Abstract
Although hypertension is common among American-style football players, the presence of concomitant vascular dysfunction has not previously been characterized. We sought to examine the impact of American-style football participation on arterial stiffness and to compare metrics of arterial function between collegiate American-style football participants and non-athletic collegiate controls. Newly matriculated collegiate athletes were studied longitudinally during a single season of American-style football participation and were then compared to healthy undergraduate controls. Arterial stiffness was characterized by use of applanation tonometry (SphygmoCor®). American-style football participants (N = 32, 18.4 ± 0.5 years old) were evenly comprised of Caucasians (N = 14, 44%) and African-Americans (N = 18, 56%). A single season of American-style football participation led to an increase in central aortic pulse pressure (27 ± 4 vs. 34 ± 8 mm Hg, P <0.001). Relative to controls (N = 47), pulse wave velocity was increased among ASF participants (5.6 ± 0.7 vs. 6.2 ± 0.9 m/s, P = 0.002). After adjusting for height, weight, body-mass index, systolic blood pressure, and diastolic blood pressure, American-style football participation was independently predictive of increased pulse wave velocity (β = 0.33, P = 0.04). In conclusion, American-style football participation leads to changes in central hemodynamics and increased arterial stiffness.
Keywords: arterial stiffness, hypertension, athlete
INTRODUCTION
American-style football (ASF) is the most popular high school team sport in the United States with more than 1 million annual participants.1,2 Hypertension is common among professional ASF athletes3 and recently, the development of hypertension and corollary concentric left ventricular hypertrophy has been prospectively documented during collegiate ASF participation.4 Hypertension is a well-established cardiovascular risk factor,5 and increased arterial stiffness serves as an important mechanistic precursor to the development of overt hypertension.6,7 While hypertension and other cardiovascular risk factors have been documented in ASF players,3,4,8,9 rigorous assessment of arterial function and its association with blood pressure has not been performed in this population. We hypothesized that collegiate ASF participants would demonstrate arterial stiffening during their initial season of collegiate ASF participation. To address this hypothesis, we conducted a prospective, longitudinal, case-controlled study to evaluate arterial elasticity and central blood pressure in collegiate ASF participants.
METHODS
ASF participants representing two National Collegiate Athletic Association Division I programs (Harvard University and Georgia Institute of Technology) were recruited for this study. Freshman ASF participants were recruited from the Harvard Athletic Initiative, an ongoing research program designed to address numerous issues relevant to athletic health and exercise physiology,4,10,11 and from a newly formed research initiative between Emory University and Georgia Tech. Anthropometric data, demographics, clinical characteristics, blood pressure, and indices of arterial function were assessed before and after their first competitive collegiate ASF season. An ethnically matched control group was similarly assessed. The Partners Human Research Committee and the Emory Institutional Review Board approved all aspects of the study before initiation, and all subjects provided written informed consent.
The pre-season was defined as the time of university matriculation. The post-season was defined as the immediate conclusion of the fall-season for each team involved. Anthropometric and clinical data collected at pre- and post-season study visits included age (years), height (cm), weight (kg), body-mass index (kg/m2), current medication use, personal/family history of hypertension,12 and pre-study period strength and endurance exercise volume (hours/week). Systolic blood pressure (SBP), diastolic blood pressure (DBP), and indices of arterial function, as measured by applanation tonometry, were assessed as described below. ASF participants and control subjects were required to abstain from exercise for ≥24 hours prior to data collection time points.
Exercise training volume data during the pre-study period, defined as the 8 weeks before baseline assessment, were collected. Training volumes during this pre-study period were characterized by total number of exercise hours per week dedicated to either endurance or strength activities. Endurance activity was defined as running, cycling, swimming, rowing, or use of an aerobic machine at an effort sustainable for ≥20 minutes. Strength activity was defined as weight lifting, plyometric exercise, and sprint running drills. Field position for each ASF participant was classified as either lineman (LM) or non-lineman (NLM) as previously proposed.13 Each ASF participant was subject to testing for performance enhancing drugs as dictated by NCAA standards.
