Abstract
Voluntary surface electromyogram (EMG) signals from neurological injury patients are often corrupted by involuntary background interference or spikes, imposing difficulties for myoelectric control. We present a novel framework to suppress involuntary background spikes during voluntary surface EMG recordings. The framework applies a Wiener filter to restore voluntary surface EMG signals based on tracking a priori signal to noise ratio (SNR) by using the decision-directed method. Semi-synthetic surface EMG signals contaminated by different levels of involuntary background spikes were constructed from a database of surface EMG recordings in a group of spinal cord injury subjects. After the processing, the onset detection of voluntary muscle activity was significantly improved against involuntary background spikes. The magnitude of voluntary surface EMG signals can also be reliably estimated for myoelectric control purpose. Compared with the previous sample entropy analysis for suppressing involuntary background spikes, the proposed framework is characterized by quick and simple implementation, making it more suitable for application in a myoelectric control system toward neurological injury rehabilitation.
Keywords: Surface EMG, myoelectric control, neurologic injuries, involuntary muscle activity
1. INTRODUCTION
Surface electromyogram (EMG) has been used as a control signal for myoelectric prostheses, rehabilitation robots or other assistive devices [1]. In studies involving neurological injuries, it happens often that voluntary surface EMG signals might be contaminated by spontaneous motor activity. For instance, when recording EMG signals from paretic muscles of stroke or spinal cord injury patients, abnormal hyper-excitable motor unit discharges may induce spontaneous tonic spikes, consequently compromising the voluntary EMG signals [2] [3]. From the point of view of implementing a myoelectric (proportional or pattern recognition) control system, such involuntary background spikes will impose two difficulties. First, the involuntary background spikes make automatic detection of muscle activity a challenging task with classical EMG amplitude thresholding based methods. Second, amplitude estimation of voluntary surface EMG can be severely affected by the presence of involuntary muscle activities.
It follows that, to develop a myoelectric control system for neurological injury rehabilitation, a surface EMG filtering algorithm is required to mitigate the effects of involuntary background spikes. Thus, the system can differentiate between user’s voluntary intention and involuntary activity from the surface EMG signals. Unfortunately, involuntary background spikes and voluntary surface EMG signals usually have overlapping frequency components. Indeed, both voluntary and involuntary EMG signals have the same origins (i.e. muscle fibers), making it very difficult to apply conventional digital filters to remove involuntary spikes.
In this study, toward developing a myoelectric control system for patients with neurological injuries, we present a novel denoising framework for mitigating the effects of involuntary background spikes in voluntary surface EMG signals. The framework applies a Wiener filter [4] to restore voluntary surface EMG signals based on tracking a priori signal to noise ratio (SNR) by the decision-directed method [5] [6]. We demonstrated that after the processing, the onset detection of voluntary muscle activity can be significantly improved against involuntary background spikes. Furthermore, using the processed signals the magnitude of voluntary surface EMG can be reliably estimated for myoelectric control purpose.
2. METHODS
2.1 Background Theory
2.1.1 Intergration of Wiener filtering and a priori SNR
Wiener filtering is a linear technique consisting of a Fourier filter in the frequency domain, where the original Fourier coefficients are rescaled according to the ratio between the desired and actual signal spectrum [4]. A measured signal y(t) in the time domain is considered as the linear summation of the true signal x(t) and the noise s(t), i.e.
| (1) |
The estimate of expected signal can be obtained in the frequency domain by filtering the measured signal, assuming known stationary signal and noise spectra:
| (2) |
where X̄ is the estimate of X, which is the frequency domain representation of x(t), Y is the frequency domain representation of y(t), G is a gain or filtering function which minimizes the mean square error between the estimated and desired processes. According to [5], the concept of a priori SNR ξk(n) can be integrated into the Wiener filtering (here n is the index of the processed frame for a specific k th frequency bandwidth), by assuming that E{γk(n)} = ξk(n)+1, where E{γk(n)} denotes the estimator of the a posteriori SNR γk(n). Thus, we have
| (3) |
The Wiener amplitude estimator is given by
| (4) |
where Âk(n) denotes the corresponding k th spectral component of the true signal x(t) in the n th frame. ξ̂k(n) is the estimator of ξk(n), Yk(n) is the k th spectral component of the noisy observations y(t) in the n th frame. In order to proceed with a Wiener filter, it is critical to extract the noise spectrum to get ξ̂k(n). In the current study, the noise spectrum for the Wiener filter is estimated from the noisy signal spectrum by tracking a priori SNR as described below.
