Abstract
The efficacy of n-3 PUFAs in preventing recurrence of atrial fibrillation (AF) is controversial and their effects on inflammation and oxidative stress in this population are not known. This study examined the effects of high-dose marine omega-3 polyunsaturated fatty acids (n-3 PUFAs) added to conventional therapy on the recurrence of AF and on markers of inflammation and oxidative stress. Patients with paroxysmal or persistent AF were randomized to n-3 PUFAs (4g/d) (n=126) or placebo (n=64) in a 2:1 ratio in a prospective, double-blind, placebo-controlled, parallel group study. The primary outcome was time to recurrence of AF. Secondary outcomes were changes in biomarkers of inflammation (serum interleukin (IL)-6, IL-8, IL-10, tissue necrosis factor alpha (TNFα), monocyte chemoattractant protein-1 (MCP-1), and vascular endothelial growth factor (VEGF)), N-terminal-pro-brain type natriuretic peptide (NTpBNP), and oxidative stress (urinary F2–isoprostanes (IsoPs)). Atrial fibrillation recurred in 74 (58.7%) patients randomized to n3-PUFAs and in 30 (46.9%) who received placebo; time to recurrence of AF did not differ significantly in the two groups (hazard ratio 1.20; 95% CI 0.76 - 1.90, adjusted P=0.438). Compared to placebo, n3-PUFAs did not result in clinically meaningful changes in concentrations of inflammatory markers, NTpBNP or F2-Isops. In conclusion, in patients with paroxysmal or persistent AF treatment with n3-PUFAs 4g/day did not reduce the recurrence of AF, nor was it associated with clinically important effects on concentrations of markers of inflammation and oxidative stress.
Keywords: atrial fibrillation, fish oil, fatty acids, omega-3, oxidative stress, inflammation
Introduction
Few studies have specifically addressed the hypothesis that inflammation and oxidative stress play a direct role in AF, and that treatment targeted at these mechanisms may be beneficial. Omega-3 polyunsaturated fatty acids (n-3 PUFAs) are rich in eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) and have antiarrhythmic,1, 2 anti-inflammatory,3, 4 and antioxidant3, 5 effects. Thus, n-3 PUFAs are attractive as a potential therapy for AF. In vitro studies,6 as well as some epidemiological 7 and interventional studies, 8, 9 support a role for n-3 PUFAs in the prevention of AF, but others do not.10-14 There is little information about the efficacy and effects on inflammation and oxidative stress of high doses of n-3 PUFAs added to conventional AF therapy. Accordingly, we designed this randomized, placebo-controlled trial to examine the effects of high-dose n-3 PUFAs added to conventional AF therapy on the recurrence of AF and on markers of inflammation and oxidative stress.
Methods
This was a prospective, double-blind, placebo-controlled, parallel group, 6 month study with randomization to n-3 PUFAs or placebo in a 2:1 ratio. The study was conducted at 4 centers in Nashville, TN and at the Marshfield Clinic in Madison, WI and was approved by the Institutional Review Boards of all participating organizations. All patients provided written informed consent.
We studied patients ≥21 years of age with a history of at least two occurrences of AF or atrial flutter, at least one of which was AF, and an electrocardiogram within the previous 12 months showing AF or atrial flutter. Patients were in sinus rhythm at randomization. Those patients taking antiarrhythmic drugs continued to do so. Key exclusion criteria included: permanent AF, New York Heart Association class III or IV heart failure or Canadian Cardiovascular Society class III or IV angina pectoris, cardiothoracic surgery, myocardial infarction or stroke within the previous 3 months, reversible causes of AF, cancer, currently taking fish oil, allergy to fish, serious bleeding in the previous year, and dialysis or renal transplantation.
Participants received 4g/day of n-3 PUFAs (Lovaza, GlaxoSmithKline, Research Triangle Park, NC) or an identical corn oil placebo. Each 1g n-3 PUFA capsule contained approximately 465 mg of EPA and 375 mg of DHA. Randomization was performed according to a computer-generated permuted block scheme with 4 strata according to baseline anti-arrhythmic therapy: i) no anti-arrhythmic therapy, ii) amiodarone, iii) class I anti-arrhythmic drugs, and iv) sotalol or dofetilide.
