Abstract
Objectives
Pediatric acute liver failure (PALF) is a rare, but serious event with poorly understood functional outcomes. The goal was to determine the prevalence of reduced neuropsychological (NP) functioning and health-related quality of life (HRQOL) following PALF.
Methods
This multi-center study examined NP functioning and HRQOL 1 - 6 (median=3.8) years after PALF. Participants age 6 - 16 (median=9.9) years were recruited from the PALF registry and administered measures of intelligence (IQ), visual spatial/visual motor coordination, attention, executive function (EF), depression, and adaptive skills. HRQOL and fatigue were assessed using the Pediatric Quality of Life Inventory 4.0 Generic Core Scales (PedsQL™4.0) and PedsQL™ Multidimensional Fatigue Scale.
Results
36 patients participated;50% were male and 67% were white. Median age at PALF was 5.6 years. A history of grade 3 or 4 hepatic encephalopathy was reported in 5/36 (14%) participants and 23/36 (64%) received a liver transplant. Visual spatial ability was significantly better than norms (p=0.009), but motor coordination was worse (p=0.04). Teachers (p=0.04 to p < 0.0001) and parents (p=0.005) reported more executive deficits versus norms, and participants had worse attention (p=0.02). Participants did not differ significantly from norms on IQ, depression, or adaptive functioning. All child self-report PedsQL™ Generic Core and Fatigue scales were significantly lower than a matched healthy sample (p=0.001 to p < 0.0001) and parent proxy-report was lower on the Fatigue scales (p=0.001 to p < 0.0001).
Conclusions
Long-term PALF survivors demonstrate average IQ and visual spatial ability, but greater than expected impairments in motor skills, attention, EF, HRQOL, and fatigue.
Keywords: Cognition disorders, executive functions, pediatric liver disease, pediatric liver transplantation, fatigue
Introduction
Pediatric acute liver failure (PALF) is a rare condition associated with spontaneous survival in approximately 50% of children1. Little is known about neuropsychological (NP) functioning or health-related quality of life (HRQOL) status among PALF survivors. History of central nervous system (CNS) insult such as hepatic encephalopathy (HE)and recovery from critical illness or liver transplantation (LT)2 may place children with PALF at risk for worse NP and HRQOL outcomes. However, these outcomes have not previously been studied in children with PALF.
PALF is often associated with HE, hyperammonemia, varying degrees of cytotoxic and vasogenic cerebral edema, and associated risk of intracranial hypertension and brain herniation 3–5. Increasingly, infection, inflammation, and oxidative stress are being recognized as modulators of the impact of hyperammonemia on the brain6,7. Even in the absence of clinically detectable cerebral edema, increased ammonia can lead to disruption of neurotransmitters, and impaired autoregulation of cerebral blood flow and metabolism 3.
A recent study of children with acute viral hepatitis found cerebral edema, increased ammonia, and serum pro-inflammatory cytokines in PALF as compared to controls8. Cognitive performance at 6 week follow-up was below controls, with some improvement at follow-up testing 5 months later. This study suggests the potential for prolonged, and perhaps incomplete, recovery of cognitive functioning following PALF. However, larger, more methodologically rigorous studies examining all types of PALF survivors are needed to provide a clearer picture of functional outcomes such as NP performance and HRQOL.
Only recently has multi-center collaboration provided the infrastructure necessary for a more thorough examination of functional outcomes of PALF. The Pediatric Acute Liver Failure Study Group is a multi-center study which was initiated in 1999, and currently has 12 participating sites and nearly 1,000 patients enrolled in the registry. The PALF registry collects detailed clinical and laboratory data daily until recovery, death, or LT. Using the infrastructure of the PALF registry for recruitment and clinical history, the current multi-center study examined the hypothesis that PALF survivors would evidence a higher prevalence of cognitive deficits, depressed mood, and adaptive skill deficits than expected compared to the normal population as well as lower HRQOL and more fatigue versus a matched healthy sample. We also explored the association between maximal level of HE and transplant status with cognitive outcomes, hypothesizing that higher HE and LT would impart greater risk for poor outcomes. This is the first multi-center study to examine NP functioning and HRQOL in PALF survivors.
Materials and Methods
Study Population
PALF registry participants must be under the age of 18, not known to have a chronic liver disease, have biochemical evidence of acute liver injury, and have a liver-based coagulopathy not corrected by parenteral vitamin K. In addition, registry participants must have liver synthetic failure with INR ≥ 1.5 with HE, or INR ≥ 2.0 without HE.
Eligible patients for the current study were drawn from the PALF registry and recruited at 12 medical centers in the U.S and Canada between June 2011 and August 2012.PALF registry participants age 6 years 0 months 0 days to 16 years 11 months 29 days, who had survived between 12 and 72 months following PALF enrollment were eligible. Patients who had received LT were eligible for this study one year after transplantation (if re-transplanted, at least 1 year from most recent transplant). Participants and parent(s)/guardian(s) were also required to be fluent in English. Exclusions included unstable medical status at the time of testing (i.e., hospitalization within 4 weeks prior to testing; awaiting LT; uncontrolled seizures) or medical factors that could independently impact functioning or invalidate testing (i.e., cancer diagnosis; weakness or abnormality of muscle tone or coordination, such as cerebral palsy, sufficiently severe that it impaired the ability to perform the physical tasks required for testing; no intelligible speech and/or inability to follow simple commands).
The Data Coordinating Center identified PALF registry participants meeting preliminary inclusion criteria. Study coordinators at each site then contacted eligible participants by telephone or at a scheduled clinic visit to determine their willingness to participate in the current study and to ensure they met full inclusion criteria. Participants were recruited, consented, and tested at the center where they received treatment for PALF. Written, informed consent was obtained from the child’s parent/legal guardian before participation in the study. Assent was obtained from children as required by individual institutions. The study protocol was approved by the institutional review boards at all PALF study sites.
Study Design
Participants underwent a neuropsychological assessment that included standardized measures of IQ, visual spatial/visual motor abilities, and attention. In addition, parent, teacher, and self-report surveys were administered to assess EF, mood, adaptive skills, HRQOL, and fatigue. Demographic and clinical data were retrieved following initial enrollment into the PALF registry. Additional information regarding demographics and medical history, current medical status, and variables related to LT was collected at the time of testing by review of medical records and parent survey.
Instruments and Testing Procedure
IQ
To assess general intellectual reasoning abilities, participants completed the Wechsler Intelligence Scales for Children-4th edition (WISC-IV)9.
