Abstract
Importance
Diagnostic coronary angiography in asymptomatic patients may lead to inappropriate percutaneous coronary intervention (PCI) due to a diagnostic to therapeutic cascade. Understanding the relationship between patient selection for coronary angiography and PCI appropriateness may inform strategies to minimize inappropriate procedures.
Objective
To determine if hospitals that frequently perform coronary angiography in asymptomatic patients, a clinical scenario wherein the benefit of angiography is less clear, are more likely to perform inappropriate PCI.
Design, Setting and Participants
Multicenter observational study of 544 hospitals participating in the CathPCI Registry® between July 2009 and September 2013.
Measures
Hospital proportion of asymptomatic patients at diagnostic coronary angiography and a hospital's rate of inappropriate PCI, as defined by 2012 Appropriate Use Criteria for coronary revascularization.
Results
Of 1,225,562 patients who underwent elective coronary angiography, 308,083 (25.1%) were asymptomatic. The hospital proportion of angiograms in asymptomatic patients ranged from 1.0% to 73.6% (median 24.7%, interquartile range 15.9% to 35.9%). By hospital quartiles of asymptomatic patients at angiography, hospitals with higher rates of asymptomatic patients at angiography had higher median rates of inappropriate PCI (14.8% vs. 20.2% vs. 24.0 vs. 29.4% from lowest to highest quartile, P<.001 for trend). This was attributable to more frequent use of PCI in asymptomatic patients at hospitals with higher rates of angiography in asymptomatic patients (inappropriate and asymptomatic PCI; 5.4% vs. 9.9% vs. 14.7% vs. 21.6% from lowest to highest quartile, P<.001 for trend). Hospitals with higher rates of asymptomatic patients at angiography also had lower rates of appropriate PCI (38.6% vs. 33.0% vs. 32.3% vs. 32.9%% from lowest to highest quartile, P<.001 for trend).
Conclusions and Relevance
In a national sample of hospitals, performing coronary angiography on asymptomatic patients was associated with higher rates of inappropriate PCI and lower rates of appropriate PCI. Improving pre-procedure risk stratification and thresholds for coronary angiography may be one strategy to improve the appropriateness of PCI.
Increasing attention is being given to proper patient selection for coronary procedures to avoid unnecessary procedural risks and costs. This is evident in the proliferation of Appropriate Use Criteria (AUC) to assess patient selection for coronary procedures.1,2 Application of the AUC for coronary revascularization has demonstrated wide facility-level variation in the quality of patient selection for elective percutaneous coronary intervention (PCI), with hospital rates of inappropriate PCI ranging from 0 to 55%.3 These findings suggest opportunities to improve patient selection for coronary procedures as part of improving healthcare quality.
In light of these findings, strategies to minimize inappropriate PCI have emphasized the interventional cardiologist by ensuring revascularization is warranted after completion of the diagnostic angiogram, particularly when PCI is considered in the same session (i.e. ad hoc PCI).4,5 This approach fails to address the potential importance of patient selection for diagnostic coronary angiography—an invasive procedure requested by a range of provider types and specialties. As an example, the clinical benefit of coronary angiography and PCI is unclear among patients without ischemic symptoms.1,2,6 Given the potential for a diagnostic to therapeutic cascade in which an initial diagnostic test triggers subsequent treatments regardless of anticipated clinical benefit,7–9 it is possible greater use of angiography in asymptomatic patients leads to more frequent AUC-defined inappropriate PCI. Alternatively, as the decision to proceed with PCI can be made independently of the decision to undertake coronary angiography, patient selection for angiography and PCI may be unrelated. Understanding the relationship between patient selection for coronary angiography and appropriate use of PCI may guide future strategies to improve patient selection for both procedures.
We sought to determine if hospitals’ rates of performing elective coronary angiography in asymptomatic patients are associated with their rates of PCI appropriateness in a national sample of hospitals participating in the CathPCI Registry. We determined the hospital proportion of asymptomatic patients at coronary angiography as a facility-level measure of patient selection for elective angiography. We emphasized symptom status in assessing patient selection for coronary angiography given the implications of symptoms on the appropriateness of PCI.
METHODS
Data Source
The CathPCI Registry is the largest registry of diagnostic cardiac catheterization and PCI in the U.S., with more than 1,400 participating centers.10,11 Captured data includes patient and hospital characteristics, procedural indication, findings, interventions, and outcomes based on pre-specified data elements.12 Data quality assurance is achieved through automatic system validation and reporting of data completeness, education and training for site data managers, and random on-site auditing. The audit process includes more than 50 fields (with fields rotating in a 3-year cycle) with between 300 to 625 records audited annually at 25 randomly identified sites (i.e., 12 to 25 records per audited site).13,14
Study Population
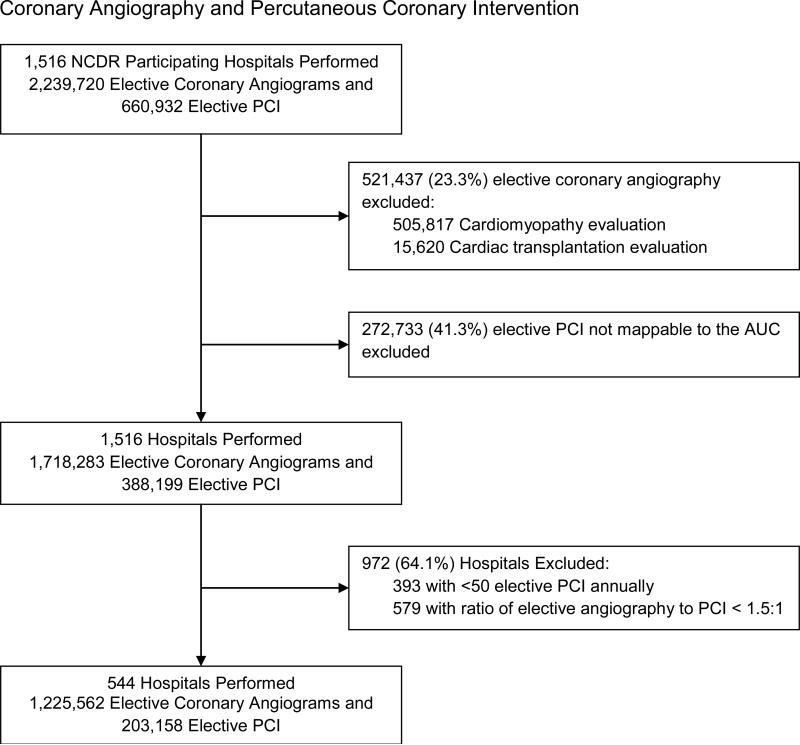
We identified 2,239,720 patients undergoing elective (non-acute) diagnostic coronary angiography and 660,932 patients undergoing elective PCI at 1,516 CathPCI participating hospitals between July 2009 and September 2013. Coronary angiography and PCI for acute indications, including acute coronary syndromes, acute myocardial infarction, or cardiogenic shock, were not included. Of patients with elective indications for coronary angiography, we excluded 521,437 (23.3%) patients undergoing angiography performed in consideration of transplantation, prior cardiac transplantation, or evaluation of cardiomyopathy. Of patients undergoing elective PCI, we excluded 272,733 (41.3%) that could not be mapped to the AUC due to missing necessary data elements (e.g. noninvasive stress test results). We also excluded 393 (25.9%) sites with annual non-acute PCI volume < 50 to avoid inflation of variance due to small numbers. Additionally, as not all participating hospitals report angiograms, we excluded 579 (38.2%) hospitals reporting fewer than 50% more elective diagnostic coronary angiograms relative to elective PCI (ratio of coronary angiography to PCI less than 1.5 to 1). Our final analytic cohort included 544 hospitals that performed 1,225,562 elective coronary angiograms and 203,158 elective PCI.