2.1.2 A priori SNR estimation
We adopted the decision-directed approach to estimate a priori SNR for noise reduction in the frequency domain [5] [6]. After applying a short-time Fourier transform of the measured signal y(t), it can be expressed in the frequency domain as:
| (5) |
where k denotes k th spectral component, n is the analysis frame index. Let ξk(n), Ak(n), λS(k,n) and γk(n) denote the a priori SNR, the amplitude, the noise variance, and the a posteriori SNR, respectively, of the corresponding k th spectral component in the n th analysis frame of the noisy input signal y(t). According to [6], the a priori SNR estimator is based on the definition of ξk(n), and its relation to the a posteriori SNR γk(n), hence the a priori SNR can be estimated by
| (6) |
| (7) |
Where E{·} denotes the estimator.
The estimator ξ̂k(n) of ξk(n) is given by
| (8) |
| (9) |
where 0 ≤ α <1, the nonlinear gain of the signal G(·,·) is the Wiener noise suppression function, as given below:
| (10) |
| (11) |
| (12) |
where L is the smoothing factor used for the noise updating.
In summary, the gain of the signal G(·,·) is updated using ξ̂k(n), and ξ̂k(n) is updated based on a previous estimate ξ̂k(n−1) and γk(n−1) according to (8) and (3), thus Âk(n) is calculated as (4). In brief, the gain of the signal G(·,·) is updated using the SNRs.
2.2. Framework Implementation
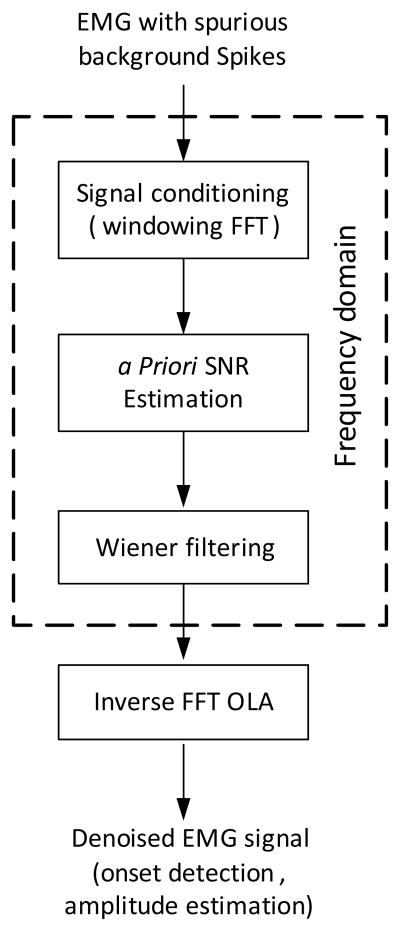
Figure 1 summarizes the framework used in this study for suppressing the involuntary background spikes contaminating the voluntary surface EMG. The EMG signal was processed in 25 ms frames with a Hamming window and a 40% overlap between successive frames. The Fast Fourier Transform (FFT) of each frame was calculated, and then the phase component of the FFT was calculated. The decision-directed approach was used to estimate the a priori SNR using the noise power spectrum. Subsequently, the Wiener amplitude estimator was applied in the frequency domain to obtain an estimate of the voluntary EMG signal. Finally, to reconstruct the EMG signal in the time domain from its spectrogram after denoising, we applied an inverse FFT and synthesized the signal using the overlap-add (OLA) method [7].
Fig. 1.
The denoising framework, including signal conditioning, a priori SNR estimation, Wiener filtering and time domain signal reconstruction modules.
2.3. Performance Evaluation
2.3.1 Testing dataset description
The testing data of this study were selected from the previous database recorded from the partially paralyzed muscles of 9 subejcts with incomplete spinal cord injury (6 male, 3 female; age range 31–62 year; Neurological injury level C4–C8; ASIA class C or D), approved by the Institutional Review Board of Northwestern University (Chicago, USA). As detailed in [8], over 50 channels of surface EMG signals were recorded from the forearm and hand muscles with a Refa EMG system (TMS International B.V., Netherlands) at a sampling rate of 2 kHz per channel, during the subject’s actuating or trying to actuate a series of hand movements.