Visual Spatial and Visual Motor Ability
Participants completed The Beery-Buktenica Developmental Test of Visual-Motor Integration, 6th Edition (VMI-6)10. The Beery VMI-6 is divided into 3 parts using the same abstract designs. Visual-Motor Integration is a subtest that involves both visual spatial and visual motor abilities to perceive the spatial relationships in designs and recreate them (copying line drawings).The Visual Perception subtest emphasizes visual spatial ability (without a significant motor response component) to quickly recognize small differences in similar designs (timed matching via multiple choice). Lastly, the Motor Coordination subtest also requires visual spatial ability, but emphasizes motor speed and precision (timed tracing of designs by staying “within the roads”.)
Attention and EF
Participants completed the Conners’ Continuous Performance Test II (CPT-II), a computerized test of sustained attention11 which involves responding to targets (all letters except “x”) by pressing the space bar as quickly as possible and inhibiting responses to infrequent distracters (“x”). Various aspects of performance are recorded including reaction time speed, variability, and standard error, number of omissions (missed targets), and commissions (erroneous responses to distracters).
The cognitive domain of EF is closely related to attention and involves the regulation and management of cognitive processing from moment to moment. EF encompasses many sub-components such as “working memory” (short term memory), multitasking, flexible thinking and problem-solving, cognitive efficiency, attention to detail, inhibition of unwanted responses, planning, and organization. Parents and teachers (primary teacher for elementary students or English/Language Arts teacher for middle and high school students) completed the Behavior Rating Inventory of Executive Function (BRIEF)12. This age-normed, standardized survey quantifies difficulties with EF in real life situations such as forgetting to hand in completed homework or complete multi-step directions, having difficulty with organization and planning, and losing track in conversation or task completion in comparison to same age peers. Participants age 11 and up also completed the BRIEF-Self Report (BRIEF-SR)13.
Mood and Adaptive Skills
The Children’s Depression Inventory, Second Edition (CDI-2)14, an age-normed, standardized survey assessing symptoms of depression , was completed by parents, as well as children who were at least 7 years of age. Simply worded, multiple-choice questions asked respondents to rate the presence/severity of various indicators of depressed mood such as difficulties with sleep, appetite, feelings of sadness, low self-esteem, hopelessness, and interpersonal difficulties.
Parents also completed the Adaptive Behavior Assessment System-Second Edition (ABAS-II)15. Adaptive skills, also referred to as “independent living skills”, are life tasks that can be completed with increasing degrees of independence as the child develops. On the ABAS-II, adaptive skills are assessed across a number of areas including communication, community functioning, social skills, self-care, and home living skills.
HRQOL and Fatigue
We administered the child self-report and parent proxy-report versions of the 23-item PedsQL™ 4.0 Generic Core Scales16 (Mapi Research Institute, Lyon, France) and the PedsQL™ Multidimensional Fatigue Scale (MFS).17,18 The MFS is an 18-item instrument encompassing 3 scales: (1) General Fatigue (6 items, e.g., “I feel tired.”; “I feel too tired to do things that I like to do.”), (2) Sleep/Rest Fatigue (6 items, e.g., “I feel tired when I wake up in the morning.”; “I rest a lot.”), and (3) Cognitive Fatigue (6 items, e.g., “It is hard for me to keep my attention on things.”; “It is hard for me to remember what people tell me”).
Statistical Analysis
To test the hypothesis that participants were representative of the broader PALF registry, we compared participants and eligible non-participants on select demographics and medical variables by Pearson Chi-square or Exact Pearson Chi-square test. Comparisons of cognitive, mood, and adaptive variables to the normal population were made using chi square test for goodness of fit due to non-normal distribution of the data. Variables were categorized by intervals of one standard deviation (SD). For the WISC-IV, VMI-6, and ABAS-II (mean=100, SD=15 for test norms), categories were: < 85, 85-100, 101-114, and ≥115. For the BRIEF, CPT-II, and CDI-2 (mean=50, SD=10 for test norms; higher scores are worse), categories were ≥60, 51-59, 40-50, and < 40.The Wilcox on rank sums test was used to test the hypothesis that the distribution of cognitive functioning differed by HE (0-1 vs. 2-4) and by history of LT.
To assess whether participants had worse HRQOL and symptoms of fatigue than average, independent-samples t tests were used to compare our sample to a sample of healthy children matched by age, gender and race/ethnicity on the PedsQL™ 4.0 Generic Core Scales and the PedsQL™ MFS. Effect sizes were calculated to determine the magnitude of the differences. Effect size as utilized in these analyses was calculated by taking the differences between the groups’ sample means, divided by the pooled standard deviation. Effect sizes for differences in means are designated as small (0.20), medium (0.50), and large (0.80) 19.
The overall Type 1 error rate was maintained at 0.05 by the Hochberg adjustment for multiple comparisons20. An adjustment was made separately for each instrument, and separately by respondent on survey measures. Statistical analyses were conducted using SAS Version 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
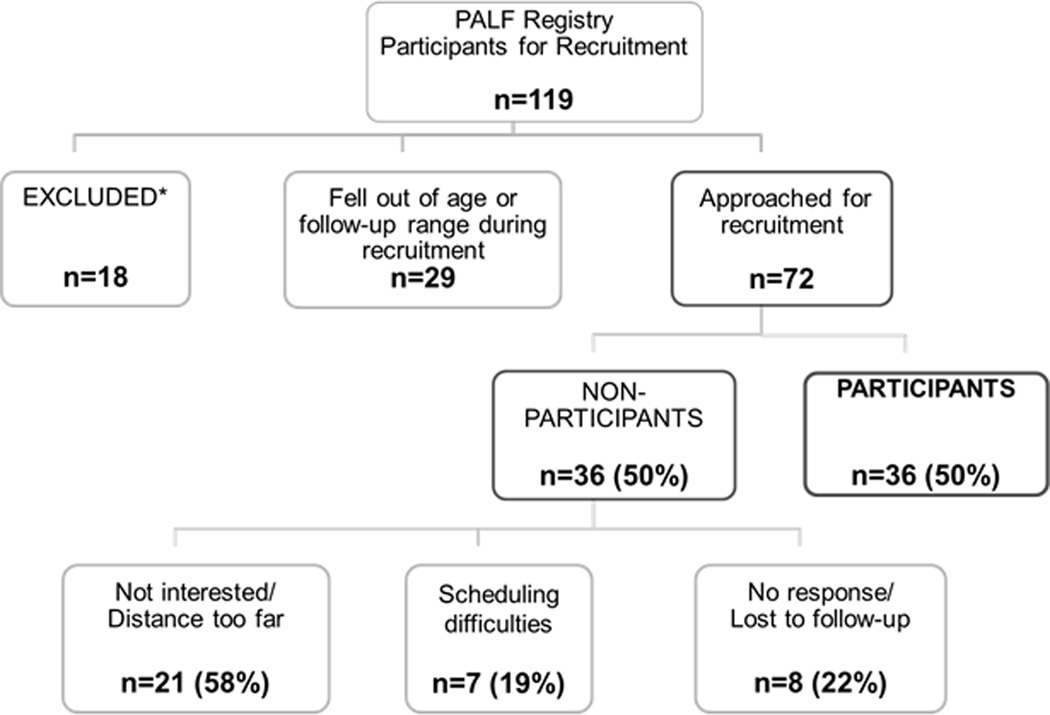
Of the 414 participants in the PALF registry at the time of this study, the Data Coordinating Center identified 119 who met basic inclusion criteria of age and time since PALF enrollment. Of these, 18 were excluded based on updated information provided by the site coordinators, and 29 fell out of the age or follow-up window during the recruitment period. Thus, 72 PALF registry participants were approached about the study, and 36 (50%) participated. Figure 1 details the recruitment algorithm and the reasons for non-participation.
