Abstract
Objective
Examine factors that mediate parent-infant relationships 12 months after positive newborn screening (NBS).
Method
We examined effects of infant diagnosis, parents’ perceptions of child vulnerability and child attachment, parental depression and anxiety on parent-infant feeding interactions for 131 mothers and 118 fathers of 131 infants whose NBS and diagnostics confirmed cystic fibrosis (CF, n=23), congenital hypothyroidism (CH, n=35), CF carrier status (CF-C, n=38), or healthy, normal NBS (H, n=35).
Results
Separate composite indicator structural equation models for mothers and fathers showed neonatal diagnosis was not associated with increased anxiety or depression. In comparison to the H group, CF group parents reported higher perceptions of child vulnerability (p< 0.001, p=0.002); and CF-C group fathers viewed their children as more attached (p=0.021). High maternal perception of child vulnerability was associated with low perceptions of child attachment (p=0.001) which was associated with task-oriented feeding behavior (p=0.016, p=0.029). Parental task-oriented feeding behavior was associated with less positive (p< 0.001, p< 0.001) and more negative interactions (p< 0.001, p= 0.001) with their infants. High paternal perception of child vulnerability was associated with negative parent interactions (p< 0.001). High parental affective involvement and verbalization was associated with high infant affective expressiveness, communicative skills, and social responsiveness (mothers’ p< 0.001, fathers’ p< 0.001). High parental negative affect and/or inconsistent and intrusive behavior was associated with infant dysregulation and irritability (mothers’ p< 0.001, fathers’ p< 0.001).
Conclusion
The severity of conditions identified through NBS can affect parents’ perceptions of their child’s vulnerability and attachment. Infant feeding problems in the context of chronic health conditions, like CF, could represent signs of more deeply rooted concerns regarding the parent-child relationship that merit additional clinical evaluation.
Keywords: attachment, cystic fibrosis, congenital hypothyroidism, newborn screening, parent-child relationship
Newborn Screening
Newborn screening (NBS) programs identify pre-symptomatic infants with serious genetic or congenital conditions to facilitate early intervention, thus preventing morbidity and mortality.1 NBS was first implemented in the United States during the 1960’s with guidelines that required conditions to be well-characterized and effective treatments readily available.2 Advances in medical technologies prompted expansion of NBS criteria to include conditions with clinical courses that are less well-documented and prognoses that are more uncertain.3 Application of genetic testing to NBS also allows for identification of neonates who are heterozygote carriers of one defective gene and reap no health benefits from early detection. Expansion of NBS to include conditions with more ambiguous outcomes and incidental findings associated with genetic testing raise questions about the short and long-term psychosocial consequences on parent-infant relationships following positive NBS results.
Newborn Screening for Cystic Fibrosis and Congenital Hypothyroidism
This study focused on NBS for cystic fibrosis (CF) and congenital hypothyroidism (CH) for several reasons. NBS for CH, consistent with Wilson and Junger 1968 guidelines, has been implemented successfully nationally for more than five decades; whereas, CF represents the more recently expanded NBS criteria. The two conditions are similar in that signs and symptoms are not readily detected in neonates, which can lead to serious complications. However, the treatment and prognosis for these conditions are considerably different. Although the severity of CF symptoms is influenced by genetic and environmental factors, defects in the cellular chloride channel lead to progressive lung disease and pancreatic insufficiency in most patients. Time and labor intensive care involve daily medications, specialized respiratory treatments, and frequent evaluations by inter-professional CF specialists.4 Serious CF complications, such as pulmonary exacerbation, can require hospitalization. Even with early detection and intervention, patient life-spans are typically shortened. By contrast, the cognitive impairment and growth delay from hormone deficiencies associated with CH can be prevented by early diagnosis and prompt initiation (within first month of life) of daily oral thyroid supplements.5 Many children with CH have thyroid levels monitored by primary care providers. Thus, examination of psychosocial outcomes for these two conditions might offer insights about the effects of the newer NBS criteria on families with implications for public policies regarding further expansion of NBS.
Parent Depression and Anxiety
Research shows an association between CF diagnosed in early infancy and parental depression and anxiety.6, 7 Mothers (35%) and fathers (23%) of children with CF report symptoms of depression which is associated with depression and treatment non-adherence in their children.8 A met-analysis of maternal depression research in the general population notes that depressed mothers tend to be less sensitive, more disengaged, and less communicative with their children than non-depressed mothers.9,10 Maternal depression has long been associated with insecure attachment behavior in infants and young children.11 A longitudinal study of children with asthma and eczema shows that maternal depression and anxiety are associated with internalizing symptoms and externalizing symptoms in the children.12 The combination of maternal depression and attachment anxiety (concerns that attachment feelings are not reciprocated) can attenuate the benefits of interventions designed to enhance maternal sensitivity to infant cues in families at-risk for child maltreatment.13 Results of a recent study suggest that children of clinically depressed fathers, as compared to mothers, have fewer depressive symptoms.14 The literature suggests that depression and anxiety are potential mediating factors in the study of parent-child relationships.
Attachment and Child Vulnerability
The grief and trauma associated with learning that one’s child has a serious diagnosis can affect caregiving behaviors, child attachment,15 and parental perceptions of child vulnerability.16, 17 Such perceptions might adversely affect the quality of parents’ interactions with their children, particularly during important care-giving activities, e.g., feeding. In a study that preceded CF NBS, toddlers diagnosed with CF in early infancy had higher rates of insecure mother-infant attachments than children diagnosed later in infancy.17 More recently, children identified as CF carriers through NBS and children with CF had significantly more primary health care encounters during the first year than children with CH and children with normal NBS results.16 NBS research has similarly documented associations between abnormal NBS results and parental concerns about their infants’ well-being disproportionate to the actual results.18–22 Thus, parents’ perceptions of child vulnerability as well as attachment may play an important role in the evolving parent-child relationship following positive NBS results.
Mealtime as Context for Relationships
Mealtime interactions are particularly salient to children with CF because these children can be at risk for malnutrition due to pancreatic insufficiency. Parent education generally emphasizes the importance of nutrition to optimize the child’s health and prevent CF complications.23 Treatment regimens typically include calorically dense diets, daily vitamin supplements, and oral enzyme replacement with meals. Children’s growth is closely monitored at specialized CF Centers. Given the critical role of nutrition in the health of children with CF, parents often go to great lengths to encourage their children to consume large volumes of food. Consequently, mealtimes can involve escalating negative interactions in which parents’ attempts to persuade their children to eat are met by children’s refusal of food.24–28 Feeding difficulties are among the most common problems for which parents of children with CF seek professional assistance.29 Feeding problems have been linked to attachment disturbances among young children with CF.17 Additionally, mothers of infants with CF are more likely to bottle feed their infants than mothers of infants with no health problems.30 Bottle feeding, regardless of the child’s health status, has been associated with more task-oriented, less responsive maternal interactions with their infants than breastfeeding.31 To our knowledge, there are no studies that examined the feeding/eating behaviors of children with CH. We speculated that growth delays historically associated with untreated CH might lead parents of children with CH to emphasize food intake more than parents of children with no health problems.
At 12 months, children typically increase their expressions of individuation from their primary care-givers. For parents of children with CF and CH, concerns for their children’s nutritional status might override consideration of the child’s autonomy. Feedings could become task-oriented rather than mutually enjoyable interactions. Therefore, we chose mealtime as the context for assessing the quality of parents’ interactions with their 12 month-olds.
Study Purpose and Hypotheses
This study was designed to examine potential parent factors affecting parent-child relationships across a continuum of severity in diagnostic results stemming from NBS. From most to least serve, study groups included: cystic fibrosis (CF), congenital hypothyroidism (CH), CF carrier (CF-C), and healthy with normal NBS results (H). The CF carrier group was included because these incidental NBS genetic findings have been associated with short-term parental distress, confusion about results, and misperceptions about the children’s vulnerability.16, 32
We hypothesized that parental anxiety and depression, as well as perception of child vulnerability and attachment, would serve as mediating factors between the child’s diagnostic status and quality of parent-child interactions. We also hypothesized that the level of task-oriented parent feeding behavior would be positively associated with the overall quality of those parent-child interactions. To test these hypotheses, we compared the three groups with abnormal NBS results (CF, CH, CF-C) to a reference group of families with healthy infants (H) who had normal NBS results.
