Abstract
Nuclear receptor (NR) subfamily 4 group A (NR4A) is a family of three highly homologous orphan nuclear receptors that have multiple physiological and pathological roles, including some in cancer. These NRs are reportedly dysregulated in multiple cancer types, with many studies demonstrating pro-oncogenic roles for NR4A1 (Nur77) and NR4A2 (Nurr1). Additionally, NR4A1 and NR4A3 (Nor-1) are described as tumor suppressors in leukemia. The dysregulation and functions of the NR4A members are due to many factors, including transcriptional regulation, protein-protein interactions, and post-translational modifications. These various levels of intracellular regulation result from the signaling cross-talk of the NR4A members with various signaling pathways, many of which are relevant to cancer and likely explain the family members' functions in oncogenesis and tumor suppression. In this review, we discuss the multiple functions of the NR4A receptors in cancer and summarize a growing body of scientific literature that describes the interconnectedness of the NR4A receptors with various oncogene and tumor suppressor pathways.
Keywords: nuclear receptor, NR4A, Nur77, Nurr1, oncogene, tumour suppressor
1. Introduction
The human nuclear receptor (NR) family is a group of structurally related transcription factors that regulate specific gene expression in a ligand-dependent manner. This superfamily of receptors constitutes an important group of drug targets that are useful in identifying compounds that affect a wide range of physiological and pathological events [1]. NRs share a common structural arrangement that consists of an N-terminal domain containing an activation function–1 (AF-1) region, a DNA-binding domain, a hinge region, and a C-terminal ligand-binding domain (LBD) that can also encode an AF-2 domain. NR subfamily 4 group A (NR4A) is composed of three members: Nur77 (NR4A1, also known as nerve growth factor IB or NGFIB), Nurr1 (NR4A2), and Nor-1 (NR4A3).
Members of the NR4A subgroup respond to various stimuli, and their expression can be induced by mitogens, stress, and apoptotic signals, implicating their roles in multiple biological processes [2, 3]. The NR4A receptors are classified as orphan receptors, having no known physiological ligands, and do not contain a typical ligand-binding domain structure common to other NRs [2, 4–6] although recent evidence suggests that unsaturated fatty acid metabolites could serve as the missing ligand for Nur77 [7]. Typical NRs have a ligand-binding domain containing a hydrophobic cleft for ligand- and coactivator-binding, but structural studies show that the NR4A subgroup contains an atypical ligand-binding groove that is hindered by bulky side groups of hydrophobic residues. Thus, the NR4A receptors are believed to be regulated in a ligand-independent manner, and a growing amount of literature supports the notion that these receptors are regulated largely by post-translational modifications and protein-protein interactions and that their expression and localization within the cell influences their cellular functions.
2. NR4A receptors in cancer
The NR4A receptors promote or suppress tumors depending on specific cellular context. For example, Nur77 is overexpressed in cancer cell and tissue samples of multiple origins, causing increased proliferation and survival in these cells and tissues [8] at least partly via upregulation of several target genes, including cyclin D2 [9], E2F1 [10], survivin [11], and thioredoxin domain–containing 5 (TXNDC5) [12], which are mediators of cell cycle progression, apoptotic inhibition and reactive oxygen species (ROS) regulation (Figure 1). In addition, loss-of-function studies of Nur77 have demonstrated its importance in cell proliferation and survival [13], with the consensus being that Nur77 knockdown reduces cellular growth rate and angiogenesis and induces intrinsic and extrinsic apoptotic pathways. It is important to note that many loss-of-function studies are performed on non-stimulated cells to determine the role of basal, endogenous Nur77. Conversely, in cells stimulated with various apoptosis-inducing agents, Nur77 plays a role in cell death through both transcription-dependent and -independent mechanisms (Figure 1). Because of the dual and opposite roles of Nur77 in cell proliferation and death, many studies have been focused on therapeutically targeting Nur77 to impede its oncogenic functions while coaxing it to activate the cellular death program [14]. These efforts would rely on the fact that non-tumor tissue will express Nur77 at much lower levels, making these tissues less responsive to Nur77-mediated apoptosis-inducing agents.
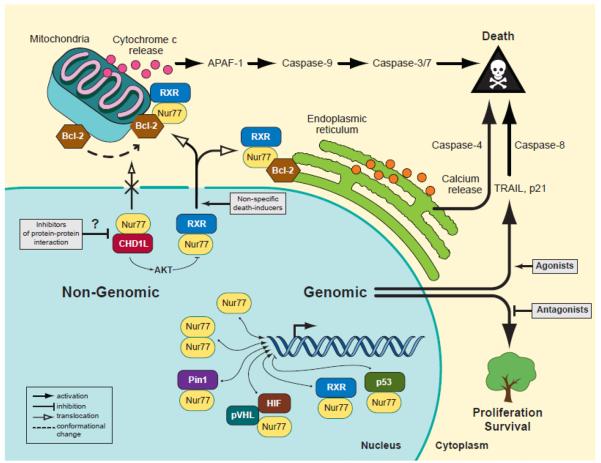
Fig. 1.
Nur77 mediates cell death or survival through localization-dependent and -independent mechanisms. As a nuclear transcription factor, Nur77 largely promotes cell proliferation and survival through regulation of specific target genes (i.e., cyclin D2, E2F1, survivin, TXNDC5). Additionally, some agonists of Nur77 transactivation are able to mediate transcription-dependent cell death. A major mechanism of Nur77-mediated cell death is the nuclear export of Nur77-RXRα heterodimers, which is suppressed by the CHD1L oncogene. Cytoplasmic Nur77 can activate mitochondrial- or ER-associated cell death by interacting with membrane-bound Bcl-2.
Nurr1 has been implicated in cancer progression although its cancer-related target genes have not been characterized. Nurr1 knockdown decreases anchorage-independent growth, suggesting that Nurr1 plays a role in cell transformation [15, 16]. The protein promotes migration but not overall proliferation in bladder cancer [17], although it does affect cell proliferation in lung and breast cancer [18, 19]. Nurr1 expression is higher in squamous cell carcinoma (SCC) samples than in normal tissues of patients with SCC, and induction of Nurr1 expression in SCC leads to increased resistance to 5-fluorouracil [20], suggesting a role for Nurr1 in drug resistance [20, 21]. Additionally, Nurr1 overexpression contributes to protection from doxorubicin-induced apoptosis by diminishing the p53 response [22].
In patients with breast cancer, Nurr1 expression in normal breast epithelium is higher than that in tumor tissue and has been positively correlated with favorable prognosis [19]. Conversely, the same study found that knockdown of Nurr1 in breast cancer cell lines diminished xenograft tumor growth. The different roles of Nurr1 in different tissues point to possible context-dependent effects of Nurr1, which might also depend on the intracellular localization of Nurr1 protein as cytoplasmic expression of Nurr1 in bladder cancer was correlated with decreased patient survival [17]. However, other studies using either stimulated endogenous or overexpressed exogenous Nurr1 have not clearly determined its subcellular localization.
Less is known about Nor-1's functions in cancer, although some key findings have been made. For example, Nr4a1−/−;Nr4a3−/− double-knockout mice develop acute myeloid leukemia (AML) with very rapid onset, dying within 2 to 4 weeks [23]. The myeloid cells from these mice have more S- and G2/M-phase populations and fewer annexin V–positive cells than do those of wild-type mice. The decrease in apoptotic cells was attributed to a reduction in extrinsic cell death signaling, as indicated by a decrease in Fas ligand and TRAIL expression. Expression of Nur77 and Nor-1 were dramatically reduced in AML patient samples. Together, these data suggest that these two NR4A receptors can play overlapping tumor suppressive roles in leukemia, as NR4A single-knockout mice do not develop cancer [24, 25]. The functional redundancy of Nur77 and Nor-1 was further confirmed in a follow-up study investigating genome-wide transcriptional changes in response to NR4A restoration in AML [26]. Nur77 and Nor-1 shared overlapping gene signatures by regulating 97% of the same transcripts, and re-expression of either NR4A receptor was able to elicit tumor-suppressive functions by reducing proliferation and increasing apoptosis. Furthermore, NR4A re-expression suppressed MYC and its accompanying oncogenic signature in multiple AML cells.
Another perturbation of Nor-1 function occurs in extraskeletal myxoid chondrosarcoma (EMC). Researchers identified a recurrent translocation of Nor-1 (also called TEC, Translocated in Extraskeletal Chondrosarcoma) with the EWS gene that encodes a novel EWS-TEC fusion protein in EMC tumors [27]. This oncogenic fusion protein binds to and regulates the NGFI-B Response Element (NBRE), with 250-fold greater transactivation capacity than that of wild-type Nor-1 [28]. Because EWS-TEC fusion protein can bind to the NBRE, several studies have been focused on the differential transactivation of target genes of EWS-TEC and Nor-1 [29, 30]. Analysis of 16 EMC tumors showed that 15 cases contained EWS-TEC fusion transcripts [31]. Introduction of the EWS-TEC oncogenic fusion protein into a chondrogenic cell line did not increase the proliferation rate but allowed cells to grow past contact inhibition to form small clusters of cells and increased anchorage-independent growth [32]. Nor-1 also forms fusion proteins with other proteins in EMC [33, 34].
3. NR4A receptors and MAPK
3.1. MAPK and cancer
The mitogen-activated protein kinase (MAPK) pathway is induced by a wide range of signals, including growth factors, cytokines, and stress, and is responsible for almost every cell function, including proliferation, differentiation, cell death, and migration. The six different MAPK pathways include extracellular signal-regulated kinase (ERK) 1/2, ERK 3/4, ERK5, ERK 7/8, Jun N-terminal kinase (JNK) 1/2/3, and p38 (ERK6), as reviewed in [35]. Typically, an external signal triggers the signaling cascade through a membrane-bound receptor, followed by recruitment of G-proteins such as Ras, Rac, and Rho and, subsequently, of downstream kinases such as Raf and MAPK kinase kinase (MEKK), which phosphorylate and activate MAPK kinase (MEK). Activated MEKs then phosphorylate and activate MAPKs [35], which will phosphorylate either transcription factors to modulate target gene expression or other kinases to regulate critical cellular events. Because the MAPK pathway is involved in almost every cellular process, it is understandable that dysregulation of this pathway could cause cancer.
3.2. Regulation of NR4A receptors by MAPK
Phosphorylation of Nur77 by MEK-ERK signaling has been described by several groups. Epidermal growth factor (EGF), an activating signal for ERK2, causes phosphorylation of Nur77 at threonine 142 [36], a phosphorylation site that stabilizes Nur77 [37], consistent with the phosphorylation-mediated stability of other nuclear receptors. Interestingly, EGF enhances the interaction of Nur77 with prolyl isomerase Pin1, and the isomerization of phosphorylated Nur77 requires ERK2- (and JNK1)-mediated phosphorylation [38]. This phosphorylation-dependent isomerization prevents degradation of Nur77, thereby increasing its transactivation activity and promoting its pro-mitogenic effect. ERK2 is responsible for phosphorylation of Nur77 at serine 431, a phospho-residue important for both the Nur77-Pin1 interaction and increased transactivation mediated by Pin1.