Subjects took the first dose of study medication under direct observation and this was considered the time of randomization. Patients were seen at baseline and then at weeks 2, 4, 8, 12, 18, and 24. At each visit adverse events were recorded and adherence monitored by capsule count and heart rhythm was monitored. Patients were provided with a transtelephonic electrocardiographic monitoring (TTM) device (eCardio Diagnostics, Houston, TX) and made routine transmissions every 2 weeks, and in addition, if they had symptoms suggestive of arrhythmia. Electrocardiograms were coded, de-identified and evaluated blindly by two cardiac electrophysiologists (DD and KM). Patients were followed for 6 months or until AF recurred. Patients who changed antiarrhythmic drug or dose during the study were withdrawn.
Venous blood was collected, separated immediately and stored in aliquots frozen at −70° C until assayed. Serum IL-6, IL-8, IL-10, tissue necrosis factor alpha (TNFα), monocyte chemoattractant protein-1 (MCP-1), vascular endothelial growth factor (VEGF) and N-terminal-pro-brain type natriuretic peptide (NTpBNP) were measured by multiplex enzyme-linked immunosorbent assay (Linco Research/Millipore Corp, St. Louis, MO) as previously described.15 Urine samples for F2 and F3–isoprostanes (IsoPs), markers of oxidative stress, were quantified using gas chromatography and mass spectroscopy and expressed as ng/mg creatinine (ng/mg Cr). 16, 17
The primary outcome was the time to documented recurrence of AF (symptomatic or asymptomatic). Secondary outcomes included changes in measures of inflammation and oxidative stress and their relationship with recurrence of AF. Initial sample size estimations (n=450) sought to provide not only excellent power for the primary and secondary outcomes but also for determining whether efficacy was related to changes in secondary outcomes. In 2010, because of slow enrollment, the sample size was recalculated to focus on the primary outcome and indicated that a sample size of n=180 would provide 94% power to detect a difference of 30% in the recurrence rate of AF between groups. We estimated that 10% of patients would drop out, and thus sought to enroll approximately 198 subjects.
Baseline demographic and clinical characteristics were examined using median and interquartile ranges or frequencies and proportions and compared using the Wilcoxon rank sum test for continuous variables or Chi-square test for categorical variables. The cumulative probability of not having a recurrence of AF was estimated using the Kaplan-Meier product limit method for n-3 PUFAs and placebo groups. Comparisons between the two survival curves were made using the logrank test. For the primary outcome, time to recurrence of AF, we used a Cox proportional hazard regression model to evaluate the effect of treatment after adjusting for covariates. Clinically relevant covariates were selected a priori as potential risk factors for AF and included: age, race, sex, duration of AF, coronary heart disease, congestive heart failure and randomization stratification factor. Duration of AF was included as a flexible smooth parameter using splines. A pre-specified analysis was performed in the subpopulation of patients who remained in the study 30 days after randomization. Subgroup analyses were adjusted for only age, sex and race due to smaller sample sizes. Proportional hazard assumptions were checked using the Schoenfeld residuals method.18 Hazard ratios (HRs) and their 95% confidence intervals (95% CIs) are reported. For biomarkers we calculated HRs per increase in interquartile range. We used intent-to-treat as the primary analysis.
To assess differences between n-3 PUFAs and placebo groups for the secondary outcomes (biomarkers), we performed analysis of covariance multiple linear regression analysis (ANCOVA) using the end-of-study value of the response variable with the baseline value as a covariate; additional covariates were as described for the primary outcome.
Additionally, we evaluated whether baseline or end-of-study concentration of biomarkers were differentially associated with recurrence of AF by treatment status. We used Cox proportional hazard models and a cross-product term between biomarker (baseline or end-of-study) and treatment status. In adjusted analyses, covariates included were limited to age, race and sex. All statistical analyses were performed using open source R statistical software version 3.0.2 (http://www.r-project.org).
Results
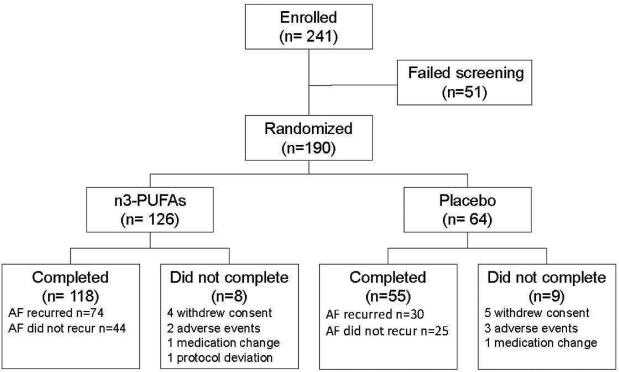
Between December 2007 and December 2012, 241 patients were enrolled and 190 (178 at Vanderbilt, 12 at other sites) ultimately randomized to n-3 PUFAs (n=126) or placebo (n=64) (Figure 1). The two groups were well matched with regard to age, race, sex and baseline anti-arrhythmic therapy, but coronary heart disease was more frequent in the control group (Table 1). Of the 190 patients randomized, 173 (91%) completed the study.