METHODS
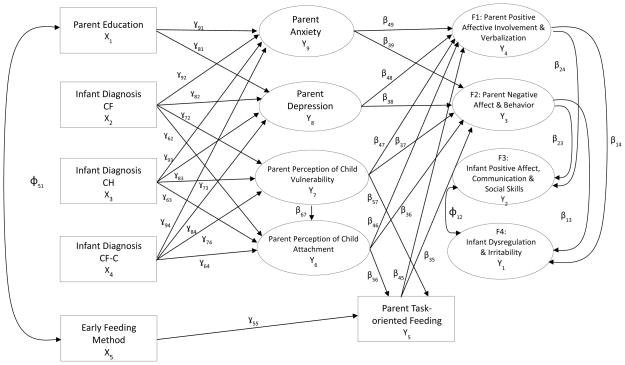
This article is one of several to report findings from a mixed-method longitudinal study conducted between 2002 and 2009 that examined psychosocial issues associated with abnormal NBS results on parents and their effects on relationships with their infants.7 As illustrated in Figure 1, exogenous variables included parent education, infant NBS diagnostic status and early feeding method (breast versus bottle). Mediating factors included parent self-reports of anxiety, depression, perceptions of child vulnerability and attachment as well as observations of the degree of task-oriented feeding behavior. Endogenous outcomes included observations of the quality of parents and their children interactions with each other.
Figure 1.
Heuristic Model for Mothers and Fathers
CF, Cystic Fibrosis; CH, Congenital Hypothyroidism; CF-C, Cystic Fibrosis Carrier; H, Healthy
Recruitment
Health professionals from four sites recruited families during regularly scheduled appointments in primary and specialty clinics. The Wisconsin State Laboratory of Hygiene assisted in recruiting the CH group because only about half of those infants in our state receive care from endocrinology specialists, while almost all infants with CF receive specialty care through CF centers or affiliates. Recruitment materials were sent from the State Laboratory to primary care providers who distributed them to parents of infants with CH. All participants provided written informed consent. This study was approved by the Institutional Review Boards of all participating sites.
Sample
The sample included 131 mothers and 118 fathers of 131 infants whose NBS and diagnostic results indicated CF (n=23), CH (n=35), CF-C (n=38), or H (n=35). The CF group included five infants with intermediate or normal diagnostic results and/or two CF mutations suggesting a CF diagnosis. Intermediate diagnostic sweat test results are a relatively new phenomena occurring more frequently as a consequence of CF NBS; long-term prognostic implications remains unclear. We also included one infant with CF identified through prenatal testing plus abnormal NBS results, one infant with borderline thyroid levels, and one infant with CH who had false-negative NBS results but subsequently diagnosed because of family history. Infants with serious co-morbid diagnoses, significant perinatal complications, APGAR scores less than 4, or < 32 weeks gestation were excluded. Sample size was congruent with the minimal subject-to-parameter ratio of 5:1 suggested by Tabachnick and Fidell to provide sufficient stability in the estimates.33
Data Collection and Measures
Data collection involved parent self-reports, videotaped observations of parent-child interactions, developmental assessments, and semi-structured interviews with parents conducted in families’ homes when infants were 2–8 weeks, 6 months, and 12 months. This report includes only parent self-reports and observational data from the 12 month data point. Demographic information was obtained upon entry into the study and is based on parent self-report of age, ethnicity, educational level, marital status, and income.
The Parent-Child Early Relational Assessment (PCERA) is a 65-item instrument that involves five-minute video-captured observations of parent-child exchanges during feeding, free play, or structured task to assess affective and behavioral quality of interactions between parents and their children from birth to 5 years.34 Items are divided into parent, infant, and dyadic domains. The PCERA has been used in more than 400 programs and research projects internationally. Psychometric properties of the PCERA have been documented in high-risk and normative populations with Cronbach’s alphas ranging from 0.78 to 0.91.34–36 Based on the PCERA author’s recommendations, we evaluated configural invariance using confirmatory factor analysis to determine if domains derived from our data were consistent with the PCERA domains.
In an effort to record typical feeding interactions, data collections were scheduled at infants’ usual meal times and conducted in families’ homes. We avoided scheduling data collections when infants or parents were ill or within 24 hours of infant immunizations. Initiation of video recording was based on parents’ appraisals of their infants’ readiness to eat. Data collectors followed the instructions in the PCERA manual34 to introduce the video recording by stating, “This is a snapshot of one point in time. We’ll be interested in your sharing with us how it is alike or different from how things usually go. We are interested in seeing (infant’s name) and you during a feeding together. Please be with (infant’s name) just as you usually would.” We also encouraged parents to feed their infants in their usual situations, e.g., highchair, room, etc. Most parents described the feedings as very typical; none thought it very atypical.
We included a task-oriented feeding item used in our earlier work that was associated with less positive and more negative parent behaviors during feeding interactions.31 This item was designed to assess the degree to which the parent provides nurturance, social engagement, and responsiveness to the infant’s cues during feeding. If parental behaviors primarily focused on getting the child to ingest food, thus overlooking socio-emotional aspects of feeding, the interaction was coded as highly task-oriented (low numerical rating). Parental behaviors that presented mealtime as an opportunity to connect with their child socially and emotionally were coded as not task-oriented (high numerical rating).
Specially trained coders followed the PCERA manual instructions to code the amount, duration, and intensity of interactions during five-minute video captured mother-infant feeding segments using a Likert scale (1=area of concern; 5=area of strength). High scores indicated favorable ratings on all PCERA factors and the task-orientation item. Coders had educational backgrounds in infant and child development. Although coders remained blind to study groups, they were informed of each infant’s age (corrected for prematurity when relevant) and carefully considered the developmental appropriateness of the infant’s behaviors for each item. The task-oriented item was included in inter-rater reliability evaluations for the PCERA items. Inter-rater agreement was established through 40 hours of reliability training. Twenty percent of segments were coded by two raters to maintain reliability. Inter-rater reliability ranged from 81% for individual items to 89% for clustered ratings within one point on the 5-point scale. Coders reviewed and discussed discrepant ratings to attain consensus scores.
The Center for Epidemiologic Studies Depression Scale (CES-D) is a 20-item self-report screening tool that measures the frequency of cognitive, affective, and, to a lesser extent, physical depressive symptoms during the past week (0=rarely; 3=most of the time).37 Scores ≥ 16 suggest clinical levels of symptoms. Internal consistency coefficients have been 0.85 in non-clinical samples and 0.90 in clinical samples, with a test-retest reliability coefficient of 0.54.
