level light therapy.
 Rui Yin, M.D., Ph.D. is an Associate Professor of Dermatology, Southwest Hospital, Third Military Medical University, and a visiting associate professor of Wellman Center for Photomedicine at Massachusetts General Hospital. Her research interests lie in the areas of photodynamic therapy for infections and cancer, the electron transfer mechanisms of photodynamic reaction. She has published 17 peer-reviewed articles in English, over 40 peer-reviewed articles in Chinese, over 30 conference proceeding, 2 book chapters. She is a reviewer for 7 journals and serves on National Natural Science Foundation of China as a grant reviewer. She is also a committee member of China Dermatologist Association and China Medical Association. In 2011, Dr. Yin was honored as one of Top 10 National Outstanding Young Dermatologist by China Dermatologist Association.
Rui Yin, M.D., Ph.D. is an Associate Professor of Dermatology, Southwest Hospital, Third Military Medical University, and a visiting associate professor of Wellman Center for Photomedicine at Massachusetts General Hospital. Her research interests lie in the areas of photodynamic therapy for infections and cancer, the electron transfer mechanisms of photodynamic reaction. She has published 17 peer-reviewed articles in English, over 40 peer-reviewed articles in Chinese, over 30 conference proceeding, 2 book chapters. She is a reviewer for 7 journals and serves on National Natural Science Foundation of China as a grant reviewer. She is also a committee member of China Dermatologist Association and China Medical Association. In 2011, Dr. Yin was honored as one of Top 10 National Outstanding Young Dermatologist by China Dermatologist Association.
 Tanupriya Agrawal, M.D., Ph.D. is a postdoctoral fellow at Dr. Hamblin’s lab at The Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA. Prior to this, she finished medical school at N.S.C.B Medical College, Jabalpur, India followed by Ph.D. in Biomedical Sciences at Creighton University, Omaha, NE. She has completed United States Medical Licensing Examination (USMLE) and certified by Educational Commission for Foreign Medical Graduates (ECFMG). She has published 6 peer-reviewed articles and 12 conference proceedings. She is investigating the role and underlying molecular mechanisms of low level laser therapy in neurogenesis and synaptogenesis in mouse model of traumatic brain injury.
Tanupriya Agrawal, M.D., Ph.D. is a postdoctoral fellow at Dr. Hamblin’s lab at The Wellman Center for Photomedicine, Massachusetts General Hospital, Boston, MA. Prior to this, she finished medical school at N.S.C.B Medical College, Jabalpur, India followed by Ph.D. in Biomedical Sciences at Creighton University, Omaha, NE. She has completed United States Medical Licensing Examination (USMLE) and certified by Educational Commission for Foreign Medical Graduates (ECFMG). She has published 6 peer-reviewed articles and 12 conference proceedings. She is investigating the role and underlying molecular mechanisms of low level laser therapy in neurogenesis and synaptogenesis in mouse model of traumatic brain injury.
 Long Y. Chiang, Ph.D. is a Professor at the Department of Chemistry, University of Massachusetts Lowell. His research interests lie in the areas of design and synthesis of ultrafast broadband photoresponsive linear and nonlinear multiphoton energy absorptive fullerenyl nanostructures and polycationic, nano-PDT drugs in combination of photoenergy, in one-photon absorptive 1PA-PDT and NIR two-photon absorptive 2PA-PDT, to kill multiantibiotic-resistant bacteria and cancer/tumor cells. He has published 269 peer-reviewed articles, book chapters, review articles, and awarded 39 patents. He was a chairman of several MRS symposia and a member of international advisory committee of ICSM. He was a member of editorial board and regional editor of two journals and is for one journal.
Long Y. Chiang, Ph.D. is a Professor at the Department of Chemistry, University of Massachusetts Lowell. His research interests lie in the areas of design and synthesis of ultrafast broadband photoresponsive linear and nonlinear multiphoton energy absorptive fullerenyl nanostructures and polycationic, nano-PDT drugs in combination of photoenergy, in one-photon absorptive 1PA-PDT and NIR two-photon absorptive 2PA-PDT, to kill multiantibiotic-resistant bacteria and cancer/tumor cells. He has published 269 peer-reviewed articles, book chapters, review articles, and awarded 39 patents. He was a chairman of several MRS symposia and a member of international advisory committee of ICSM. He was a member of editorial board and regional editor of two journals and is for one journal.
 Michael R. Hamblin, Ph.D. is a Principal Investigator at the Wellman Center for Photomedicine at Massachusetts General Hospital, an Associate Professor of Dermatology at Harvard Medical School and is a member of the affiliated faculty of the Harvard-MIT Division of Health Science and Technology. His research interests lie in the areas of photodynamic therapy (PDT) for infections and cancer, and in low-level light therapy (LLLT) for wound healing, arthritis, traumatic brain injury and hair regrowth. He has published 250 peer-reviewed articles, over 150 conference proceedings, book chapters, has edited 3 major textbooks, and holds 8 patents. He is Associate Editor for 7 journals and serves on NIH Study Sections. In 2011 Dr Hamblin was honored by election as a Fellow of SPIE.