Fig. 1.
Recruitment consort diagram and reasons for non-participation. *Of note, one child was excluded due to severe neurological abnormalities in motor skills and speech precluding the ability to engage in the testing process.
Missing data on the WISC-IV, VMI-6, CPT-II, CDI-2, and PedsQL™ child forms were due to two participants who were either unable to complete the tasks or their raw scores were too low to calculate standard scores. This was largely due to difficulties with comprehension/low functioning. The BRIEF was completed by 36 parents (0 missing), 32 teachers (2 not returned, 1 child home schooled, 1 child not in school yet), and 12 participants who were at least 11 years old (0 missing). The CDI-2 Parent was completed for 24 children age 7 and over (0 missing).The ABAS-2 was completed for all participants, but parents of 3 left too many blank responses to calculate the General Adaptive Composite score.
Demographic and Medical Characteristics
Participants and eligible non-participants did not differ significantly with respect to age at PALF diagnosis, gender, ethnicity, race, maximal HE, transplantation, or final diagnosis. Table 1 provides descriptive statistics for participants on select medical and demographic variables. The final diagnosis was indeterminate for 47% (n=17). A maximal HE level of grade 3 or 4 was documented in 14% of the sample (n=5) within the first 30 days of PALF registry enrollment. Of those who spontaneously recovered, 77% (10/13) had maximal HE of 0 or 1, and 1 each had HE of 2, 3, and 4. In contrast, only 39% (9/23) of transplanted patients had a maximal HE of 0 (n=4) or 1 (n=5); 48% (11/23) had HE of 2, 9% (2/23) had HE of 3, and 1 patient had HE of 4.
Table 1.
Participant Demographic and Medical Variables
| Total (n = 36) | ||
|---|---|---|
| n | % | |
| Male | 18 | 50.0 |
| Hispanic | 5 | 13.9 |
| Race | ||
| Caucasian | 24 | 66.7 |
| African-American | 4 | 11.1 |
| Other or missing (n = 1) | 8 | 22.2 |
| Maternal Education | ||
| Some High School or less | 2 | 5.6 |
| High School diploma/GED | 6 | 16.7 |
| Some College or more | 28 | 77.7 |
| Mother’s Household Status | ||
| Two person household | 27 | 75.0 |
| One person household | 9 | 25.0 |
| Current Insurance | ||
| Private Insurance | 21 | 58.3 |
| Medicare/Medicaid/Provincial | 10 | 27.8 |
| Other | 5 | 13.9 |
| Final Diagnosis | ||
| Acetaminophen Toxicity | 3 | 8.3 |
| Autoimmune | 4 | 11.1 |
| Metabolic* | 4 | 11.1 |
| Viral Infection† | 3 | 8.3 |
| Indeterminate | 17 | 47.2 |
| Other | 5 | 13.9 |
| Maximal Hepatic Encephalopathy (in first 30 days of PALF registry enrollment) | ||
| 0 | 11 | 30.6 |
| 1 | 8 | 22.2 |
| 2 | 12 | 33.3 |
| 3 | 3 | 8.3 |
| 4 | 2 | 5.6 |
| Liver Transplant | ||
| Yes | 23 | 63.9 |
| No | 13 | 36.1 |
| Median | Range | |
| Age at PALF enrollment (years) | 5.6 | 0.1–15.4 |
| Interval from PALF to Testing (years) | 3.8 | 1.3–6.0 |
| Age at Testing (years) | 9.9 | 6.0–16.6 |
| Hospital days in past 12 months | 0 | 0–17 |
Wilson’s disease (n= 3); Other metabolic disease (n = 1)
Other viral hepatitis (n = 3) [not A, B, C, E, EBV, CMV, or Herpes Simplex]
Medical record review indicated 64% of participants (n=23)had received LT; all transplants occurred between 2006 – 2010.The majority were split (35%, n=8), followed by reduced (26%, n=6), living related (22%, n=5), and whole (17%, n=4).At the time of transplant, 61% of liver recipients (n=14) were intubated, and recipients spent a median of 20 (range = 6 – 44) days hospitalized from transplant to discharge. At the time of testing, 91% (n=21) were taking Tacrolimus, 9% (n=2) steroids, and 1 was taking seizure medication.
Of note, 1 participant who received a liver transplant and 1 participant who spontaneously recovered underwent continuous veno-venous hemofiltration (CVVH) within the first 30 days. Also, 1 LT recipient required dialysis within the first 30 days. At the time of testing, all participants had normal liver function and no evidence of portal hypertension. None experienced cardiac arrest in the first 30 days of PALF registry enrollment.
Regarding language use in the home, 72% of parents (n=26) reported speaking English “all of the time”, 17% (n=6) “most of the time”, and 2 families each reported “about half of the time” and “sometimes”. None of the participants had a hearing impairment requiring hearing aids. Of note, two participants had seizures by parent report.