A control cohort comprised of ethnically matched, but non-athletic, male undergraduate students from one undergraduate institution (Emory University) was recruited and studied in similar fashion. Recruitment was accomplished using study advertisement brochures that were distributed at various campus locations. The control cohort was studied at a single time point that occurred 3 months into the academic calendar to facilitate comparison with ASF participants at the post-season time point. This approach was chosen to ensure that potential determinants of blood pressure and vascular function inherent in both high school (i.e. pubertal development) and the initial collegiate experience (i.e, access to institutional dining services, changes in sleep patterns, etc.,) would be reflected in the control group data set.
Blood pressure was measured by one of the study physicians at all study time points using a manual aneroid sphygmomanometer and an appropriately sized cuff. The average of 3 measurements occurred with participants in a seated position after at least 10 minutes of rest. Blood pressure was classified in accordance with Joint National Commission-8 definitions as follows: optimal (SBP <120 mm Hg and DBP <80 mm Hg), prehypertensive (SBP = 120 to 139 mm Hg and/or DBP = 80 to 89 mm Hg), and stage 1 or greater hypertension (SBP ≥140 mm Hg and/or DBP ≥90 mm Hg).5
Indices of arterial stiffness were measured with participants in the supine position by two study investigators (J.H.K. and S.S.) using a high fidelity applanation tonometer (SphygmoCor®, Atcor Medical, Australia), which records sequential high-quality pressure waveforms at peripheral pulse sites. Full details of tonometer technology and measurement algorithms have been previously detailed.14 Vascular function was characterized using measurements of central aortic pulse pressure (CPP) and pulse wave velocity (PWV) as these are well validated surrogates of arterial stiffness and corollary cardiovascular disease risk.15–20 PWV, the gold standard index of arterial stiffness,14 was measured by acquisition of pressure waveforms within the carotid and femoral arteries and calculated using the “foot-to-foot” method.21 Adequate tonometric analysis was defined as PWA derivation > than 80% of the Operator Index and PWV with < than 10% standard deviation. Studies not meeting these criteria were excluded from the final analysis. Reproducibility studies in our laboratory on 9 subjects on consecutive days demonstrated a coefficient of variation of 3.8% for PWV.22
Categorical variables are presented as proportions and continuous variables mean ± standard deviation. The Shapiro-Wilk test was used to determine the normality of distribution. Categorical variables were compared by use of the Fisher exact test. Continuous variables were assessed with the Student paired t-test for normally distributed variables or the Mann-Whitney test for non-normally distributed variables. Linear regression analyses were used to identify factors associated with the post-season PWV. Univariate covariates tested included age, ASF participation, program affiliation, player position (NLM or LM), ethnicity, height, weight, body-mass index, pre-study weekly strength and endurance exercise volume, family history of hypertension, SBP, DBP, and heart rate. Univariate covariates with a P-value <0.10 were included in a multivariable linear regression analysis. Analyses were performed with SPSS software (version 21.0, SPSS Inc, Chicago, Illinois). A P-value of ≥0.05 was considered significant.
RESULTS
Of the 50 ASF participants recruited for this study (Harvard, N = 29; Georgia Tech, N = 21), 18 were excluded secondary to the lack of post-season data due to injury (N = 15; 11 from Harvard and 4 from Georgia Tech) or to inadequate tonometric analysis (N = 3; all Harvard). Thus, 32 ASF participants (15 from Harvard and 17 from Georgia Tech) were included in the final longitudinal analysis. No ASF participants crossed over from a LM to a NLM (or vice versa) field position during the study period. Three of 50 control subjects were excluded from analysis due to inadequate tonometric data yielding a final control cohort comprised of 47 subjects. Baseline characteristics of the ASF cohort are shown in Table 1. In the pre-season 9/32 (28%) ASF participants had pre-hypertension. The ASF cohort was evenly comprised of LM (N = 16) and NLM (N = 16). As anticipated, LM were taller (193 ± 4 vs. 184 ± 6 cm, P <0.001) and heavier (32.7 ± 4 vs. 27 ± 3 kg/m2, P <0.001) than NLM. No ASF participants were taking prescription medications at the time of college matriculation.
Table 1.