Michael R. Hamblin, Ph.D. is a Principal Investigator at the Wellman Center for Photomedicine at Massachusetts General Hospital, an Associate Professor of Dermatology at Harvard Medical School and is a member of the affiliated faculty of the Harvard-MIT Division of Health Science and Technology. His research interests lie in the areas of photodynamic therapy (PDT) for infections and cancer, and in low-level light therapy (LLLT) for wound healing, arthritis, traumatic brain injury and hair regrowth. He has published 250 peer-reviewed articles, over 150 conference proceedings, book chapters, has edited 3 major textbooks, and holds 8 patents. He is Associate Editor for 7 journals and serves on NIH Study Sections. In 2011 Dr Hamblin was honored by election as a Fellow of SPIE.
References
- 1.Henderson BW, Dougherty TJ. How does photodynamic therapy work? Photochem Photobiol. 1992;55:145. doi: 10.1111/j.1751-1097.1992.tb04222.x. [DOI] [PubMed] [Google Scholar]
- 2.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J. Photodynamic therapy of cancer: An update. CA Cancer J Clin. 2011;61:250. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle RW, Dolphin D. Structure and biodistribution relationships of photodynamic sensitizers. Photochem Photobiol. 1996;64:469. doi: 10.1111/j.1751-1097.1996.tb03093.x. [DOI] [PubMed] [Google Scholar]
- 4.Sternberg ED, Dolphin D, Brückner C. Porphyrin-based photosensitizers for use in photodynamic therapy. Tetrahedron. 1998;54:4151. [Google Scholar]
- 5.Ravindra K, Pandey RK, Zheng G. In: Porphyrins as photosensitizers in photodynamic therapy, The Porphyrin Handbook. Kadish K, Guilard R, Smiths KM, editors. Vol. 6. Elsevier; San Diego: 2000. pp. 157–161. [Google Scholar]
- 6.Allison RR, Downie GH, Cuenca R, Hu XH, Childs CJ, Sibata CH. Photosensitizers in clinical PDT. Photodiagnosis Photodyn Ther. 2004;1:27. doi: 10.1016/S1572-1000(04)00007-9. [DOI] [PubMed] [Google Scholar]
- 7.Weersink RA, Bogaards A, Gertner M, Davidson SR, Zhang K, Netchev G, Trachtenberg J, Wilson BC. Techniques for delivery and monitoring of TOOKAD (WST09)-mediated photodynamic therapy of the prostate: Clinical experience and practicalities. J Photochem Photobiol B: Biol. 2005;79:211. doi: 10.1016/j.jphotobiol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Hopper C, Niziol C, Sidhu M. The cost-effectiveness of Foscan mediated photodynamic therapy (Foscan-PDT) compared with extensive palliative surgery and palliative chemotherapy for patients with advanced head and neck cancer in the UK. Oral Oncol. 2004;40:372. doi: 10.1016/j.oraloncology.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Krammer B, Plaetzer K. ALA and its clinical impact, from bench to bedside. Photochem Photobiol Sci. 2008;7:283. doi: 10.1039/b712847a. [DOI] [PubMed] [Google Scholar]
- 10.Allen CM, Sharman WM, Van Lier JE. Current status of phthalocyanines in the photodynamic therapy of cancer. J Porphyrins Phthalocyanines. 2001;5:161. [Google Scholar]
- 11.St Denis TG, Hamblin MR. Synthesis, bioanalysis and biodistribution of photosensitizer conjugates for photodynamic therapy. Bioanalysis. 2013;5:1099. doi: 10.4155/bio.13.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leonard KA, Nelen MI, Simard TP, Davies SR, Gollnick SO, Oseroff AR, Gibson SL, Hilf R, Chen LB, Detty MR. Synthesis and evaluation of chalcogenopyrylium dyes as potential sensitizers for the photodynamic therapy of cancer. J Med Chem. 1999;42:3953. doi: 10.1021/jm990245q. [DOI] [PubMed] [Google Scholar]
- 13.Harris F, Chatfield LK, Phoenix DA. Phenothiazinium based photosensitisers–photodynamic agents with a multiplicity of cellular targets and clinical applications. Curr Drug Targets. 2005;6:615. doi: 10.2174/1389450054545962. [DOI] [PubMed] [Google Scholar]
- 14.Lin CW, Shulok JR, Wong YK, Schanbacher CF, Cincotta L, Foley JW. Photosensitization, uptake, and retention of phenoxazine Nile blue derivatives in human bladder carcinoma cells. Cancer Res. 1991;51:1109. [PubMed] [Google Scholar]
- 15.Olivo M, Chin W. Perylenequinones in photodynamic therapy: Cellular versus vascular response. J Environ Pathol Toxicol Oncol. 2006;25:223. doi: 10.1615/jenvironpatholtoxicoloncol.v25.i1-2.140. [DOI] [PubMed] [Google Scholar]