IQ and Visual Spatial /Visual Motor Ability
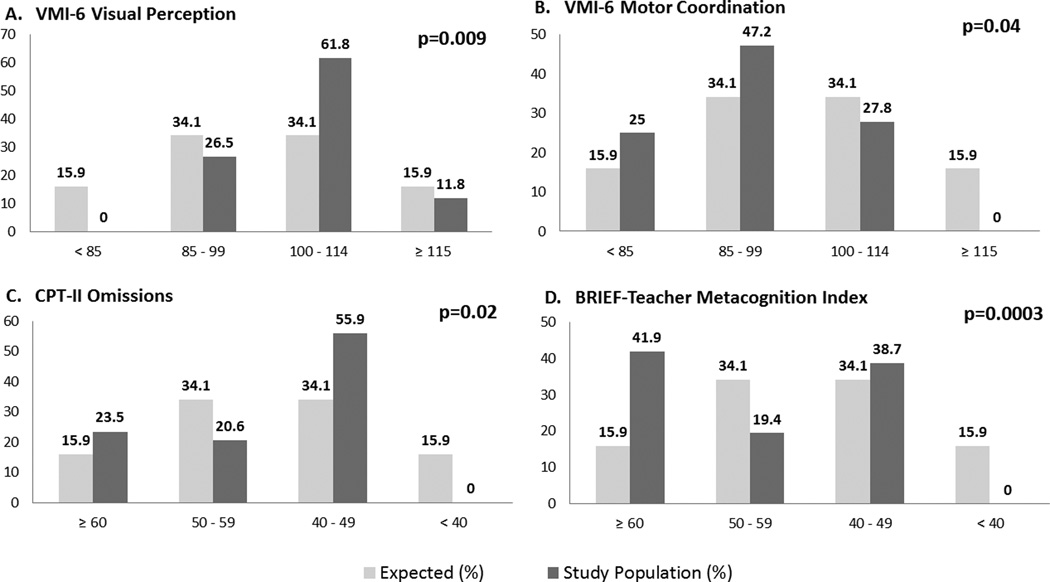
Compared to test norms, the distribution of participant scores was shifted towards the right (better performance) for the VMI-6 Visual Perception subscale (p=0.009), but towards the left (worse performance) for the Motor Coordination subscale (p=0.04). This indicates that more participants than expected have average or better visual spatial ability, but worse motor skills than in the normal population. Figure 2 depicts the observed versus expected distribution of scores. The distribution of scores did not differ from test norms on the VMI-6 Visual Motor Integration subscale or the WISC-IV composites (IQ). Means are reported in Table 2.
Fig. 2.
Comparison of observed (study population) to expected (normal population) distribution for select variables. A) More participants scored average or better than expected. B) More participants scored average or worse than expected. C) More participants scored average or worse than expected. D) More participants scored worse than expected. Note that C) and D) are T scores, with higher scores representing worse performance, while A) and B) are standard scores, with lower scores representing worse performance.
Table 2.
Neuropsychological Performance of Participants Compared to Test Norms.
| PALF STUDY SAMPLE | ||||
|---|---|---|---|---|
| N | Mean | SD | Adjusted significance level* |
|
| WISC-IV↑ | ||||
| Full Scale IQ (FSIQ) | 34 | 101.3 | 9.9 | NS |
| Verbal Comprehension (VC) | 34 | 100.9 | 10.6 | NS |
| Perceptual Reasoning (PR) | 35 | 103.2 | 14.9 | NS |
| Working Memory (WM) | 35 | 96.3 | 14.5 | NS |
| Processing Speed (PS) | 34 | 97.7 | 12.8 | NS |
| VMI-6 ↑ | ||||
| Visual Motor Integration | 36 | 90.9 | 18.5 | NS |
| Visual Perception | 34 | 104.8 | 9.0 | 0.009 |
| Motor Coordination | 35 | 91.2 | 14.5 | 0.04 |
| CPT-II ¥ | ||||
| Omission Errors | 34 | 52.5 | 9.2 | 0.02 |
| Commission Errors | 34 | 50.7 | 10.8 | NS |
| Hit Reaction Time (RT) | 34 | 53.5 | 11.9 | NS |
| RT Standard Error | 34 | 54.0 | 8.7 | NS |
| Variability | 34 | 54.1 | 8.7 | NS |
| CDI-2 Self Report – Total ¥ | 23 | 54.2 | 10.6 | NS |
| CDI-2 Parent Report - Total¥ | 24 | 50.6 | 10.1 | NS |
| ABAS-II General Adaptive Composite ↑ | 33 | 97.1 | 17.2 | NS |
Chi-square Test for Goodness of Fit
Test norms: Mean=100±15
Test norms: Mean=50±10 – higher is worse
Wechsler Intelligence Scales for Children-4th edition (WISC-IV)
The Beery-Buktenica Developmental Test of Visual-Motor Integration, 6th Edition (VMI-6)
Conners’ Continuous Performance Test II (CPT-II)
Children’s Depression Inventory, Second Edition (CDI-2)
Adaptive Behavior Assessment System-Second Edition (ABAS-II)
Attention and EF
CPT-II
More participants had excessive Omission Errors on the CPT-II compared to norms (p=0.02), indicating difficulties with sustained attention. However, participants did not differ significantly from norms on the remainder of the CPT-II (Figure 2 and Table 2).
Parent BRIEF
There were no significant differences between the study sample and the test norms for the parent BRIEF composite scores. However, participants did demonstrate a higher proportion of elevated concerns on the Inhibit subscale compared to test norms (p=0.005) (Table 3). This reflects greater difficulties with inhibiting inappropriate behaviors, as reported by parents.
Table 3.