The State-Trait Anxiety Inventory (STAI) contains two 20-item self-report scales based on a two-factor model of anxiety present or absent.38 The State scale, used in this analysis, measures levels of worry or apprehension in the present. Respondents rate symptom frequency on a Likert scale (1=not at all; 4=very much so). The median internal consistency reliability coefficients for the State Scale and Trait Scale have been 0.92 and 0.90 respectively. Item remainder correlation coefficients for both scales have been consistently high (≥ .90) (27). Scores ≥ 40 have been considered within a clinical range.39, 40
The Child Vulnerability Scale (CVS) is an 8-item self-report that uses a 4-point Likert scale (0=strongly disagree; 3=strongly agree) to assess parents’ perceptions of child vulnerability to illness.41 Total scores range from 0–24. Scores ≥ 10 suggest elevated PPCV. Although the instrument was standardized with a sample of mothers of children ages 4 to 8 years, it has been used with parents of children as young as one month.42 Forsyth et al. reported total scale and internal consistency of item-total as having good reliability (Cronbach alpha=.74; Pearson correlation coefficients=.51 to .68).41 The Cronbach alpha for the CVS derived from our study sample was .82 suggesting good internal consistency. Significant correlations between the scores on the CVS and the Achenbach Child Behavior Check List (p<.001) and the number of acute care clinic visits (p<.001) support the validity of the CVS.41 A recent confirmatory factor analysis was conducted on CVS data obtained from 226 parents including mothers, fathers, and custodial grandmothers of children age 8 months to 18 years. Results found a good fit for a single factor of vulnerability.43
The Attachment Q-Sort (AQS) is an observational procedure based on Attachment Theory designed to examine “secure-base” behavior in children 12-months to 5 years.44 Each parent is asked to review 90 cards containing descriptors of the child, e.g., when child is near mother and sees something he wants to play with, he fusses or tries to drag mother over to it. Parents sort cards into 9 piles ranging from most to least characteristic of their child. This 20–30-minute procedure has been correlated with the Strange Situation in differentiating secure from insecure child attachments age 12 months.44
Analytic Strategies
Demographics
We used parametric and non-parametric tests to assess mothers’ and fathers’ data separately for group differences in age, ethnicity, educational level, marital status, income, and infant gender. Some of the categorical conditions were small. Therefore, the exact Type I error was obtained using StatXact 8.45 Due to the large number of group contrasts in demographic and major study variables, we adjusted the Type 1 error rate using Sidak’s family-wise error.46
Factor analysis
Initial psychometrics (configural invariance) were conducted by confirmatory factor analysis of categorical items using a Weighted Least Squares (WLSMV) estimator in Mplus Version 7.11.47 Assessment of fit was conducted using χ2/df ratio, the Tucker-Lewis index (TLI), the comparative fit index (CFI), root mean square error of approximation (RMSEA), and weighted root mean square residual (WRMR). Reliability estimates of Cronbach’s alpha, composite reliability and average variance extracted were also assessed. Hu and Bentler suggest the following fit index cut values for good model fit: TLI > .95, CFI> .95, RMSEA < .06, SRMR < .08, and χ2/df < 2.48
Structural Model
Structural equation modeling was used to examine parent and infant factors that might contribute to the quality of parent-child interactions. Our analysis used a subset of structural equation modeling known as single composite indicator structure equation modeling (CISE). This approach is based on item parceling, allowing us to examine a recursive set of linear relationships between exogenous variables, i.e., variables that are not caused by another variable in the model, and endogenous variables, i.e., variables that are caused by one or more variables in the model, using maximum likelihood estimation.49 CISE was used to improve the normality of indicators, reduce the number of parameters to be estimated, improve the internal consistency of parameters, and improve the variable to sample size ratio.50, 51 Measurement error for composite indicators was fixed to an estimate of the measurement error based on an estimate of reliability [(1 - Cronbach’s alpha)*σ2 of composite variable]52, while measurement error for individual items in the model was set to zero. Our model (Figure 1) was constructed using the Mplus software (Version 7.11).47
Assessing Fit of the Model
Our CISE model was assessed for fit using the following indices; χ2/df ratio, the Tucker-Lewis index (TLI), the comparative fit index (CFI), the root mean square error of approximation (RMSEA), and the standardized root mean square residual (SRMR).
RESULTS
Missing Data
No PCERA data were missing. Initial appraisal of missing data for the other measures indicated < 5% total items missing per scale. All missingness met the conditions of missing completely at random (MCAR) based on Little’s test.53 We used the Expectancy Maximum (EM) algorithm with NORM software to impute missing items.54
Demographics and Major Study Variables
Correlations between income and education were significant for mothers (r = 0.517) and fathers (r = 0.404). Therefore, to limit the number of variables in the model relative the sample size, we used education as a measure of socio-economic status. Using Sidak’s family-wise error rate, 46 no significant group differences were found for any parent demographic variables or infant gender. As shown in Table 1, mean ages of mothers and fathers were 30.1 (SD=5.3) and 32.3 (SD=6.1) years respectively. Most mothers (87%) and fathers (94%) were married, European American (96%), college educated (88%, 76%), and had annual family incomes ≥ $41,000 (81%, 83%). Other racial/ethnic participant backgrounds included African American, Hispanic American, Asian American, and American Indian. Infant gender was fairly evenly divided for the total sample (53% female). In comparison to publicly available data for Wisconsin from the 2010 United States Census, 55 the racial/ethnic distribution of our sample was consistent with data showing 86.2% residents to be non-Hispanic white. Our sample was more highly educated (22.4% of state residents have college degrees), but the income of our sample was comparable (median statewide household income in 2008=$52,103). Table 2 shows mothers’ and fathers’ means and standard deviations for study variables by group.
Table 1.
Sample Demographics by Group
| H | CF-C | CH | CF | Total N= 236(%) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Mothers n= 36(%) |
Fathers n= 29(%) |
Mothers n= 38(%) |
Fathers n= 31(%) |
Mothers %) |
Fathers n= 27(%) |
Mother s n= 23(%) |
Fathers n= 18(%) |
||
| Age in Years | 30.47 | 31.62 | 28.76 | 30.19 | 30.97 | 34.22 | 30.35 | 33.22 | 31.23 |
| SD=4.33 | SD=3.96 | SD=5.42 | SD=5.60 | SD=4.69 | SD=6.37 | SD=6.75 | SD=8.57 | SD=5.71 | |
| Married | 35 (97.2) | 29 (100) | 30 (79.0) | 26 (83.9) | 30 (88.2) | 26 (92.9) | 19 (82.6) | 18 (100) | 213 (90.3) |
| Ethnicity | |||||||||
| European American | 35 (97.2) | 27 (93.1) | 38 (100) | 31 (100) | 30 (88.2) | 27 (96.4) | 21 (91.3) | 18 (100) | 227 (96.2) |
| Native American | 0 (0) | 1 (3.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.5) |
| African American | 1 (2.8) | 1 (3.5) | 0 (0) | 0 (0) | 1 (2.9) | 0 (0) | 2 (8.7) | 0 (0) | 3 (1.3) |
| Hispanic American | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (5.7) | 1 (3.6) | 0 (0) | 0 (0) | 2 (1.0) |
| Asian American | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.9) | 0 (0) | 0 (0) | 0 (0) | 1 (0.5) |
| Mixed | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.5) |
| Education | |||||||||
| High School/GED | 1 (2.8) | 5 (17.2) | 6 (15.8) | 9 (29.0) | 3 (8.8) | 5 (17.9) | 5 (21.7) | 6 (33.3) | 40 (16.5) |
| Community College/Trade School | 3 (8.3) | 3 (10.3) | 11 (29.0) | 8 (25.8) | 13 (38.2) | 8 (28.6) | 6 (26.1) | 7 (38.9) | 59 (25.0) |
| Baccalaureate Degree | 23 (63.9) | 12 (41.4) | 14 (36.8) | 10 (32.3) | 13 (38.2) | 9 (32.1) | 7 (30.4) | 4 (22.2) | 92 (39.0) |
| Graduate/Professional Degree | 9 (25.0) | 9 (31.0) | 7 (18.4) | 4 (12.9) | 5 (14.7) | 6 (21.4) | 5 (21.7) | 1 (5.6) | 46 (19.5) |
| Income | |||||||||
| Less than $20,000 | 0 (0) | 0 (0) | 4 (10.5) | 3 (9.7) | 3 (8.8) | 0 (0) | 3 (13.0) | 0 (0) | 13 (5.5) |
| $21,000 – $40,000 | 5 (13.9) | 3 (10.3) | 10 (26.3) | 7 (22.6) | 6 (17.7) | 5 (17.9) | 4 (17.4) | 3 (16.7) | 43 (18.2) |
| $41,000 – $60,000 | 10 (27.8) | 9 (31.0) | 8 (21.1) | 6 (19.4) | 8 (23.5) | 9 (32.1) | 6 (26.1) | 5 (27.8) | 61 (25.8) |
| $61,000 – $80,000 | 10 (27.8) | 8 (27.6) | 6 (15.8) | 6 (19.4) | 4 (11.8) | 3 (10.7) | 4 (17.4) | 4 (22.2) | 45 (19.1) |
| $81,000 – $100,000 | 3 (8.3) | 2 (6.9) | 5 (13.2) | 3 (9.7) | 3 (8.8) | 4 (14.2) | 2 (8.7) | 2 (11.1) | 10 (10.2) |
| More than $100,000 | 8 (22.2) | 7 (24.1) | 5 (13.2) | 5 (16.1) | 10 (29.4) | 7 (25.0) | 4 (17.4) | 4 (22.2) | 25 (21.2) |
H, Healthy Group; CF-C, Cystic Fibrosis Carrier Group; CH, Congenital Hypothyroidism Group; CF, Cystic Fibrosis Group
Table 2.