ERK2 activation upon EGF treatment causes Nur77 nuclear localization and prevents its cytosolic induction of apoptosis [39], but the opposite phenotype occurs in T-cells. Inhibiting MEK1 decreases expression of Nur77 in T-cells [40] and prevents its nuclear export and mitochondrial localization [41], demonstrating the role of ERK signaling in Nur77-induced cell death. Ribosomal S6 kinase 2 (RSK2), an effector kinase of MEK-ERK, phosphorylates Nur77 at serine 354 [41, 42] and positively regulates Nur77's nuclear export and subsequent mediation of apoptosis. Other groups have reported phosphorylation of Nur77 by ERK5, but not by ERK2, in T-cells that caused increased Nur77 transcription and induction of apoptosis [43, 44].
Activation of ERK1/2 signaling is positively associated with the nuclear localization of Nur77 and negatively associated with the ability of fenretinide to induce apoptosis [45]. Inhibiting MEK-ERK and treating with fenretinide enhance the cytosolic localization of Nur77 in fenretinide-resistant HepG2 cells, but activating MEK-ERK prevents this event in fenretinide-sensitive HuH-7 cells, demonstrating a role for ERK signaling in the drug resistance of liver cancer cells. In another study, constitutive signaling through BRAF-MEK-ERK was shown to positively regulate the expression of both Nur77 and Nurr1 via inhibition of BRAF and MEK1/2 [46], implicating this commonly mutated and hyperactive pathway as one of the causes of increased Nur77 levels in cancers. In addition, the p38 MAPK pathway can also modulate the activity of Nurr1. For example, upon activation by apoptosis signal–regulating kinase 1, p38 phosphorylates Nurr1, leading to synthesis of melanin, a pigment important in protecting skin, hair, and eyes from harmful elements [47].
In another study, fibroblast growth factor 8b (FGF-8b) induced the expression of all three NR4As in MC3T3-E1 preosteoblastic cells [48]. Furthermore, the effect of FGF-8b was mediated through the MAPK, phosphatidylinositol 3-kinase (PI3K), and protein kinase C (PKC) pathways. Proliferation of these cells can be increased by FGF-8b, and overexpression of Nur77 and Nurr1 further enhance this proliferative effect and decrease apoptosis. Therefore, the MAPK pathways regulate the levels, sub-cellular localizations, and activities of the NR4A receptors.
4. NR4A receptors, PI3K-AKT, and mTOR in proliferation and survival
4.1. PI3K-AKT and cancer
The PI3K-AKT signaling axis is a major regulator of cell proliferation and survival, acting downstream of growth factors and receptor tyrosine kinases in parallel with MAPK signaling [49]. PI3K heterodimers consist of regulatory and catalytic subunits, which are responsible for PI3K regulation and downstream signaling, respectively. Upon receptor tyrosine kinase activation and recruitment of PI3K to the plasma membrane, the primary function of PI3K is to add a phosphate group to phosphatidylinositol 4,5-bisphosphate (PIP2), converting it to phosphatidylinositol 3,4,5-trisphosphate (PIP3) [50] to flip the “on” switch for downstream signaling. PIP3 recruits AKT kinase to the plasma membrane, enabling its phosphorylation and activation by 3-phosphoinositide-dependent protein kinase-1 (PDK1) [51, 52]. Conversely, AKT signaling can be turned off through the phosphatase PTEN, which converts PIP3 back to PIP2 [53, 54]. AKT can inhibit apoptosis to promote cell survival by phosphorylating and inhibiting both pro-apoptotic Bad [55] and caspase-9 [56]; enhance cell-cycle progression by phosphorylating and inactivating glycogen synthase kinase 3 beta (GSK3B), leading to stabilization of cyclin D1 [57]; and increase cell growth by stimulating the mechanistic target of rapamycin (mTOR) pathway to promote protein synthesis [58].
Alterations in the PI3K-AKT pathway have been identified in various cancer types and include mutations that directly increase PI3K and AKT activity [49, 59] and inactivate the tumor suppressor PTEN [60–62]. Mice that are heterozygous for PTEN develop an array of tumor types [63] due to uncontrolled PI3K-AKT signaling. Aberrant AKT signaling can also lead to inactivation of tumor suppressor p53 [64], with the hyperactivation of PI3K-AKT in cancer ultimately leading to increased cell survival and proliferation and contributing to tumor growth, metastasis, and angiogenesis through modulation of downstream effectors, including NR4A receptors.
4.2. The interplay of NR4A receptors and PI3K-AKT
AKT phosphorylates Nur77 [65–67] to negatively regulate its function in mediating cell death. Specifically, AKT phosphorylates human Nur77 at Ser-351 (Ser-350 in rats) in the DNA-binding domain [67], a phosphorylation site that inhibits Nur77 transcriptional activity [68]. AKT-mediated phosphorylation of Nur77 occurs in the cytoplasm in a PI3K-dependent manner. More importantly, phosphorylation of Nur77 by AKT decreases the transcriptional activity of Nur77 by 50%–85%. Additionally, the other two NR4A members, Nurr1 and Nor-1, also have a similar phosphorylation motif, so it is likely that AKT also phosphorylates Nurr1 and Nor-1, although this has not been investigated. Furthermore, AKT directly inhibits Nur77's DNA binding activity [66].
In addition to inhibiting its DNA binding and transcriptional activity, AKT also prevents Nur77 from inducing apoptosis in T-cell hybridomas [66]. To inhibit apoptosis and increase cell survival, AKT phosphorylation of Nur77 can be considered to be a “priming” step for an interaction with 14-3-3 protein, which recognizes the phosphorylated motif near the Ser-351 residue. This protein-protein interaction, which only occurs with wild-type Nur77 following AKT phosphorylation, is similar to AKT-mediated phosphorylation of Bad and its subsequent interaction with 14-3-3: both protein-protein interactions prevent the protein (Nur77 or Bad) from interacting with Bcl-2 and causing subsequent apoptosis [55]. By using a DNA-binding domain deletion mutant of Nur77 that readily localizes to the mitochondria and induces apoptosis [69], researchers showed that overexpressing AKT blocks mitochondrial association with Bcl-2 and causes a diffuse cytoplasmic localization of Nur77 [65]. Because the Nur77 DNA-binding domain deletion mutant lacks the Ser-351 residue, this experiment also showed that AKT can phosphorylate cytoplasmic Nur77 at the N-terminus.
AKT can also act on nuclear Nur77, preventing its nuclear export and subsequent apoptosis [65, 70]. Overexpression of constitutively active AKT can overcome the effects of MEKK1-induced nuclear export of Nur77, retaining it within the nucleus [70]. The effect of AKT depends on Ser-351 of Nur77: if Ser-351 is replaced with alanine, the Nur77 mutant migrates to and remains in the cytoplasm in response to MEKK1 activation, regardless of AKT status. Inhibition of PI3K-AKT or knockdown of AKT restores Nur77's cytoplasmic localization, whereas PI3K activation by insulin or AKT overexpression efficiently blocks TPA-induced Nur77 nuclear export, cytochrome c release, and apoptosis in gastric cancer cells [65]. In addition to Ser-351, the N-terminal region of Nur77, specifically residues 51–105, are also shown critical for AKT binding and phosphorylation-dependent regulation [65]. AKT's inhibition of Nur77 has also been credited with mediating cisplatin-induced apoptosis in ovarian cancer cells [71].
4.3. The interplay of NR4A receptors and mTOR
AKT acts on multiple downstream proteins, including the mTOR kinase complex (mTORC1). The PI3K-AKT-mTOR axis could be considered to be a single pathway [58] in which AKT activates mTOR to control cell proliferation and growth in response to environmental stimuli by phosphorylating and inactivating the mTOR suppressors tuberous sclerosis protein 1 (TSC1) and TSC2 [72, 73]. Another upstream negative regulator of mTOR, liver kinase B1 (LKB1), activates AMP-activated protein kinase (AMPK) [74] to suppresses the mTORC1 complex [75]. PTEN also negatively regulates mTOR by turning off PI3K-AKT signaling. The major effect of mTOR activation is increased protein synthesis, leading to increased expression of proteins involved in proliferation, survival, and angiogenesis [58].
Nur77 indirectly activates mTOR signaling by attenuating AMPK signaling, and knockdown of Nur77 in non-small cell lung cancer cells decreased proliferation and enhanced apoptosis [76]. Whereas AMPKα phosphorylation was increased and mTOR phosphorylation decreased in that study, AKT phosphorylation status remained unchanged, suggesting that the effect on mTOR occurs downstream of AKT. A previous microarray study by the same group demonstrated that sestrin-2 expression increased after knockdown of Nur77 [77]. Sestrin-2, a target gene of p53 [78], serves to activate AMPK signaling. Knowing that Nur77 has been shown to interact with and inhibit p53 [79], the authors further showed that knockdown of Nur77 enhanced sestrin-2–mediated AMPK activation only in cells with wild-type p53. Further support for the role of Nur77 in mTOR activation comes in a report demonstrating Nur77's suppression of AMPK signaling [80], wherein knockdown or overexpression of Nur77 increased or decreased AMPK phosphorylation, respectively. This relationship was not seen in LKB1-null HeLa cells unless LKB1 was cotransfected with Nur77. Furthermore, Nur77-LBD interacts with and sequesters LKB1 in the nucleus, away from cytoplasmic AMPK, leading to decreased AMPK phosphorylation. A subsequent chemical screen found that the small molecule ethyl 2-[2,3,4-trimethoxy-6-(1-octanoyl)phenyl]acetate (TMPA) enhanced AMPK phosphorylation by disrupting the Nur77-LKB1 interaction; this effect was not seen in LKB1-null cells, further confirming LKB1-dependency. These studies demonstrate interesting perspectives of Nur77 in which multiple signaling nodes are interconnected through Nur77, as PI3K-AKT suppresses the pro-apoptotic functions of Nur77 and Nur77 itself enhances downstream mTOR signaling to promote tumor progression, possibly in the context of LKB1 or p53.
5. NR4A receptors, hypoxia, and angiogenesis
5.1. HIF-1 and cancer
The hypoxia-inducible factors (HIFs) are a family of transcription factors that mediate the balance of oxygen within tissues, having functions in multiple diseases, both in protective and pathogenic roles [81]. At normoxic conditions (i.e., normal oxygen levels), the HIF-1α subunit undergoes rapid proteolysis to maintain a low protein level, and the HIF-1β subunit remains at a relatively constant level [82, 83]. The HIF-1α subunit is maintained in its suppressed state by hydroxylation of specific prolyl residues [84, 85], which promotes an interaction with the von Hippel-Lindau tumor suppressor protein (pVHL) [86], a key protein responsible for targeting HIF-1α for proteasomal degradation by recruiting ubiquitin ligases [87]. The suppression of HIF-1α is released under hypoxic conditions (i.e., low oxygen levels) due to the unavailability of oxygen for the hydroxylation step, leading to loss of pVHL recognition of HIF-1α and an accumulation of HIF-1α protein. Additionally, a hydroxylation of HIF-1α by factor-inhibiting HIF prevents binding of the coactivator p300 to the transactivation domain of HIF-1α, preventing its transactivation [88]. Once at sufficient levels, HIF-1α migrates to the nucleus where it forms a heterodimeric transcriptional complex with HIF-1β to regulate the expression of target genes, including those encoding erythropoietin (EPO) [89], vascular endothelial growth factor (VEGF) [90], and proteins involved in glucose uptake and metabolism [91–94]. The HIF-1 transcription factors are able to mediate key processes within hypoxic regions deep within newly established tumor sites to control the balance of oxygen consumption and oxygen delivery. HIF-1 effectively controls these processes by regulating the expression of hundreds of genes, many of which are involved in proliferation, metabolic adaption, and angiogenesis [95, 96], which are key hallmarks of cancer [97]. As summarized by Semenza [95], loss-of-function and gain-of-function studies have revealed that the HIF-1 target gene products are tumor-promoting and, thus, increase proliferation, angiogenesis, and metastasis in multiple tumor models. Many primary tumors and metastases have increased HIF-1 activation as indicated by increased staining of the HIF-1 protein in tissue samples when compared to adjacent normal tissue [98, 99], and its expression is associated with increased tumor vascularization and aggressiveness [100]. Increased HIF-1α expression and activity in cancer can be attributed to cancer-associated hypoxia, loss of tumor suppressor function (i.e. pVHL, p53 or PTEN), or contributions of growth factor or oncogene signaling (i.e. PI3K or MAPK), either through blocking degradation or enhancing synthesis of HIF-1α [96]. Patients with clear cell renal carcinoma and loss of pVHL have enhanced HIF-1α expression due to the decreased degradation of HIF-1α, defining pVHL as a tumor suppressor [101, 102].