- 16.Kamkaew A, Lim SH, Lee HB, Kiew LV, Chung LY, Burgess K. BODIPY dyes in photodynamic therapy. Chem Soc Rev. 2013;42:77. doi: 10.1039/c2cs35216h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Avirah RR, Jayaram DT, Adarsh N, Ramaiah D. Squaraine dyes in PDT: From basic design to in vivo demonstration. Org Biomol Chem. 2012;10:911. doi: 10.1039/c1ob06588b. [DOI] [PubMed] [Google Scholar]
- 18.Kavarnos GJ, Turro NJ. Photosensitization by reversible electron transfer: Theories, experimental evidence, and examples. Chem Rev. 1986;86:401. [Google Scholar]
- 19.Accorsi G, Armaroli N. Taking advantage of the electronic excited states of [60]-fullerenes. J Phys Chem C. 2010;114:1385. [Google Scholar]
- 20.Mroz P, Pawlak A, Satti M, Lee H, Wharton T, Gali H, Sarna T, Hamblin MR. Functionalized fullerenes mediate photodynamic killing of cancer cells: Type I versus Type II photochemical mechanism. Free Radic Biol Med. 2007;43:711. doi: 10.1016/j.freeradbiomed.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang M, Nalla V, Jeon S, Mamidala V, Ji W, Tan LS, Cooper T, Chiang LY. Large femtosecond two-photon absorption cross sections of fullerosome vesicle nanostructures derived from a highly photoresponsive amphiphilic C60-light-harvesting fluorene dyad. J Phys Chem C. 2011;115:18552. doi: 10.1021/jp207047k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Culotta L, Koshland DE., Jr Buckyballs: Wide open playing field for chemists. Science. 1991;254:1706. doi: 10.1126/science.254.5039.1706. [DOI] [PubMed] [Google Scholar]
- 23.Meng J, Wang DL, Wang PC, Jia L, Chen C, Liang XJ. Biomedical activities of endohedral metallofullerene optimized for nanopharmaceutics. J Nanosci Nanotechnol. 2010;10:8610. doi: 10.1166/jnn.2010.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brant JA, Labille J, Robichaud CO, Wiesner M. Fullerol cluster formation in aqueous solutions: Implications for environmental release. J Colloid Interface Sci. 2007;314:281. doi: 10.1016/j.jcis.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Angelini G, De Maria P, Fontana A, Pierini M, Maggini M, Gasparrini F, Zappia G. Study of the aggregation properties of a novel amphiphilic C60 fullerene derivative. Langmuir. 2001;17:6404. [Google Scholar]
- 26.Baffreau J, Leroy-Lhez S, Vân Anh N, Williams RM, Hudhomme P. Fullerene C60–Perylene-3, 4:9, 10-bis (dicarboximide) light-harvesting dyads: Spacer-length and bay-substituent effects on intramolecular singlet and triplet energy transfer. Chem Eur J. 2008;14:4974. doi: 10.1002/chem.200800156. [DOI] [PubMed] [Google Scholar]
- 27.Tuchin VV, Wang RK, Yeh AT. Optical clearing of tissues and cells. J Biomed Opt. 2008;13:021101. doi: 10.1117/1.2903745. [DOI] [PubMed] [Google Scholar]
- 28.Hirshburg J, Choi B, Nelson JS, Yeh AT. Correlation between collagen solubility and skin optical clearing using sugars. Lasers Surg Med. 2007;39:140. doi: 10.1002/lsm.20417. [DOI] [PubMed] [Google Scholar]
- 29.Jiang J, Boese M, Turner P, Wang RK. Penetration kinetics of dimethyl sulphoxide and glycerol in dynamic optical clearing of porcine skin tissue in vitro studied by Fourier transform infrared spectroscopic imaging. J Biomed Opt. 2008;13:021105. doi: 10.1117/1.2899153. [DOI] [PubMed] [Google Scholar]
- 30.Kogan A, Garti N. Microemulsions as transdermal drug delivery vehicles. Adv Colloid Interface Sci. 2006;123–126:369. doi: 10.1016/j.cis.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Bhawalkar JD, Kumar ND, Zhao CF, Prasad PN. Two-photon photodynamic therapy. J Clin Laser Med Surg. 1997;15:201. doi: 10.1089/clm.1997.15.201. [DOI] [PubMed] [Google Scholar]
- 32.Karotki A, Khurana M, Lepock JR, Wilson BC. Simultaneous two-photon excitation of photofrin in relation to photodynamic therapy. Photochem Photobiol. 2006;82:443. doi: 10.1562/2005-08-24-RA-657. [DOI] [PubMed] [Google Scholar]
- 33.Samkoe KS, Clancy AA, Karotki A, Wilson BC, Cramb DT. Complete blood vessel occlusion in the chick chorioallantoic membrane using two-photon excitation photodynamic therapy: Implications for treatment of wet age-related macular degeneration. J Biomed Opt. 2007;12:034025. doi: 10.1117/1.2750663. [DOI] [PubMed] [Google Scholar]