Longitudinal Profile of the American-Style Football Player Cohort
| Variable | Pre-Season ASF (N=32) | Post Season ASF (N=32) | P-Value |
|---|---|---|---|
| Age (years) | 18.4 ± 0.5 | - | - |
| Height (cm) | 189 ± 7 | - | - |
| Weight (kg) | 107 ± 21 | 108 ± 20 | 0.77 |
| Body-Mass Index (kg/m2) | 29.8 ± 4.6 | 30 ± 4.3 | 0.78 |
| Caucasian/African-American | 14 (44%)/18 (56%) | - | - |
| Hypertension | 0/32 (0%) | - | - |
| Endurance Exercise Volume (hours/week) | 4.1 ± 2.3 | - | - |
| Strength Exercise Volume (hours/week) | 5.8 ± 2.2 | - | - |
| Family History of Hypertension | 10/32 (31%) | - | - |
| Tobacco | 0/32 (0%) | - | - |
| Heart Rate (bpm) | 66 ± 12 | 63 ± 11 | 0.22 |
| Systolic Blood Pressure (mmHg) | 114 ± 13 | 123 ± 9 | <0.001 |
| Diastolic Blood Pressure (mmHg) | 65 ± 11 | 71 ± 9 | <0.001 |
| Central Aortic Pulse Pressure (mmHg) | 27 ± 4 | 34 ± 8 | <0.001 |
| Pulse Wave Velocity (m/sec) | 6.4 ± 1.1 | 6.2 ± 0.9 | 0.27 |
ASF: American-style football
Values expressed as the mean ± SD or n (%)
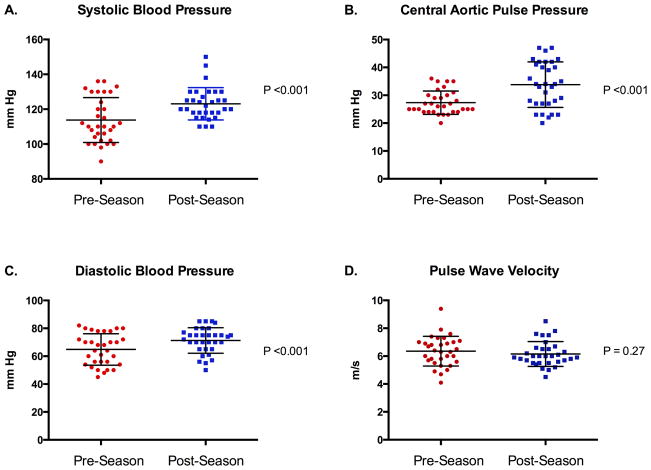
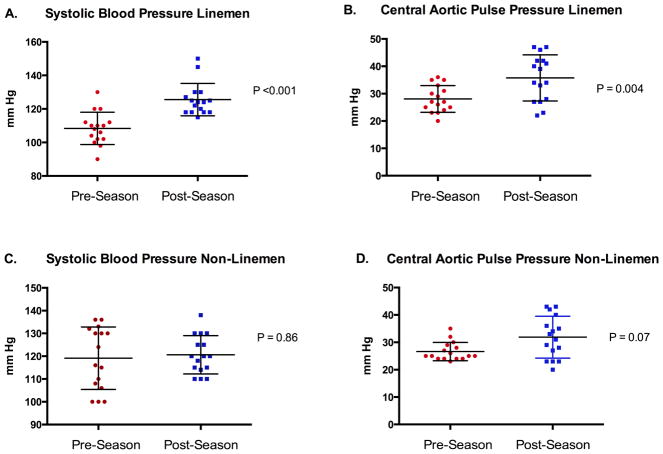
Metrics of blood pressure and arterial stiffness among ASF participants following completion of a single ASF season are shown in Table 1 and Figure 1. Following the completion of the ASF season, participants demonstrated significant increases in CPP, SBP and DBP with a resultant increase in the percentage of participants with either pre-hypertension or stage I hypertension [pre-season = 9/32 (28%) vs. post-season = 19/32 (59%), P = 0.02)]. Group mean changes in both peripheral blood pressure and CPP were driven by changes among ASF participants who played a LM field position (SBP = 108 ± 10 vs. 126 ± 10 mm Hg, P <0.001; CPP = 28 ± 5 vs. 36 ± 8 mm Hg, P = 0.004; Figure 2).