Means (Standard Deviations) for Mothers’ and Fathers’ Variables by Study Group
| Mothers | Fathers | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Variable | Infants with CF Diagnosis | Infants with CH Diagnosis | Infants with CF-C | H-Group Infants No Diagnosis | Infants with CF Diagnosis | Infants with CH Diagnosis | Infants with CF-C | H-Group Infants No Diagnosis |
| Parent Anxiety | 27.91 (6.18) | 29.27 (8.53) | 30.43 (9.39) | 29.37(8.40) | 29.06 (8.97) | 27.47 (7.07) | 30.47 (9.85) | 28.71 (7.95) |
| Parent Depression | 7.97 (5.54) | 7.07 (7.77) | 8.60 (6.45) | 5.70 (5.83) | 7.65 (9.63) | 5.73 (5.46) | 5.73 (4.44) | 7.00 (7.05) |
| Scores≥Clinical Cut | 8.5% | 12.5% | 13.0% | 5.4% | 12.5%% | 5.2% | 0.0% | 11.4% |
| Parent Perception of Child Vulnerability | 4.37 (3.90) | 4.02 (3.69) | 8.04 (4.97) | 3.05 (2.91) | 3.65 (3.14) | 2.97 (3.42) | 7.00 (3.75) | 4.02 (3.44) |
| Parent Perception of Child Attachment | 0.55 (0.22) | 0.46 (0.29) | 0.37 (0.22) | 0.52 (0.17) | 0.43 (0.17) | 0.48 (0.22) | 0.47 (0.22) | 0.38 (0.24) |
| Parent Task-Oriented feeding | 2.76 (1.01) | 3.44 (1.05) | 2.52 (0.73) | 2.86 (1.12) | 3.00 (1.03) | 3.12 (0.92) | 2.94 (0.80) | 2.75 (0.95) |
| Parent Positive Affective Involvement & Verbalization | 3.55 (0.62) | 3.75 (0.71) | 3.51 (0.70) | 3.65 (0.66) | 3.45 (0.96) | 3.66 (0.67) | 3.37 (0.59) | 3.38 (0.78) |
| Parent Negative Affect & Behavior | 3.97 (0.66) | 4.32 (0.64) | 4.06 (0.53) | 4.23 (0.57) | 4.37 (0.42) | 4.17 (0.55) | 4.09 (0.58) | 4.32 (0.59) |
| Infant Positive Affect, Communication & Social Skills | 3.72 (0.55) | 3.84 (0.68) | 3.74 (0.62) | 3.63 (0.56) | 3.70 (0.65) | 3.95 (0.55) | 3.95 (0.42) | 3.78 (0.45) |
| Infant Dysregulation & Irritability | 4.27 (0.60) | 4.31 (0.66) | 4.45 (0.55) | 4.44 (0.51) | 4.30 (0.51) | 4.36 (0.47) | 4.35 (0.46) | 4.35 (0.54) |
PCERA Factor Analysis
PCERA factors were standardized with a community sample and the instrument has been used in research on many clinical and non-clinical populations. As recommended by the PCERA author, we applied analytic procedures to identify factors with the best fit for our study sample. We conducted an exploratory factor analysis and eliminated items with loadings < 0.40 on mothers’, fathers’, or combined mother-father data. Each item was exclusively added to the factor for which it had the highest loading and a confirmatory factor analysis was conducted (Table 3 available on-line). Finally, we conducted measurement configural invariance analyses56 (Table 4 available on-line). Results showed the four-factor PCERA measurement model to be a good fit for this sample and therefore invariant.
Table 3.
Standardized Loadings for PCERA ratings of Mothers’, Fathers’, and Combined Data
| PCERA Factor | Mothers | Fathers | Combined |
|---|---|---|---|
| Factor 1: Parent Positive Affective Involvement and Verbalization | |||
| 2. expressive, non-flat voice tone | 0.749 | 0.854 | 0.782 |
| 3. warm, kind tone of voice | 0.504 | 0.758 | 0.633 |
| 4. expressed positive affect | 0.799 | 0.921 | 0.834 |
| 7. lack of depressed, withdrawn mood | 0.841 | 0.971 | 0.854 |
| 9. enthusiastic, animated, cheerful mood | 0.930 | 0.968 | 0.956 |
| 12. enjoyment, pleasure | 0.824 | 0.917 | 0.874 |
| 15. visual contact | 0.720 | 0.743 | 0.680 |
| 16. amount of verbalization | 0.827 | 0.849 | 0.893 |
| 17. quality of verbalizations | 0.716 | 0.793 | 0.810 |
| 18. social initiative | 0.711 | 0.896 | 0.833 |
| 19. contingent responsivity | 0.727 | 0.631 | 0.693 |
| 21. lack of structuring/mediating | 0.661 | 0.735 | 0.676 |
| 22. sensitivity/responsiveness to child’s cues | 0.565 | 0.654 | 0.585 |
| 23. connectedness | 0.769 | 0.932 | 0.852 |
| 24. mirroring | 0.841 | 0.862 | 0.848 |
| 26. creativity | 0.745 | 0.938 | 0.863 |
| Factor 2: Parent Negative Affect, Inconsistent and Intrusive Behavior | |||
| 1. angry, hostile tone of voice | 0.928 | 0.983 | 1.013 |
| 5. expressed negative affect | 0.980 | 1.013 | 0.983 |
| 6. angry, hostile mood | 0.956 | 1.000 | 0.978 |
| 11. displeasure | 0.851 | 0.872 | 0.827 |
| 14. quality and amount of physical contact: Neg. | 0.556 | 0.570 | 0.544 |
| 20. contingent responsivity to negative behavior | 0.781 | 0.827 | 0.768 |
| 27. intrusiveness | 0.828 | 0.739 | 0.503 |
| 28. inconsistency/unpredictability | 0.630 | 0.787 | 0.600 |
| Factor 3: Infant Positive Affect, Communication, and Social Skills | |||
| 30. expressed positive affect | 0.887 | 0.922 | 0.891 |
| 32. happy, pleasant, cheerful mood | 0.808 | 0.954 | 0.848 |
| 33. no apathetic, withdrawn mood | 0.806 | 0.926 | 0.818 |
| 36. no sober/serious mood | 0.711 | 0.648 | 0.702 |
| 38. alertness/interest | 0.690 | 0.717 | 0.699 |
| 39. social initiative | 0.823 | 0.809 | 0.775 |
| 40. social behavior of infant/child-responds | 0.680 | 0.810 | 0.722 |
| 41. avoiding, averting, resistance | 0.516 | 0.830 | 0.608 |
| 45. quality of exploratory play | 0.714 | 0.648 | 0.619 |
| 47. robustness | 0.669 | 0.413 | 0.634 |
| 53. passivity, lethargy | 0.631 | 0.711 | 0.687 |
| 55. visual contact | 0.500 | 0.663 | 0.559 |
| 56. communicative competence | 0.780 | 0.772 | 0.747 |
| 57. readability | 0.567 | 0.585 | 0.503 |
| Factor 4: Infant Dysregulation and Irritability | |||
| 31. expressed negative affect | 0.948 | 0.963 | 0.960 |
| 35. irritability/angry mood | 0.966 | 0.928 | 0.976 |
| 37. emotional lability | 0.893 | 0.775 | 0.779 |
| 43. willful/controlling | 0.918 | 0.582 | 0.723 |
| 46. inattentiveness | 0.450 | 0.501 | 0.505 |
| 49. impulsivity | 0.932 | 0.970 | 0.950 |
| 50. lack of self-regulation/organization | 0.790 | 0.891 | 0.848 |
| 51. consolability, soothability | 0.412 | 0.772 | 0.632 |
| 54. hyperactivity, over-active | 0.685 | 0.708 | 0.509 |
Table 4.
Configural Invariance Factor Fit Indices for PCERA 12- month Feedings Scales (N=249)
| Factor | χ2/df Ratio | CFI | TLI | Cronbach Alpha | Composite Reliability | AVE | RMSEA | WRMR |
|---|---|---|---|---|---|---|---|---|
| 1. Parent Positive Affective Involvement and Verbalization | 4.99 | 0.965 | 0.960 | 0.953 | 0.953 | 0.568 | 0.127 | 1.415 |
| 2. Parent Negative Affect, Inconsistent and Intrusive Behavior | 2.99 | 0.996 | 0.994 | 0.904 | 0.911 | 0.582 | 0.089 | 0.746 |
| 3. Infant Positive Affect, Communicative and Social Skills | 10.07 | 0.880 | 0.894 | 0.858 | 0.904 | 0.441 | 0.188 | 2.203 |
| 4. Infant Dysregulation and Irritability | 2.74 | 0.995 | 0.993 | 0.878 | 0.926 | 0.624 | 0.084 | 0.897 |
|
| ||||||||
| Optimal values | < 2.3 | > 0.95 | > 0.95 | > 0.95 | > 0.95 | > 0.50 | < 0.06 | < 0.90 |
PCERA, Parent-Child Early Relational Assessment; df, degrees of freedom; CFI, Comparative Fit Index; TLI, Tucker-Lewis Index; AVE, average variance extracted; RMSEA, root mean square error of approximation; WRMR, weighted root square residual.