Figure 1.
Study flowchart
Table 1.
Demographic and clinical characteristics of subjects at randomization
| Baseline Characteristics | n-3-PUFA (N=126) | Placebo (N=64) | P value |
|---|---|---|---|
| Age (years) | 62 (12) | 61 (11) | 0.57 |
| Women | 59 (47%) | 22 (34%) | 0.10 |
| White | 119 (94%) | 61 (95%) | 0.29 |
| Body mass index (kg/m2) | 30.1 (7.2) | 31.9 (7.3) | 0.04 |
| Hypertension | 78 (62%) | 44 (69%) | 0.35 |
| Diabetes mellitus | 23 (18%) | 13 (20%) | 0.73 |
| Coronary heart disease | 9 (7%) | 14 (22%) | 0.003 |
| Prior myocardial infarction | 7 (6%) | 8 (12%) | 0.09 |
| Heart failure | 14 (11%) | 13 (20%) | 0.09 |
| Lone atrial fibrillation | 20 (16%) | 7 (11%) | 0.36 |
| Duration of atrial fibrillation (months) | 50 [13,111] | 54 [22,112] | 0.32 |
| Ejection fraction (%*) | 58 ± 7 | 57 ± 6 | 0.31 |
| Left atrial size (mm*) | 40 ± 7 | 42 ± 7 | 0.13 |
| Therapy | |||
| No anti-arrhythmic therapy | 46 (37%) | 21 (33%) | 0.91** |
| Amiodarone | 12 (10%) | 8 (12%) | |
| Class I agent | 30 (24%) | 15 (23%) | |
| Sotalol or dofetilide | 38 (30%) | 20 (31%) | |
| Beta blocker | 71 (56%) | 41 (64%) | 0.31 |
| Statin | 45 (36%) | 28 (44%) | 0.28 |
| ACE inhibitor | 33 (26%) | 16 (25%) | 0.86 |
| Angiotensin receptor blocker | 22 (17%) | 6 (9%) | 0.14 |
| Warfarin | 63 (50%) | 28 (44%) | 0.41 |
Data are shown as number (%) or mean (standard deviation) or median [interquartile range].
Measurements for left atrial size and ejection fraction were available in 152 and 169 patients, respectively.
P-value represents comparison of the prevalence of 4 anti-arrhythmic therapies (no therapy, amiodarone, class I agent, and sotalol or dofetilide) in the two groups.
Lone atrial fibrillation was defined as atrial fibrillation with no underlying structural or functional heart disease, no hypertension, no diabetes and younger than 65 yrs.
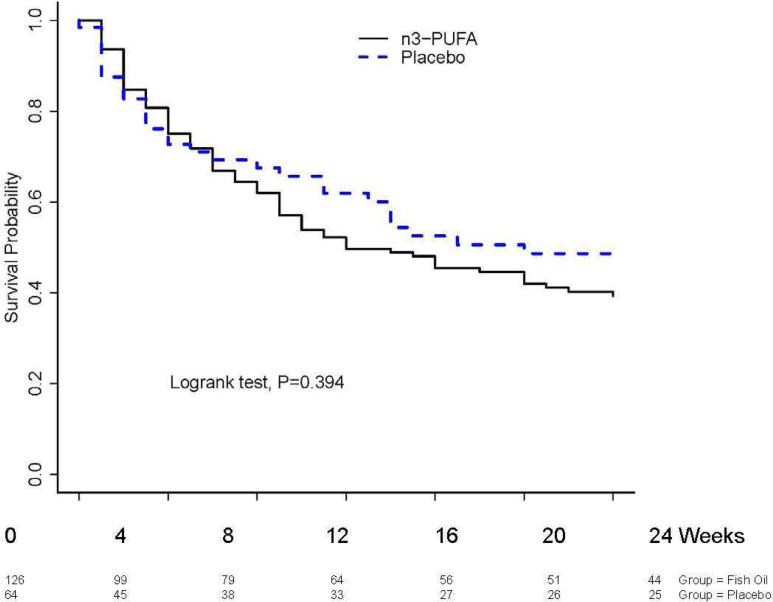
AF recurred in 74 (59%) of 126 patients randomized to n3-PUFAs and in 30 (47%) of those who received placebo; the majority (81 of 104 recurrences, 78%) were symptomatic. There was no statistically significant difference in the primary outcome of time to recurrence of AF among patients randomized to receive n3-PUFAs or placebo (HR 1.20; 95% CI 0.79-1.84, P=0.39) (Figure 2). Statistical adjustment for age, race, sex, randomization stratum, coronary artery disease, congestive heart failure and duration of AF did not alter the findings materially (HR 1.20; 95% CI 0.76 - 1.90, P=0.438). There was no statistically significant treatment effect in patients who remained in the study 30 days after randomization (HR: 1.65; 95% CI 0.88 - 3.09, P=0.119 age, race, and sex adjusted).