5.2. The interplay of NR4A receptors and HIF-1
Hypoxia increases the expression of all three NR4A family members at both mRNA and protein levels in a HIF-1α-dependent manner [103–107]. HIF-1α, but not HIF-2α, can directly regulate Nur77 and Nor-1 expression by binding to hypoxia-response elements in each gene's promoter [103, 104, 106]. Additionally, the Nur77 target gene proopiomelanocortin (POMC) is induced under hypoxic conditions through HIF-1α-dependent regulation of Nur77. Furthermore, Nur77 itself regulates HIF-1α, implicating this as a possible feedback mechanism in cancer progression. Expression of wild-type but not of dominant negative Nur77 can activate a hypoxia response element–containing promoter, increasing nuclear localization of HIF-1α [104, 108]. Additionally, when examining this HIF-1 response to Nur77, the HIF-1 target gene VEGF increased at both the mRNA and protein levels, but HIF-1α increased at only the protein level and underwent attenuated ubiquitination [104, 108], suggesting stabilization of transcriptionally active HIF-1α by Nur77. Two reports have suggested different Nur77 domains as being requirements for HIF-1α stabilization and transactivation, either through the N-terminal [104] or ligand-binding domain [108]. These domain-specific stabilizations of HIF-1α depend on different Nur77 protein interactions, although neither affects HIF-1α binding with pVHL. The N-terminal domain of Nur77 can block Mdm2 from binding to and degrading HIF-1α and also leads to decreased Mdm2 expression [104]. However, another group demonstrated that Nur77-LBD can interact with the α-domain of pVHL, forcing elongin C dissociation and blocking pVHL-mediated degradation of HIF-1α [108]. The fact that pVHL was required for Nur77-mediated stabilization indicates that pVHL serves as an adaptor protein to form a Nur77-pVHL-HIF-1α complex. Interestingly, Nurr1 and Nor-1 positively regulate HIF-1α expression [105, 108], and HIF-1α– induced Nor-1 protects endothelial cells exposed to hypoxia, possibly by regulating cellular inhibitor of apoptosis 2 [106].
The regulation and function of Nur77 within hypoxic environments may be critical factors to consider and are addressed by a recent report showing crosstalk between Nur77 and β-catenin signaling during hypoxia [107], in which both were induced and required to positively regulate each other through transcription-independent mechanisms. Although Nur77 is typically considered to be a nuclear protein that regulates target gene expression in the context of cancer, hypoxia-induced Nur77 is highly expressed in the cytoplasm, and this localization is required for stabilization of β-catenin [107]. This finding is similar to one in bladder cancer patients that indicated cytoplasmic Nurr1 level was correlated with poor prognosis, although other possible factors were not described [17]. Hypoxia can increase AKT phosphorylation in a Nur77-dependent manner [107], presenting a unique regulatory mechanism, as Nur77 is phosphorylated by AKT to prevent its mitochondrial localization [65], thus preventing cell death and enhancing its pro-oncogenic functions. The feed-forward loop of Nur77 and β-catenin promotes cell growth, migration, and invasion and alters epithelial-to-mesenchymal transition (EMT) markers [107]. Together, these studies reveal that hypoxia affects both the level and function of NR4A receptors, which in turn play positive feedback roles that affect hypoxia-induced signaling.
5.3. NR4A receptors as mediators of VEGF-induced angiogenesis
Given the reported roles of the NR4A receptors in hypoxia, a reasonable hypothesis might be that hypoxia-induced upregulation of NR4A receptors will positively regulate HIF-1α, thus increasing HIF-1–regulated genes and promoting survival, angiogenesis, and tumor promotion. The anti-metabolite 6-mercaptopurine (6-MP), known to increase NR4A transactivation through its AF-1 domain [109], induces the expression of all three NR4A members, HIF-1α, and VEGF and increases the stability, nuclear localization, and transactivation of HIF-1α protein in a Nur77-dependent manner [105]. The effects of 6-MP on NR4A and HIF-1α depend on p44/p42 ERK phosphorylation and can be abolished using a MEK inhibitor, a previously reported regulatory mechanism of Nur77 in hypoxia [104]. Furthermore, 6-MP enhances the HIF-1α response in endothelial cells and promotes capillary tube formation [105], indicating that induction of Nur77 and its regulation of HIF-1α can promote angiogenesis.
One main mediator of angiogenesis is VEGF, which is induced by deoxycholic acid (DCA) through enhancing the expression of Nur77 in colon cancer cells [110]. VEGF stimulation of cells rapidly induces expression of all three NR4A members [111–114], which mediate VEGF-induced effects on proliferation and angiogenesis. Similar to VEGF, Nur77 expression increases the proliferative rate of endothelial cells and protects cells from apoptosis [113], suggesting that VEGF exerts its effect by upregulating Nur77 expression. Nur77 knockdown increases apoptosis, which cannot be rescued by addition of VEGF, further suggesting that Nur77 operates downstream of VEGF in this context. In addition, the effect of VEGF depends on Nur77, and both VEGF and Nur77 induce the expression of the cell cycle- related genes cyclin A, cyclin D1, proliferating cell nuclear antigen (PCNA), and E2F [113]. Inactivation of Nur77 reduces capillary tube formation, and mutants lacking the DNA-binding domain undergo no tube formation [113]. Compared to wild-type mice, Nur77−/− mice form fewer xenograft tumors, with reduced angiogenesis within the tumors [113]. These data suggest that VEGF-induced angiogenesis is mediated through Nur77.
Induction of Nor-1 by VEGF has been attributed to VEGF receptor 2 (VEGFR-2), and its expression is modulated at the Nor-1 promoter through CBP [112]. Knockdown of Nor-1 in endothelial cells attenuates DNA synthesis and progression of cells into S-phase following VEGF stimulation, suggesting that Nor-1 mediates the effects of VEGF and confirming a previous finding of Nor-1's role in vascular smooth muscle proliferation [115]. Similarly, VEGF-induced Nurr1 expression occurs rapidly, within 1 hour, and is mediated at the promoter level by NF-κB and CREB response elements [114]. Upon VEGF stimulation, CREB becomes phosphorylated and binds to the Nurr1 promoter, and this action can be blocked by inhibiting protein kinase D. Nurr1 knockdown can inhibit VEGF-mediated proliferation, migration, and in vivo angiogenesis [114]. These studies demonstrate that NR4A receptors are critical mediators of VEGF-mediated signaling.
6. NR4A receptors, p53, and cell death
6.1. p53 and cancer
As the main arbitrator of determining cell cycle progression, DNA repair, and apoptosis, the tumor suppressor p53 is a central hub in regulating cell fate [116, 117]. In response to a stress stimulus, such as DNA damage, p53 is quickly induced. Induction of p53 typically occurs at the protein level through inhibition of p53 degradation, known as derepression, by the blocking of a critical ubiquitination by the E3 ligase Mdm2 and, ultimately, the enhancement of p53 protein stability. With increased p53 protein levels, the cell's fate can now be regulated by tetramerization of p53 proteins and transcription of target genes. For example, p53 can drive the expression of cyclin-dependent kinase p21 [118, 119] and Gadd45 [120] to block cell-cycle progression, allowing the cell enough time to undergo DNA repair to correct any lesions. If the damage received proves to be too extensive, then p53 can initiate the cell death program through induction of genes such as Puma [121], Noxa [122], and Bax [123] to prevent the outgrowth of cells with damaged genomes. Additionally, p53 can execute the cell death program through DNA-binding–independent mechanisms by forming complexes with other signaling molecules such as B-cell lymphoma 2 (Bcl-2) at the mitochondrial membrane, which compromises the integrity of the outer mitochondrial membrane, resulting in cytochrome c release [124]. Through these multiple mechanisms of halting cell expansion, p53 serves as a critical tumor suppressor to prevent the formation of malignant lesions.
Since the discovery of p53, an overwhelming amount of reports have suggested that somatic p53 mutations occur in at least half of all cancers, with higher frequencies in certain malignancies, making it the most commonly mutated gene in cancer [125]. Mutations of p53, which commonly occur in its DNA-binding domain, serve to hijack its function as a tumor suppressor, making cells vulnerable to malignant transformation. Also, because many p53 mutations occur in the DNA-binding domain, mutated p53, acting in a dominant-negative manner, is still able to form tetramers with wild-type p53 protein to render them inactive [126]. Accumulating evidence also points to oncogenic functions of mutated p53 [127]. Additionally, in cancers with wild-type p53, the function of p53 can still be altered through overexpression of the negative regulator Mdm2 [128]. The importance of p53 in tumor suppression is evident in studies of p53 knockout mice in which all mice lacking p53 eventually succumb to disease, mostly due to sarcomas and lymphomas [129].
6.2. The interplay of NR4A receptors and p53
Both Nur77 and Nurr1 interact with p53 and regulate critical p53-dependent signaling, which could at least partially explain the oncogenic functions of NR4A receptors. On the heels of a finding that Nur77 could mediate Mdm2 degradation to promote HIF-1α stabilization [104] despite the lack of a Nur77-Mdm2 interaction, a direct interaction of Nur77 with p53 was demonstrated [79] that could explain this negative regulation of Mdm2. The Nur77-p53 interaction leads to a blockade of p53 acetylation, resulting in loss of p53-dependent transactivation and subsequent decreased expression of the target genes Mdm2 and cyclin-dependent kinase inhibitor p21. Interestingly, Nur77 can enhance p53-dependent apoptosis with and without UV irradiation, suggesting non-genomic regulation within the Mdm2-p53 axis. Ubiquitination of p53 by Mdm2 is also obstructed by Nur77, enhancing its stability. The results of these studies suggest that the pool of available p53 protein might play an important role in its transcriptional regulation, protection from Mdm2-mediated destruction, and enhancement of apoptosis conferred by Nur77. More recent findings show that the Nur77-mediated enhancement of p53-dependent apoptosis is due to phosphorylation of Nur77 by DNA-dependent protein kinase (DNA-PK) [130]. This phosphorylation of Nur77 enhances that of p53 by DNA-PK and, ultimately, increases the potential for induction of apoptosis upon DNA damage.