Composite Indicator Structural Equation Models for Mothers and Fathers
CISE modeling was used to construct separate models for mothers’ and fathers’ data. Within each model, we used diagnostic classification as the predictor of outcomes with the H group as the reference. Table 5 (available on-line) details unstandardized estimates (partial regression coefficients) that show relationships between feeding patterns and PCERA factors. Negative signs indicate inverse relationships; no sign indicates positive/direct relationships. All significant relationships between feeding patterns and parent-child interactions were in the hypothesized directions.
Table 5.
Effect Paths For Mother and Father Models
| Direct Effect Paths | Mother | Father | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Model Parameter |
Unstandardized Estimates |
SE | Unstandardized 95% CI |
p | Standardized Estimates |
Unstandardized Estimate |
SE | Unstandardized 95% CI |
p | Standardized Estimates |
|
| F1 → F4 | β14 | 0.036 | 0.082 | [−0.125, 0.196] | 0.664 | 0.041 | −0.100 | 0.061 | [−0.219, 0.019] | 0.101 | −0.164 |
| F2 → F4 | β13 | 0.441 | 0.092 | [0.261, 0.620] | < 0.001* | 0.469 | 0.448 | 0.094 | [0.263, 0.632] | < 0.001* | 0.492 |
| F1 → F3 | β24 | 0.540 | 0.079 | [0.385, 0.695] | < 0.001* | 0.598 | 0.500 | 0.055 | [0.393, 0.607] | < 0.001* | 0.739 |
| F2 → F3 | β23 | −0.055 | 0.088 | [−0.228, 0.118] | 0.533 | −0.056 | −0.157 | 0.084 | [−0.322, 0.009] | 0.064 | −0.154 |
| CVS → F1 | β47 | −0.003 | 0.015 | [−0.033, 0.027] | 0.860 | −0.016 | −0.022 | 0.019 | [−0.058, 0.015] | 0.248 | −0.092 |
| QSORT → F1 | β46 | −0.037 | 0.269 | [−0.565, 0.491] | 0.891 | −0.013 | −0.461 | 0.309 | [−1.067, 0.145] | 0.136 | −0.123 |
| CESD → F1 | β48 | −0.010 | 0.014 | [−0.038, 0.017] | 0.467 | −0.091 | −0.007 | 0.014 | [−0.034, 0.021] | 0.641 | −0.055 |
| STAI → F1 | β49 | −0.004 | 0.010 | [−0.024, 0.016] | 0.686 | −0.048 | −0.014 | 0.010 | [−0.034, 0.007] | 0.183 | −0.142 |
| CVS → F2 | β37 | −0.010 | 0.015 | [−0.040, 0.020] | 0.510 | −0.065 | −0.055 | 0.016 | [−0.088, −0.023] | 0.001* | −0.358 |
| QSORT → F2 | β36 | −0.028 | 0.271 | [−0.560, 0.504] | 0.917 | −0.010 | −0.224 | 0.279 | [−0.772, 0.323] | 0.422 | −0.090 |
| CESD → F2 | β38 | −0.009 | 0.014 | [−0.036, 0.017] | 0.497 | −0.089 | −0.007 | 0.012 | [−0.031, 0.018] | 0.588 | −0.085 |
| STAI → F2 | β39 | 0.004 | 0.010 | [−0.015, 0.023] | 0.657 | 0.056 | −0.008 | 0.009 | [−0.026, 0.010] | 0.392 | −0.122 |
| CVS → QSORT | β67 | −0.020 | 0.006 | [−0.032, −0.008] | 0.001* | −0.349 | −0.007 | 0.007 | [−0.021, 0.008] | 0.350 | −0.111 |
| Task → F1 | β45 | 0.362 | 0.047 | [0.270, 0.454] | < 0.001* | 0.593 | 0.592 | 0.061 | [0.471, 0.712] | < 0.001* | 0.724 |
| Task → F2 | β35 | 0.260 | 0.048 | [0.166, 0.354] | < 0.001* | 0.461 | 0.172 | 0.054 | [0.066, 0.279] | 0.001* | 0.317 |
| Education → CESD | γ81 | −1.619 | 0.611 | [−2.816, −0.422] | 0.008* | −0.264 | −0.772 | 0.626 | [−1.999, 0.456] | 0.218 | −0.126 |
| CH Group → CESD | γ83 | 1.548 | 1.499 | [−1.390, 4.485] | 0.302 | 0.118 | 0.436 | 1.704 | [−2.904, 3.776] | 0.798 | 0.029 |
| CF-C Group → CESD | γ84 | 0.547 | 1.460 | [−2.314, 3.409] | 0.708 | 0.044 | −1.657 | 1.663 | [−4.917, 1.603] | 0.319 | −0.120 |
| CF Group → CESD | γ82 | 1.992 | 1.692 | [−1.324, 5.309] | 0.239 | 0.130 | −1.807 | 2.026 | −5.778, 2.163] | 0.372 | −0.106 |
| Education → STAI | γ91 | −1.435 | 0.772 | [−2.948, 0.079] | 0.063 | −0.175 | −0.508 | 0.737 | [−1.952, 0.936] | 0.490 | −0.067 |
| CH Group → STAI | γ93 | −1.146 | 1.895 | [−4.859, 2.568] | 0.545 | −0.065 | 0.188 | 2.000 | [−3.732, 4.107] | 0.925 | 0.010 |
| CF-C Group → STAI | γ94 | 0.136 | 1.847 | [−3.484, 3.755] | 0.941 | 0.008 | −1.503 | 1.951 | [−5.327, 2.322] | 0.441 | −0.089 |
| CF Group → STAI | γ92 | 1.250 | 2.140 | [−2.945, 5.444] | 0.559 | 0.061 | 1.401 | 2.379 | [−3.262, 6.063] | 0.556 | 0.067 |
| CH Group → CVS | γ73 | 1.301 | 0.885 | [−0.433, 3.034] | 0.142 | 0.150 | −0.364 | 0.821 | [−1.974, 1.246] | 0.658 | −0.048 |
| CF-C Group → CVS | γ74 | 0.947 | 0.857 | [−0.733, 2.626] | 0.269 | 0.114 | −0.934 | 0.789 | [−2.479, 0.612] | 0.236 | −0.132 |
| CF Group → CVS | γ72 | 4.988 | 0.996 | [3.035, 6.941] | < 0.001* | 0.493 | 2.977 | .958 | [1.100, 4.855] | 0.002* | 0.338 |
| CH Group → QSORT | γ63 | −0.030 | 0.054 | [−0.135, 0.076] | 0.581 | −0.059 | 0.078 | 0.054 | [−0.027, 0.183] | 0.147 | 0.165 |
| CF-C Group → QSORT | γ64 | 0.060 | 0.052 | [−0.043, 0.162] | 0.253 | 0.124 | 0.121 | 0.052 | [0.018, 0.223] | 0.021* | 0.275 |
| CF Group → QSORT | γ62 | −0.041 | 0.069 | [−0.177, 0.095] | 0.551 | −0.071 | 0.116 | 0.067 | [−0.016, 0.248] | 0.085 | 0.213 |
| QSORT → Task | β56 | 1.160 | 0.484 | [0.212, 2.108] | 0.016* | 0.243 | 1.057 | 0.483 | [0.111, 2.004] | 0.029* | 0.230 |
| CVS → Task | β57 | −0.022 | 0.029 | [−0.079, 0.034] | 0.436 | −0.081 | 0.042 | 0.031 | [−0.018, 0.103] | 0.167 | 0.149 |
| Feeding → Task | γ55 | 0.213 | 0.192 | [−0.163, 0.589] | 0.267 | 0.100 | −0.278 | 0.195 | [−0.661, 0.104] | 0.154 | −0.146 |
|
| |||||||||||
| Correlations | |||||||||||
| F3 ↔ F4 | Φ12 | 0.087 | 0.025 | [0.038, 0.135] | < 0.001* | 0.375 | 0.071 | 0.019 | [0.034, 0.108] | < 0.001* | 0.485 |
| Feeding ↔ Education | Φ51 | 0.120 | 0.041 | [0.039, 0.201] | 0.004* | 0.259 | 0.165 | 0.048 | [0.071, 0.260] | 0.001* | 0.323 |
|
| |||||||||||
| Indirect Effect Paths | |||||||||||
| CF Group → CVS → Task | −0.112 | 0.145 | [−0.396, 0.172] | 0.441 | −0.040 | 0.126 | 0.099 | [−0.068, 0.321] | 0.203 | 0.050 | |
| CF Group → QSORT → Task | −0.048 | 0.083 | [−0.210, 0.114] | 0.562 | −0.017 | 0.123 | 0.089 | [−0.052, 0.298] | 0.169 | 0.049 | |
| CF Group → CVS → QSORT → Task | −0.117 | 0.065 | [−0.245, 0.011] | 0.073 | −0.042 | −0.022 | 0.026 | [−0.073, 0.030] | 0.407 | −0.009 | |
Note. F1, Factor 1: Parent Positive Affective Involvement & Verbalization; F2, Factor 2: Parent Negative Affect & Behavior; F3, Factor 3; Infant Positive Affect, Communication & Social Skills; F4, Factor 4: Infant Dysregulation & Irritability; CVS, Child Vulnerability Scale; QSORT, Parent-Child Attachment Assessment; CESD, Center for Epidemiologic Studies Depression Scale; STAI, State Trait Anxiety Index; Task, Parent Task-Oriented Feeding; Education, Parent Education; Feeding, Early Feeding Method; CH-group, congenital hypothyroidism group; CF-C group, cystic fibrosis carrier group; CF group, cystic fibrosis group; CI, confidence interval; USE, unstandardized error.