- 34.Samkoe KS, Cramb DT. Application of an ex ovo chicken chorioallantoic membrane model for two-photon excitation photodynamic therapy of age-related macular degeneration. J Biomed Opt. 2003;8:410. doi: 10.1117/1.1577117. [DOI] [PubMed] [Google Scholar]
- 35.Oberdorster G. Safety assessment for nanotechnology and nanomedicine: Concepts of nanotoxicology. J Intern Med. 2010;267:89. doi: 10.1111/j.1365-2796.2009.02187.x. [DOI] [PubMed] [Google Scholar]
- 36.Arbogast JW, Darmanyan AP, Foote CS, Diederich FN, Whetten R, Rubin Y, Alvarez MM, Anz SJ. Photophysical properties of sixty atom carbon molecule (C60) J Phys Chem A. 1991;95:11. [Google Scholar]
- 37.Haddon R, Brus LE, Raghavachari K. Electronic structure and bonding in icosahedral C60. Chem Phys Lett. 1986;125:459. [Google Scholar]
- 38.Wu W, Zhao J, Sun J, Guo S. Light-harvesting fullerene dyads as organic triplet photosensitizers for triplet–triplet annihilation upconversions. J Org Chem. 2012;77:5305. doi: 10.1021/jo300613g. [DOI] [PubMed] [Google Scholar]
- 39.Yamakoshi Y, Umezawa N, Ryu A, Arakane K, Miyata N, Goda Y, Masumizu T, Nagano T. Active oxygen species generated from photoexcited fullerene (C60) as potential medicines: O2−* versus 1O2. J Am Chem Soc. 2003;125:12803. doi: 10.1021/ja0355574. [DOI] [PubMed] [Google Scholar]
- 40.Xie Q, Perez-Cordero E, Echegoyen L. Electrochemical detection of C606-and C706-: Enhanced stability of fullerides in solution. J Am Chem Soc. 1992;114:3978. [Google Scholar]
- 41.Guldi DM, Prato M. Excited-state properties of C(60) fullerene derivatives. Acc Chem Res. 2000;33:695. doi: 10.1021/ar990144m. [DOI] [PubMed] [Google Scholar]
- 42.Ohkubo K, Kitaguchi H, Fukuzumi S. Activation of electron-transfer reduction of oxygen by hydrogen bond formation of superoxide anion with ammonium ion. J Phys Chem A. 2006;110:11613. doi: 10.1021/jp064115m. [DOI] [PubMed] [Google Scholar]
- 43.Andrievsky GV, Bruskov VI, Tykhomyrov AA, Gudkov SV. Peculiarities of the antioxidant and radioprotective effects of hydrated C60 fullerene nanostuctures in vitro and in vivo. Free Radic Biol Med. 2009;47:786. doi: 10.1016/j.freeradbiomed.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 44.Avdeev MV, Khokhryakov AA, Tropin TV, Andrievsky GV, Klochkov VK, Derevyanchenko LI, Rosta L, Garamus VM, Priezzhev VB, Korobov MV, Aksenov VL. Structural features of molecular-colloidal solutions of C60 fullerenes in water by small-angle neutron scattering. Langmuir. 2004;20:4363. doi: 10.1021/la0361969. [DOI] [PubMed] [Google Scholar]
- 45.de La Vaissière B, Sandall JP, Fowler PW, de Oliveira P, Bensasson RV. Regioselectivity in radical reactions of C60 derivatives. J Chem Soc Perkin. 2001;2:821. [Google Scholar]
- 46.Gharbi N, Pressac M, Hadchouel M, Szwarc H, Wilson SR, Moussa F. [60]fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett. 2005;5:2578. doi: 10.1021/nl051866b. [DOI] [PubMed] [Google Scholar]
- 47.Kato S, Aoshima H, Saitoh Y, Miwa N. Fullerene-C60/liposome complex: Defensive effects against UVA-induced damages in skin structure, nucleus and collagen type I/IV fibrils, and the permeability into human skin tissue. J Photochem Photobiol B. 2010;98:99. doi: 10.1016/j.jphotobiol.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 48.Doi Y, Ikeda A, Akiyama M, Nagano M, Shigematsu T, Ogawa T, Takeya T, Nagasaki T. Intracellular uptake and photodynamic activity of water-soluble [60]- and [70]fullerenes incorporated in liposomes. Chemistry. 2008;14:8892. doi: 10.1002/chem.200801090. [DOI] [PubMed] [Google Scholar]
- 49.Yan A, Von Dem Bussche A, Kane AB, Hurt RH. Tocopheryl polyethylene glycol succinate as a safe, antioxidant surfactant for processing carbon nanotubes and fullerenes. Carbon N Y. 2007;45:2463. doi: 10.1016/j.carbon.2007.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akiyama M, Ikeda A, Shintani T, Doi Y, Kikuchi J, Ogawa T, Yogo K, Takeya T, Yamamoto N. Solubilisation of [60]fullerenes using block copolymers and evaluation of their photodynamic activities. Org Biomol Chem. 2008;6:1015. doi: 10.1039/b719671g. [DOI] [PubMed] [Google Scholar]
- 51.Kojima C, Toi Y, Harada A, Kono K. Aqueous solubilization of fullerenes using poly(amidoamine) dendrimers bearing cyclodextrin and poly(ethylene glycol) Bioconjug Chem. 2008;19:2280. doi: 10.1021/bc8001503. [DOI] [PubMed] [Google Scholar]