Similarly, Nurr1 interacts with p53 and inhibits p53-dependent apoptosis by inhibiting transactivation [22]. Nurr1 interacts with the C-terminal domain of p53 to attenuate doxorubicin-induced expression of the proapoptotic protein Bax, and cells lacking p53 do not exhibit doxorubicin resistance in the presence of Nurr1. The effects of Nurr1 on p53 are attributed to a reduction in the tetramerization of p53, which is required for its transcriptional activity. Although Nurr1 also has roles in DNA-PK–mediated DNA repair [131], the role of a Nurr1-p53 interaction in this process is unknown. Interestingly, a recent study found Nurr1 expression to be inversely correlated with p53 expression in primary breast cancer tissues [19]. The effects of p53 interaction with Nurr1, and Nur77 likewise, have not been addressed, and it remains to be determined whether p53 can interfere with the oncogenic effects of the NR4A receptors through direct protein interactions or other signaling mechanisms.
NR4A receptors not only affect the immediate responses of p53 but also affect other downstream pathways in a p53-dependent context. One such pathway is the AMPK-mTOR axis. As discussed in section 4.3, Nur77 can indirectly activate mTORC1 signaling by inhibiting p53 [76]. In p53 wild-type cells, but not in p53-null cells, Nur77 inhibited p53-mediated transactivation of the sestrin-2 promoter and subsequent expression of sestrin-2, a known target of p53 that inhibits mTOR signaling [78].
6.3. Nur77 in cell death
An intricate review by Moll and colleagues [132] draws parallels between p53 and Nur77 as cell death mediators that act through the intrinsic cell death pathway. Regardless of the route in which p53 activates cell death (i.e., transcription-dependent or -independent), the ultimate outcome is a mitochondria-induced cell death either through p53 target genes such as PUMA or BAX or through direct interactions with other molecules such as Bcl-2 at the mitochondrial outer membrane. Similarly, increasing evidence points to multiple mechanisms of Nur77-mediated cell death through nuclear and cytosolic Nur77 functions (Figure 1). A well-characterized mechanism of Nur77-mediated cell death is that in which Nur77 translocates from the nucleus to begin the apoptosis cascade, independently of transactivation, in response to certain death-inducing compounds [133, 134]. Nur77 heterodimerizes with retinoid X receptor (RXR) alpha and translocates from the nucleus to the cytosol, where the complex can target the mitochondria [135–137]. Bcl-2, a protein that is anti-apoptotic under most conditions, serves as a receptor on the mitochondrial outer membrane and is the downstream effector of Nur77 through an interaction with its ligand-binding domain [138]. Anti-apoptotic Bcl-2 is converted to a proapoptotic molecule through a conformational change of its Bcl-2 homology (BH) domains after Nur77 binding [138]. The conformational change exposes the buried pro-apoptotic BH3 domain of Bcl-2, resulting in a release of cytochrome c [69] and further activation of apoptosis. Deletion of the BH3 domain of Bcl-2 inhibits Nur77-mediated apoptosis. Another report suggests that Nur77 can mediate stress-induced apoptosis by targeting Bcl-2 at the endoplasmic reticulum (ER), leading to a release and depletion of ER calcium and activation of caspase-4 and -8 [139]. Therefore, cytosolic Nur77, by interacting with Bcl-2 either at the mitochondrial outer membrane or ER, is pro-apoptotic.
Although Nur77 exerts its pro-apoptotic effect largely through a translocation-dependent mechanism, it also regulates specific target genes involved in cell cycle regulation, survival, and apoptosis. Therefore, small-molecule agonists [77, 140–145] or antagonists [11, 12, 140] of Nur77 could be useful in modulating its transcription factor functions. One group has identified methylene-substituted diindolylmethanes (DIMs) as being agonists or antagonists of Nur77 and has shown that in vitro and in vivo treatment of colon, pancreatic, and bladder cancer cells with DIM-C-pPhOCH3 activates Nur77 through its ligand-binding domain [140], leading to growth inhibition, cell cycle arrest, and/or cell death [77, 140–143]. Treatment with DIM-C-pPhOCH3 causes a Nur77-dependent increase in TNF-related apoptosis-inducing ligand (TRAIL/TNFSF10), p21, and other genes, implicating DIM-C-pPhOCH3 in regulating the cell cycle and apoptosis [77, 140–142] (Table 1). The DIM-C-pPhOCH3–mediated effects are independent of Nur77's translocation to mitochondria, and Nur77 remains localized within the nucleus. TRAIL is implicated in the extrinsic pathway of apoptosis, a cell death pathway that bypasses the intrinsic mitochondrial pathway, further supporting a translocation-independent mechanism. As expected, cleavage of caspase-8 is detected after treatment with DIM-C-pPhOCH3 [77, 140, 141, 143]. Another compound in this series of DIM analogs, DIM-C-pPhOH, is described as a Nur77 antagonist, capable of inhibiting both basal and agonist-induced Nur77 transactivation through its N-terminal domain [11, 12, 140, 142]. Interestingly, DIM-C-pPhOH can also inhibit in vitro and in vivo cancer cell growth and induce apoptosis [11, 12, 76, 140], mimicking the effects seen after RNAi-mediated knockdown of Nur77, primarily through a Nur77-dependent regulation of target gene BIRC5/Survivin (Table 1). Nur77 knockdown or DIM-C-pPhOH treatment also cause morphological changes indicative of ER stress, which are accompanied by upregulation of ER stress genes and proteins (Table 1) and downregulation of the Nur77 target gene TXNDC5, a gene responsible for maintaining proper ROS levels [12]. The structure-dependent effects of C-DIMs on Nur77-regulated pro-apoptotic and anti-apoptotic genes, although not completely straightforward, are attributed to interactions with specific cofactors (i.e., p300) and other transcription factors (i.e., Sp1) [11, 142], but other genes are regulated through direct NBRE- and NuRE-binding sites.
Table 1.
Summary of gene expression changes after treatment with NR4A agonists or antagonists.
| Symbol | Gene name | Compound | Detectiona | NR4A-dependent? | Direct? | Cancer type | Ref | ||
|---|---|---|---|---|---|---|---|---|---|
| ATF3 | Activating transcription factor 3 | DIM-C-pPhOCH3 | Agonist | Increased | M,G | No | n.d. | Colon | 141 |
| M,G, P | Yes; Nur77 | n.d. | Pancreatic | 143 | |||||
| ATF4 | Activating transcription factor 4 | DIM-C-pPhOH | Antagonist | Increased | P | Yes; Nur77 | n.d. | Pancreatic | 12 |
| BCL2 | B-cell lymphoma 2 | DIM-C-pPhOH | Antagonist | Decreased | P | n.d.b | n.d. | Pancreatic, Lung | 11, 76 |
| BIRC5 | Baculoviral IAP repeat containing 5 (Survivin) | DIM-C-pPhOH | Antagonist | Decreased | G, P, R, C | Yes; Nur77 | Yes | Pancreatic, Lung | 11, 76 |
| BRE | Brain and reproductive organ-expressed | Cytosporone B | Agonist | Decreased | M, G, P, R, C | Yes; Nur77 | Yes | Gastric | |
| CDKN1A | Cyclin-dependent kinase inhibitor 1A (p21, Cip1) | DIM-C-pPhOCH3 | Agonist | Increased | M, G, P, R, C | Yes; Nur77 | Yes | Pancreatic, Bladder | 77, 142, 143 |
| DIM-C-Ph | Agonist | Increased | G | n.d. | n.d. | Pancreatic | 142 | ||
| DDIT3 | DNA-damage-inducible transcript 3 (CHOP) | DIM-C-pPhOH | Antagonist | Increased | G, P | Yes; Nur77 | n.d. | Pancreatic | 12 |
| MYC | V-Myc avian myelocytomatosis viral oncogene homolog (c-Myc) | DIM-C-pPhOH | Antagonist | Decreased | P | n.d. | n.d. | Lung | 76 |
| CTH | Cystathionase (cystathionine gamma-lyase) | DIM-C-pPhOCH3 | Agonist | Increased | M, G | No | n.d. | Colon | 141 |
| M, G | Yes; Nur77 | n.d. | Bladder, Pancreatic | 77, 143 | |||||
| DUSP1 | Dual specificity phosphatase 1 | DIM-C-pPhOCH3 | Agonist | Increased | M, G | Yes; Nur77 | n.d. | Pancreatic | 143 |
| EGFR | Epidermal growth factor receptor | DIM-C-pPhOH | Antagonist | Decreased | P | n.d. | n.d. | Lung | 76 |
| FASLG | Fas ligand (TNF superfamily, member 6) | DIM-C-pPhOCH3 | Agonist | Increased | P | Yes; Nur77 | n.d. | Pancreatic | 143 |
| GDF15 | Growth differentiation factor 15 (NAG-1) | DIM-C-pPhOCH3 | Agonist | Increased | M, G | No | n.d. | Bladder, Pancreatic | 77, 143 |
| PDCD1 | Programmed cell death 1 | DIM-C-pPhOCH3 | Agonist | Increased | M, G | Yes; Nur77 | n.d. | Colon | 141 |
| NUPR1 | Nuclear protein, transcriptional regulator 1 (p8) | DIM-C-pPhOCH3 | Agonist | Increased | M, G | Yes; Nur77 | n.d. | Bladder | 77 |
| SESN2 | Sestrin 2 | DIM-C-pPhOCH3 | Agonist | Increased | M, G | Yes; Nur77 | n.d. | Bladder | 77 |
| DIM-C-pPhOH | Antagonist | Increased | G, P, R, C | Yes; Nur77 | No | Lung | 76 | ||
| TNFSF10 | Tumor necrosis factor (ligand) superfamily, member 10 (TRAIL) | DIM-C-pPhOCH3 | Agonist | Increased | G, P | Yes; Nur77 | n.d. | Pancreatic, Colon, Bladder | 77, 140, 141, 143 |
| DIM-C-pPhCH3 | Agonist | Increased | P | n.d. | n.d. | Pancreatic | 140 | ||
| DIM-C-Ph | Agonist | Increased | G, P | n.d. | n.d. | Pancreatic, Colon, Bladder | 77, 140, 141 | ||
| DIM-C-pPhCI | Agonist | Increased | G, P | Yes; Nurri | n.d. | Bladder | 146 | ||
| TXNDC5 | Thioredoxin domain containing 5 | DIM-C-pPhOH | Antagonist | Decreased | M, G, P, R, C | Yes; Nur77 | Yes | Pancreatic | 12 |
| XBP1 | X-box binding protein 1 | DIM-C-pPhOH | Antagonist | Increased | P | Yes; Nur77 | n.d. | Pancreatic | 12 |
Detection key: M=microarray, G=gene, P=protein, R=promoter reporter, C=chromatin IP
not determined
Another DIM analog, DIM-C-pPhCl, was found to be a Nurr1-specific agonist [146]. This compound can inhibit TRAIL induction, apoptosis, and proliferation of bladder cancer cell lines, can block in vivo tumor growth, and can increase overall survival. These data suggest that Nurr1, similar to Nur77, may regulate both proliferative and survival genes (or death-inducing genes) depending on specific stimuli or structure-dependent small-molecule features and that agonists or antagonists could be developed to regulate this orphan receptor.