Assessing PCI Appropriateness
Each PCI in our cohort was mapped to an AUC clinical indication using algorithms to assign procedural appropriateness of “appropriate”, “uncertain”, or “inappropriate”, based upon the 2012 publication of the AUC.1 In these criteria, PCI are considered inappropriate when the procedure is unlikely to improve the patient's health status (symptoms, function, or quality of life) or survival.1,3,15 The 2012 AUC were applied as they provide greater specificity in defining non-acute indications than 2009 criteria.1,16
Statistical Analysis
For each hospital, we determined the proportion of asymptomatic patients (“no symptoms, no angina” per CathPCI Registry data element #5000) among those undergoing elective diagnostic coronary angiography. We compared patient and hospital level characteristics across quartiles of hospitals’ proportions of asymptomatic patients at angiography. We also compared baseline patient characteristics at elective PCI by hospital quartiles of asymptomatic patients at angiography. Comparisons of patient characteristics were completed using linear trend tests for continuous variables and Mantel-Haenszel trend test for categorical variables. Comparisons of hospital-level characteristics were completed using Mantel-Haenszel trend tests, with the exception of median procedural volumes that were compared using Kruskal-Wallis test.
We then plotted each hospital's proportion of inappropriate PCI against their proportion of angiography performed in asymptomatic patients and assessed the relationship using Spearman's correlation coefficient. We compared hospital median PCI appropriateness ratings by hospital quartiles of asymptomatic patients at angiography using the Kruskal-Wallis test. Further, we compared the proportion of inappropriate PCI attributable to being performed in asymptomatic patients by hospital quartiles of asymptomatic patients at angiography using Mantel-Haenszel trend tests.
Sensitivity Analysis
To ensure findings from our primary analysis did not reflect inclusion of hospitals that did not report all angiograms to CathPCI Registry (in the absence of performing PCI), we repeated our analyses after excluding facilities with less than twice as many elective angiograms reported than elective PCI (ratio less than 2 to 1).
All statistical analyses were performed with SAS 9.2 (SAS Institute, Inc, Cary, NC) and evaluated at a significance level of 0.05. Waiver of written informed consent and authorization for this study was granted by Chesapeake Research Review Incorporated.
RESULTS
Elective Diagnostic Coronary Angiography
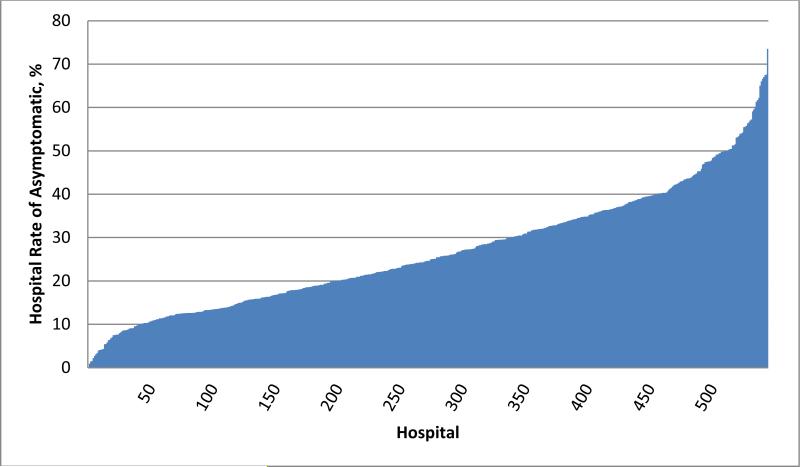
Of the 1,225,562 patients who underwent elective diagnostic coronary angiography, 308,083 (25.1%) were asymptomatic at the time of angiography. The median hospital proportion of angiography performed in asymptomatic patients was 24.7%, with an interquartile range from 15.9% to 35.9% and a range from 1.0% to 73.6% (Figure 2). Categorized by quartiles, the median proportion of asymptomatic patients was 12.1% in lowest-quartile hospitals, 20.3% in second lowest-quartile, 30.2% in second highest-quartile, and 43.2% in highest-quartile hospitals (Table 1).
Figure 2.
Variation in Hospital Rates of Asymptomatic Patients at Elective Diagnostic Coronary Angiography
Table 1.