- 52.Pan B, Cui D, Xu P, Ozkan C, Feng G, Ozkan M, Huang T, Chu B, Li Q, He R, Hu G. Synthesis and characterization of polyamidoamine dendrimer-coated multi-walled carbon nanotubes and their application in gene delivery systems. Nanotechnology. 2009;20:125101. doi: 10.1088/0957-4484/20/12/125101. [DOI] [PubMed] [Google Scholar]
- 53.Hooper JB, Bedrov D, Smith GD. Supramolecular self-organization in PEO-modified C60 fullerene/water solutions: Influence of polymer molecular weight and nanoparticle concentration. Langmuir. 2008;24:4550. doi: 10.1021/la703057y. [DOI] [PubMed] [Google Scholar]
- 54.Nitta N, Seko A, Sonoda A, Ohta S, Tanaka T, Takahashi M, Murata K, Takemura S, Sakamoto T, Tabata Y. Is the use of fullerene in photodynamic therapy effective for atherosclerosis? Cardiovasc Intervent Radiol. 2008;31:359. doi: 10.1007/s00270-007-9238-8. [DOI] [PubMed] [Google Scholar]
- 55.Liu J, Ohta S, Sonoda A, Yamada M, Yamamoto M, Nitta N, Murata K, Tabata Y. Preparation of PEG-conjugated fullerene containing Gd3+ ions for photodynamic therapy. J Control Release. 2007;117:104. doi: 10.1016/j.jconrel.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 56.Tabata Y, Murakami Y, Ikada Y. Photodynamic effect of polyethylene glycol-modified fullerene on tumor. Jpn J Cancer Res. 1997;88:1108. doi: 10.1111/j.1349-7006.1997.tb00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bali V, Ali M, Ali J. Novel nanoemulsion for minimizing variations in bioavailability of ezetimibe. J Drug Target. 2010;18:506. doi: 10.3109/10611860903548362. [DOI] [PubMed] [Google Scholar]
- 58.Amani A, York P, Chrystyn H, Clark BJ. Factors affecting the stability of nanoemulsions–use of artificial neural networks. Pharm Res. 2010;27:37. doi: 10.1007/s11095-009-0004-2. [DOI] [PubMed] [Google Scholar]
- 59.Bansal T, Mustafa G, Khan ZI, Ahmad FJ, Khar RK, Talegaonkar S. Solid self-nanoemulsifying delivery systems as a platform technology for formulation of poorly soluble drugs. Crit Rev Ther Drug Carrier Syst. 2008;25:63. doi: 10.1615/critrevtherdrugcarriersyst.v25.i1.20. [DOI] [PubMed] [Google Scholar]
- 60.Shakeel F, Faisal MS. Nanoemulsion: A promising tool for solubility and dissolution enhancement of celecoxib. Pharm Dev Technol. 2010;15:53. doi: 10.3109/10837450902967954. [DOI] [PubMed] [Google Scholar]
- 61.Filippone S, Heimann F, Rassat A. A highly water-soluble 2:1 beta-cyclodextrin-fullerene conjugate. Chem Commun (Camb) 2002:1508. doi: 10.1039/b202410a. [DOI] [PubMed] [Google Scholar]
- 62.Zhao B, He YY, Bilski PJ, Chignell CF. Pristine (C60) and hydroxylated [C60(OH)24] fullerene phototoxicity towards HaCaT keratinocytes: Type I versus type II mechanisms. Chem Res Toxicol. 2008;21:1056. doi: 10.1021/tx800056w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakamura E, Isobe H. Functionalized fullerenes in water. The first 10 years of their chemistry, biology, and nanoscience. Acc Chem Res. 2003;36:807. doi: 10.1021/ar030027y. [DOI] [PubMed] [Google Scholar]
- 64.Yurovskaya MA, Trushkov IV. Cycloaddition to buckmin-sterfullerene C60: Advancements and future prospects. Russ Chem Bull. 2002;51:367. [Google Scholar]
- 65.Maggini M, Scorrano G, Prato M. Addition of azomethine ylides to C60: Synthesis, characterization, and functionalization of fullerene pyrrolidines. J Am Chem Soc. 1993;115:9798. [Google Scholar]
- 66.Wang M, Huang L, Sharma SK, Jeon S, Thota S, Sperandio FF, Nayka S, Chang J, Hamblin MR, Chiang LY. Synthesis and photodynamic effect of new highly photostable decacationically armed [60]- and [70]fullerene decaiodide monoadducts to target pathogenic bacteria and cancer cells. J Med Chem. 2012;55:4274. doi: 10.1021/jm3000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sperandio FF, Sharma SK, Wang M, Jeon S, Huang YY, Dai T, Nayka S, de Sousa SC, Chiang LY, Hamblin MR. Photoinduced electron-transfer mechanisms for radical-enhanced photodynamic therapy mediated by water-soluble decacationic C70 and C84O2 Fullerene Derivatives. Nanomedicine. 2013;9:570. doi: 10.1016/j.nano.