The small-molecule natural product cytosporone B (Csn-B) and its analogs are also ligands for the Nur77 ligand-binding domain [144, 145]. Csn-B stimulates Nur77's transcriptional activity through an interaction with Tyr-453 without affecting the activity of other NRs. A critical hydrogen bond is formed between a hydroxyl group of Csn-B (and its analogs) and Nur77 at Tyr-453 [144]. The series of analogs depend upon Nur77 in mediating apoptosis, which is induced by the translocation of Nur77 to the mitochondria after activation of the intrinsic pathway of apoptosis [145]. Csn-B and analogs suppress the expression of brain and reproductive organ–expressed protein (BRE), an anti-apoptotic protein. The promoter of BRE contains a binding site for Nur77, suggesting that modulation of nuclear Nur77 may suppress genes containing NBRE or NuRE in their promoter, possibly by recruiting corepressors (i.e., nuclear receptor co-repressor-1). Nur77 can also upregulate BRE in colon cancer cells after DCA treatment [110].
7. NR4A, Wnt, and β-catenin
7.1. Wnt and cancer
The roles of Wnt signaling in cancer have been previously reviewed [147, 148]. Wnt binds to and activates a Frizzled receptor, which then interacts with the intracellular Dishevelled to activate downstream events, including the canonical (i.e., β-catenin–dependent) and non-canonical Wnt pathways [148]. In the canonical pathway, β-catenin accumulates within the cytoplasm and translocates to the nucleus, where it acts as a transcriptional coactivator of the TCF/LEF family of transcription factors. TCF/LEF then targets genes involved in cell proliferation, stem cell maintenance, and differentiation. These target genes include those of cyclin D1 and c-Myc, which are required for the transition from G1 to S phase in the cell cycle. In the absence of Wnt signaling, β-catenin is normally degraded by a complex of proteins including Axin, adenomatosis polyposis coli (APC), Glycogen synthase kinase 3β (GSK3β), and casein kinase 1α (CK1α). These proteins mediate phosphorylation of β-catenin, leading to ubiquitination and subsequent proteasomal degradation, thus preventing β-catenin from accumulating in the cytoplasm and performing its coactivator function.
Dysregulation of Wnt signaling has been linked to cancer development [147]. Wnt1 ligand is a proto-oncogene in a mouse model of breast cancer. Multiple signaling dysregulations lead to elevated β-catenin, which is strongly correlated with poor prognosis of breast cancer patients, and have been implicated in other cancers such as colorectal cancer, melanoma, prostate cancer, lung cancer, glioblastoma, esophageal cancer, ovarian cancer, and familial adenomatous polyposis. The initial consensus was that increased Wnt signaling always correlates with negative patient outcomes, however recent evidence shows otherwise. Enhanced Wnt signaling can either promote or inhibit cancer formation and progression, and this is strongly dependent on the type and stage of cancer.
7.2. The interplay of NR4A receptors and Wnt signaling
Nuclear receptors, including the NR4A receptors, modulate the Wnt pathway [149, 150]. Nurr1 inhibits Wnt signaling by blocking β-catenin transactivation in both 293F and MC3TC-E1 osteoblastic cells. A similar observation was made in U2OS osteosarcoma cells in which the NR4A receptors block the transcriptional activity of β-catenin through a mechanism involving the DNA-binding domain of the NR4A receptors [150]. In addition, Nur77 promotes β-catenin degradation in the cytoplasm and inhibits tumor formation in vivo through transcriptional inhibition of the Wnt pathway [8, 151]. This finding would seem contradictory because Nur77 is overexpressed in most solid tumors; however, analysis of tissue samples from patients with colon cancer revealed that Nur77 is hyperphosphorylated by GSK-3β, which may impede its inhibition of the Wnt pathway [151]. Conversely, the NR4A receptors can indirectly increase β-catenin in melanoma cells. Nur77 targets CBP/p300-interacting transactivator 1 (CITED1) and Nur77/Nurr1 targets Dishevelled-binding antagonist of beta-catenin 1 (DACT1), both of which are negative regulators of the Wnt pathway. CITED1 inhibits β-catenin transactivation, and DACT1 interacts with the Wnt activator Dishevelled to promote its degradation, leading to inhibition of the Wnt pathway [46]. Therefore, NR4A receptors inhibit CITED1 and DACT1 to increase Wnt activity.
Alternatively, the Wnt pathway can either upregulate or repress the NR4A receptors depending on the cellular context. In 293F cells, β-catenin and Nurr1 directly bind, disrupting an interaction with the corepressor Lef-1. This enables Nurr1 and β-catenin to activate their transcriptional targets. This interaction is important for normal neuron development and the survival of dopaminergic neurons [8, 150]. Nur77 can also be upregulated upon the addition of the colon carcinogen DCA, which stabilizes β-catenin and allows it to form a transcriptional complex with AP-1 that can then bind to the Nur77 promoter to enhance transcription of Nur77 [8, 150, 151]. Conversely, overexpression of β-catenin in U2OS and HeLa cells inhibits NR4A transcriptional activity through a mechanism involving the ligand-binding domain of the NR4A receptors [150].
As discussed in section 5.2, a positive feedback loop between Nur77 and β-catenin has been identified under hypoxic conditions in colorectal cancer cells [107]. β-catenin induces Nur77 expression through HIF-1α. However, Nur77 can increase β-catenin's protein levels by increasing its half-life in the cytoplasm. Furthermore, the growth, migration, and invasion of colorectal cancer cells increase upon overexpression of β-catenin or Nur77, and these effects are further enhanced when β-catenin and Nur77 are coexpressed. The authors of these findings argued that previous studies on the interaction between Nur77 and β-catenin are conflicting because of the normoxic conditions used in those studies and that it is, therefore, more realistic to perform these experiments under hypoxic conditions, which more closely mimic the environment of a tumor. Overall, it is clear that the tissue type and environmental conditions play an important role in how NR4A receptors interact with the Wnt pathway.
8. CHD1L oncogene and Nur77
8.1. CHD1L and cancer
Chromodomain helicase/ATPase DNA binding protein 1-like, or CHD1L, is a member of the Snf2-like family of chromatin remodelers and modifiers [152]. Unlike other members of this family, CHD1L does not contain a chromodomain that recognizes methylated histone tails but, instead, harbors a macro domain containing a Poly (ADP-ribose) (PAR)-binding element [153], which allows binding with Parp1 [154, 155]. Several groups have shown that CHD1L has macro domain–dependent ATPase activity in the presence of DNA and nucleosomes that is enhanced by Parp1 [154, 155]. CHD1L also interacts with proteins involved in DNA repair in a Parp1-dependent manner and is recruited to DNA damage break points through its macro domain [154, 155]. In addition to its function in chromatin remodeling and DNA repair, CHD1L also has DNA-binding and transcription factor capabilities [156]. Confirmed target genes of CHD1L include ARHGEF9 [156], TCTP [157] and SPOCK1 [158].
The CHD1L gene, also called ALC1 (Amplified in Liver Cancer 1), is an oncogene, residing in the frequent 1q21 amplicon found in some solid tumors [159], including hepatocellular carcinoma (HCC) [160, 161]; amplification of the 1q21 locus has been found in 58%–78% of HCC cases [161, 162]. Gain-of-function and loss-of-function studies have confirmed the role of CHD1L as an oncogene, having the ability to enhance in vitro cell transformation and in vivo tumor formation and tumor size, which can be attributed to its ability to promote the G1/S phase transition [161]. In addition to its growth-promoting effects, CHD1L can protect cells from apoptosis [161] and 5-fluorouracil [163]. Analyses of patient samples revealed that approximately 50% of patients with HCC have CHD1L overexpression [161, 163] and that 68% of metastatic tumor sites have higher levels of CHD1L than are found in the matching primary tumors [156]. Indeed, overexpression of CHD1L is associated with resistance to chemotherapy in patients with HCC [163]. Studies of CHD1L-transgenic mice further demonstrate the oncogenic ability of CHD1L, with about 25% of mice forming spontaneous tumors, including some cases of HCC [164].
The effects of CHD1L on oncogenesis can be attributed to its transcription factor function and target genes. The first target gene identified for CHD1L was ARHGEF9 [156], a guanine nucleotide exchange factor that activates Cdc42, which is a GTPase involved in epithelial-to-mesenchymal transition (EMT) and metastasis [165]. Indeed, CHD1L overexpression can induce an AHGEF9-Cdc42–dependent EMT, resulting in increased in vivo tumor invasiveness and metastasis. Target gene TCTP is overexpressed in about 40% of HCC patient samples and is associated with advanced tumor stage; its overexpression increases in vivo tumor formation via faster mitotic exit and cell division [157]. Similarly, CHD1L target gene SPOCK1 is associated with clinical stage and metastasis and can protect cells from staurosporine-induced apoptosis in an AKT-dependent manner [158].
8.2. The interplay of Nur77 and CHD1L
Recent evidence demonstrates that Nur77 interacts directly with the CHD1L protein [166]. The C-terminal macro domain of CHD1L interacts with Nur77, inhibiting its nuclear-to-mitochondrial translocation and subsequent induction of apoptosis. CHD1L expression in a panel of HCC cell lines negatively correlates with induction of apoptosis following staurosporine treatment, further supporting CHD1L's role as an inhibitor of apoptosis and a potential mediator of drug resistance. It remains to be determined which residues of Nur77 are critical in the interaction with CHD1L and whether this interaction prevents binding of other proteins, such as RXR, or specific post-translational modifications of Nur77. Additionally, CHD1L is involved in chromatin remodeling and DNA repair [154, 155], which is mediated by its C-terminal macro domain through interactions with Ku70 and DNA-dependent protein kinase, catalytic subunit (DNA-PKcs). Given that Nur77 interacts with Ku80 to suppress DNA repair [130], it is plausible that a Nur77-CHD1L interaction could also repress chromatin remodeling and subsequent DNA repair, making the interaction mutually inhibitory. Also of interest is that CHD1L-mediated expression of SPOCK1 can activate AKT to maintain mitochondrial membrane potential, which prevents cytochrome c release and apoptosis, all of which is blocked by pretreatment with an AKT1 inhibitor [158]. This scenario raises the possibility that CHD1L might inhibit Nur77 translocation through both a direct protein-protein interaction and through activation of AKT, which is known to be inhibitory to Nur77's mitochondrial association [65]. The regulation of Nur77 by CHD1L might offer a useful therapeutic avenue in which a small molecule could be developed to disrupt this interaction, allowing Nur77 to become fully functional in the cell death program.
9. Concluding remarks
In summary, the NR4A family, represented by three highly homologous orphan receptors, plays multiple roles in cancer, with most studies highlighting the pro-oncogenic functions of Nur77 and Nurr1. In addition, Nur77 and Nor-1 have been characterized as being tumor suppressors in AML, likely due to their regulation of apoptosis in hematopoietic cells. This finding, in combination with other confounding results, as indicated by overexpression or downregulation in cancer cell lines and patient samples, shows the need to determine the cellular context in which the NR4A receptors contribute to oncogenesis or tumor suppression. It appears that multiple nuances can determine the role of NR4As in cancer, including but not limited to cell and tissue type, subcellular localization, external stimuli, protein-protein interactions, and post-translational modifications.