Figure 2. Time to recurrence of atrial fibrillation.
There was no statistically significant difference in time to recurrence of atrial fibrillation among patients randomized to receive n3-PUFAs and those who received placebo (hazard ratio 1.20; 95% CI 0.79 - 1.84; P=0.393 unadjusted and after adjustment for age, race, sex, randomization stratum, coronary artery disease, congestive heart failure and duration of atrial fibrillation HR: 1.20; 95% CI 0.76 - 1.90; P=0.438).
Concentrations of cytokines and NTpBNP (Table 2) were similar to those we previously reported in a different group of patients with AF and higher than would be expected in the general population;15 however, n3-PUFA therapy did not affect concentrations of IL-6, IL-8, IL-10, MCP-1 or NTpBNP significantly (Table 2). There were small differences in TNF-α and VEGF concentrations of marginal significance that were not statistically significant after adjustment for covariates (P>0.05 for both). Concentrations of markers of inflammation and NTpBNP at baseline were not significantly associated with time to recurrence of AF (all P values > 0.05), although there was a differential association between higher levels of IL-6 at baseline and time to recurrence of AF between subjects who received n3-PUFAs and those that did not (P interaction =0.019; n3-PUFAs HR 1.23; 95% CI 0.94 -1.59; placebo HR 0.61; 95%CI 0.35-1.08, adjusted for age, sex and race).
Table 2.
Measures of inflammation and oxidative stress at baseline and end of study in patients who received omega-3 polyunsaturated fatty acids or placebo
| Baseline | End of Study | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Biomarker | n3-PUFAs | Placebo | n3-PUFAs | Placebo | P value* | P** value |
| IL-6 (pg/ml) | 2.8 (1.5-4.4) | 2.5 (1.3-4.5) | 2.2 (1.3-4.0) | 2.4 (1.5-4.7) | 0.212 | 0.77 |
| IL-8 (pg/ml) | 7.2 (5.5-10.0) | 5.6 (4.1-8.4) | 6.8 (5.0-9.2) | 5.8 (3.8-8.0) | 0.793 | 0.41 |
| IL-10 (pg/ml) | 2.0 (0-5.9) | 1.5 (0-8.4) | 1.4 (0-5.3) | 1.6 (0-6.3) | 0.634 | 0.92 |
| TNF-α (pg/ml) | 8.5 (6.0-11.2) | 7.8 (6.5-9.9) | 8.0 (5.5-10.9) | 7.6 (6.2-9.8) | 0.049 | 0.10 |
| MCP-1 (pg/ml) | 148 (97-210) | 142(110-176) | 131 (102-188) | 129 (104-172) | 0.941 | 0.62 |
| VEGF (pg/ml) | 95 (35-240) | 103(30-189) | 90 (33-228) | 69 (18-145) | 0.046 | 0.33 |
| NTpBNP (pg/ml) | 124.0 (0.35-386.0) | 157.5 (9.2-441.0) | 192 (11-723) | 288 (27-752) | 0.768 | 0.85 |
| Urinary F2-IsoPs (ng/mg Cr) | 1.41 (0.91-2.06) | 1.29 (1.05-1.83) | 1.25 (0.87-1.81) | 1.08 (0.83-1.98) | 0.991 | 0.95 |
| Urinary F3-IsoPs (ng/mg Cr) | 0.134 (0.089-0.23) | 0.141 (0.085-0.177) | 0.31 (0.19-0.53) | 0.12 (0.06-0.18) | <0.0001 | <0.0001 |
Values are shown as median with interquartile range.