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brettreich M, Burghardt S, Bottcher C, Bayerl T, Bayerl S, Hirsch A. Globular amphiphiles: Membrane-forming hexaadducts of C(60) Angew Chem Int Ed Engl. 2000;39:1845. doi: 10.1002/(sici)1521-3773(20000515)39:10<1845::aid-anie1845>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 69.Tegos GP, Demidova TN, Arcila-Lopez D, Lee H, Wharton T, Gali H, Hamblin MR. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem Biol. 2005;12:1127. doi: 10.1016/j.chembiol.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang M, Maragani S, Huang L, Jeon S, Canteenwala T, Hamblin MR, Chiang LY. Synthesis of decacationic [60]fullerene decaiodides giving photoinduced production of superoxide radicals and effective PDT-mediation on antimicrobial photoinactivation. Eur J Med Chem. 2013;63:170. doi: 10.1016/j.ejmech.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thota S, Wang M, Jeon S, Maragani S, Hamblin MR, Chiang LY. Synthesis and characterization of positively charged pentacationic [60]fullerene monoadducts for antimicrobial photodynamic inactivation. Molecules. 2012;17:5225. doi: 10.3390/molecules17055225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Constantin C, Neagu M, Ion RM, Gherghiceanu M, Stavaru C. Fullerene-porphyrin nanostructures in photodynamic therapy. Nanomedicine (Lond) 2010;5:307. doi: 10.2217/nnm.09.111. [DOI] [PubMed] [Google Scholar]
- 73.Chiang LY, Padmawar PA, Rogers-Haley JE, So G, Canteenwala T, Thota S, Tan LS, Pritzker K, Huang YY, Sharma SK, Kurup DB, Hamblin MR, Wilson B, Urbas A. Synthesis and characterization of highly photoresponsive fullerenyl dyads with a close chromophore antenna-C(60) contact and effective photodynamic potential. J Mater Chem. 2010;20:5280. doi: 10.1039/C0JM00037J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.El-Khouly ME, Padmawar P, Araki Y, Verma S, Chiang LY, Ito O. Photoinduced processes in a tricomponent molecule consisting of diphenylaminofluorene-dicyanoethylenemethano[ 60]fullerene. J Phys Chem A. 2006;110:884. doi: 10.1021/jp055324u. [DOI] [PubMed] [Google Scholar]
- 75.Tegos GP, Demidova TN, Arcila-Lopez D, Lee H, Wharton T, Gali H, Hamblin MR. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem Biol. 2005;12:1127. doi: 10.1016/j.chembiol.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Diederich F, Gómez-López M. Supramolecular fullerene chemistry. Chem Soc Rev. 1999;28:263. [Google Scholar]
- 77.Kadish KM, Ruoff RS, editors. Fullerenes: Chemistry, Physics, and Technology. John Wiley & Sons Inc; New York: 2000. [Google Scholar]
- 78.Hu Z, Guan W, Wang W, Huang L, Xing H, Zhu Z. Protective effect of a novel cystine C(60) derivative on hydrogen peroxide-induced apoptosis in rat pheochromocytoma PC12 cells. Chem Biol Interact. 2007;167:135. doi: 10.1016/j.cbi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 79.Bingel C. ChemInform abstract: Cyclopropylation of fullerenes. ChemInform. 1994;25:1957. [Google Scholar]
- 80.Maggini M, Scorrano G, Prato M. Addition of azomethine ylides to C60–synthesis, characterization, and functionalization of fullerenes pyrrolidines. J Am Chem Soc. 1993;115:9798. [Google Scholar]
- 81.Lu Z, Dai T, Huang L, Kurup DB, Tegos GP, Jahnke A, Wharton T, Hamblin MR. Photodynamic therapy with a cationic functionalized fullerene rescues mice from fatal wound infections. Nanomedicine (Lond) 2010;5:1525. doi: 10.2217/nnm.10.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mizuno K, Zhiyentayev T, Huang L, Khalil S, Nasim F, Tegos GP, Gali H, Jahnke A, Wharton T, Hamblin MR. Antimicrobial photodynamic therapy with functionalized fullerenes: Quantitative structure-activity relationships. J Nanomed Nanotechnol. 2011;2:1. doi: 10.4172/2157-7439.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang M, Huang L, Sharma SK, Jeon S, Thota S, Sperandio FF, Nayka SN, Chang J, Hamblin MR, Chiang LY. Synthesis and photodynamic effect of new highly photostable decacationically armed [60]-and [70] fullerene decaiodide monoadducts to target pathogenic bacteria and cancer cells. J Med Chem. 2012;55:4274. doi: 10.1021/jm3000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bhonsle JB, Chi Y, Huang JP, Shiea J, Chen BJ, Chiang LY. Novel water-soluble hexa(sulfobutyl)fullerenes as potent free radical scavengers. Chem Lett. 1998;27:465. [Google Scholar]