To perform quantitative evaluation, semi-synthetic signals were constructed by combining two types of signals selected from the database: “clean” voluntary surface EMG data free of spontaneous background interference and “pure” involuntary background interference recorded during the subject’s rest period. Specifically, 10 segments (each 2 s in length) of typical involuntary background spikes and 10 segments (each 1 s in length) of surface EMG free of background spikes were selected. Each of the 10 “clean” voluntary surface EMG segments was scaled over a range of magnitudes, and summed with each of the 10 “pure” spontaneous interference segments (starting from 0.5 to 1.5 s in the 2 s period). To simulate semi-synthetic surface EMG signals with different involuntary spike levels, the signal segments were artificially scaled to generate semi-synthetic surface EMG signals in such a way that the background spike or noise level resulted in different SNRs (22, 20, 18, 15, 12, 10, 8, 5 and 2 dB, respectively).
2.3.2 Evaluation of onset detection
After denoising the EMG signal, the muscle activity onset detection was performed. Three approaches were used including:
Amplitude thresholding. Three times standard deviation (SD) of the baseline was utilized as the threshold for the conventional amplitude thresholding method for muscle onset detection.
-
Thresholding in the Teager Kaiser Energy (TKE) domain. The TKE operation is able to simultaneously highlight the instantaneous frequency and amplitude changes for muscle activity onset detection [9–11]. The TKE operator is defined as:
(13) where ψ is the discrete TKE operation in the time domain and x(i) represents the input signal. In this study, the TKE operation output was smoothed with a second order, 25 Hz Butterworth low pass filter to avoid errors induced by the rapid variations in the unsmoothed signal [11]. Threshold was set to 15 times SD of the baseline in the TKE domain for onset detection.
Double thresholding in the TKE domain. To reduce the dependence of onset detection on the baseline EMG signal, the double threshold method was used to create an alternative threshold by combining the amplitude and rate of change in the TKE domain [12]. The rate of change of the TKE output was measured by the slope of the line between the amplitudes of two successive data points. Muscle activation was determined when the TKE operation output exceeded 2.5% of its maximum amplitude and when the slope between two successive data points exceeded 10−3, for at least 22 data points in a 25 point sliding window.
For performance comparison, the onset detection was also performed on signals before suppressing involuntary spikes. The semi-synthetic surface EMG signals with preset onset time (i.e. at 500 ms for each testing signal) were used to evaluate the performance under different conditions. The onset performance was evaluated by the latency τ, defined as the absolute difference between the true onset time t0 (i.e. 500 ms) and the detected onset time td :
| (14) |
The statistical difference in onset detection accuracy from various methods was evaluated by one-way repeated-measures ANOVA. Post hoc analysis, paired t-test, was conducted to compare the differences between conditions.
2.3.3 EMG amplitude estimation
To evaluate the application of the proposed method for myoelectric control, the intensity of muscle contraction was estimated by calculating the root mean square (RMS) amplitude of the surface EMG after suppression of the involuntary background spikes. Sliding analysis windows were used to segment the EMG data and compute RMS. The length of the sliding windows was 256 ms (increment step 64 ms). The correlations between RMS amplitudes of the EMG signal after involuntary spike suppression and the corresponding “clean” voluntary EMG signal were examined.
3. RESULTS
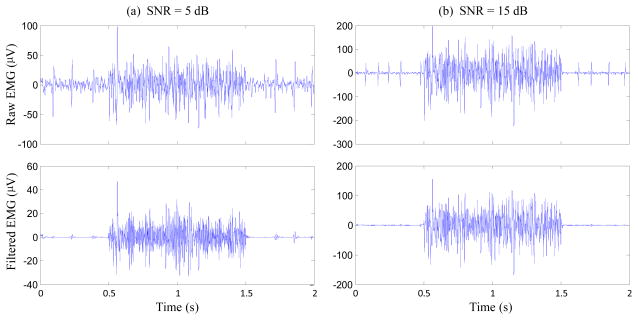
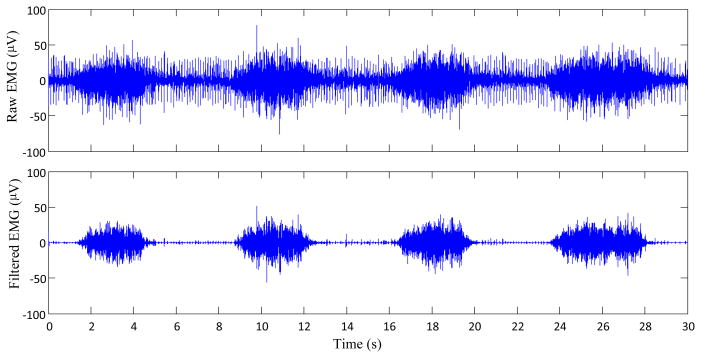
Figure 2 shows a comparison of two representative semi-synthetic surface EMG signals (with different SNRs) and the outputs after the proposed processing. It demonstrates that the Wiener filtering based on tracking a priori SNR via the decision-directed method can suppress the involuntary background spikes while the voluntary surface EMG signal can be remained. Figure 3 shows an example of one channel experimental (rather than semi-synthetic) surface EMG signal recorded from the forearm muscles of an SCI subject during his performing four repetitions of voluntary muscle contractions, and the restored voluntary surface EMG signal with the proposed framework. It was observed that the recorded surface EMG signal had significant involuntary background spikes, which were suppressed after the processing.