Behavior Rating Inventory of Executive Function (BRIEF) Composite and Subscales Scores for Parent, Teacher, and Self-Report.
| BRIEF Composite and Subscales¥ |
PALF STUDY SAMPLE | |||
|---|---|---|---|---|
| N | Mean | SD | Adjusted significance level* |
|
| BRIEF – Parent Report | ||||
| Global Executive Composite | 36 | 51.4 | 12.0 | NS |
| Behavioral Regulation Index | 36 | 51.3 | 12.2 | NS |
| Inhibit | 36 | 50.9 | 12.4 | 0.005 |
| Shift | 36 | 51.6 | 11.2 | NS |
| Emotional Control | 36 | 51.2 | 11.5 | NS |
| Metacognition Index | 36 | 51.2 | 11.8 | NS |
| Initiate | 36 | 49.8 | 10.3 | NS |
| Working Memory | 36 | 52.8 | 13.0 | NS |
| Plan/Organize | 36 | 52.3 | 11.7 | NS |
| Organization Of Materials | 36 | 50.1 | 10.7 | NS |
| Monitor | 36 | 50.1 | 13.0 | NS |
| BRIEF – Teacher Report | ||||
| Global Executive Composite | 31 | 56.7 | 13.9 | 0.001 |
| Behavioral Regulation Index | 32 | 55.0 | 13.7 | NS |
| Inhibit | 32 | 51.3 | 10.8 | 0.04 |
| Shift | 32 | 55.7 | 14.7 | NS |
| Emotional Control | 32 | 53.0 | 14.0 | NS |
| Metacognition Index | 31 | 57.4 | 14.0 | 0.0003 |
| Initiate | 32 | 52.1 | 10.0 | <0.0001 |
| Working Memory | 32 | 52.8 | 8.8 | 0.002 |
| Plan/Organize | 31 | 51.6 | 7.4 | 0.0002 |
| Organization Of Materials | 31 | 49.8 | 11.2 | 0.01 |
| Monitor | 31 | 53.8 | 8.8 | NS |
| BRIEF – Self-Report | ||||
| Global Executive Composite | 12 | 53.2 | 11.1 | NS |
| Behavioral Regulation Index | 12 | 53.7 | 13.8 | NS |
| Inhibit | 12 | 51.3 | 10.8 | NS |
| Shift | 12 | 55.7 | 14.7 | NS |
| Emotional Control | 12 | 53.0 | 14.0 | NS |
| Monitor | 12 | 53.8 | 10.0 | NS |
| Metacognition Index | 12 | 52.1 | 8.6 | NS |
| Working Memory | 12 | 52.8 | 8.8 | NS |
| Plan/Organize | 12 | 51.6 | 7.4 | NS |
| Organization Of Materials | 12 | 49.8 | 11.2 | NS |
| Task Completion | 12 | 53.8 | 8.8 | NS |
Chi-square Test for Goodness of Fit
Test norms: Mean=50±10 – higher is worse
Note: BRIEF-Teacher Report study population is affected by 1 report where missing responses resulted in some incomplete scale calculations.
Teacher BRIEF
Teachers reported significantly more participants than expected with elevated EF concerns (Meta cognition Index (MI); p=0.0003) (Figure 2 and Table 3). In addition, a higher proportion of participants compared to test norms was reported to have EF problems on the Global Executive Composite (p=0.001) and several subscales: Inhibit (p=0.04), Initiate (<0.0001), Working Memory (0.002), Plan/Organize (0.0002), and Organization of Materials (0.01) (Table 3). These subscales are primarily reflected on the MI, and indicate more problems than expected with inhibiting behaviors, getting started on tasks, utilizing working memory to briefly hold information in short term memory in order to manipulate it, planning and organization of information, materials, and task completion. BRIEF scores ≥ 65 indicate a “clinically significant” level of concerns12. In our sample 26% (8/31)had a BRIEF Teacher MI score at or above 65, well above the expected rate of 7% in the normal population (p=0.0007).
Self-report BRIEF
There were no significant differences between the PALF study sample and the normative population on the BRIEF self-report composite or subscale scores (Table 3).
Mood and Adaptive Skills
The distribution of scores for parent and self-report on the CDI-2 Total, as well as parent report on the ABAS-II General Adaptive Composite did not differ significantly from test norms (see Table 2). This indicates that our sample did not have elevated rates of depressed mood or adaptive skill deficits compared to the normal population.
HRQOL and Fatigue
Table 4 presents the means and standard deviations of the PedsQL™ 4.0 Generic Core Scales and the PedsQL™ MFS for the study population and the sample of matched healthy children. Participants self-reported generic HRQOL and fatigue that were significantly worse than the normative sample for all scales. Large effect sizes were seen across both measures, with the largest effect sizes found in Cognitive Fatigue (1.46) and Total Fatigue (1.44).
Table 4.
PedsQL™ 4.0 Generic Core Scales and Multidimensional Fatigue Scale Comparisons to a Healthy Sample Matched for Age, Gender, and Race/Ethnicity
| PALF Study Sample | Healthy | Adjusted significance level |
Effect Size |
|||||
|---|---|---|---|---|---|---|---|---|
| Scale | n | Mean | SD | n | Mean | SD | ||
| Child Self-Report Generic | ||||||||
| Total Score | 34 | 72.19 | 14.48 | 796 | 84.25 | 12.42 | <0.0001 | 0.96 |
| Physical Health | 34 | 77.11 | 13.50 | 795 | 88.93 | 12.40 | <0.0001 | 0.95 |
| Psychosocial Health | 34 | 69.61 | 16.87 | 794 | 81.75 | 14.17 | <0.0001 | 0.85 |
| Emotional Functioning | 34 | 68.82 | 23.49 | 796 | 79.69 | 18.41 | 0.001 | 0.58 |
| Social Functioning | 34 | 74.41 | 20.70 | 794 | 85.12 | 16.79 | 0.0008 | 0.63 |
| School Functioning | 34 | 65.59 | 20.77 | 786 | 80.35 | 16.65 | <0.0001 | 0.88 |
| Child Self-Report Fatigue | ||||||||
| Total Fatigue | 34 | 63.04 | 16.19 | 147 | 82.43 | 12.72 | <0.0001 | 1.44 |
| General Fatigue | 34 | 66.18 | 23.32 | 147 | 86.73 | 13.79 | <0.0001 | 1.29 |
| Sleep Fatigue | 34 | 65.07 | 18.35 | 147 | 77.49 | 16.43 | 0.0002 | 0.74 |
| Cognitive Fatigue | 34 | 57.87 | 21.68 | 147 | 83.07 | 16.10 | <0.0001 | 1.46 |
| Parent Report Generic | ||||||||
| Total Score | 36 | 78.59 | 17.48 | 998 | 83.77 | 13.94 | NS | 0.37 |
| Physical Health | 36 | 80.64 | 19.30 | 999 | 87.26 | 16.77 | NS | 0.39 |
| Psychosocial Health | 36 | 77.50 | 19.08 | 999 | 81.83 | 14.62 | NS | 0.29 |
| Emotional Functioning | 36 | 76.94 | 17.41 | 998 | 80.80 | 16.81 | NS | 0.23 |
| Social Functioning | 36 | 82.08 | 22.44 | 996 | 85.35 | 17.74 | NS | 0.18 |
| School Functioning | 36 | 73.47 | 22.77 | 966 | 79.15 | 18.26 | NS | 0.31 |
| Parent Report Fatigue | ||||||||
| Total Fatigue | 36 | 78.74 | 16.66 | 192 | 89.16 | 10.69 | <0.0001 | 0.88 |
| General Fatigue | 36 | 80.21 | 17.06 | 192 | 89.43 | 12.33 | 0.0003 | 0.70 |
| Sleep Fatigue | 36 | 80.21 | 17.00 | 192 | 88.64 | 13.09 | 0.001 | 0.61 |
| Cognitive Fatigue | 36 | 75.81 | 23.92 | 192 | 89.41 | 14.38 | <0.0001 | 0.84 |
Higher scores represent better HRQOL or lower fatigue. Effect sizes are designated as small (0.20), medium (0.50), and large (0.80). SD equals standard deviation.