Baseline Patient Characteristics at Angiography by Hospital Quartile of Asymptomatic Patients at Angiography
| Hospital Quartile of Asymptomatic Patients at Angiography | ||||||
|---|---|---|---|---|---|---|
| Patient Characteristics | All patients (n=1,225,562) | 1 – Lowest (n=358,265) | 2 (n=317,106) | 3 (n=282,219) | 4 – Highest (n=267,972) | P value |
| Hospital median proportion of asymptomatic, % (IQR) | 24.7 (15.9, 35.9) | 12.1 (1.0, 13.6) | 20.3 (18.1, 22.3) | 30.2 (27.4, 32.9) | 43.2 (39.0, 49.9) | |
| Hospital range of asymptomatic, % | 1.0-73.6 | 1.0-15.9 | 15.9-24.7 | 24.7-35.8 | 36.0-73.6 | |
| Demographics | ||||||
| Age, mean (SD), y | 63.8 ± 12.0 | 63.2 ± 12.1 | 63.7 ± 12.0 | 64.0 ± 12.0 | 64.4 ± 12.1 | <.001 |
| Male (%) | 696379 (56.8) | 197153 (55.0) | 179704 (56.7) | 162931 (57.7) | 156591 (58.4) | <.001 |
| White (%) | 319884 (89.3) | 319884 (89.3) | 277919 (87.6) | 242214 (85.8) | 229731 (85.7) | <.001 |
| Insurance | ||||||
| Private | 832703 (67.9) | 234933 (65.6) | 212255 (66.9) | 195780 (69.4) | 189735 (70.8) | |
| Public only | 352732 (28.8) | 110089 (30.7) | 94537 (29.8) | 77696 (27.5) | 70410 (26.3) | <.001 |
| None | 40127 (3.3) | 13243 (3.7) | 10314 (3.3) | 8743 (3.1) | 7827 (2.9) | <.001 |
| Cardiac History | ||||||
| Prior MI | 213461 (17.4) | 60401 (16.9) | 57041 (18.0) | 50552 (17.9) | 45467 (17.0) | 0.04 |
| Prior PCI | 314541 (25.7%) | 96442 (26.9) | 81959 (25.9) | 70481 (25.0) | 65659 (24.5) | <.001 |
| Prior CABG | 155012 (12.6) | 46452 (13.0) | 39584 (12.5) | 34690 (12.3) | 34286 (12.8) | 0.001 |
| Clinical Risk Factors and Comorbidities, No. (%) | ||||||
| Diabetes | 396783 (32.4) | 116474 (32.5) | 103094 (32.5) | 92140 (32.7) | 85075 (31.8) | <.001 |
| Family history of premature CAD | 316242 (25.8) | 99297 (27.7) | 88066 (27.8) | 67896 (24.1) | 60983 (22.8) | <.001 |
| Dialysis | 26097 (2.1) | 5264 (1.5) | 5275 (1.7) | 7039 (2.5) | 8519 (3.2) | <.001 |
| Clinical Presentation | ||||||
| Symptoms, No. (%) | ||||||
| No symptoms, no angina | 308083 (25.1) | 38885 (10.9) | 64889 (20.5) | 84808 (30.1) | 119501 (44.6) | |
| Symptom unlikely to be ischemic | 321683 (26.2) | 102442 (28.6) | 91690 (28.9) | 72035 (25.5) | 55516 (20.7) | <.001 |
| Stable angina | 595796 (48.6) | 216938 (60.6) | 160527 (50.6) | 125376 (44.4) | 92955 (34.7) | |
| Anginal Classification | ||||||
| No angina | 398508 (32.6) | 63622 (17.8) | 89360 (28.2) | 109830 (39.0) | 135696 (50.7) | |
| CCS I | 219734 (18.0) | 76500 (21.5) | 58208 (18.4) | 46699 (16.6) | 38327 (14.3) | |
| CCS II | 444507 (36.4) | 159391 (44.7) | 128609 (40.6) | 92960 (33.0) | 63547 (23.7) | <.001 |
| CCS III | 128258 (10.5) | 49034 (13.8) | 32679 (10.3) | 23862 (8.5) | 22683 (8.5) | |
| CCS IV | 31396 (2.6) | 7931 (2.2) | 7722 (2.4) | 8291 (2.9) | 7452 (2.8) | |
| Any anti-anginal medication | 710075 (58.0) | 208004 (58.1) | 187082 (59.0) | 166121 (58.9) | 148868 (55.6) | <.001 |
| Noninvasive testing, No. (%) | ||||||
| Performed | 756926 (61.8) | 215221 (60.1) | 201748 (63.6) | 176817 (62.7) | 163140 (60.9) | <.001 |
| Noninvasive Result, No (%) | ||||||
| Low-risk | 229700 (30.3) | 62775 (29.2) | 61605 (30.5) | 55543 (31.4) | 49777 (30.5) | |
| Intermediate-risk | 203935 (26.9) | 55920 (26.0) | 51835 (25.7) | 50962 (28.8) | 45218 (27.7) | <.001 |
| High-risk | 70672 (9.3) | 19141 (8.9) | 17389 (8.7) | 17900 (10.1) | 16242 (10.0) | |
| Missing | 252619 (33.4) | 77385 (36.0) | 70919 (35.2) | 52412 (29.6) | 51903 (31.8) | |
| Preoperative Evaluation Before Non-Cardiac Surgery | 100901 (8.2) | 20263 (5.7) | 24250 (7.6) | 25571 (9.1) | 30817 (11.5) | < .001 |
| Angiographic Findings, No. (%) | ||||||
| Obstructive CAD | 549285 (44.8) | 156279 (43.6) | 141675 (44.7) | 128603 (45.6) | 122728 (45.8) | <.001 |
| 1 vessel CAD | 242088 (19.8) | 69487 (19.4) | 62874 (19.8) | 56305 (20.0) | 53422 (19.9) | <.001 |
| 2 vessel CAD | 160019 (13.1) | 45199 (12.6) | 41380 (13.0) | 37738 (13.4) | 35702 (13.3) | <.001 |
| 3 vessel CAD | 147178 (12.0) | 41593 (11.6) | 37421 (11.8) | 34560 (12.2) | 33604 (12.5) | <.001 |
Abbreviations: CABG, coronary artery bypass graft; CAD, coronary artery disease; CCS, Canadian Cardiovascular Society; IQR, interquartile range; MI, myocardial infarction; PCI, percutaneous coronary intervention; SD, standard deviation
Comparisons across hospital quartiles of asymptomatic patients at angiography were statistically significant for all baseline characteristics given our large sample size; however, most differences were small. Results of coronary angiography demonstrated slightly higher rates of obstructive coronary disease among patients at hospitals with higher rates of angiography in asymptomatic patients (43.6% vs. 44.7% vs. 45.6% vs. 45.8%, P<.001 for trend).
In the evaluation of hospital characteristics, hospitals with a higher proportion of asymptomatic patients at angiography were more likely to be a teaching hospital (32.4% vs. 43.4 vs. 47.1 vs. 53.7%, P<.001). Procedural volumes were slightly lower at hospitals with higher rates of asymptomatic patients at angiography, although this was only statistically significant for elective angiography volumes (Table 2).
Table 2.