The NR4A family is intertwined with many relevant cancer signaling pathways, which likely explains the dysregulated expression of these NRs in cancer, as well as their functions in tumorigenic hallmarks, including proliferation and survival (Figure 2). As an emerging research topic, it is highly likely that microRNAs are able to regulate the expression of NR4As in cancer, making their regulation and function much more interesting yet complex. Determining the contributions of microRNAs to NR4A regulation would provide further insight into NR4A dysregulation, as it is likely that a deleted or silenced tumor suppressor–like microRNA could target NR4A, explaining the general consensus of NR4A overexpression. Restoration of microRNA expression by using chemically modified and/or lipid-encapsulated mimics could lead to suppressed NR4A expression, ultimately reducing proliferation and inducing apoptosis.
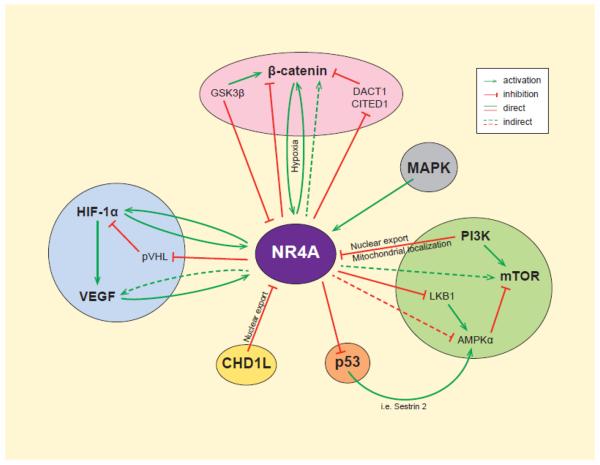
Fig. 2.
The NR4A family and key signaling pathways regulate each other. Both the expression and function of the NR4A members are mediated by activation or inhibition of multiple signaling pathways. Growth factor signaling upregulates the expression and nuclear localization of NR4A members and inhibits NR4A nuclear export and cell death. Additionally, NR4A members can either positively regulate oncogenic signaling pathways (i.e. HIF, β-catenin, mTOR) or overcome tumor suppressor signaling (i.e., pVHL, p53, LKB1).
Lastly, due to its dual functions in cell proliferation and death, Nur77 remains a unique drug target that several groups are targeting using small molecule approaches. One promising approach using small molecules would be to target the Nur77-CHD1L interface in hepatocellular carcinoma with 1q21 amplification to release nuclear-retained Nur77 from CHD1L; presumably, this type of small molecule could be used in combination with compounds that induce Nur77 nuclear export to yield a higher apoptotic response.
Highlights.
The expression and function of NR4As are dysregulated in multiple cancer types
NR4As are positively regulated by oncogenic signaling pathways
NR4As are capable of inhibiting tumor suppressor signaling
The connectedness of NR4As with these pathways mediate their functions in cancer
NR4A agonists and antagonists offer therapeutic strategies for cancer treatment
Acknowledgements
This work was supported by the American Lebanese Syrian Associated Charities, St. Jude Children's Research Hospital, National Institutes of Health National Institute of General Medical Sciences [Grants GM086415 & GM110034], and National Cancer Institute [Grant P30-CA21765]. The authors thank members of the Chen group for their valuable discussions, Klo Spelshouse for assistance with artwork, and Cherise Guess PhD, ELS, for editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen T. Nuclear receptor drug discovery. Curr Opin Chem Biol. 2008;12(4):418–26. doi: 10.1016/j.cbpa.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Maxwell MA, Muscat GE. The NR4A subgroup: immediate early response genes with pleiotropic physiological roles. Nucl Recept Signal. 2006;4:e002. doi: 10.1621/nrs.04002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearen MA, Muscat GE. Minireview: Nuclear hormone receptor 4A signaling: implications for metabolic disease. Mol Endocrinol. 2010;24(10):1891–903. doi: 10.1210/me.2010-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker KD, et al. The Drosophila orphan nuclear receptor DHR38 mediates an atypical ecdysteroid signaling pathway. Cell. 2003;113(6):731–42. doi: 10.1016/s0092-8674(03)00420-3. [DOI] [PubMed] [Google Scholar]
- 5.Flaig R, et al. Structural basis for the cell-specific activities of the NGFI-B and the Nurr1 ligand-binding domain. J Biol Chem. 2005;280(19):19250–8. doi: 10.1074/jbc.M413175200. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, et al. Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature. 2003;423(6939):555–60. doi: 10.1038/nature01645. [DOI] [PubMed] [Google Scholar]
- 7.Vinayavekhin N, Saghatelian A. Discovery of a protein-metabolite interaction between unsaturated fatty acids and the nuclear receptor Nur77 using a metabolomics approach. J Am Chem Soc. 2011;133(43):17168–71. doi: 10.1021/ja208199h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohan HM, et al. Molecular pathways: the role of NR4A orphan nuclear receptors in cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(12):3223–8. doi: 10.1158/1078-0432.CCR-11-2953. [DOI] [PubMed] [Google Scholar]
- 9.Chen HZ, et al. Prolyl isomerase Pin1 stabilizes and activates orphan nuclear receptor TR3 to promote mitogenesis. Oncogene. 2012;31(23):2876–87. doi: 10.1038/onc.2011.463. [DOI] [PubMed] [Google Scholar]
- 10.Mu X, Chang C. TR3 orphan nuclear receptor mediates apoptosis through up-regulating E2F1 in human prostate cancer LNCaP cells. J Biol Chem. 2003;278(44):42840–5. doi: 10.1074/jbc.M305594200. [DOI] [PubMed] [Google Scholar]
- 11.Lee SO, et al. Inactivation of the orphan nuclear receptor TR3/Nur77 inhibits pancreatic cancer cell and tumor growth. Cancer Res. 2010;70(17):6824–36. doi: 10.1158/0008-5472.CAN-10-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SO, et al. The orphan nuclear receptor NR4A1 (Nur77) regulates oxidative and endoplasmic reticulum stress in pancreatic cancer cells. Mol Cancer Res. 2014;12(4):527–38. doi: 10.1158/1541-7786.MCR-13-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Safe S, et al. NR4A orphan receptors and cancer. Nucl Recept Signal. 2011;9:e002. [Google Scholar]
- 14.Lee SO, et al. Targeting NR4A1 (TR3) in cancer cells and tumors. Expert Opin Ther Targets. 2011;15(2):195–206. doi: 10.1517/14728222.2011.547481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ke N, et al. Nuclear hormone receptor NR4A2 is involved in cell transformation and apoptosis. Cancer Res. 2004;64(22):8208–12. doi: 10.1158/0008-5472.CAN-04-2134. [DOI] [PubMed] [Google Scholar]
- 16.Komiya T, et al. Enhanced activity of the CREB co-activator Crtc1 in LKB1 null lung cancer. Oncogene. 2010;29(11):1672–80. doi: 10.1038/onc.2009.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inamoto T, et al. Cytoplasmic mislocalization of the orphan nuclear receptor Nurr1 is a prognostic factor in bladder cancer. Cancer. 2010;116(2):340–6. doi: 10.1002/cncr.24737. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Tai HH. Activation of thromboxane A(2) receptors induces orphan nuclear receptor Nurr1 expression and stimulates cell proliferation in human lung cancer cells. Carcinogenesis. 2009;30(9):1606–13. doi: 10.1093/carcin/bgp161. [DOI] [PubMed] [Google Scholar]
- 19.Llopis S, et al. Dichotomous roles for the orphan nuclear receptor NURR1 in breast cancer. BMC Cancer. 2013;13:139. doi: 10.1186/1471-2407-13-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shigeishi H, et al. PGE(2) targets squamous cell carcinoma cell with the activated epidermal growth factor receptor family for survival against 5-fluorouracil through NR4A2 induction. Cancer Lett. 2011;307(2):227–36. doi: 10.1016/j.canlet.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Holla VR, et al. Prostaglandin E2 regulates the nuclear receptor NR4A2 in colorectal cancer. J Biol Chem. 2006;281(5):2676–82. doi: 10.1074/jbc.M507752200. [DOI] [PubMed] [Google Scholar]
- 22.Zhang T, et al. NGFI-B nuclear orphan receptor Nurr1 interacts with p53 and suppresses its transcriptional activity. Mol Cancer Res. 2009;7(8):1408–15. doi: 10.1158/1541-7786.MCR-08-0533. [DOI] [PubMed] [Google Scholar]
- 23.Mullican SE, et al. Abrogation of nuclear receptors Nr4a3 and Nr4a1 leads to development of acute myeloid leukemia. Nat Med. 2007;13(6):730–5. doi: 10.1038/nm1579. [DOI] [PubMed] [Google Scholar]
- 24.Lee SL, et al. Unimpaired thymic and peripheral T cell death in mice lacking the nuclear receptor NGFI-B (Nur77) Science. 1995;269(5223):532–5. doi: 10.1126/science.7624775. [DOI] [PubMed] [Google Scholar]
- 25.Ponnio T, et al. The nuclear receptor Nor-1 is essential for proliferation of the semicircular canals of the mouse inner ear. Mol Cell Biol. 2002;22(3):935–45. doi: 10.1128/MCB.22.3.935-945.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boudreaux SP, et al. Genome-wide profiling reveals transcriptional repression of MYC as a core component of NR4A tumor suppression in acute myeloid leukemia. Oncogenesis. 2012;1:e19. doi: 10.1038/oncsis.2012.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Labelle Y, et al. Oncogenic conversion of a novel orphan nuclear receptor by chromosome translocation. Hum Mol Genet. 1995;4(12):2219–26. doi: 10.1093/hmg/4.12.2219. [DOI] [PubMed] [Google Scholar]
- 28.Labelle Y, et al. The EWS/TEC fusion protein encoded by the t(9;22) chromosomal translocation in human chondrosarcomas is a highly potent transcriptional activator. Oncogene. 1999;18(21):3303–8. doi: 10.1038/sj.onc.1202675. [DOI] [PubMed] [Google Scholar]
- 29.Filion C, Labelle Y. Identification of genes regulated by the EWS/NR4A3 fusion protein in extraskeletal myxoid chondrosarcoma. Tumour Biol. 2012;33(5):1599–605. doi: 10.1007/s13277-012-0415-2. [DOI] [PubMed] [Google Scholar]
- 30.Ohkura N, Nagamura Y, Tsukada T. Differential transactivation by orphan nuclear receptor NOR1 and its fusion gene product EWS/NOR1: possible involvement of poly(ADP-ribose) polymerase I, PARP-1. J Cell Biochem. 2008;105(3):785–800. doi: 10.1002/jcb.21876. [DOI] [PubMed] [Google Scholar]
- 31.Panagopoulos I, et al. Molecular genetic characterization of the EWS/CHN and RBP56/CHN fusion genes in extraskeletal myxoid chondrosarcoma. Genes Chromosomes Cancer. 2002;35(4):340–52. doi: 10.1002/gcc.10127. [DOI] [PubMed] [Google Scholar]
- 32.Filion C, Labelle Y. The oncogenic fusion protein EWS/NOR-1 induces transformation of CFK2 chondrogenic cells. Exp Cell Res. 2004;297(2):585–92. doi: 10.1016/j.yexcr.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 33.Sjogren H, et al. Fusion of the EWS-related gene TAF2N to TEC in extraskeletal myxoid chondrosarcoma. Cancer Res. 1999;59(20):5064–7. [PubMed] [Google Scholar]
- 34.Sjogren H, et al. Fusion of the NH2-terminal domain of the basic helix-loop-helix protein TCF12 to TEC in extraskeletal myxoid chondrosarcoma with translocation t(9;15)(q22;q21) Cancer Res. 2000;60(24):6832–5. [PubMed] [Google Scholar]
- 35.Dhillon AS, et al. MAP kinase signalling pathways in cancer. Oncogene. 2007;26(22):3279–90. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 36.Slagsvold HH, et al. Nuclear receptor and apoptosis initiator NGFI-B is a substrate for kinase ERK2. Biochem Biophys Res Commun. 2002;291(5):1146–50. doi: 10.1006/bbrc.2002.6579. [DOI] [PubMed] [Google Scholar]
- 37.Strom BO, Paulsen RE. Apoptosis inducer NGFI-B is degraded by the proteasome and stabilized by treatment with EGF. Biochem Biophys Res Commun. 2012;417(4):1292–7. doi: 10.1016/j.bbrc.2011.12.132. [DOI] [PubMed] [Google Scholar]
- 38.Chen HZ, et al. Prolyl isomerase Pin1 stabilizes and activates orphan nuclear receptor TR3 to promote mitogenesis. Oncogene. 2011 doi: 10.1038/onc.2011.463. [DOI] [PubMed] [Google Scholar]
- 39.Jacobs CM, et al. ERK2 prohibits apoptosis-induced subcellular translocation of orphan nuclear receptor NGFI-B/TR3. J Biol Chem. 2004;279(48):50097–101. doi: 10.1074/jbc.M409145200. [DOI] [PubMed] [Google Scholar]