P value refers to comparison between biomarker values in n3-PUFA and placebo groups at the end of study with adjustment for baseline values using analysis of covariance regression methods (ANCOVA).
The model was further adjusted for age, race, sex, randomization stratum, coronary artery disease, congestive heart failure and duration of atrial fibrillation
IL = interleukin; MCP = monocyte chemoattractant protein; NTpBNP = N-terminal pro–brain (B-type) natriuretic peptide; TNF = tumor necrosis factor; Urinary F2-IsoPs = urinary F2-isoprostanes; Urinary F3-IsoPs = urinary F3-isoprostanes; VEGF = vascular endothelial growth factor
Changes in urinary F2-Isop excretion did not differ significantly among those who received placebo or n3-PUFAs (P=0.991)(Table 2). F3-Isops are formed from EPA17, 19 and thus increased significantly after n3-PUFAs (P<0.0001) (Table 2). F3-Isop excretion at baseline (n3-PUFAs HR 1.01; 95%CI 0.76 - 1.35; placebo HR 0.91; 95%CI: 0.53 - 1.58, P interaction =0.59, adjusted for age, race and sex) and at the exit visit (n3-PUFAs HR=1.02; 95%CI: 0.69 - 1.52; placebo HR=0.99; 95%CI: 0.55 -1.78, P interaction=0.82 adjusted for age, sex and race) were not significantly associated with time to recurrence of AF.
The study intervention was tolerated well. No patients died and there were 5 serious adverse events: n3-PUFAs (n=2): pseudogout (1) non-cardiac chest pain (1); placebo (n=3): acute coronary syndrome (1), bradycardia (1), and rapid AF (1). Five patients did not complete the study because of adverse events (n3-PUFAs: gastrointestinal discomfort (2); placebo: prostate biopsy (1), worsening heart failure (1), and acute coronary syndrome (1)).
Discussion
The major findings of this study are that high doses (4g/day) of n3-PUFAs, in addition to usual antiarrhythmic therapy, did not decrease the rate of AF recurrence. Moreover, n3-PUFAs did not have clinically important effects on concentrations of inflammatory cytokines, NTpBNP, or a marker of oxidative stress (F2-Isop excretion).
Studies performed in non-human animals, and some epidemiologic and clinical studies have suggested that n3-PUFAs may prevent AF or its recurrence.8, 9, 20, 21 Our findings that n3-PUFAs as adjunctive therapy did not prevent recurrence of AF are, however, concordant with studies in postoperative AF,22, 23 and several randomized controlled clinical trials of persistent or paroxysmal AF10, 13, 14, as well as a recent meta-analysis.24 Clinical trials of n3-PUFAs to prevent AF recurrence used a range of doses and differed in design. One such difference is the duration of exposure to n3-PUFAs before efficacy was assessed. Of interest, n3-PUFAs were administered for at least 4 weeks before cardioversion in the two studies that found a protective effect,8, 9 a finding compatible with the observation that concentrations of EPA and DHA in atrial tissue accumulate slowly and reach a maximum after approximately 30 days of therapy.25 When we restricted our analysis to those patients who completed at least 30 days of treatment, we found no protective effect.
Fish oil has several antiarrhythmic mechanisms including effects on ion channels, atrial remodeling, inflammation and oxidative stress.3 Inflammation is thought to play a fundamental role in the pathogenesis of AF and not to merely represent an epiphenomenon.26, 27 Similarly, substantial evidence from animal and human studies implicates increased oxidative stress as important in the pathogenesis of AF and as a potential therapeutic target.26, 28 Therefore, the effect of n3-PUFAs on inflammation and oxidative stress in AF is of interest.
Anti-inflammatory effects of n3-PUFAs have been reported in many experimental models and human studies,3, 4 including heart failure.29 However, these effects have not been consistent. For example, although n3-PUFAs decreased IL-6 and TNFα concentrations significantly in a meta-analysis of heart failure studies, not all studies showed the decrease and there was no effect on C-reactive protein.29 In a previous study, we observed that serum IL-6, IL-8, IL-10, TNFα, MCP-1, VEGF and NTpBNP concentrations were elevated in patients with AF compared to controls.15 Therefore, we studied the effects of n3-PUFAs on the same panel of biomarkers. Despite performing a large study with high doses of n3-PUFAs, we found no clinically important effect on cytokine concentrations. This suggests that the inflammation associated with AF is not reduced by n3-PUFA therapy.