Hospital Characteristics by Hospital Quartile of Asymptomatic at Angiography
| Hospital Quartile of Asymptomatic Patients at Angiography | ||||||
|---|---|---|---|---|---|---|
| Hospital Characteristics | All Hospitals (n=544) | 1 – Lowest (n=136) | 2 (n=136) | 3 (n=136) | 4 – Highest (n=136) | P value |
| Median proportion of asymptomatic, % (range) | 24.7 (1.0-73.6) | 12.1 (1.0-15.9) | 20.3 (15.9-24.7) | 30.2 (24.7-35.8) | 43.2 (36.0-73.6) | |
| Hospital location, No. (%) | ||||||
| Rural | 80 (14.7) | 29 (21.3) | 26 (19.1) | 11 (8.1) | 14 (10.3) | |
| Suburban | 176 (32.4) | 39 (28.7) | 33 (24.3) | 54 (39.7) | 50 (36.8) | 0.08 |
| Urban | 288 (52.9) | 68 (50.0) | 77 (56.6) | 71 (52.2) | 72 (52.9) | |
| Hospital type | ||||||
| Government | 5 (0.9) | 1 (0.7) | 3 (2.2) | 0 (0) | 1 (0.7) | |
| Private/Community | 485 (89.2) | 128 (94.1) | 125 (91.9) | 121 (89.0) | 111 (81.6) | .001< |
| University | 54 (9.9) | 7 (5.1) | 8 (5.9) | 15 (11.0) | 24 (17.6) | |
| Teaching Hospital | 240 (44.1) | 44 (32.4) | 59 (43.4) | 64 (47.1) | 73 (53.7) | <.001 |
| Public Hospital | 185 (34.0) | 38 (27.9) | 48 (35.3) | 44 (32.4) | 55 (40.4) | 0.06 |
| Region | ||||||
| Midwest | 172 (31.6) | 45 (33.1) | 42 (30.9) | 39 (28.7) | 46 (33.8) | |
| Northeast | 62 (11.4) | 8 (5.9) | 19 (14.0) | 20 (14.7) | 15 (11.0) | 0.51 |
| South | 212 (39.0) | 68 (50.0) | 52 (38.2) | 51 (37.5) | 41 (30.1) | |
| West | 98 (18.0) | 15 (11.0) | 23 (16.9) | 26 (19.1) | 34 (25.0) | |
| Annual procedural volume, mean (SD) | ||||||
| Total coronary angiography | 1372 (913, 2108) | 1462 (900, 2211) | 1384 (929, 2125) | 1337 (898, 2200) | 1370 (913, 1969) | 0.75 |
| Elective coronary angiography | 460.1 (314.8, 693.9) | 549.7 (337.1, 791.5) | 477.3 (334.0, 698.6) | 418.9 (308.7, 621.5) | 390.2 (278.3, 585.6) | 0.001 |
| Total PCI Volume | 507 (341, 780) | 509 (325, 787) | 528 (359, 796) | 496 (328, 809) | 492 (354, 755) | 0.89 |
| Elective PCI Volume | 135 (93, 217) | 149 (92, 243) | 137 (98, 248) | 128 (95, 194) | 131 (86, 199) | 0.42 |
Abbreviations: PCI, percutaneous coronary intervention; SD, standard deviation
Elective PCI
In the 203,158 patients who underwent elective PCI, comparisons across hospital quartiles of asymptomatic patients at coronary angiography were statistically significant for most comorbidities and risk factors given our large sample size; however, differences were small (Table 3). Patients receiving PCI at hospitals with more asymptomatic patients were slightly less likely to receive anti-anginal medications prior to PCI (2 or more anti-anginals 25.1% vs. 23.9% vs. 23.7% vs. 22.9%, P<.001). Use of pre-procedural stress testing was higher among patients receiving PCI at hospitals with higher rates of angiography in asymptomatic patients (61.4% vs. 66.8% vs. 69.6% vs. 68.8%, P<.001 for trend).
Table 3.
Baseline Patient Characteristics at Elective PCI by Hospital Quartile of Asymptomatic Patients at Angiography
| Hospital Quartile of Asymptomatic Patients at Angiography | ||||||
|---|---|---|---|---|---|---|
| Patient Characteristics | All patients (n=203,158) | 1 – Lowest (n=57,721) | 2 (n=51,544) | 3 (n=48,286) | 4 – Highest (n=45,607) | P value |
| Demographics | ||||||
| Age, mean ± SD, y | 66.2 ± 11.0 | 65.9 ± 10.9 | 66.2 ± 11.0 | 66.1 ± 10.9 | 66.5 ± 11.1 | <.001 |
| Male (%) | 138033 (67.9) | 38108 (66.0) | 35060 (68.0) | 33277 (68.9) | 31588 (69.3) | <.001 |
| White (%) | 179669 (88.4%) | 52058 (90.2%) | 46121 (89.5%) | 41594 (86.1%) | 39896 (87.5%) | <.001 |
| Insurance | ||||||
| Private | 137498 (67.7) | 37615 (65.2) | 34644 (67.2) | 33222 (68.8) | 32017 (70.2) | <.001 |
| Public only | 60221 (29.6) | 18445 (32.0) | 15459 (30.0) | 15064 (31.2) | 12509 (27.4) | <.001 |
| None | 5439 (2.7) | 1661 (2.9) | 1441 (2.8) | 1256 (2.6) | 1081 (2.4) | <.001 |
| Clinical Risk Factors and Comorbidities, No. (%) | ||||||
| Use of tobacco | 42071 (20.7) | 13420 (23.3) | 10711 (20.8) | 9403 (19.5) | 8537 (18.7) | <.001 |
| Hypertension | 174694 (86.0) | 49908 (86.5) | 44398 (86.2) | 41428 (85.8) | 38960 (85.4) | <.001 |
| Dyslipidemia | 171307 (84.4) | 48020 (83.3) | 43866 (85.2) | 40977 (84.9) | 38444 (84.3) | <.001 |
| Family history of CAD | 53603 (26.4) | 17027 (29.5) | 14410 (28.0) | 11680 (24.2) | 10486 (23.0) | <.001 |
| Prior MI | 56086 (27.6) | 14993 (26.0) | 15010 (29.1) | 13710 (28.4) | 12373 (27.1) | <.001 |