Parentproxy-report was not significantly different from the normative sample on the PedsQL™ 4.0Generic Core Scales. However, all scales on the parentproxy-report PedsQL™ MFS were significantly worse than the normative sample, with effect sizes in the medium to large range. Consistent with child self-report, the largest effect sizes were for Cognitive Fatigue (0.84) and Total Fatigue (0.88).
Relationship to Medical Factors
Cognitive Functioning and Level of Hepatic Encephalopathy
Participants were divided into two groups: HE=0-1 (n=19) and HE=2-4 (n=17). When the groups were compared across the cognitive variables (WISC-IV, VMI-6, CPT-II, parent and teacher BRIEF composites), no significant differences emerged.
Cognitive Functioning and Transplant Status
We also compared participants who survived spontaneously (n=13) with those who received LT (n=23) on the same cognitive variables. There were no statistically significant differences between the groups on any of these measures.
Discussion
This is the only study of NP and HRQOL outcomes in PALF survivors, apart from the few studies that have examined cognitive functioning in specific disease groups such as acute viral hepatitis8, Wilson’s Disease21, and Reye Syndrome22. Prior studies had significant limitations such as small sample size, single center design, a narrowly defined patient group, and lack of commonly used cognitive tools. Most importantly, prior studies lack a big picture perspective on PALF as an entity. Using a multi-center design and the infrastructure of the PALF registry, we provide the most detailed description of NP and HRQOL outcomes following PALF thus far, as well as an exploratory look at risk factors.
The current findings indicate that 1 to 6 years following PALF, many survivors are doing well cognitively. Participants did not differ from the normal population in IQ, unlike non-PALF LT recipients2, and performed better than expected in terms of visual spatial processing (recognizing and matching designs without a motor component). However, as predicted, the distribution of participant scores was shifted downward compared to the normal population in motor coordination (tracing designs with speed and precision) as well as attention and EF. Participants also demonstrated significantly lower generic HRQOL by child self-report, and fatigue by both participant self-report and parent proxy-report, compared to a healthy matched sample.
Whereas a greater number of PALF survivors performed better than expected on simple visual spatial processing without motor demands, the reverse was true when motor coordination was emphasized. While the Visual Motor Integration subtest of the VMI-6 (copying designs) was not significantly different from test norms, this task is untimed, and may not have been sensitive enough to detect the motor difficulties seen in this population. The stronger performance compared to norms on the visual spatial matching task may reflect, in part, the generally high socioeconomic status (SES) of our sample, with over three quarters reporting maternal education levels of some college or greater. This makes the finding of more patients with below average functioning on the Motor Coordination subtest even more noteworthy in comparison. Deficits in motor speed and control are hallmarks of HE 23. Further, HE has been associated with disruption of basal ganglia networks, which are involved in motor control 24. Motor slowing and basal ganglia abnormalities have also been seen in Wilson’s Disease 21.
On a computerized test of sustained attention, we found one indicator of increased prevalence of difficulties with concentration. More participants than expected had excessive omission errors (missed more targets) relative to test norms. This provides relatively subtle evidence of problems with sustained attention given that other variables on the CPT-II were not significantly abnormal (commission errors, RT, RT standard error, and variability).
In the related area of EF, parents reported more concerns regarding inhibition than expected compared to the normal population, suggesting difficulties with behavioral dysregulation and impulsivity (e.g., blurting out answers, engaging in silly, off task behaviors). Even more striking, teachers confirmed a higher prevalence of concerns regarding inhibition, but also endorsed greater concerns than expected in several meta cognitive skills, including difficulty initiating tasks, utilizing working memory (short term memory) to keep track of information briefly, planning and organizing information, materials, and time. A quarter of our sample, more than 3 times as many as expected, had clinically elevated MI score son the BRIEF by teacher report. Teachers have a unique opportunity to observe students in relation to their peers and in the context of arguably greater EF requirements than in the home setting, making EF deficits even more evident. Participants did not indicate greater concerns about EF by self-report. However, only 12 participants were old enough (age 11 and up) to complete this survey. Attention and EF are particularly vulnerable to brain insult generally 25,26, and symptoms of confusion and poor concentration are hallmarks of HE23. EF deficits have been reported in children with extra hepatic portal vein thrombosis and evidence of portal-systemic encephalopathy 27, hepatitis C 28, and following pediatric LT for chronic liver disease2.
In our sample, PALF survivors reported significantly diminished generic HRQOL compared to a matched healthy sample, and both parents and participants reported greater levels of fatigue, especially cognitive fatigue, than a matched healthy sample. Reduced HRQOL compared to healthy controls has been reported in many pediatric populations with acute or chronic illness, including pediatric LT 29,30. In a sample of 1000 pediatric LT recipients which primarily included patients transplanted for chronic liver disease indications, HRQOL was significantly below average, especially in school function and cognitive fatigue 29. As in the current study, variability between participant self-report and parentproxy-report data has been found previously in multiple prior studies using the PedsQL™, as well as other instruments that include both self and proxy report, in a variety of populations.31, 32 This suggests that patient self-report and parent proxy-report of HRQOL are not equivalent, especially for less observable or internal symptoms, such as fatigue and emotional distress. While patient self-report is of primary interest, these two perspectives are complimentary, and both should be assessed to provide the most complete picture possible.33
The concept of cognitive fatigue is particularly noteworthy, since it is found to correlate with EF deficits and particularly working memory on the BRIEF34,35. Higher levels of cognitive fatigue relative to healthy controls on the PedsQL™ MFS have also been reported in children with cancer17 and traumatic brain injury 34,35, consistent with attention and EF deficits in these populations.