Fig.2.
Semi-synthetic surface EMG signals (top panel) and the voluntary surface EMG signals obtained after the processing (bottom panel).
Fig. 3.
An example of experimental surface EMG signal corrupted by significant involuntary background spikes (top panel) and the voluntary surface EMG signal after the processing (bottom panel).
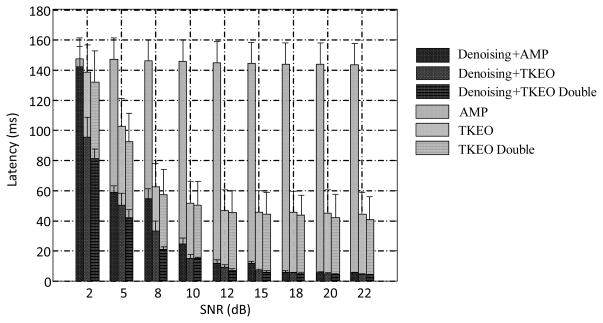
The muscle onset detection performance with different conditions or methods is summarized in Figure 4, using a series of semi-synthetic surface EMG signals. We observed that after the denoising, the onset detection of voluntary muscle activity was significantly improved against involuntary background spikes (paired samples t-test, P < 0.001 for each method before and after denoising, for all the tested SNRs). We also observed that as the SNR of the signal decreased from 20 to 2 dB, the latency of the onset detection for all the methods tended to increase. For each signal condition, significant differences were observed between different methods after the denoising (one-way repeated measures ANOVA, F[2, 16] = 4.78, P = 0.02). Paired samples t-tests were used to make post hoc comparisons between conditions. Across all SNRs, there was a significant difference in the detection accuracy of the double threshold in the TKE domain versus any of the other two methods (P < 0.03). Among all the methods, the double threshold in the TKE domain after the denoising with the proposed framework exhibited the best performance at all the SNR levels.
Fig. 4.
Comparison of onset detection performance under different conditions (mean ± standard error). AMP: the conventional amplitude thresholding method before the processing; Denoising+AMP: the conventional amplitude thresholding method after the processing; TKEO: the TKEO thresholding method before the processing; Denoising+TKEO: the TKEO thresholding method after the processing; TKEO Double: double threshold algorithm in the TKE domain before the processing; Denoising+TKEO Double: the double threshold algorithm in the TKE domain after the processing. For each SNR level, the mean latency was averaged over 100 trials of semi-synthetic surface EMG signals.
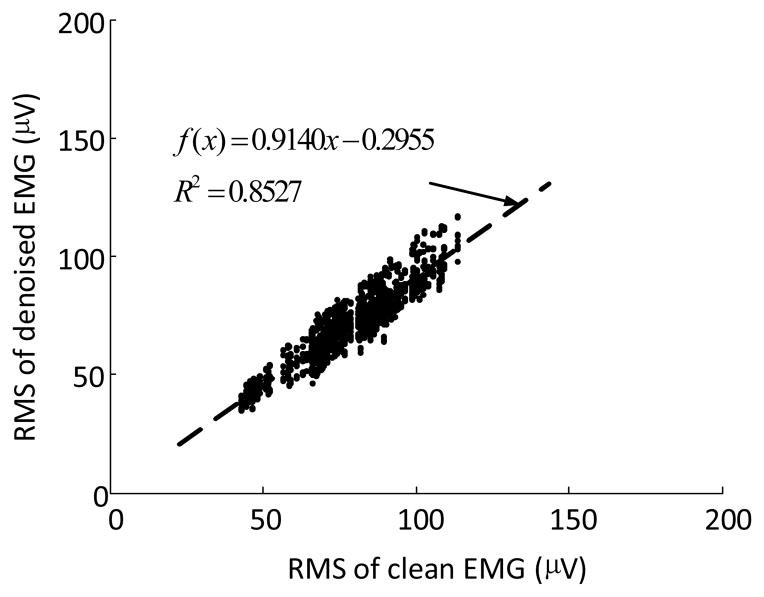
The EMG amplitude estimation indicated that after processing using the proposed framework it was feasible to estimate RMS amplitudes of the EMG signals contaminated by background spikes. To evaluate the performance, the RMS values of each analysis window of the semi-synthetic signals during EMG bursts were calculated for both the signals after the processing and the corresponding “clean” voluntary surface EMG signals. Figure 5 shows the correlation between the RMS amplitude of the two signals (SNR = 5 in this example). Using linear regression analysis, the RMS of the processed EMG signals was highly positively correlated with the RMS of the “clean” voluntary EMG signals (R = 0.92, P < 0.05).