| Heart Failure | 25119 (12.4) | 6855 (11.9) | 6396 (12.4) | 5932 (12.3) | 5936 (13.0) | <.001 |
| Prior Valve Surgery | 3077 (1.5) | 727 (1.3) | 828 (1.6) | 724 (1.5) | 798 (1.8) | <.001 |
| Prior PCI | 85520 (42.1) | 25174 (43.6) | 21819 (42.3) | 19966 (41.4) | 18561 (40.7) | <.001 |
| Prior CABG | 27456 (13.5) | 7569 (13.1) | 6908 (13.4) | 6546 (13.6) | 6433 (14.1) | <.001 |
| Hemodialysis | 5617 (2.8) | 1130 (2.0) | 1122 (2.2) | 1623 (3.4) | 1742 (3.8) | <.001 |
| Cerebrovascular Disease | 25733 (12.7) | 7592 (13.2) | 6536 (12.7) | 5966 (12.4) | 5639 (12.4) | <.001 |
| Peripheral Arterial Disease | 28152 (13.9) | 8652 (15.0) | 6493 (12.6) | 6401 (13.3) | 6606 (14.5) | 0.03 |
| Chronic Lung Disease | 31060 (15.3) | 9687 (16.8) | 8133 (15.8) | 6918 (14.3) | 6322 (13.9) | <.001 |
| Diabetes Mellitus | 79503 (39.1) | 22575 (39.1) | 20032 (38.9) | 19046 (39.5) | 17850 (39.1) | 0.49 |
| Clinical Presentation, No. (%) | ||||||
| Symptoms | ||||||
| No symptoms | 48872 (24.1) | 6131 (10.6) | 10141 (19.7) | 13704 (28.4) | 18896 (41.4) | |
| Symptom unlikely to be ischemic | 22832 (11.2) | 6442 (11.2) | 6469 (12.6) | 5592 (11.6) | 4329 (9.5) | <.001 |
| Stable angina | 131454 (64.7) | 45148 (78.2) | 34934 (67.8) | 28990 (60.0) | 22382 (49.1) | |
| Angina Severity | ||||||
| No angina | 53606 (26.4) | 7646 (13.2) | 11757 (22.8) | 15002 (31.1) | 19201 (42.1) | |
| CCS I | 26119 (12.9) | 7745 (13.4) | 6734 (13.1) | 6456 (13.4) | 5184 (11.4) | |
| CCS II | 79939 (39.3) | 27159 (47.1) | 21940 (42.6) | 18020 (37.3) | 12820 (28.1) | <.001 |
| CCS III | 37450 (18.4) | 13534 (23.4) | 9582 (18.6) | 7323 (15.2) | 7011 (15.4) | |
| CCS IV | 6044 (3.0) | 1637 (2.8) | 1531 (3.0) | 1485 (3.1) | 1391 (3.0) | |
| Anti-anginal medications | ||||||
| 0 | 57867 (28.5) | 16620 (28.8) | 14031 (27.2) | 13362 (27.7) | 13854 (30.4) | |
| 1 | 96555 (47.5) | 26598 (46.1) | 25211 (48.9) | 23461 (48.6) | 21285 (46.7) | <.001 |
| ≥ 2 | 48716 (24.0) | 14495 (25.1) | 12299 (23.9) | 11458 (23.7) | 10464 (22.9) | |
| Noninvasive testing | ||||||
| Performed | 134804 (66.4) | 35421 (61.4) | 34428 (66.8) | 33591 (69.6) | 31364 (68.8) | <.001 |
| 9 | 5 | 2 | 2 | |||
| Noninvasive Result when Performed | ||||||
| Low-risk | 38068 (28.2) | 9920 (28.0) | 9999 (29.0) | 9202 (27.4) | 8947 (28.5) | |
| Intermediate-risk | 58815 (43.6) | 15444 (43.6) | 14890 (43.2) | 14852 (44.2) | 13629 (43.5) | <.01 |
| High-risk | 31209 (23.2) | 7922 (22.4) | 7853 (22.8) | 8105 (24.1) | 7329 (23.4) | |
| Missing | 6712 (5.0) | 2135 (6.0) | 1686 (4.9) | 1432 (4.3) | 1432 (4.6) | |
| Coronary artery stenoses | ||||||
| 1 | 114305 (56.3) | 33988 (58.9) | 28630 (55.5) | 26630 (55.2) | 25057 (54.9) | |
| 2 | 56443 (27.8) | 15250 (26.4) | 14562 (28.3) | 13715 (28.4) | 12916 (28.3) | <.001 |
| 3 | 29198 (14.4) | 7622 (13.2) | 7402 (14.4) | 7309 (15.1) | 6865 (15.1) | |
| Significant proximal LAD stenosis | 45612 (22.5) | 12150 (21.0) | 11679 (22.7) | 11063 (22.9) | 10720 (23.5) | <.001 |
Abbreviations: CABG, coronary artery bypass graft; CAD, coronary artery disease; CCS, Canadian Cardiovascular Society; IQR, interquartile range; LAD, left anterior descending; MI, myocardial infarction; PCI, percutaneous coronary intervention;
PCI Appropriateness by Hospital Rate of Asymptomatic at Angiography
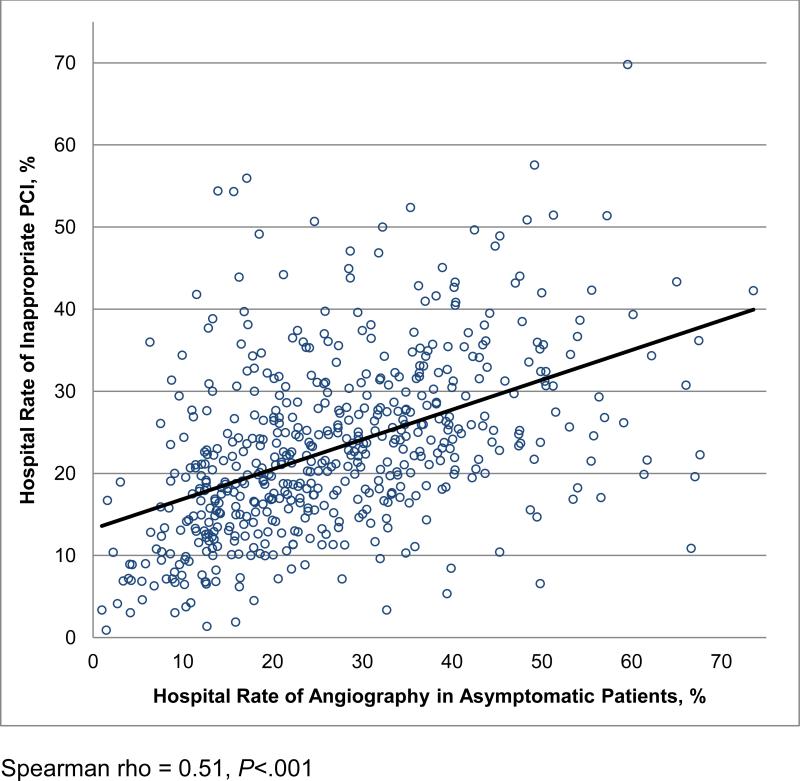
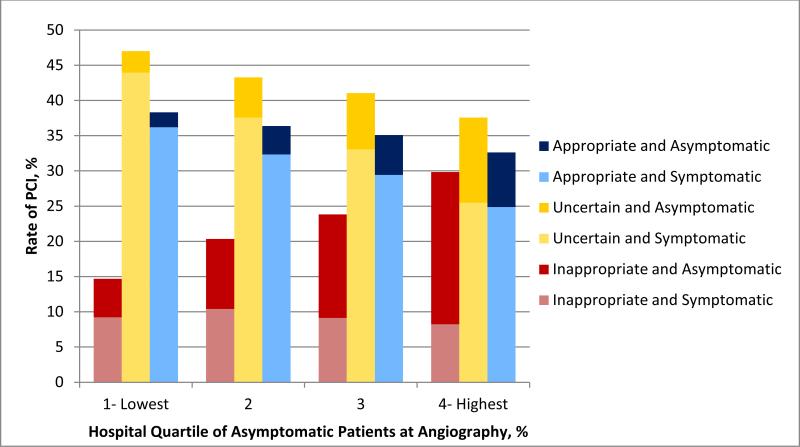
The hospital proportion of angiography performed in asymptomatic patients was positively associated with rates of inappropriate PCI (Figure 3) (Spearman rho = 0.51; P<.001). Similarly, by hospital quartiles of asymptomatic patients at angiography, hospitals with higher rates of asymptomatic patients at angiography had higher median rates of inappropriate PCI (14.8% vs. 20.2% vs. 24.0% vs. 29.4%, P for trend <.001) (Table 4). Hospitals with higher rates of asymptomatic patients at angiography also had lower rates of ‘uncertain’ PCI and lower rates of appropriate PCI (38.6% vs. 33.0% vs. 32.3% vs. 32.9%, P for trend <.001). At the patient level, the rate of inappropriate PCI was attributable to more frequent use of PCI in asymptomatic patients at hospitals with higher rates of angiography performed in asymptomatic patients (Figure 4). These findings were unchanged in our sensitivity analysis of hospitals reporting at least twice as many elective coronary angiograms as elective PCI (Online Supplement).