Although participants reported significantly more psychosocial concerns on the PedsQL™ 4.0 compared to healthy peers, including emotional distress, neither children nor parents reported greater than expected depressive symptoms on the CDI-2. These two instruments measure somewhat different constructs; the CDI-2 is specifically focused on mood disorder symptoms, while the PedsQL™ Emotional Functioning Scale measures subclinical levels of overall emotional distress. The current findings therefore suggest that while some subtle emotional distress may be present following PALF, most survivors do not demonstrate mood disorders in the long-term. This is certainly encouraging, considering PALF survivors’ experience of an unexpected life-threatening illness. Likewise, parents reported age-appropriate adaptive skills overall compared to the normal population, consistent with the finding of average IQ in our sample.
A final goal of the study was to explore the relationship of potential risk factors to cognitive outcomes. Given the small sample size, we elected to perform univariate analysis on two factors, HE and transplant status. Neither was found to be significant in the current study. Maximal level of HE was recorded within the first 30 days following enrollment in the PALF registry, and was retrospectively obtained for this study by careful medical record review. However, it is possible that data was incomplete, or affected by more subjective determination of HE in very young patients. We also did not find differences in cognitive functioning between patients who had recovered spontaneously versus those who received LT. However, about two thirds of our sample were transplant recipients, who were more easily recruited given their ongoing relationship with the transplant centers. Selection bias is also possible in this small sample despite the lack of significant differences between participants and eligible non-participants.
Other factors may also play an important role in cognitive outcomes. The impact of inflammatory response, oxidative stress, and disturbances in neurotransmitter function and auto regulation of cerebral blood flow/metabolism during ALF is becoming increasingly clear6,7,36. A recent study in pediatric acute viral hepatitis patients, while very small, is consistent with adult literature in identifying cognitive deficits tied to indicators of cerebral edema, pro-inflammatory cytokines, and ammonia levels8. More detailed analysis of medical and demographic factors (such as SES)in a larger, longitudinally assessed cohort will be a priority to tease apart several potential contributors to cognitive outcomes in PALF.
Several limitations must be considered when interpreting the current findings. Our sample is relatively small but heterogeneous in terms of PALF presentation, and therefore our findings need to be replicated in a larger study. We examined long-term outcomes using a cross-sectional design and cannot speak to the longitudinal nature of NP functioning and HRQOL in PALF. Our ability to tease apart predictors of cognitive outcomes was limited by sample size, and due to retrospective study design, we may have missed the opportunity to capture some important variables. Further, given the high proportion of LT recipients in this sample, it is not clear whether the findings are more representative of PALF survivors with LT, or whether the findings truly do characterize PALF survivors as a whole. Additionally, the BRIEF self-report was completed by a small number of participants due to the age requirement, and therefore results of this measure are limited.
Despite these limitations, this study made use of the unique infrastructure of the PALF registry and multi-center collaboration to obtain the only sample of PALF survivors focusing on cognitive and HRQOL outcomes to date. Notwithstanding the rarity of this devastating disease and high mortality, we amassed a sample of 36 PALF survivors. The current study is an important first step in characterizing functional outcomes in children with PALF. We have demonstrated apparently circumscribed concerns in areas of attention and EF, motor coordination, as well as HRQOL and fatigue (especially cognitive fatigue). In contrast, IQ and visual spatial processing without motor demands appear to be largely intact in this group. However, additional data is clearly needed to more fully characterize functional outcomes and associated predictors in this rare group of patients in order to inform clinical care, minimize modifiable risks, and support patients/families through this challenging experience. NP functioning and HRQOL are currently being examined in a much larger, prospective, multi-center study through the PALF study group. Participants are assessed and extensive medical data are collected at 6 and 12 months following presentation with PALF, which will allow a more thorough investigation of NP and HRQOL outcomes and their predictors.
Supplementary Material
Acknowledgments
Source of Funding: This study was supported by 3U01DK072146-05S2of the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. The sponsoring agency was not involved in the collection, analysis, or interpretation of data or the generation of the report. L.G.S. wrote the first draft of the manuscript and received salary support through the grant mechanism, but no honorarium or other form of payment was given. J.W.V. holds the copyright and trademark for the PedsQL™ and receives financial compensation from the Mapi Research Trust, a nonprofit research institute that charges distribution fees to for-profit companies that use the Pediatric Quality of Life Inventory™.
Footnotes
Conflicts of Interest: The remaining authors declare no financial relationships. The authors declare no conflict of interest.