Fig. 5.
Correlation between the RMS amplitudes of the voluntary surface EMG signals after the processing and the corresponding “clean” EMG of the semi-synthetic signals. The RMS amplitude were obtained from analysis windows of the semi-synthetic signals (SNR=5). The linear function was tested for fitting the data.
4. DISCUSSION
It is common to observe involuntary background activity from paretic muscles of neurological injury patients. Such involuntary activity might distort voluntary surface EMG processing, thus imposing challenges for implementing a myoelectric control system. This study presents a novel framework for suppressing the effects of the involuntary background spikes by combining a Wiener filter [4] and a priori SNR estimation via the decision-directed method (using the power spectrum of the noisy EMG signal) [5] [6].
The results indicate that the combination of a Wiener filter and tracking of a priori SNR can be used as an effective tool to suppress involuntary background spikes towards facilitating myoelectric control using surface EMG signals recorded from paretic muscles. After the processing, previously developed onset detection methods can be used to detect the onset of voluntary muscle activity [13–17]. The amplitude of voluntary muscle activity can also be reliable estimated, demonstrated as a strong positive linear correlation between RMS amplitudes of the EMG signals after involuntary spike suppression and the corresponding “clean” voluntary EMG signals.
The proposed framework has several advantages for myoelectric control compared with previous studies. For example, a Wiener filtering based approach was used to remove noise in surface EMG signal, composed by non-stationary sharp spectral line of variable intensity and frequency [18]. The approach required simultaneous acquisition of the environmental noise via a separate channel. In contrast, the current study combines the Wiener filter with a noise estimator. The noise spectrum for the Wiener filter is extracted by tracking a prior SNR of the processed signal via the decision-directed approach.
To suppress the effect of spontaneous background spikes on surface EMG processing, the sample entropy (SampEn) analysis has been used to perform muscle activity onset detection [19]. A primary limitation of the SampEn based method is the high computational cost imposed by the algorithms for computing the SampEn. The method also requires estimation of the global tolerance for SampEn calculation. The framework of the current study requires low computational load. For example, It took approximately 1.2 s to calculate the SampEn of a 2 s semi-synthetic surface EMG signals by a series of analysis windows (window length of 32 ms and a window increment of 4 ms [19]). In contrast, it took less than 0.05 s to denoise the same signal by using the proposed framework (performed on a 3.30-GHz Core i5 based PC using a Windows 7 operating system with 8-GB Memory). Such an advantage makes it suitable for real time implementation toward overcoming involuntary background spikes for myoelectric control.
Highlights.
Voluntary surface EMG from neurological injury patients are often corrupted by involuntary background interference or spikes.
We develop a novel framework to suppress involuntary motor activity contaminating voluntary surface EMG.
The framework applies a Wiener filter based on tracking a priori SNR.
The framework is characterized by quick and simple implementation.
Acknowledgments
Funding: This work was supported in part by the National Institutes of Health of the U.S. Department of Health and Human Services under Grants R21NS075463 and R01NS080839, and in part by the Memorial Hermann Foundation.
Footnotes
Competing interests: None declared.