Figure 3.
Hospital Rate of Inappropriate PCI by Rate of Angiography Performed in Asymptomatic Patients
Table 4.
Hospital PCI Appropriateness for Non-Acute Indications by Hospital Quartile of Asymptomatic Patients at Angiography
| Hospital Quartile of Asymptomatic Patients | ||||||
|---|---|---|---|---|---|---|
| Hospital Characteristics | All Hospitals (n=539) | 1 – Lowest (n=134) | 2 (n=135) | 3 (n=135) | 4 – Highest (n=135) | P value |
| Median proportion of asymptomatic, % (IQR) | 24.7 (15.9, 35.9) | 12.1 (1.0, 13.6) | 20.3 (18.1, 22.3) | 30.2 (27.4, 32.9) | 43.2 (39.0, 49.9) | |
| Range of asymptomatic, % | 1.0-73.6 | 1.0-15.9 | 15.9-24.7 | 24.7-35.8 | 36.0-73.6 | |
| PCI Appropriateness, median (IQR) % | ||||||
| Inappropriate | 22.0 (15.7, 29.4) | 14.8 (9.7, 19.9) | 20.2 (15.7, 26.3) | 24.0 (18.2, 28.3) | 29.4 (22.9, 35.7) | <.001 |
| Uncertain | 41.3 (34.2, 48.2) | 45.1 (35.3, 52.6) | 44.2 (36.8, 50.5) | 40.2 (33.6, 47.6) | 36.8 (32.6, 42.4) | <.001 |
| Appropriate | 33.9 (25.1, 43.7) | 38.7 (27.4, 50.4) | 33.0 (25.1, 44.1) | 32.3 (24.9, 42.7) | 32.9 (24.2, 40.3) | <.001 |
Abbreviations: IQR, interquartile range
Figure 4.
PCI Appropriateness by Quartile of Asymptomatic Patients at Angiography
COMMENT
We sought to determine if hospital-level patient selection for diagnostic coronary angiography, as assessed by symptom status at the time of the procedure, is associated with PCI appropriateness. In 544 hospitals participating in the CathPCI Registry that performed elective coronary angiography on more than 1 million patients, 25% of patients were asymptomatic at the time of coronary angiography. We observed marked variation in the hospital rate of angiography performed in asymptomatic patients, ranging from 1.0% to 73.6%. Hospitals with higher rates of asymptomatic patients at angiography also had higher rates of inappropriate PCI, due to greater use of PCI in asymptomatic patients. Hospitals with higher rates of asymptomatic patients at angiography also had lower rates of appropriate PCI. These findings suggest patient selection for coronary angiography is associated with the quality of patient selection for PCI as determined by Appropriate Use Criteria.
In a prior study from the CathPCI Registry, the hospital rate of inappropriate PCI was observed to vary from 0 to 55%.3 This variation has subsequently been observed in other regional PCI quality improvement programs.17,18 In these studies, PCI performed in asymptomatic patients was found to account for nearly half of the procedures categorized as inappropriate.3 Other factors accounting for PCI being classified as inappropriate included submaximal anti-anginal therapy or low ischemic risk by pre-procedural stress testing.3,17 These findings prompted suggestions by interventional cardiology associations to ensure the patient's symptom status, medical regimen, and ischemic risk are assessed upon completion of the coronary angiogram to confirm revascularization is warranted prior to proceeding with PCI.4
Although a strategy of clinical assessment immediately prior to PCI may minimize inappropriate use of the procedure, our findings suggest an opportunity to address patient selection upstream of the catheterization laboratory to optimize use of both angiography and PCI. Our findings of hospital variation in patient selection for angiography as determined by symptom status complement a prior study in which hospital rates of obstructive coronary disease were used as a measure of patient selection for the procedure.19 In this prior study, the median hospital rate of obstructive coronary angiography (defined as stenosis > 50% left main or > 70% in any other epicardial coronary) was 30% and ranged from 15% to 100%. Together, these findings suggest strategies are needed to improve patient selection for coronary angiography, a procedure requested by a range of provider types and specialties for more than 1 million U.S. patients annually at an average cost of $9000 per procedure.20,21
Concerns for a diagnostic to therapeutic cascade have long been present in the use of coronary angiography and PCI.7–9 In this cascade, PCI is performed for obstructive coronary lesions identified at angiography, regardless of whether revascularization is indicated.22 However, prior studies were limited to comparison of rates of diagnostic and therapeutic coronary procedures as indirect evidence of this cascade. Our study provides more direct evidence of this cascade based on assessment of the clinical scenarios for angiography and PCI.
While proposals to reassess the indication for PCI at the completion of angiography may inhibit the so called “oculostenotic reflex” leading to a therapeutic cascade,4,5,23 optimal patient selection for coronary angiography may reduce the opportunity for this cascade altogether. As a corollary, ensuring the indication for both coronary angiography and potential PCI are addressed prior to the catheterization laboratory may reduce barriers to appropriate use of ad hoc PCI; a procedural strategy that reduces patient inconvenience and cost.22,23 Thus, the onus on proper patient selection for PCI rests not only with the interventional cardiologist, but equally with the referring physicians (e.g. cardiologists, internists, family physicians) for coronary angiography.
Possible reasons for a diagnostic to therapeutic cascade in the use of coronary procedures include perceived patient expectations, 26 a belief in the benefits of PCI for ischemia, and the open artery hypothesis.22 We observed greater use of pre-procedural stress testing among patients receiving PCI at hospitals with higher rates of angiography in asymptomatic patients; a finding that is consistent with use of screening stress testing to identify silent ischemia leading to angiography and PCI despite uncertain benefit.1,27,28 An emphasis on screening of asymptomatic patients with clinical risk factors may also explain the minimal differences in patient characteristics across hospital quartiles. It is worth noting the AUC for Coronary Revascularization considers PCI in asymptomatic patients with CAD involving the left main coronary, proximal left anterior descending coronary, or 3-vessel CAD to be at worst of “uncertain” appropriateness. Thus, our findings do not reflect greater use of PCI in patients with high-risk CAD at hospitals performing more angiography in asymptomatic patients. Potential approaches to addressing gaps in proper patient selection for invasive coronary procedures include greater patient involvement in the decision process,29 patient decision support,30,31 and application of the AUC in measurement, reporting, and clinical decision support.
Previously, the rate of normal coronary angiography has been proposed as an indirect measure of the quality of patient selection for angiography.32–34 We observed a rate of obstructive coronary disease that was slightly higher at hospitals with a larger proportion of asymptomatic patients at angiography. This suggests the results of angiography may not accurately reflect the quality of patient selection as determined by pre-procedural characteristics of clinical decision-making. In addition, the facility rate of obstructive coronary disease lacks a target for quality improvement in patient selection. Alternatively, an emphasis on procedural indication reflected by symptoms, ischemic risk, and the potential implications of angiographic findings may support strategies to ensure patients selected for angiography are anticipated to benefit from the procedure.