p < .05
Diagnostic Group Differences
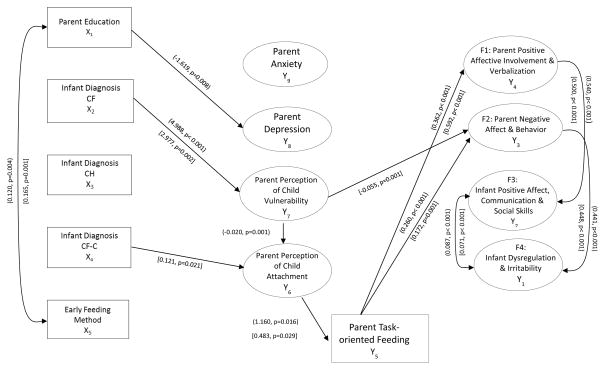
As illustrated in Figure 2, mothers with lower educational levels were more likely to report depressive symptoms than higher educated mothers. Neonatal diagnosis was not associated with increased anxiety or depression in mothers or fathers relative to the H group. Mothers and fathers of children with CF reported significantly higher perceptions of child vulnerability (p< 0.001, p=0.002) than parents of healthy children. Fathers of infants identified as CF carriers through NBS tended to view their children as significantly more attached than H group fathers (p=0.021).
Figure 2.
Significant Paths in (Mothers’) and [Fathers’] Model
CF, Cystic Fibrosis; CH, Congenital Hypothyroidism; CF-C, Cystic Fibrosis Carrier; H, Healthy
Parent Perceptions of Child Vulnerability and Attachment
Mothers who perceived their children as highly vulnerable, regardless of diagnostic status, were significantly more likely to also perceive their children as less attached than mothers who viewed their infants as less vulnerable (p=0.001).
Task-orient Feeding Behavior and Parent-Child Interactions
Mothers and fathers (regardless of diagnostic status) who perceived their infants as less attached were significantly more likely to engage in task-oriented feeding behavior than parents who viewed their infants as more attached (p=0.016, p=0.029). Mothers and fathers who engaged in more task-oriented behavior were also observed to show significantly less overall positive (p< 0.001, p< 0.001) and significantly more overall negative interactions (p< 0.001, p= 0.001) with their infants than parents who used less task-oriented feeding strategies. Fathers who perceived their infants as more vulnerable showed significantly more negative overall interactions with their infants than fathers who perceived their infants as less vulnerable (p< 0.001). Mothers and fathers who demonstrated high levels of positive affective involvement and verbalization had infants who exhibited significantly more positive affective expressiveness, communicative skills, and social responsiveness (p< 0.001, p< 0.001). Mothers and fathers who demonstrated more negative affect and/or inconsistent and intrusive behavior had infants who were significantly more dysregulated and irritable (p< 0.001, p< 0.001). As predicted there was a significant positive association between the two infant observational factors. There were no significant differences between the CH group and H group on any parameters; no indirect paths were significant.
DISCUSSION
Parental Depression and Anxiety
Although research shows an association between a CF diagnosis in early infancy and parental depression or anxiety,6, 7 our findings at 12 months post diagnosis suggest that such symptoms may diminish within one year after diagnosis. Still, these findings could be a consequence of our study protocol which involved re-contacting parents after each data collection (time of diagnosis, 6 months, and 12 months) if their depression scores were within a clinical range. We encouraged these parents to obtain additional assessment and we provided them with mental health referral information. The protocol also included qualitative interviews at each data point that afforded parents opportunities to reflectively share their thoughts and feelings about their children’s health. Parents reported that these discussions were very helpful. Though this study was not designed to be an intervention per se, there might have been some unintended therapeutic value that affected parents’ psychological profiles at 12 months.
Parent Perceptions of Child Vulnerability and Attachment
As hypothesized, mothers and fathers of infants with CF tended to view their children as more vulnerable than parents of healthy infants with no chronic health problems. However, there were no significant indirect paths from CF to parent perception of child attachment. The small CF sample size could be responsible for this lack of statistical significance. It is still plausible that the presence of a positive CF diagnosis can lead some parents to emotionally distance themselves from their infants. This point was illustrated by interview data from one mother in this study. She explained that upon hearing the news of her newborn’s CF diagnosis, she considered giving the child up for adoption stating, “I just didn’t want to get attached” because “they (children with CF) could die at any age.” At 12 months she also reported that she now, “could not imagine life without him/her (child’s sex not included to protect confidentiality).” Nevertheless, such ambivalence towards one’s child so early in life raises serious concerns about the long-term impact on their relationship and the child’s socio-emotional development.
Maternal perceptions of child vulnerability (regardless of study group) were associated with lower appraisals of her child’s attachment. Perhaps, infants perceived as vulnerable have more health problems that render them less socially engaging and reinforcing than healthy infants. Some mothers might misinterpret such behavior as a lack of attachment. These findings raise additional questions about whether such perceptions of vulnerability and/or low attachment lead to over-protective parenting practices that thwart normative child development.
Fathers’ perceptions of child vulnerability were associated with negative interactions. Mothers of infants with health problems sometimes act as gatekeepers to the child’s care,57 thus precluding fathers’ involvement in caregiving tasks such as feeding. Consequently, these fathers might lack competence derived from experience in such activities resulting in more negative interactions. More research is needed to explore how much engagement fathers actually have in the care of children with special health care needs and how that involvement might affect their perceptions of and relationships with their children.
An unexpected finding was that CF-C group fathers viewed their infants as more attached than their counterparts in the reference H group. However, this observation is consistent with interview data from our earlier work32 in which parents explained how the emotional trauma of hearing one’s newborn might have CF followed by the relief of a normal diagnostic test engendered a deeper sense of closeness to that child. Such feelings might motivate fathers to attend more to their children’s expressions of affection and interact with their children in ways that promote child attachment behavior.
Task-Oriented Feeding and Parent-Child Interactions
The level of task-oriented feeding behavior represented a critical link between the parent perceptions of attachment and the overall quality of parent-child dyadic interactions in the mothers’ model and the fathers’ model. Feeding problems are well-documented in children with CF. Our findings suggest that practitioners can support parent-child feeding interactions by encouraging parents to view mealtime as an opportunity for them to enjoy their child, teaching parents how to read and respond to the child’s cues about hunger and satiety, and cautioning them to avoid cajoling, bribing or pleading with the child to eat.25–27 When problems are identified, families might benefit from a multidisciplinary assessment that includes an appraisal of the parent-child relationship and possible support for the developing relationship. Further research is needed to determine whether relational interventions designed to enhance the overall quality of parent-child interactions can also improve children’s health outcomes.