Furthermore, we found no relation between the markers of inflammation at baseline and risk of recurrence of AF in either treatment group, although there appeared to be a differential association between higher levels of IL-6 at baseline and a shorter time to recurrence of AF in subjects who received n3-PUFAs. However, this was an exploratory analysis and the P value was not adjusted for multiplicity.
We also found that n3-PUFAs had no effect on F2-IsoP excretion, considered by many to be the gold standard for measurement of systemic oxidative stress. Despite the suggestion that AF is associated with increased oxidative stress, we have previously reported that F2-IsoP excretion did not differ among patients with AF and controls.15 Thus, it is possible that an antioxidant effect of n3-PUFAs in AF could have been difficult to detect in the absence of increased F2-IsoP excretion. We did, however, find that F3-IsoP excretion more than doubled after n3-PUFA therapy. F3-IsoPs are formed from the oxidation of EPA, just as F2-IsoPs are formed from the peroxidation of arachidonic acid.17, 19 Thus, the increase in F3-IsoPs after n3-PUFA therapy is expected.
The biological significance of the increase in F3-IsoPs that occurs after n3-PUFA therapy is unknown. Two possibilities have been proposed.19 First, because EPA is more susceptible to lipid peroxidation than arachidonic acid, it could scavenge free radicals and form F3-IsoPs that are likely less biologically active than F2-IsoPs; thus, differential formation of F3-IsoPs rather than F2-IsoPs could contribute to the beneficial effects of n-PUFAs.19 We found that F3-IsoPs increased, but there was no concomitant decrease in F2-IsoPs after n3-PUFA therapy. This finding does not support the hypothesis that free radicals are differentially directed away from the F2-IsoP pathway by the formation of F3-IsoPs. Second, increased F3-IsoPs might have deleterious effects that antagonize beneficial effects of n3-PUFAs.19 Our study did not address this hypothesis directly; however, there was no relationship between risk of recurrence of AF and F3-IsoP excretion.
We and many others have previously reported that NTpBNP concentrations are increased in patients with AF.15 The effect of n3-PUFAs on NTpBNP concentrations in AF are not well-established, but treatment with 2g/day for 3 months decreased NTpBNP concentrations in 38 patients with heart failure.30 We found no significant effect of n3-PUFAs on NTpBNP concentrations in patients with AF, concordant with the overall lack of effect on AF recurrence.
Our study had several limitations. We determined the recurrence of AF by both routine and symptomatic TTM transmissions, but we did not examine the effect of therapy on total AF burden. Our sample size was relatively small and included a heterogeneous patient population, including patients receiving different antiarrhythmic therapies in stable doses. Although this approach provided the advantage that our findings could be generalizable to a wide population, it did not allow us to study specific populations such as patients receiving amiodarone, in whom efficacy of n3-PUFAs was previously reported.9
Acknowledgements
We thank Dr. Edward Pritchett for providing advice regarding study design and implementation; Drs. Peng-Sheng Chen, Dan Roden, Jeff Rottman, and Dan Byrne for serving on the Data Safety Monitoring Board; GlaxoSmithKline for providing omega-3 fatty acid capsules and matching placebo; and eCardio for providing transtelephonic monitoring devices and diagnostic services.