- 85.Yu C, Canteenwala T, El-Khouly ME, Araki Y, Pritzker K, Ito O, Wilson BC, Chiang LY. Efficiency of singlet oxygen production from self-assembled nanospheres of molecular micelle-like photosensitizers FC4S. J Mater Chem. 2005;15:857. [Google Scholar]
- 86.Jeng US, Lin TL, Tsao CS, LCH, Canteenwala T, Wang LY, Chiang LY, Han CC. Study of aggregates of fullerene-based ionomers in aqueous solutions using small angle neutron and X-ray scattering. J Phys Chem B. 1999;103:1059. [Google Scholar]
- 87.Guldi DM, Asmus KD. Photophysical properties of mono-and multiply-functionalized fullerene derivatives. J Phys Chem B. 1997;101:1472. [Google Scholar]
- 88.Bensasson RV, Berberan-Santos MN, Bretreich M, Frederiksen J, Göttinger H, Hirsch A, Land EJ, Leach S, McGarvey DJ, Schönberger H, Schröder C. Triplet state properties of malonic acid C60 derivatives C60[C(COOR)2]n; R H, Et; n = 1–6. Phys Chem Chem Phys. 2001;3:4679. [Google Scholar]
- 89.Foley S, Crowley C, Smaihi M, Bonfils C, Erlanger BF, Seta P, Larroque C. Cellular localisation of a water-soluble fullerene derivative. Biochem Biophys Res Commun. 2002;294:116. doi: 10.1016/S0006-291X(02)00445-X. [DOI] [PubMed] [Google Scholar]
- 90.Porter AE, Gass M, Muller K, Skepper JN, Midgley P, Welland M. Visualizing the uptake of C60 to the cytoplasm and nucleus of human monocyte-derived macrophage cells using energy-filtered transmission electron microscopy and electron tomography. Environ Sci Technol. 2007;41:3012. doi: 10.1021/es062541f. [DOI] [PubMed] [Google Scholar]
- 91.Tokuyama H, Yamago S, Nakamura E, Shiraki T, Sugiura Y. Photoinduced biochemical activity of fullerene carboxylic acid. J Am Chem Soc. 1993;115:7918. [Google Scholar]
- 92.Burlaka AP, Sidorik YP, Prylutska SV, Matyshevska OP, Golub OA, Prylutskyy YI, Scharff P. Catalytic system of the reactive oxygen species on the C60 fullerene basis. Exp Oncol. 2004;26:326. [PubMed] [Google Scholar]
- 93.Rancan F, Rosan S, Boehm F, Cantrell A, Brellreich M, Schoenberger H, Hirsch A, Moussa F. Cytotoxicity and photocytotoxicity of a dendritic C(60) mono-adduct and a malonic acid C(60) tris-adduct on Jurkat cells. J Photochem Photobiol B. 2002;67:157. doi: 10.1016/s1011-1344(02)00320-2. [DOI] [PubMed] [Google Scholar]
- 94.Rancan F, Helmreich M, Molich A, Jux N, Hirsch A, Roder B, Witt C, Bohm F. Fullerene-pyropheophorbide a complexes as sensitizer for photodynamic therapy: Uptake and photo-induced cytotoxicity on Jurkat cells. J Photochem Photobiol B. 2005;80:1. doi: 10.1016/j.jphotobiol.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 95.Li R, Bounds DJ, Granville D, Ip SH, Jiang H, Margaron P, Hunt DW. Rapid induction of apoptosis in human keratinocytes with the photosensitizer QLT0074 via a direct mitochondrial action. Apoptosis. 2003;8:269. doi: 10.1023/a:1023624922787. [DOI] [PubMed] [Google Scholar]
- 96.Gupta S, Ahmad N, Mukhtar H. Involvement of nitric oxide during phthalocyanine (Pc4) photodynamic therapy-mediated apoptosis. Cancer Res. 1998;58:1785. [PubMed] [Google Scholar]
- 97.Elisa Milanesio M, Alvarez MG, Rivarola V, Silber JJ, Durantini EN. Porphyrin-fullerene C60 dyads with high ability to form photoinduced charge-separated state as novel sensitizers for photodynamic therapy. Photochem Photobiol. 2005;81:891. doi: 10.1562/2005-01-24-RA-426. [DOI] [PubMed] [Google Scholar]
- 98.Alvarez MG, Prucca C, Milanesio ME, Durantini EN, Rivarola V. Photodynamic activity of a new sensitizer derived from porphyrin-C60 dyad and its biological consequences in a human carcinoma cell line. Int J Biochem Cell Biol. 2006;38:2092. doi: 10.1016/j.biocel.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 99.Elisa Milanesio M, Gabriela Alvarez M, Durantini EN. Methoxyphenyl porphyrin derivatives as phototherapeutic agents. Curr Bioact Compd. 2010;6:97. [Google Scholar]
- 100.Mroz P, Tegos GP, Gali H, Wharton T, Sarna T, Hamblin MR. Photodynamic therapy with fullerenes. Photochem Photobiol Sci. 2007;6:1139. doi: 10.1039/b711141j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu C, Canteenwala T, Chen HH, Jeng U-S, Lin T-L, Chiang LY. Free radical scavenging and photodynamic functions of micelle-like hydrophilic hexa (sulfobutyl) fullerene (FC4S) In: Ōsawas E, editor. Perspectives of Fullerene Nanotechnology. Springer; Netherlands: 2002. pp. 165–183. [Google Scholar]