Our results suggest that the level of infant responsivity may be contingent upon the quality of parenting behavior. Patterns of parent-child interactions are established early in the child’s life with long-term consequences for the parent-child relationship. A recent study showed that sub-optimal parental caregiving observed when children were 12 months old was associated with disorganized attachment and similar parenting tended to continue into middle childhood.58 However, Carlson and Sroufe suggest that both parental caregiving behavior and the attachment relationship are malleable at various stages of development.59 Our findings suggest that when an infant is diagnosed with a serious medical condition, such as CF, reducing parents’ perceptions of child vulnerability and increasing their perceptions of the child’s attachment might help parents develop normative relationship with their children. These findings build upon our previous work that focused on parent perceptions of child vulnerability and child illness status by demonstrating links between these factors and parent perceptions of child attachment and parent-child interactions.16
Study Limitations
Limitations of this study include a small subject-parameter ratio that could have affected the sensitivity and stability of the analysis in this study. A larger sample might have produced significant indirect relationships among variables. The use of convenience sampling could have produced selection bias. Our sample was demographically homogenous relative to racial/ethnic background. Thus, caution should be exercised in generalizing these results. Future research might also include measures of child development that could also influence parents’ perceptions of child vulnerability, attachment and quality of interactions.
Implications for NBS
It is notable that only the CH group resembled the healthy reference group on all variables. This observation suggests that the seriousness of the screened condition found to be abnormal, even if proven to be false-positive, can affect parents’ perceptions of their infants in ways that may influence the quality of parents’ interactions with their infants and perhaps the parent-child relationship. With growing interest and capacity to apply genomic/genetic technologies to NBS, ever increasing numbers of families will be affected by neonatal diagnoses of serious health conditions or incidental genetic findings, e.g., infant’s carrier status. Results from our study underscore the importance of including family-centered services that address both parental well-being and parent-child relationships in clinical infrastructures designed to provide follow-up care for families affected by the neonatal diagnosis of serious conditions, such as CF.
CONCLUSION
Findings suggest that the severity of conditions identified through NBS can affect parents’ perceptions of their children’s vulnerability and attachment. Such perceptions can affect caregiving behavior, such as feeding. Thus, infant feeding problems particularly in the presence of a serious health condition, like CF, could represent an important sign of more deeply rooted concerns regarding the overall parent-child relationship that merit additional clinical evaluation. Follow-up services for affected families should include information regarding infant vulnerability and support for the well-being of parent-infant relationships. Additional research is needed to explicate how best to promote normative parent-child interactions and prevent relational disturbances within the context of NBS programs.
Acknowledgments
This project was supported by the National Institute of Child Health and Human Development (K23HD42098), the University of Wisconsin-Madison School of Nursing Research Committee, and the University of Wisconsin-Madison Graduate School.
We are grateful to the families who participated in this study and the research teams from the University of Wisconsin-Madison, the American Family Children’s Hospital, the Children’s Hospital of Wisconsin, the Marshfield Clinic, the Gundersen-Lutheran Medical Center, and the Wisconsin State Laboratory of Hygiene.
Footnotes
Conflict of Interest
None of the authors have any conflicts of interest.
References
- 1.American Academy of Pediatrics Newborn Screening Task Force. Serving the family from birth to the medical home: Newborn screening: A blueprint for the future - a call for a national agenda on state newborn screening programs. Pediatrics. 2000;106:389–422. [PubMed] [Google Scholar]
- 2.Wilson JM, Junger G. Public Health Papers. Geneva: World Health Organization; 1968. Principles and practice of screening for disease; p. 34. Retrieved from http://whqlibdoc.who.int/php/WHO_PHP_34.pdf. [Google Scholar]
- 3.Andermann A, Blancquaert I, Beauchamp S, Déry V. Revisiting Wilson and Jungner in the genomic age: a review of screening criteria over the past 40 years. Bulletin of the World Health Organization. 2008;84:241–320. doi: 10.2471/BLT.07.050112. http://www.who.int/bulletin/volumes/86/4/07-050112/en/index.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howenstine M, Montegomery GS. Cystic fibrosis. Pediatrics in Review. 2009;30:302–310. doi: 10.1542/pir.30-8-302. [DOI] [PubMed] [Google Scholar]
- 5.Rastogi MV, LaFranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis. 2010;5:1–22. doi: 10.1186/1750-1172-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glasscoe C, Lancaster GA, Smyth RL, et al. Parental depression following the early diagnosis of cystic fibrosis: a matched, prospective study. J Pediatr. 2007;150:185–191. doi: 10.1016/j.jpeds.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Tluczek A, Clark R, Kosick RL, et al. Mother-infant relationship in the context of neonatal CF diagnosis: Preliminary findings. Pediatr Pulmonol Suppl. 2005;28:179–180. [Google Scholar]
- 8.Smith BA, Modi AC, Quittner AL, Wood BL. Depressive symptoms in children with cystic fibrosis and parents and its effects on adherence to airway clearance. Pediatr Pulmonol. 2010;45:756–63. doi: 10.1002/ppul.21238. [DOI] [PubMed] [Google Scholar]
- 9.Goodman SH. Depression in mothers. Annu Rev Clin Psychol. 2007;3:107–135. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- 10.Lovejoy C, Graczyk PA, O’Hare E, Neuman G. Maternal depression and parenting behaviour: a meta-analytic review. Clin Psychol Rev. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- 11.Atkinson L, Paglia A, Coolbear J. Attachment security: a meta-analysis of maternal mental health correlates. Clin Psychol Rev. 2000;20:1019–1040. doi: 10.1016/s0272-7358(99)00023-9. [DOI] [PubMed] [Google Scholar]
- 12.Teyhan A, Galobardes B, Henderson J. Child allergic symptoms and mental well-being: The role of maternal anxiety and depression. J Pediatr. 2014 doi: 10.1016/j.jpeds.2014.05.023. S0022–3476(14)00450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duggan AK, Berlin LJ, Cassidy J, Burrell L, Tandon SD. Examining maternal depression and attachment insecurity as moderators of the impacts of home visiting for at-risk mothers and infants. J Consult Clin Psychol. 2009;77:788–99. doi: 10.1037/a0015709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pilowsky DJ, Wickramaratne P, Poh E, et al. Psychopathology and functioning among children of treated depressed fathers and mothers. J Affect Disord. 2014;164:107–11. doi: 10.1016/j.jad.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madigan S, Bakermans-Kranenburg MJ, Van IJzendoorn MH, et al. Unresolved states of mind, anomalous parental behavior, and disorganized attachment: A review and meta-analysis of a transmission gap. Attach Hum Dev. 2006;8:89–111. doi: 10.1080/14616730600774458. [DOI] [PubMed] [Google Scholar]
- 16.Tluczek A, McKechnie AC, Brown RL. Factors associated with parental perception of child vulnerability 12 months after abnormal newborn screening results. Res Nurs Health. 2011;34:389–400. doi: 10.1002/nur.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simmons RJ, Goldberg S, Washington J, et al. Infant-mother attachment and nutrition in children with cystic fibrosis. J Dev Behav Pediatr. 1995;16:183–186. [PubMed] [Google Scholar]