Our study should be considered in light of the following limitations. First, the decision to proceed to coronary angiography often incorporates an understanding of the patient's global coronary risk, noninvasive study results, and symptoms. This approach is the basis for recently published AUC for diagnostic coronary angiography.2 In our approach to ascertaining patient selection for coronary angiography, we were unable to use these AUC due to high rates of missingness for elements necessary to estimate Framingham risk (99% missing) or noninvasive study results (38% not performed, 33% missing results). As patient symptoms were uniformly collected, we emphasized this pre-procedural data element as our primary mode of ascertaining patient selection for the procedure. As the decision to proceed with coronary angiography is not a mandate to proceed to PCI, these findings are not a tautology of the AUC. Second, our study does not address the clinical outcomes associated with a strategy of performing PCI in asymptomatic patients and although the clinical benefit of coronary angiography in asymptomatic patients is less clear, this does not equate to an inappropriate procedure. Application of the AUC for coronary angiography as a more inclusive measure of patient selection in diagnostic coronary angiography is an area for future research. Third, we are unable to ensure hospitals included in the analysis reported all coronary angiograms to CathPCI Registry, as reporting of coronary angiography is voluntary in the program. However, sensitivity analysis suggests our findings were not influenced by inclusion of hospitals with incomplete reporting of angiography data. Fourth, CathPCI Registry data elements do not include all possible indications for coronary angiography, with a notable example being angiography performed prior to consideration of valve surgery. However, failure to exclude these patients would likely bias our association toward the null as evidence of obstructive coronary disease in this population would likely lead to coronary bypass at the time of valve surgery rather than PCI. Fifth, the generalizability of our findings may be impacted by the limitation of our analysis to hospitals that participated in the CathPCI Registry and performed at least 50 elective PCI annually. However, studies have demonstrated the appropriateness of coronary procedures performed in non-CathPCI Registry hospitals are comparable to those observed within the registry.17 Sixth, we cannot exclude potential misclassification of patient symptoms. However, we are reassured by findings congruent with more aggressive use of coronary procedures at hospitals with more asymptomatic patients, including lower use of anti-anginal medications and lower symptom severity among those patients with angina. Seventy, although we explored facility-level factors associated with rates of angiography in asymptomatic patients, our analysis does not define hospital factors that are correlated with rates of inappropriate PCI. Eighth, current AUC consider the implications of fractional flow reserve in assessing the physiologic significance of CAD in a limited number of PCI scenarios.1,35,36 However, recent data from the CathPCI Registry demonstrates fractional flow reserve is performed in only 6% of patients with intermediate coronary lesions (40-70% stenosis).37 Finally, we are unable to comment on the potential for underuse of invasive coronary procedures as we lacked data on patients who do not undergo the procedure.
In conclusion, at 544 U.S. hospitals participating in the CathPCI Registry, approximately 1 in 4 patients were asymptomatic at the time of elective coronary angiography. We observed wide hospital-level variation in the rate of asymptomatic patients at angiography, and higher hospital rates were associated with higher rates of inappropriate PCI and lower rates of appropriate PCI. Current emphasis on proper patient selection for PCI alone fails to address the dramatic variation in the use of upstream diagnostic coronary angiography. Furthermore, strategies upstream of the cardiac catheterization laboratory to improve patient selection for coronary angiography may concurrently reduce inappropriate use of PCI and barriers to appropriate use of ad hoc PCI.
Supplementary Material
Figure 1.
Identification of Hospitals Performing Elective (Non-acute) Diagnostic Coronary Angiography and Percutaneous Coronary Intervention
ACKNOWLEDGEMENTS
Dr. Bradley had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bradley, Spertus, Chan, Patel, and Rumsfeld.
Analysis and interpretation of data: Bradley, Spertus, Kennedy, Nallamothu, Chan, Patel, Bryson, Malenka, Rumsfeld.
Drafting of the manuscript: Bradley.
Critical revision of the manuscript for important intellectual content: Bradley, Spertus, Kennedy, Nallamothu, Chan, Patel, Bryson, Malenka, Rumsfeld.
Statistical analysis: Kennedy.
Study supervision: Bradley and Rumsfeld.
Conflict of interest disclosures: Dr. Spertus reports serving as PI of a contract with the American College of Cardiology Foundation (ACCF) to conduct analyses of the National Cardiovascular Data Registry (NCDR). The other authors report no relevant disclosures. Funding sources/support: This research was supported by the ACCF's NCDR. Dr. Bradley is supported by VA Health Services Research & Development Career Development Award (HSR&D-CDA2 10-199).
Role of the sponsors: Representatives of the CathPCI Research and Publications committee approved the final manuscript.
Footnotes
Disclaimer: The views expressed in this manuscript represent those of the authors, and do not necessarily represent the official views, position, or policy of the NCDR, its associated professional societies identified at www.ncdr.com, the Department of Veterans Affairs, or the United States government.
REFERENCES
- 1.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012 Appropriate use criteria for coronary revascularization focused update: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, American Society of Nuclear Cardiology, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2012;59(9):857–881. doi: 10.1016/j.jacc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Patel MR, Bailey SR, Bonow RO, et al. ACCF/SCAI/AATS/AHA/ASE/ASNC/HFSA/HRS/SCCM/SCCT/SCMR/STS 2012 appropriate use criteria for diagnostic catheterization: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;59(22):1995–2027. doi: 10.1016/j.jacc.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Chan PS, Patel MR, Klein LW, et al. Appropriateness of percutaneous coronary intervention. JAMA. 2011;306(1):53–61. doi: 10.1001/jama.2011.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blankenship JC, Gigliotti OS, Feldman DN, et al. Ad hoc percutaneous coronary intervention: a consensus statement from the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv Off J Soc Card Angiogr Interv. 2013;81(5):748–758. doi: 10.1002/ccd.24701. [DOI] [PubMed] [Google Scholar]
- 5.Nallamothu BK, Krumholz HM. Putting ad hoc PCI on pause. JAMA. 2010;304(18):2059–2060. doi: 10.1001/jama.2010.1509. [DOI] [PubMed] [Google Scholar]
- 6.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60(24):e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Verrilli D, Welch HG. The impact of diagnostic testing on therapeutic interventions. JAMA. 1996;275(15):1189–1191. [PubMed] [Google Scholar]
- 8.Wennberg DE, Kellett MA, Dickens JD, Malenka DJ, Keilson LM, Keller RB. The association between local diagnostic testing intensity and invasive cardiac procedures. JAMA. 1996;275(15):1161–1164. [PubMed] [Google Scholar]
- 9.Lucas FL, Siewers AE, Malenka DJ, Wennberg DE. Diagnostic-therapeutic cascade revisited: coronary angiography, coronary artery bypass graft surgery, and percutaneous coronary intervention in the modern era. Circulation. 2008;118(25):2797–2802. doi: 10.1161/CIRCULATIONAHA.108.789446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College of Cardiology-National Cardiovascular Data Registry (ACC NCDR): building a national clinical data repository. J Am Coll Cardiol. 2001;37(8):2240–2245. doi: 10.1016/s0735-1097(01)01372-9. [DOI] [PubMed] [Google Scholar]
- 11.Weintraub WS, McKay CR, Riner RN, et al. The American College of Cardiology National Database: progress and challenges. American College of Cardiology Database Committee. J Am Coll Cardiol. 1997;29(2):459–465. doi: 10.1016/s0735-1097(96)00545-1. [DOI] [PubMed] [Google Scholar]
- 12. [June 13, 2013];NCDR® CathPCI Registry®. Available at: https://www.ncdr.com/webncdr/cathpci.