- 40.van den Brink MR, et al. The extracellular signal-regulated kinase pathway is required for activation-induced cell death of T cells. J Biol Chem. 1999;274(16):11178–85. doi: 10.1074/jbc.274.16.11178. [DOI] [PubMed] [Google Scholar]
- 41.Wang A, et al. Phosphorylation of Nur77 by the MEK-ERK-RSK cascade induces mitochondrial translocation and apoptosis in T cells. J Immunol. 2009;183(5):3268–77. doi: 10.4049/jimmunol.0900894. [DOI] [PubMed] [Google Scholar]
- 42.Wingate AD, et al. Nur77 is phosphorylated in cells by RSK in response to mitogenic stimulation. Biochem J. 2006;393(Pt 3):715–24. doi: 10.1042/BJ20050967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teixeiro E, Daniels MA. ERK and cell death: ERK location and T cell selection. FEBS J. 2010;277(1):30–8. doi: 10.1111/j.1742-4658.2009.07368.x. [DOI] [PubMed] [Google Scholar]
- 44.Fujii Y, et al. ERK5 is involved in TCR-induced apoptosis through the modification of Nur77. Genes Cells. 2008;13(5):411–9. doi: 10.1111/j.1365-2443.2008.01177.x. [DOI] [PubMed] [Google Scholar]
- 45.Yang H, et al. Induction and intracellular localization of Nur77 dictate fenretinide-induced apoptosis of human liver cancer cells. Biochem Pharmacol. 2010;79(7):948–54. doi: 10.1016/j.bcp.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith AG, et al. Regulation of NR4A nuclear receptor expression by oncogenic BRAF in melanoma cells. Pigment Cell Melanoma Res. 2011;24(3):551–63. doi: 10.1111/j.1755-148X.2011.00843.x. [DOI] [PubMed] [Google Scholar]
- 47.Sekine Y, et al. p38 MAPKs regulate the expression of genes in the dopamine synthesis pathway through phosphorylation of NR4A nuclear receptors. J Cell Sci. 2011;124(Pt 17):3006–16. doi: 10.1242/jcs.085902. [DOI] [PubMed] [Google Scholar]
- 48.Lammi J, Aarnisalo P. FGF-8 stimulates the expression of NR4A orphan nuclear receptors in osteoblasts. Mol Cell Endocrinol. 2008;295(1–2):87–93. doi: 10.1016/j.mce.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 49.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 50.Whitman M, et al. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988;332(6165):644–6. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- 51.Alessi DR, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7(4):261–9. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 52.Stephens L, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279(5351):710–4. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 53.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273(22):13375–8. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 54.Stambolic V, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95(1):29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 55.Datta SR, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 56.Cardone MH, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282(5392):1318–21. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 57.Diehl JA, et al. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12(22):3499–511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Porta C, Paglino C, Mosca A. Targeting PI3K/Akt/mTOR Signaling in Cancer. Front Oncol. 2014;4:64. doi: 10.3389/fonc.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 60.Chalhoub N, Baker SJ. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol. 2009;4:127–50. doi: 10.1146/annurev.pathol.4.110807.092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li J, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275(5308):1943–7. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 62.Steck PA, et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15(4):356–62. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 63.Di Cristofano A, et al. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19(4):348–55. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 64.Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci U S A. 2001;98(20):11598–603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen HZ, et al. Akt phosphorylates the TR3 orphan receptor and blocks its targeting to the mitochondria. Carcinogenesis. 2008;29(11):2078–88. doi: 10.1093/carcin/bgn197. [DOI] [PubMed] [Google Scholar]
- 66.Masuyama N, et al. Akt inhibits the orphan nuclear receptor Nur77 and T-cell apoptosis. J Biol Chem. 2001;276(35):32799–805. doi: 10.1074/jbc.M105431200. [DOI] [PubMed] [Google Scholar]
- 67.Pekarsky Y, et al. Akt phosphorylates and regulates the orphan nuclear receptor Nur77. Proc Natl Acad Sci U S A. 2001;98(7):3690–4. doi: 10.1073/pnas.051003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hirata Y, et al. The phosphorylation and DNA binding of the DNA-binding domain of the orphan nuclear receptor NGFI-B. J Biol Chem. 1993;268(33):24808–12. [PubMed] [Google Scholar]
- 69.Li H, et al. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 2000;289(5482):1159–64. doi: 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- 70.Han YH, et al. Regulation of Nur77 nuclear export by c-Jun N-terminal kinase and Akt. Oncogene. 2006;25(21):2974–86. doi: 10.1038/sj.onc.1209358. [DOI] [PubMed] [Google Scholar]
- 71.Wilson AJ, et al. TR3 modulates platinum resistance in ovarian cancer. Cancer Res. 2013;73(15):4758–69. doi: 10.1158/0008-5472.CAN-12-4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao X, et al. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat Cell Biol. 2002;4(9):699–704. doi: 10.1038/ncb847. [DOI] [PubMed] [Google Scholar]
- 73.Potter CJ, Pedraza LG, Xu T. Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol. 2002;4(9):658–65. doi: 10.1038/ncb840. [DOI] [PubMed] [Google Scholar]
- 74.Lizcano JM, et al. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J. 2004;23(4):833–43. doi: 10.1038/sj.emboj.7600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shaw RJ, et al. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6(1):91–9. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 76.Lee SO, et al. The nuclear receptor TR3 regulates mTORC1 signaling in lung cancer cells expressing wild-type p53. Oncogene. 2012;31(27):3265–76. doi: 10.1038/onc.2011.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cho SD, et al. Activation of nerve growth factor-induced B alpha by methylene-substituted diindolylmethanes in bladder cancer cells induces apoptosis and inhibits tumor growth. Mol Pharmacol. 2010;77(3):396–404. doi: 10.1124/mol.109.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134(3):451–60. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao BX, et al. p53 mediates the negative regulation of MDM2 by orphan receptor TR3. EMBO J. 2006;25(24):5703–15. doi: 10.1038/sj.emboj.7601435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhan YY, et al. The orphan nuclear receptor Nur77 regulates LKB1 localization and activates AMPK. Nat Chem Biol. 2012;8(11):897–904. doi: 10.1038/nchembio.1069. [DOI] [PubMed] [Google Scholar]
- 81.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang GL, et al. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92(12):5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270(3):1230–7. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 84.Ivan M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292(5516):464–8. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 85.Jaakkola P, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 86.Maxwell PH, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–5. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 87.Ohh M, et al. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol. 2000;2(7):423–7. doi: 10.1038/35017054. [DOI] [PubMed] [Google Scholar]
- 88.Lando D, et al. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16(12):1466–71. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12(12):5447–54. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Forsythe JA, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16(9):4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen C, et al. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem. 2001;276(12):9519–25. doi: 10.1074/jbc.M010144200. [DOI] [PubMed] [Google Scholar]
- 92.Semenza GL, et al. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271(51):32529–37. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 93.Kim JW, et al. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3(3):177–85. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 94.Papandreou I, et al. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3(3):187–97. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 95.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29(5):625–34. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15(4):621–7. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- 97.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 98.Talks KL, et al. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157(2):411–21. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhong H, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59(22):5830–5. [PubMed] [Google Scholar]
- 100.Zagzag D, et al. Expression of hypoxia-inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer. 2000;88(11):2606–18. [PubMed] [Google Scholar]
- 101.Latif F, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260(5112):1317–20. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 102.Moore LE, et al. Von Hippel-Lindau (VHL) inactivation in sporadic clear cell renal cancer: associations with germline VHL polymorphisms and etiologic risk factors. PLoS Genet. 2011;7(10):e1002312. doi: 10.1371/journal.pgen.1002312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Choi JW, et al. Nur77 activated by hypoxia-inducible factor-1alpha overproduces proopiomelanocortin in von Hippel-Lindau-mutated renal cell carcinoma. Cancer Res. 2004;64(1):35–9. doi: 10.1158/0008-5472.can-03-0145. [DOI] [PubMed] [Google Scholar]
- 104.Yoo YG, et al. Novel function of orphan nuclear receptor Nur77 in stabilizing hypoxia-inducible factor-1alpha. J Biol Chem. 2004;279(51):53365–73. doi: 10.1074/jbc.M408554200. [DOI] [PubMed] [Google Scholar]
- 105.Yoo YG, et al. 6-Mercaptopurine, an activator of Nur77, enhances transcriptional activity of HIF-1alpha resulting in new vessel formation. Oncogene. 2007;26(26):3823–34. doi: 10.1038/sj.onc.1210149. [DOI] [PubMed] [Google Scholar]
- 106.Martorell L, et al. The hypoxia-inducible factor 1/NOR-1 axis regulates the survival response of endothelial cells to hypoxia. Mol Cell Biol. 2009;29(21):5828–42. doi: 10.1128/MCB.00945-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.To SK, et al. Hypoxia triggers a Nur77-beta-catenin feed-forward loop to promote the invasive growth of colon cancer cells. Br J Cancer. 2014;110(4):935–45. doi: 10.1038/bjc.2013.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim BY, et al. Nur77 upregulates HIF-alpha by inhibiting pVHL-mediated degradation. Exp Mol Med. 2008;40(1):71–83. doi: 10.3858/emm.2008.40.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wansa KD, et al. The AF-1 domain of the orphan nuclear receptor NOR-1 mediates trans-activation, coactivator recruitment, and activation by the purine anti-metabolite 6-mercaptopurine. J Biol Chem. 2003;278(27):24776–90. doi: 10.1074/jbc.M300088200. [DOI] [PubMed] [Google Scholar]