- 18.Poulakis Z, Barker M, Wake M. Six month impact of false positives in an Australian infant hearing screening programme. Arch Dis Child. 2003;88:20–24. doi: 10.1136/adc.88.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Ocampo AC, Macias MM, Saylor CF, et al. Caretaker perception of child vulnerability predicts behavior problems in NICU graduates. Child Psychiatry Hum Dev. 2003;34:83–96. doi: 10.1023/a:1027384306827. [DOI] [PubMed] [Google Scholar]
- 20.Gurian EA, Kinnamon DD, Henry JJ, et al. Expanded newborn screening for biochemical disorders: the effect of a false-positive result. Pediatrics. 2006;117:1915–1921. doi: 10.1542/peds.2005-2294. [DOI] [PubMed] [Google Scholar]
- 21.Waisbren SE, Albers S, Amato S, et al. Effect of expanded newborn screening for biochemical genetic disorders on child outcomes and parental stress. JAMA. 2003;290:2564–2572. doi: 10.1001/jama.290.19.2564. [DOI] [PubMed] [Google Scholar]
- 22.Kerruish NJ, Campbell-Stokes PL, Gray A, et al. Maternal psychological reaction to newborn genetic screening for type 1 diabetes. Pediatrics. 2007;120:e324–e335. doi: 10.1542/peds.2006-1381. [DOI] [PubMed] [Google Scholar]
- 23.Kalnins D, Wilschanski M. Maintenance of nutritional status in patients with cystic fibrosis: new and emerging therapies. Drug Des Devel Ther. 2012;6:151–61. doi: 10.2147/DDDT.S9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell MJ, Powers SW, Byars KC, et al. Family functioning in young children with cystic fibrosis: observations of interactions at mealtime. J Dev Behav Pediatr. 2004;25:335–346. doi: 10.1097/00004703-200410000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Powers SW, Patton SR, Byars KC, et al. Caloric intake and eating behavior in infants and toddlers with cystic fibrosis. Pediatrics. 2002;109:e75. doi: 10.1542/peds.109.5.e75. [DOI] [PubMed] [Google Scholar]
- 26.Powers SW, Mitchell MJ, Patton SR, et al. Mealtime behaviors in families of infants and toddlers with cystic fibrosis. J Cystic Fibrosis. 2005;4:175–182. doi: 10.1016/j.jcf.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 27.Stark LJ, Jelalian E, Powers SW, et al. Parent and child mealtime behavior in families of children with cystic fibrosis. J Pediatr. 2000;136:195–200. doi: 10.1016/s0022-3476(00)70101-6. [DOI] [PubMed] [Google Scholar]
- 28.Stark LJ, Opipari LC, Jelalian E, et al. Child behavior and parent management strategies at mealtime in families with a school-age child with cystic fibrosis. Health Psychol. 2005;24:274–280. doi: 10.1037/0278-6133.24.3.274. [DOI] [PubMed] [Google Scholar]
- 29.Sheehan J, Massie J, Hay M, et al. The natural history and predictors of persistent problem behaviours in cystic fibrosis: a multicentre, prospective study. Arch Dis Child. 2012;97:625–631. doi: 10.1136/archdischild-2011-301527. [DOI] [PubMed] [Google Scholar]
- 30.Parker EM, O’Sullivan BP, Shea JC, et al. Survey of breast-feeding practices and outcomes in the cystic fibrosis population. Pediatr Pulmonol. 2004;37:362–367. doi: 10.1002/ppul.10450. [DOI] [PubMed] [Google Scholar]
- 31.Tluczek A, Clark R, McKechnie AC, et al. Task-oriented and bottle feeding adversely affect the quality of mother-infant interactions after abnormal newborn screens. J Dev Behav Pediatr. 2010;31:414–26. doi: 10.1097/DBP.0b013e3181dd5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tluczek A, Orland KM, Cavanagh L. Psychosocial consequences of false-positive newborn screen for cystic fibrosis. Qual Health Res. 2011;21:174–86. doi: 10.1177/1049732310382919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabachnick BG, Fidell LS. Using multivariate statistics. 2. New York, NY: Harper & Row; 1989. [Google Scholar]
- 34.Clark R. The Parent-Child Early Relational Assessment: Instrument and manual. Madison: University of Wisconsin Medical School, Department of Psychiatry; 1985. [Google Scholar]
- 35.Clark R. The parent-child early relational assessment: a factorial validity study. Educ Psychol Meas. 1999;59:821–846. [Google Scholar]
- 36.Clark R, Tluczek A, Gallagher KC. Assessment of Parent-Child Early Relational Disturbances. In: DelCarmen-Wiggins R, Carter A, editors. Handbook of Infant, Toddler, and Preschool Mental Health Assessment. Vol. 3. New York, NY: Oxford University Press; 2004. pp. 25–60. [Google Scholar]
- 37.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- 38.Spielberger CD, Gorsuch RL, Lushene RE, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 39.Grant KA, McMahon C, Austin MP. Maternal anxiety during the transition to parenthood: a prospective study. J Affect Disord. 2008;108:101–111. doi: 10.1016/j.jad.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Kvaal K, Macijauskiene J, Engedal K, et al. High prevalence of anxiety in hospitalized geriatric patients. Int J Geriatr Psychiatry. 2001;16:690–693. doi: 10.1002/gps.405. [DOI] [PubMed] [Google Scholar]
- 41.Forsyth BW, Horwitz SM, Leventhal JM, et al. The child vulnerability scale: an instrument to measure parental perceptions of child vulnerability. J Pediatr Psychol. 1996;21:89–101. doi: 10.1093/jpepsy/21.1.89. [DOI] [PubMed] [Google Scholar]
- 42.Dogan DG, Ertem IO, Karaaslan T, et al. Perception of vulnerability among mothers of healthy infants in a middle-income country. Child Care Health Dev. 2009;35:868–872. doi: 10.1111/j.1365-2214.2009.01015.x. [DOI] [PubMed] [Google Scholar]
- 43.Fedele DA, Grant DM, Wolfe-Christensen C, et al. An examination of the factor structure of parenting capacity measures in chronic illness populations. J Pediatr Psychol. 2010;35:1083–1092. doi: 10.1093/jpepsy/jsq045. [DOI] [PubMed] [Google Scholar]
- 44.Waters E. Unpublished instrument. State University of New York at Stony Brook, Department of Psychology; 1987. Attachment behavior Q-Set (revision 3.0) [Google Scholar]
- 45.StatXact8 [computer program] Cambridge, MA: Cytel Inc; 2009. [Google Scholar]
- 46.Sidak Z. Rectangular confidence regions for the means of multivariate normal distributions. J Am Stat Assoc. 1967;62:626–633. [Google Scholar]
- 47.Muthén LK, Muthén BO. Mplus: Statistical Analysis with Latent Variables User’s Guide. 7. Los Angeles, CA: Muthén and Muthén; 1998–2012. [Google Scholar]
- 48.Hu L, Bentler P. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 49.McDonald RA, Behson SJ, Seifert CF. Strategies for Dealing with Measurement Error in Multiple Regression. Journal of Academy of Business and Economics. 2005;5:80. [Google Scholar]
- 50.Bandalos DL, Finney SJ. Item parceling issues in structural equation modeling. In: Marcoulides GA, Schumacker RE, editors. New Developments and Techniques in structural equation modeling. Mahwah, NJ: Lawrence Erlbaum Associates Publishers; 2001. pp. 269–296. [Google Scholar]
- 51.Bandalos DL. The effects of item parceling on goodness-of-fit and parameter estimate bias in structural equation modeling. Struct Equ Modeling. 2002;9:78–102. [Google Scholar]
- 52.Hayduk LA. Structural Equation Modeling with LISREL: Essentials and Advances. Baltimore, MD: Johns Hopkins University Press; 1988. [Google Scholar]
- 53.Little RJA. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc. 1988;83:1198–1202. [Google Scholar]
- 54.NORM: multiple imputation of incomplete multivariate data under a normal model. [computer software]. Version 2.03. Schafer JL: 1999. [Google Scholar]
- 55.United States Census Bureau. [Accessed, January 10, 2014.];State by State Quick Facts: Wisconsin, 2014. 2014 Jan 6; Available at: http://quickfacts.census.gov/qfd/states/55000.html.
- 56.Milfont TL, Fischer R. Testing measurement invariance across groups: Applications in cross-cultural research. International Journal of Psychological Research. 2010;3:112–131. [Google Scholar]
- 57.Fagan J, Barnett M. The Relationship between Maternal Gatekeeping, Paternal Competence, Mothers’ Attitudes about the Father Role, and Father Involvement. J Fam Issues. 2003;24:1020–1043. [Google Scholar]
- 58.Madigana S, Voci S, Benoit D. Stability of atypical caregiver behaviors over six years and associations with disorganized infant–caregiver attachment. Attach Hum Dev. 2011;13:237–252. doi: 10.1080/14616734.2011.562410. [DOI] [PubMed] [Google Scholar]
- 59.Carlson EA, Sroufe LA. Contribution of attachment theory to developmental psychopathology. In: Cicchetti D, Cohen D, editors. Developmental processes and psychopathology, Vol. 1: Theoretical perspectives and methodological approaches. New York, NY: Cambridge University Press; 1995. pp. 581–617. [Google Scholar]