- 13.CathPCI Registry Companion Guide to Your NCDR Data Quality Report. American College of Cardiology Foundation; Washington, DC: 2008. [Google Scholar]
- 14.Messenger JC, Ho KKL, Young CH, et al. The National Cardiovascular Data Registry (NCDR) Data Quality Brief: the NCDR Data Quality Program in 2012. J Am Coll Cardiol. 2012;60(16):1484–1488. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Patel MR, Spertus JA, Brindis RG, et al. ACCF proposed method for evaluating the appropriateness of cardiovascular imaging. J Am Coll Cardiol. 2005;46(8):1606–1613. doi: 10.1016/j.jacc.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 16.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness Criteria for Coronary Revascularization: a report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2009;53(6):530–553. doi: 10.1016/j.jacc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Bradley SM, Maynard C, Bryson CL. Appropriateness of Percutaneous Coronary Interventions in Washington State. Circ Cardiovasc Qual Outcomes. 2012;5(4):445–53. doi: 10.1161/CIRCOUTCOMES.111.964320. [DOI] [PubMed] [Google Scholar]
- 18.Hannan EL, Cozzens K, Samadashvili Z, et al. Appropriateness of coronary revascularization for patients without acute coronary syndromes. J Am Coll Cardiol. 2012;59(21):1870–1876. doi: 10.1016/j.jacc.2012.01.050. [DOI] [PubMed] [Google Scholar]
- 19.Douglas PS, Patel MR, Bailey SR, et al. Hospital variability in the rate of finding obstructive coronary artery disease at elective, diagnostic coronary angiography. J Am Coll Cardiol. 2011;58(8):801–809. doi: 10.1016/j.jacc.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 20.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agency for Healthcare Research and Quality [June 30, 2013];Healthcare Cost and Utilization Project (HCUPnet) Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp.
- 22.Lin GA, Dudley RA, Redberg RF. Cardiologists’ use of percutaneous coronary interventions for stable coronary artery disease. Arch Intern Med. 2007;167(15):1604–1609. doi: 10.1001/archinte.167.15.1604. [DOI] [PubMed] [Google Scholar]
- 23.Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92(8):2333–2342. doi: 10.1161/01.cir.92.8.2333. [DOI] [PubMed] [Google Scholar]
- 24.Krone RJ, Shaw RE, Klein LW, Blankenship JC, Weintraub WS. American College of Cardiology - National Cardiovascular Data Registry. Ad hoc percutaneous coronary interventions in patients with stable coronary artery disease--a study of prevalence, safety, and variation in use from the American College of Cardiology National Cardiovascular Data Registry (ACC-NCDR). Catheter Cardiovasc Interv. 2006;68(5):696–703. doi: 10.1002/ccd.20910. [DOI] [PubMed] [Google Scholar]
- 25.Adele C, Vaitkus PT, Wells SK, Zehnacker JB. Cost advantages of an ad hoc angioplasty strategy. J Am Coll Cardiol. 1998;31(2):321–325. doi: 10.1016/s0735-1097(97)00512-3. [DOI] [PubMed] [Google Scholar]
- 26.Lin GA, Dudley RA, Redberg RF. Why physicians favor use of percutaneous coronary intervention to medical therapy: a focus group study. J Gen Intern Med. 2008;23(9):1458–1463. doi: 10.1007/s11606-008-0706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. J Am Coll Cardiol. 2009;53(23):2201–2229. doi: 10.1016/j.jacc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Douglas PS, Garcia MJ, Haines DE, et al. ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Society of Echocardiography, American Heart Association, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Critical Care Medicine, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance Endorsed by the American College of Chest Physicians. J Am Coll Cardiol. 2011;57(9):1126–1166. doi: 10.1016/j.jacc.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Pines JM, Szyld D. Risk tolerance for the exclusion of potentially life-threatening diseases in the ED. Am J Emerg Med. 2007;25(5):540–544. doi: 10.1016/j.ajem.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Rothberg MB, Sivalingam SK, Ashraf J, et al. Patients’ and cardiologists’ perceptions of the benefits of percutaneous coronary intervention for stable coronary disease. Ann Intern Med. 2010;153(5):307–313. doi: 10.7326/0003-4819-153-5-201009070-00005. [DOI] [PubMed] [Google Scholar]
- 31.Hess EP, Knoedler MA, Shah ND, et al. The chest pain choice decision aid: a randomized trial. Circ Cardiovasc Qual Outcomes. 2012;5(3):251–259. doi: 10.1161/CIRCOUTCOMES.111.964791. [DOI] [PubMed] [Google Scholar]
- 32.Bashore TM, Bates ER, Berger PB, et al. American College of Cardiology/Society for Cardiac Angiography and Interventions Clinical Expert Consensus Document on cardiac catheterization laboratory standards. A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37(8):2170–2214. doi: 10.1016/s0735-1097(01)01346-8. [DOI] [PubMed] [Google Scholar]
- 33.Douglas P, Iskandrian AE, Krumholz HM, et al. Achieving quality in cardiovascular imaging: proceedings from the American College of Cardiology-Duke University Medical Center Think Tank on Quality in Cardiovascular Imaging. J Am Coll Cardiol. 2006;48(10):2141–2151. doi: 10.1016/j.jacc.2006.06.076. [DOI] [PubMed] [Google Scholar]
- 34.Bradley SM, Maddox TM, Stanislawski MA, et al. Normal Coronary Rates for Elective Angiography in the VA Health Care System: Insights from the VA CART Program. J Am Coll Cardiol. 2014;63(5):417–26. doi: 10.1016/j.jacc.2013.09.055. [DOI] [PubMed] [Google Scholar]
- 35.Tonino PAL, De Bruyne B, Pijls NHJ, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360(3):213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 36.De Bruyne B, Pijls NHJ, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367(11):991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 37.Dattilo PB, Prasad A, Honeycutt E, Wang TY, Messenger JC. Contemporary patterns of fractional flow reserve and intravascular ultrasound use among patients undergoing percutaneous coronary intervention in the United States: insights from the National Cardiovascular Data Registry. J Am Coll Cardiol. 2012;60(22):2337–2339. doi: 10.1016/j.jacc.2012.08.990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.