- 110.Wu H, et al. Regulation of Nur77 expression by beta-catenin and its mitogenic effect in colon cancer cells. FASEB J. 2011;25(1):192–205. doi: 10.1096/fj.10-166462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu D, et al. Vascular endothelial growth factor-regulated gene expression in endothelial cells: KDR-mediated induction of Egr3 and the related nuclear receptors Nur77, Nurr1, and Nor1. Arterioscler Thromb Vasc Biol. 2003;23(11):2002–7. doi: 10.1161/01.ATV.0000098644.03153.6F. [DOI] [PubMed] [Google Scholar]
- 112.Rius J, et al. NOR-1 is involved in VEGF-induced endothelial cell growth. Atherosclerosis. 2006;184(2):276–82. doi: 10.1016/j.atherosclerosis.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 113.Zeng H, et al. Orphan nuclear receptor TR3/Nur77 regulates VEGF-A-induced angiogenesis through its transcriptional activity. J Exp Med. 2006;203(3):719–29. doi: 10.1084/jem.20051523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao D, Desai S, Zeng H. VEGF stimulates PKD-mediated CREB-dependent orphan nuclear receptor Nurr1 expression: role in VEGF-induced angiogenesis. Int J Cancer. 2011;128(11):2602–12. doi: 10.1002/ijc.25600. [DOI] [PubMed] [Google Scholar]
- 115.Martinez-Gonzalez J, et al. Neuron-derived orphan receptor-1 (NOR-1) modulates vascular smooth muscle cell proliferation. Circ Res. 2003;92(1):96–103. doi: 10.1161/01.es.0000050921.53008.47. [DOI] [PubMed] [Google Scholar]
- 116.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88(3):323–31. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 117.Zilfou JT, Lowe SW. Tumor suppressive functions of p53. Cold Spring Harb Perspect Biol. 2009;1(5):a001883. doi: 10.1101/cshperspect.a001883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.el-Deiry WS, et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54(5):1169–74. [PubMed] [Google Scholar]
- 119.el-Deiry WS, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75(4):817–25. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 120.Kastan MB, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71(4):587–97. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 121.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7(3):683–94. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 122.Oda E, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288(5468):1053–8. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 123.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80(2):293–9. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 124.Mihara M, et al. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11(3):577–90. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 125.Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9(10):749–58. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.de Vries A, et al. Targeted point mutations of p53 lead to dominant-negative inhibition of wild-type p53 function. Proc Natl Acad Sci U S A. 2002;99(5):2948–53. doi: 10.1073/pnas.052713099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev. 2012;26(12):1268–86. doi: 10.1101/gad.190678.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Oliner JD, et al. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992;358(6381):80–3. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- 129.Jacks T, et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4(1):1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 130.Zhao BX, et al. Orphan receptor TR3 enhances p53 transactivation and represses DNA double-strand break repair in hepatoma cells under ionizing radiation. Mol Endocrinol. 2011;25(8):1337–50. doi: 10.1210/me.2011-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Malewicz M, et al. Essential role for DNA-PK-mediated phosphorylation of NR4A nuclear orphan receptors in DNA double-strand break repair. Genes Dev. 2011;25(19):2031–40. doi: 10.1101/gad.16872411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Moll UM, Marchenko N, Zhang XK. p53 and Nur77/TR3 - transcription factors that directly target mitochondria for cell death induction. Oncogene. 2006;25(34):4725–43. doi: 10.1038/sj.onc.1209601. [DOI] [PubMed] [Google Scholar]
- 133.Wu Q, et al. Dual roles of Nur77 in selective regulation of apoptosis and cell cycle by TPA and ATRA in gastric cancer cells. Carcinogenesis. 2002;23(10):1583–92. doi: 10.1093/carcin/23.10.1583. [DOI] [PubMed] [Google Scholar]
- 134.Zhang XK. Targeting Nur77 translocation. Expert Opin Ther Targets. 2007;11(1):69–79. doi: 10.1517/14728222.11.1.69. [DOI] [PubMed] [Google Scholar]
- 135.Cao X, et al. Retinoid X receptor regulates Nur77/TR3-dependent apoptosis by modulating its nuclear export and mitochondrial targeting. Mol Cell Biol. 2004;24(22):9705–25. doi: 10.1128/MCB.24.22.9705-9725.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Katagiri Y, et al. Modulation of retinoid signalling through NGF-induced nuclear export of NGFI-B. Nat Cell Biol. 2000;2(7):435–40. doi: 10.1038/35017072. [DOI] [PubMed] [Google Scholar]
- 137.Lin XF, et al. RXRalpha acts as a carrier for TR3 nuclear export in a 9-cis retinoic acid-dependent manner in gastric cancer cells. J Cell Sci. 2004;117(Pt 23):5609–21. doi: 10.1242/jcs.01474. [DOI] [PubMed] [Google Scholar]
- 138.Lin B, et al. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116(4):527–40. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- 139.Liang B, et al. Involvement of TR3/Nur77 translocation to the endoplasmic reticulum in ER stress-induced apoptosis. Exp Cell Res. 2007;313(13):2833–44. doi: 10.1016/j.yexcr.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 140.Chintharlapalli S, et al. Activation of Nur77 by selected 1,1-Bis(3'-indolyl)-1-(p-substituted phenyl)methanes induces apoptosis through nuclear pathways. J Biol Chem. 2005;280(26):24903–14. doi: 10.1074/jbc.M500107200. [DOI] [PubMed] [Google Scholar]
- 141.Cho SD, et al. Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor-dependent and nuclear receptor-independent pathways. Cancer Res. 2007;67(2):674–83. doi: 10.1158/0008-5472.CAN-06-2907. [DOI] [PubMed] [Google Scholar]
- 142.Lee SO, et al. p21 expression is induced by activation of nuclear nerve growth factor-induced Balpha (Nur77) in pancreatic cancer cells. Mol Cancer Res. 2009;7(7):1169–78. doi: 10.1158/1541-7786.MCR-08-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yoon K, et al. Activation of nuclear TR3 (NR4A1) by a diindolylmethane analog induces apoptosis and proapoptotic genes in pancreatic cancer cells and tumors. Carcinogenesis. 2011;32(6):836–42. doi: 10.1093/carcin/bgr040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu JJ, et al. A unique pharmacophore for activation of the nuclear orphan receptor Nur77 in vivo and in vitro. Cancer Res. 2010;70(9):3628–37. doi: 10.1158/0008-5472.CAN-09-3160. [DOI] [PubMed] [Google Scholar]
- 145.Zhan Y, et al. Cytosporone B is an agonist for nuclear orphan receptor Nur77. Nat Chem Biol. 2008;4(9):548–56. doi: 10.1038/nchembio.106. [DOI] [PubMed] [Google Scholar]
- 146.Inamoto T, et al. 1,1-Bis(3'-indolyl)-1-(p-chlorophenyl)methane activates the orphan nuclear receptor Nurr1 and inhibits bladder cancer growth. Mol Cancer Ther. 2008;7(12):3825–33. doi: 10.1158/1535-7163.MCT-08-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer. 2013;13(1):11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 148.Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circulation research. 2010;106(12):1798–806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- 149.Mulholland DJ, et al. Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know? Endocr Rev. 2005;26(7):898–915. doi: 10.1210/er.2003-0034. [DOI] [PubMed] [Google Scholar]
- 150.Rajalin AM, Aarnisalo P. Cross-talk between NR4A orphan nuclear receptors and beta-catenin signaling pathway in osteoblasts. Archives of biochemistry and biophysics. 2011;509(1):44–51. doi: 10.1016/j.abb.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 151.To SK, Zeng JZ, Wong AS. Nur77: a potential therapeutic target in cancer. Expert Opin Ther Targets. 2012;16(6):573–85. doi: 10.1517/14728222.2012.680958. [DOI] [PubMed] [Google Scholar]
- 152.Flaus A, et al. Identification of multiple distinct Snf2 subfamilies with conserved structural motifs. Nucleic Acids Res. 2006;34(10):2887–905. doi: 10.1093/nar/gkl295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Karras GI, et al. The macro domain is an ADP-ribose binding module. EMBO J. 2005;24(11):1911–20. doi: 10.1038/sj.emboj.7600664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ahel D, et al. Poly(ADP-ribose)-dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science. 2009;325(5945):1240–3. doi: 10.1126/science.1177321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gottschalk AJ, et al. Poly(ADP-ribosyl)ation directs recruitment and activation of an ATP-dependent chromatin remodeler. Proc Natl Acad Sci U S A. 2009;106(33):13770–4. doi: 10.1073/pnas.0906920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen L, et al. CHD1L promotes hepatocellular carcinoma progression and metastasis in mice and is associated with these processes in human patients. J Clin Invest. 2010;120(4):1178–91. doi: 10.1172/JCI40665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chan TH, et al. Translationally controlled tumor protein induces mitotic defects and chromosome missegregation in hepatocellular carcinoma development. Hepatology. 2012;55(2):491–505. doi: 10.1002/hep.24709. [DOI] [PubMed] [Google Scholar]
- 158.Li Y, et al. SPOCK1 is regulated by CHD1L and blocks apoptosis and promotes HCC cell invasiveness and metastasis in mice. Gastroenterology. 2013;144(1):179–191. e4. doi: 10.1053/j.gastro.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 159.Cheng W, Su Y, Xu F. CHD1L: a novel oncogene. Mol Cancer. 2013;12(1):170. doi: 10.1186/1476-4598-12-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen L, Chan TH, Guan XY. Chromosome 1q21 amplification and oncogenes in hepatocellular carcinoma. Acta Pharmacol Sin. 2010;31(9):1165–71. doi: 10.1038/aps.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ma NF, et al. Isolation and characterization of a novel oncogene, amplified in liver cancer 1, within a commonly amplified region at 1q21 in hepatocellular carcinoma. Hepatology. 2008;47(2):503–10. doi: 10.1002/hep.22072. [DOI] [PubMed] [Google Scholar]
- 162.Guan XY, et al. Recurrent chromosome alterations in hepatocellular carcinoma detected by comparative genomic hybridization. Genes Chromosomes Cancer. 2000;29(2):110–6. [PubMed] [Google Scholar]
- 163.Chen L, et al. Clinical significance of CHD1L in hepatocellular carcinoma and therapeutic potentials of virus-mediated CHD1L depletion. Gut. 2011;60(4):534–43. doi: 10.1136/gut.2010.224071. [DOI] [PubMed] [Google Scholar]
- 164.Chen M, et al. Transgenic CHD1L expression in mouse induces spontaneous tumors. PLoS One. 2009;4(8):e6727. doi: 10.1371/journal.pone.0006727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer. 2002;2(2):133–42. doi: 10.1038/nrc725. [DOI] [PubMed] [Google Scholar]
- 166.Chen L, et al. Chromodomain helicase/adenosine triphosphatase DNA binding protein 1-like (CHD1l) gene suppresses the nucleus-to-mitochondria translocation of nur77 to sustain hepatocellular carcinoma cell survival. Hepatology. 2009;50(1):122–9. doi: 10.1002/hep.22933. [DOI] [PubMed] [Google Scholar]