Ethical approval: The study was approved by the Institutional Review Board of Northwestern University, Chicago, IL, USA (Reference number: STU00023682).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oskoei MA, Hu HS. Myoelectric control systems-A survey. Biomedical Signal Processing and Control. 2007;2:275–94. [Google Scholar]
- 2.McKay WB, Ovechkin AV, Vitaz TW, Terson de Paleville DG, Harkema SJ. Long-lasting involuntary motor activity after spinal cord injury. Spinal Cord. 2011;49(1):87–93. doi: 10.1038/sc.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Li Y, Chen X, Li G, Rymer WZ, Zhou P. The effect of involuntary motor activity on myoelectric pattern recognition: a case study with chronic stroke patients. Journal of Neural Engineering. 2013;10(4):046015. doi: 10.1088/1741-2560/10/4/046015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wiener N. Extrapolation, interpolation, and smoothing of stationary time series. Cambridge, MA, USA: The MIT Press; 1964. [Google Scholar]
- 5.Scalart P, Vieira J. Speech enhancement based on a priori signal to noise estimation. IEEE International Conference on Acoustics, Speech, and Signal Processing, Conference Proceedings; 1996. pp. 629–32. [Google Scholar]
- 6.Ephraim Y, Malah D. Speech enhancement using a minimum mean-square error short-time spectral amplitude estimator. IEEE Transactions on Acoustics Speech and Signal Processing. 1984;32:1109–21. [Google Scholar]
- 7.Helms HD. Fast Fourier transform method of computing difference equations and simulating filters. IEEE Transactions on Audio and Electroacoustics. 1967;Au15(2):85–90. [Google Scholar]
- 8.Liu J, Zhou P. A novel myoelectric pattern recognition strategy for hand function restoration after incomplete cervical spinal cord injury. IEEE Transactions on Neural Systems and Rehabilitation Engineering. 2013;21:96–103. doi: 10.1109/TNSRE.2012.2218832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Zhou P, Aruin AS. Teager-Kaiser energy operation of surface EMG improves muscle activity onset detection. Annals of Biomedical Engineering. 2007;35:1532–8. doi: 10.1007/s10439-007-9320-z. [DOI] [PubMed] [Google Scholar]
- 10.Lauer RT, Prosser LA. Use of the Teager-Kaiser Energy operator for muscle activity detection in children. Annals of Biomedical Engineering. 2009;37:1584–93. doi: 10.1007/s10439-009-9727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solnik S, Rider P, Steinweg K, DeVita P, Hortobagyi T. Teager-Kaiser energy operator signal conditioning improves EMG onset detection. European Journal of Applied Physiology. 2010;110:489–98. doi: 10.1007/s00421-010-1521-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malone A, Meldrum D, Gleeson J, Bolger C. Reliability of surface electromyography timing parameters in gait in cervical spondylotic myelopathy. Journal of Electromyography and Kinesiology. 2011;21:1004–10. doi: 10.1016/j.jelekin.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Vannozzi G, Conforto S, D’Alessio T. Automatic detection of surface EMG activation timing using a wavelet transform based method. Journal of Electromyography and Kinesiology. 2010;20:767–72. doi: 10.1016/j.jelekin.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Bonato P, D’Alessio T, Knaflitz M. A statistical method for the measurement of muscle activation intervals from surface myoelectric signal during gait. IEEE Transactions on Biomedical Engineering. 1998;45:287–99. doi: 10.1109/10.661154. [DOI] [PubMed] [Google Scholar]
- 15.Santello M, McDonagh MJN. The control of timing and amplitude of EMG activity in landing movements in humans. Experimental Physiology. 1998;83:857–74. doi: 10.1113/expphysiol.1998.sp004165. [DOI] [PubMed] [Google Scholar]
- 16.Staude G, Wolf W. Objective motor response onset detection in surface myoelectric signals. Medical Engineering & Physics. 1999;21:449–67. doi: 10.1016/s1350-4533(99)00067-3. [DOI] [PubMed] [Google Scholar]
- 17.Staude GH. Precise onset detection of human motor responses using a whitening filter and the log-likelihood-ratio test. IEEE Transactions on Biomedical Engineering. 2001;48:1292–305. doi: 10.1109/10.959325. [DOI] [PubMed] [Google Scholar]
- 18.Aschero G, Gizdulich P. Denoising of surface EMG with a modified Wiener filtering approach. Journal of Electromyography and Kinesiology. 2010;20:366–73. doi: 10.1016/j.jelekin.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Zhou P. Sample entropy analysis of surface EMG for improved muscle activity onset detection against spurious background spikes. Journal of Electromyography and Kinesiology. 2012;22:901–7. doi: 10.1016/j.jelekin.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]