Funding Sources Supported by a grant from the National Institutes of Health HL087254, and by CTSA award UL1TR000445 from the National Center for Advancing Translational Sciences. GlaxoSmithKline provided Lovaza capsules and matching placebo free of charge. eCardio provided transtelephonic monitoring devices and diagnostic services free of charge. None of the sources of support was involved in the data analysis, interpretation or writing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures
None
References
- 1.Leaf A, Kang JX, Xiao YF, Billman GE. Clinical prevention of sudden cardiac death by n-3 polyunsaturated fatty acids and mechanism of prevention of arrhythmias by n-3 fish oils. Circulation. 2003;107:2646–2652. doi: 10.1161/01.CIR.0000069566.78305.33. [DOI] [PubMed] [Google Scholar]
- 2.Kang JX, Leaf A. Antiarrhythmic effects of polyunsaturated fatty acids. Recent studies. Circulation. 1996;94:1774–1780. doi: 10.1161/01.cir.94.7.1774. [DOI] [PubMed] [Google Scholar]
- 3.Das UN. Beneficial effect(s) of n-3 fatty acids in cardiovascular diseases: but, why and how? Prostaglandins Leukot Essent Fatty Acids. 2000;63:351–362. doi: 10.1054/plef.2000.0226. [DOI] [PubMed] [Google Scholar]
- 4.Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, Cannon JG, Rogers TS, Klempner MS, Weber PC. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320:265–271. doi: 10.1056/NEJM198902023200501. [DOI] [PubMed] [Google Scholar]
- 5.Higdon JV, Liu J, Du SH, Morrow JD, Ames BN, Wander RC. Supplementation of postmenopausal women with fish oil rich in eicosapentaenoic acid and docosahexaenoic acid is not associated with greater in vivo lipid peroxidation compared with oils rich in oleate and linoleate as assessed by plasma malondialdehyde and F(2)-isoprostanes. Am J Clin Nutr. 2000;72:714–722. doi: 10.1093/ajcn/72.3.714. [DOI] [PubMed] [Google Scholar]
- 6.Jahangiri A, Leifert WR, Patten GS, McMurchie EJ. Termination of asynchronous contractile activity in rat atrial myocytes by n-3 polyunsaturated fatty acids. Mol Cell Biochem. 2000;206:33–41. doi: 10.1023/a:1007025007403. [DOI] [PubMed] [Google Scholar]
- 7.Mozaffarian D, Psaty BM, Rimm EB, Lemaitre RN, Burke GL, Lyles MF, Lefkowitz D, Siscovick DS. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;110:368–373. doi: 10.1161/01.CIR.0000138154.00779.A5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar S, Sutherland F, Morton JB, Lee G, MORGAN J, Wong J, Eccleston DE, Voukelatos J, Garg ML, Sparks PB. Long-term omega-3 polyunsaturated fatty acid supplementation reduces the recurrence of persistent atrial fibrillation after electrical cardioversion. Heart Rhythm. 2012;9:483–491. doi: 10.1016/j.hrthm.2011.11.034. [DOI] [PubMed] [Google Scholar]
- 9.Nodari S, Triggiani M, Campia U, Manerba A, Milesi G, Cesana BM, Gheorghiade M, Dei CL. n-3 polyunsaturated fatty acids in the prevention of atrial fibrillation recurrences after electrical cardioversion: a prospective, randomized study. Circulation. 2011;124:1100–1106. doi: 10.1161/CIRCULATIONAHA.111.022194. [DOI] [PubMed] [Google Scholar]
- 10.Kowey PR, Reiffel JA, Ellenbogen KA, Naccarelli GV, Pratt CM. Efficacy and safety of prescription omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: a randomized controlled trial. JAMA. 2010;304:2363–2372. doi: 10.1001/jama.2010.1735. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe E, Sobue Y, Sano K, Okuda K, Yamamoto M, Ozaki Y. Eicosapentaenoic acid for the prevention of recurrent atrial fibrillation. Ann Noninvasive Electrocardiol. 2011;16:373–378. doi: 10.1111/j.1542-474X.2011.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozaydin M, Erdogan D, Tayyar S, Uysal BA, Dogan A, Icli A, Ozkan E, Varol E, Turker Y, Arslan A. N-3 polyunsaturated fatty acids administration does not reduce the recurrence rates of atrial fibrillation and inflammation after electrical cardioversion: a prospective randomized study. Anadolu Kardiyol Derg. 2011;11:305–309. doi: 10.5152/akd.2011.080. [DOI] [PubMed] [Google Scholar]