- 102.Mroz P, Xia Y, Asanuma D, Konopko A, Zhiyentayev T, Huang YY, Sharma SK, Dai T, Khan UJ, Wharton T, Hamblin MR. Intraperitoneal photodynamic therapy mediated by a fullerene in a mouse model of abdominal dissemination of colon adenocarcinoma. Nanomedicine. 2011;7:965. doi: 10.1016/j.nano.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Otake E, Sakuma S, Torii K, Maeda A, Ohi H, Yano S, Morita A. Effect and mechanism of a new photodynamic therapy with glycoconjugated fullerene. Photochem Photobiol. 2010;86:1356. doi: 10.1111/j.1751-1097.2010.00790.x. [DOI] [PubMed] [Google Scholar]
- 104.Maisch T. A new strategy to destroy antibiotic resistant microorganisms: Antimicrobial photodynamic treatment. Mini Rev Med Chem. 2009;9:974. doi: 10.2174/138955709788681582. [DOI] [PubMed] [Google Scholar]
- 105.Talan DA. MRSA: Deadly super bug or just another staph? Ann Emerg Med. 2008;51:299. doi: 10.1016/j.annemergmed.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 106.Hamblin MR, Hasan T. Photodynamic therapy: A new antimicrobial approach to infectious disease? Photochem Photobiol Sci. 2004;3:436. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hancock RE, Bell A. Antibiotic uptake into gram-negative bacteria. Eur J Clin Microbiol Infect Dis. 1988;7:713. doi: 10.1007/BF01975036. [DOI] [PubMed] [Google Scholar]
- 108.Martin JP, Logsdon N. The role of oxygen radicals in dye-mediated photodynamic effects in Escherichia coli B. J Biol Chem. 1987;262:7213. [PubMed] [Google Scholar]
- 109.Duncan LK, Jinschek JR, Vikesland PJ. C60 colloid formation in aqueous systems: Effects of preparation method on size, structure, and surface charge. Environ Sci Technol. 2008;42:173. doi: 10.1021/es071248s. [DOI] [PubMed] [Google Scholar]
- 110.Mashino T, Usui N, Okuda K, Hirota T, Mochizuki M. Respiratory chain inhibition by fullerene derivatives: Hydrogen peroxide production caused by fullerene derivatives and a respiratory chain system. Bioorg Med Chem. 2003;11:1433. doi: 10.1016/s0968-0896(02)00610-7. [DOI] [PubMed] [Google Scholar]
- 111.Mashino T, Okuda K, Hirota T, Hirobe M, Nagano T, Mochizuki M. Inhibition of E. coli growth by fullerene derivatives and inhibition mechanism. Bioorg Med Chem Lett. 1999;9:2959. doi: 10.1016/s0960-894x(99)00515-6. [DOI] [PubMed] [Google Scholar]
- 112.Spesia MB, Milanesio ME, Durantini EN. Synthesis, properties and photodynamic inactivation of Escherichia coli by novel cationic fullerene C60 derivatives. Eur J Med Chem. 2008;43:853. doi: 10.1016/j.ejmech.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 113.Dai T, Huang YY, Sharma SK, Hashmi JT, Kurup DB, Hamblin MR. Topical antimicrobials for burn wound infections. Recent Pat Antiinfect Drug Discov. 2010;5:124. doi: 10.2174/157489110791233522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dahl TA, Midden WR, Hartman PE. Comparison of killing of gram-negative and gram-positive bacteria by pure singlet oxygen. J Bacteriol. 1989;171:2188–2194. doi: 10.1128/jb.171.4.2188-2194.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Valduga G, Bertoloni G, Reddi E, Jori G. Effect of extracellularly generated singlet oxygen on gram-positive and gram-negative bacteria. J Photochem Photobiol B. 1993;21:81. doi: 10.1016/1011-1344(93)80168-9. [DOI] [PubMed] [Google Scholar]
- 116.Ollstein RN, McDonald C. Topical and systemic antimicrobial agents in burns. Ann Plast Surg. 1980;5:386. doi: 10.1097/00000637-198011000-00010. [DOI] [PubMed] [Google Scholar]
- 117.Saffle JR. Closure of the excised burn wound: Temporary skin substitutes. Clin Plast Surg. 2009;36:627. doi: 10.1016/j.cps.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 118.Dai T, Huang YY, Sharma SK, Hashmi JT, Kurup DB, Hamblin MR. Topical antimicrobials for burn wound infections. Recent Pat Antiinfect Drug Discov. 2010;5:124. doi: 10.2174/157489110791233522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang L, Wang M, Dai T, Sperandio FF, Huang YY, Xuan Y, Chiang LY, Hamblin MR. Antimicrobial photodynamic therapy with decacationic monoadducts and bisadducts of [70]fullerene: In vitro and in vivo studies. Nanomedicine (Lond) 2013;9:253. doi: 10.2217/nnm.13.22. [DOI] [PMC free article] [PubMed] [Google Scholar]