References
- 1.Squires RH, Jr, Shneider BL, Bucuvalas J, et al. Acute liver failure in children: The first 348 patients in the Pediatric Acute Liver Failure Study Group. J Pediatr. 2006;148:652–658. doi: 10.1016/j.jpeds.2005.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorensen LG, Neighbors K, Martz K, et al. Cognitive and academic outcomes after pediatric liver transplantation: Functional Outcomes Group (FOG) results. Am J Transplant. 2011;11:303–311. doi: 10.1111/j.1600-6143.2010.03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bemeur C, Butterworth RF. Liver-brain proinflammatory signalling in acute liver failure: role in the pathogenesis of hepatic encephalopathy and brain edema. Metab Brain Dis. 2013;28:145–150. doi: 10.1007/s11011-012-9361-3. [DOI] [PubMed] [Google Scholar]
- 4.Dhawan A. Etiology and prognosis of acute liver failure in children. Liver Transpl. 2008;14(Suppl 2):S80–S84. doi: 10.1002/lt.21641. [DOI] [PubMed] [Google Scholar]
- 5.Squires RH., Jr Acute liver failure in children. Semin Liver Dis. 2008;28:153–166. doi: 10.1055/s-2008-1073115. [DOI] [PubMed] [Google Scholar]
- 6.Coltart I, Tranah TH, Shawcross DL. Inflammation and hepatic encephalopathy. Arch Biochem Biophys. 2013;536:189–196. doi: 10.1016/j.abb.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Seyan AS, Hughes RD, Shawcross DL. Changing face of hepatic encephalopathy: role of inflammation and oxidative stress. World J Gastroenterol. 2010;16:3347–3357. doi: 10.3748/wjg.v16.i27.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava A, Yadav SK, Borkar VV, et al. Serial evaluation of children with ALF with advanced MRI, serum proinflammatory cytokines, thiamine, and cognition assessment. J Pediatr Gastroenterol Nutr. 2012;55:580–586. doi: 10.1097/MPG.0b013e31825f4c3e. [DOI] [PubMed] [Google Scholar]
- 9.Wechsler D. Wechsler Intelligence Scale for Children - Fourth Edition (WISC-IV) San Antonio: The Psychological Corporation; 2003. [Google Scholar]
- 10.Beery KE, Buktenica NA, Beery NA. Beery-Buktenica Developmental Test of Visual-Motor Integration, Sixth Edition (Beery VMI) San Antonio: Pearson; 2010. [Google Scholar]
- 11.Conners KC. Conners' Continuous Performance Test II Version 5: Multi-Health Systems. 2004 [Google Scholar]
- 12.Gioia GA, Isquith PK, Guy SC, et al. Behavior Rating Inventory of Executive Function. Odessa, FL: Psychological Assessment Services, Inc.; 2000. [Google Scholar]
- 13.Guy SC, Isquith PK, Gioia GA. Behavior Rating Inventory of Executive Function-Self Report Version (BRIEF-SR) Odessa, FL: Psychological Assessment Resources, Inc.; 2004. [Google Scholar]
- 14.Kovacs M. MHS Staff. Children's Depression Inventory 2nd Edition (CDI-2): Multi-Health Systems. 2011 [Google Scholar]
- 15.Harrison P, Oakland T. Adaptive Behavior Assessment System - Second Edition (ABAS-II) Pearson; 2003. [Google Scholar]
- 16.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Varni JW, Burwinkle TM, Katz ER, et al. The PedsQL™ in Pediatric Cancer: Reliability and Validity of the Pediatric Quality of Life Inventory™ Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer. 2002;94:2090–2106. doi: 10.1002/cncr.10428. [DOI] [PubMed] [Google Scholar]
- 18.Panepinto JA, Torres S, Bendo CB, et al. PedsQL Multidimensional Fatigue Scale in sickle cell disease: feasibility, reliability, and validity. Pediatr Blood Cancer. 2014;61:171–177. doi: 10.1002/pbc.24776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 20.Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Statistics in Medicine. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- 21.Hegde S, Sinha S, Rao SL, et al. Cognitive profile and structural findings in Wilson's disease: A neuropsychological and MRI-based study. Neurol India. 2010;58:708–713. doi: 10.4103/0028-3886.72172. [DOI] [PubMed] [Google Scholar]
- 22.Meekin SL, Glasgow JFT, McCusker CG, et al. A long-term follow-up of cognitive, emotional, and behavioural sequelae to Reye syndrome. Dev Med Child Neurol. 1999;41:549–553. doi: 10.1017/s0012162299001164. [DOI] [PubMed] [Google Scholar]
- 23.Alonso EM, Squires RH, Whitington PF. Acute liver failure in children. In: Suchy FJ, Sokol RJ, Balistreri WF, editors. Liver Disease in Children. Third Edition. Cambridge: Cambridge University Press; 2007. pp. 71–96. [Google Scholar]
- 24.Qi R, Zhang LJ, Zhong J, et al. Altered effective connectivity network of the basal ganglia in low-grade hepatic encephalopathy: a resting-state fMRI study with Granger causality analysis. PLoS One. 2013;8:e53677. doi: 10.1371/journal.pone.0053677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ris MD, Abbey R. Pediatric Brain Tumors. In: Yeates KO, Ris MD, Taylor HG, et al., editors. Pediatric Neuropsychology: Research, Theory, and Practice. Second ed. New York: The Guilford Press; 2010. pp. 92–111. [Google Scholar]
- 26.Yeates KO. Traumatic Brain Injury. In: Yeates KO, Ris MD, Taylor HG, et al., editors. Pediatric Neuropsychology: Research, Theory, and Practice. Second. New York: The Guilford Press; 2010. pp. 112–146. [Google Scholar]
- 27.Mack CL, Zelko FA, Lokar J, et al. Surgically restoring portal blood flow to the liver in children with primary extrahepatic portal vein thrombosis improves fluid neurocognitive ability. Pediatrics. 2006;117:e405–e412. doi: 10.1542/peds.2005-1177. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigue JR, Balistreri W, Haber B, et al. Impact of hepatitis C virus infection on children and their caregivers: quality of life, cognitive, and emotional outcomes. J Pediatr Gastroenterol Nutr. 2009;48:341–347. doi: 10.1097/MPG.0b013e318185998f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alonso EM, Limbers CA, Neighbors K, et al. Cross-sectional analysis of health-related quality of life in pediatric liver transplant recipients. J Pediatr. 2010;156:270–276. doi: 10.1016/j.jpeds.2009.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Limbers CA, Neighbors K, Martz K, et al. Health-related quality of life in pediatric liver transplant recipients compared with other chronic disease groups. Pediatr Transplant. 2011;15:245–253. doi: 10.1111/j.1399-3046.2010.01453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Achenbach TM, McConaughy SH, Howell CT. Child/adolescent behavioral and emotional problems: implications of cross-informant correlations for situational specificity. Psychol Bull. 1987;101:213–232. [PubMed] [Google Scholar]
- 32.Sprangers MA, Aaronson NK. The role of health care providers and significant others in evaluating the quality of life of patients with chronic disease: a review. J Clin Epidemiol. 1992;45:743–760. doi: 10.1016/0895-4356(92)90052-o. [DOI] [PubMed] [Google Scholar]
- 33.Eiser C, Varni JW. Health-related quality of life and symptom reporting: similarities and differences between children and their parents. Eur J Pediatr. 2013;172:1299–1304. doi: 10.1007/s00431-013-2049-9. [DOI] [PubMed] [Google Scholar]
- 34.McCarthy ML, MacKenzie EJ, Durbin DR, et al. The Pediatric Quality of Life Inventory: an evaluation of its reliability and validity for children with traumatic brain injury. Arch Phys Med Rehabil. 2005;86:1901–1909. doi: 10.1016/j.apmr.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 35.Varni JW, Limbers CA, Sorensen LG, et al. PedsQL Cognitive Functioning Scale in pediatric liver transplant recipients: feasibility, reliability, and validity. Qual Life Res. 2011;20:913–921. doi: 10.1007/s11136-010-9823-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kappus MR, Bajaj JS. Covert hepatic encephalopathy: not as minimal as you might think. Clin Gastroenterol Hepatol. 2012;10:1208–1219. doi: 10.1016/j.cgh.2012.05.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.