- 13.Macchia A, Grancelli H, Varini S, Nul D, Laffaye N, Mariani J, Ferrante D, Badra R, Figal J, Ramos S, Tognoni G, Doval HC. Omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: results of the FORWARD (Randomized Trial to Assess Efficacy of PUFA for the Maintenance of Sinus Rhythm in Persistent Atrial Fibrillation) trial. J Am Coll Cardiol. 2013;61:463–468. doi: 10.1016/j.jacc.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 14.Bianconi L, Calo L, Mennuni M, Santini L, Morosetti P, Azzolini P, Barbato G, Biscione F, Romano P, Santini M. n-3 polyunsaturated fatty acids for the prevention of arrhythmia recurrence after electrical cardioversion of chronic persistent atrial fibrillation: a randomized, double-blind, multicentre study. Europace. 2011;13:174–181. doi: 10.1093/europace/euq386. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Solus J, Chen Q, Rho YH, Milne G, Stein CM, Darbar D. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. 2010;7:438–444. doi: 10.1016/j.hrthm.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrow JD, Roberts LJ. Mass spectrometric quantification of F2-isoprostanes as indicators of oxidant stress. Methods Mol Biol. 2002;186:57–66. doi: 10.1385/1-59259-173-6:57. [DOI] [PubMed] [Google Scholar]
- 17.Gao L, Yin H, Milne GL, Porter NA, Morrow JD. Formation of F-ring isoprostane-like compounds (F3-isoprostanes) in vivo from eicosapentaenoic acid. J Biol Chem. 2006;281:14092–14099. doi: 10.1074/jbc.M601035200. [DOI] [PubMed] [Google Scholar]
- 18.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;14:1707–1723. doi: 10.1002/sim.4780141510. [DOI] [PubMed] [Google Scholar]
- 19.Song WL, Paschos G, Fries S, Reilly MP, Yu Y, Rokach J, Chang CT, Patel P, Lawson JA, FitzGerald GA. Novel eicosapentaenoic acid-derived F3-isoprostanes as biomarkers of lipid peroxidation. J Biol Chem. 2009;284:23636–23643. doi: 10.1074/jbc.M109.024075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Camm AJ, Savelieva I. Fish oil for secondary prevention of atrial fibrillation: should we still believe in its antiarrhythmic effect? Circulation. 2011;124:1093–1096. doi: 10.1161/CIRCULATIONAHA.111.048140. [DOI] [PubMed] [Google Scholar]
- 21.Wu JH, Lemaitre RN, King IB, Song X, Sacks FM, Rimm EB, Heckbert SR, Siscovick DS, Mozaffarian D. Association of plasma phospholipid long-chain omega-3 fatty acids with incident atrial fibrillation in older adults: the cardiovascular health study. Circulation. 2012;125:1084–1093. doi: 10.1161/CIRCULATIONAHA.111.062653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mozaffarian D, Marchioli R, Macchia A, Silletta MG, Ferrazzi P, Gardner TJ, Latini R, Libby P, Lombardi F, O'Gara PT, Page RL, Tavazzi L, Tognoni G. Fish oil and postoperative atrial fibrillation: the Omega-3 Fatty Acids for Prevention of Post-operative Atrial Fibrillation (OPERA) randomized trial. JAMA. 2012;308:2001–2011. doi: 10.1001/jama.2012.28733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mozaffarian D, Wu JH, de Oliveira Otto MC, Sandesara CM, Metcalf RG, Latini R, Libby P, Lombardi F, O'Gara PT, Page RL, Silletta MG, Tavazzi L, Marchioli R. Fish oil and post-operative atrial fibrillation: a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2013;61:2194–2196. doi: 10.1016/j.jacc.2013.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mariani J, Doval HC, Nul D, Varini S, Grancelli H, Ferrante D, Tognoni G, Macchia A. N-3 polyunsaturated fatty acids to prevent atrial fibrillation: updated systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2013;2(1):19, e005033. doi: 10.1161/JAHA.112.005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metcalf RG, James MJ, Gibson RA, Edwards JR, Stubberfield J, Stuklis R, Roberts-Thomson K, Young GD, Cleland LG. Effects of fish-oil supplementation on myocardial fatty acids in humans. Am J Clin Nutr. 2007;85:1222–1228. doi: 10.1093/ajcn/85.5.1222. [DOI] [PubMed] [Google Scholar]
- 26.Van Wagoner DR. Oxidative stress and inflammation in atrial fibrillation: role in pathogenesis and potential as a therapeutic target. J Cardiovasc Pharmacol. 2008;52:306–313. doi: 10.1097/FJC.0b013e31817f9398. [DOI] [PubMed] [Google Scholar]
- 27.Aviles RJ, Martin DO, pperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 28.Huang CX, Liu Y, Xia WF, Tang YH, Huang H. Oxidative stress: a possible pathogenesis of atrial fibrillation. Med Hypotheses. 2009;72:466–467. doi: 10.1016/j.mehy.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 29.Xin W, Wei W, Li X. Effects of fish oil supplementation on inflammatory markers in chronic heart failure: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2012;12:77. doi: 10.1186/1471-2261-12-77. doi: 10.1186/1471-2261-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao YT, Shao L, Teng LL, Hu B, Luo Y, Yu X, Zhang DF, Zhang H. Effects of n-3 polyunsaturated fatty acid therapy on plasma inflammatory markers and N-terminal pro-brain natriuretic peptide in elderly patients with chronic heart failure. J Int Med Res. 2009;37:1831–1841. doi: 10.1177/147323000903700619. [DOI] [PubMed] [Google Scholar]