Figure 1. Longitudinal Changes in Blood Pressure, Central Aortic Pulse Pressure, and Pulse Wave Velocity in the American-Style Football Cohort.
Mean ± standard deviation (error bars) systolic blood pressure (Panel A), central aortic pulse pressure (Panel B), diastolic blood pressure (Panel C), and pulse wave velocity (Panel D).
Figure 2. Longitudinal Changes in Systolic Blood Pressure and Central Aortic Pulse Pressure in the Lineman and Non-Lineman Player Cohorts.
Mean ± standard deviation (error bars) lineman systolic blood pressure (Panel A), lineman central aortic pulse pressure (Panel B), non-lineman systolic blood pressure (Panel C), and non-lineman central aortic pulse pressure (Panel D).
ASF participants were younger, taller, heavier, and endorsed more baseline exercise volume than controls (Table 2). Although there were no significant differences in SBP and DBP between ASF participants and controls, ASF participants demonstrated significantly higher CPP and PWV. Notably, the relative increase in PWV among the ASF athletes compared to controls was present prior to and remained stable during the study period of collegiate ASF exposure.
Table 2.
Comparison of the Post-Season American-Style Football and Control Cohorts
| Variable | Post-Season ASF (N=32) | Control Population (N=47) | P-Value |
|---|---|---|---|
| Age (years) | 18.4 ± 0.5 | 19.1 ± 0.9 | <0.001 |
| Height (cm) | 189 ± 7 | 177 ± 7 | <0.001 |
| Weight (kg) | 108 ± 20 | 75 ± 13 | <0.001 |
| Body-Mass Index (kg/m2) | 30 ± 4.3 | 24 ± 4 | <0.001 |
| Caucasian | 14/32 (44%) | 23/47 (49%) | 0.82 |
| African-American | 18/32 (56%) | 24/47 (51%) | 0.82 |
| Hypertension | 0/32 (0%) | 0/47 (0%) | - |
| Tobacco | 0/32 (0%) | 0/47 (0%) | - |
| Family History of Hypertension | 10/32 (31%) | 15/47 (32%) | - |
| Heart Rate (bpm) | 63 ± 11 | 65 ± 12 | 0.47 |
| Systolic Blood Pressure (mmHg) | 123 ± 9 | 118 ± 13 | 0.06 |
| Diastolic Blood Pressure (mmHg) | 71 ± 9 | 72 ±11 | 0.78 |
| Central Aortic Pulse Pressure (mmHg) | 34 ± 8 | 29 ± 6 | 0.002 |
| Pulse Wave Velocity (m/sec) | 6.2 ± 0.9 | 5.6 ± 0.7 | 0.002 |
ASF: American-style football
Values expressed as the mean ± SD or n (%)
The results of univariable and multivariable linear regression analyses designed to determine predictors of PWV in the combined cohort of post-season ASF participants and controls are shown in Table 3. African-American ethnicity, participation in ASF, height, weight, body-mass index, SBP, and DBP were significant univariate predictors of PWV. In the multivariate model adjusting for the aforementioned variables, only ASF participation (β = 0.33, P = 0.04) retained independent association with post-season PWV.
Table 3.
Significant Univariate and Multivariate Correlates of Pulse Wave Velocity
| Variable | R | P-value* |
|---|---|---|
| Age (years) | 0.05 | 0.69 |
| ASF participation | 0.33 | 0.003† |
| Program Affiliation | 0.15 | 0.41 |
| Player Position | 0.10 | 0.58 |
| African-American Ethnicity | 0.25 | 0.03 |
| Height (cm) | 0.26 | 0.02 |
| Weight (kg) | 0.25 | 0.03 |
| Body-Mass Index (kg/m2) | 0.21 | 0.06 |
| Strength Training (hours/week) | 0.01 | 0.96 |
| Endurance Training (hours/week) | 0.01 | 0.96 |
| Family History of Hypertension | 0.04 | 0.76 |
| Systolic Blood Pressure (mmHg) | 0.33 | 0.003 |
| Diastolic Blood Pressure (mmHg) | 0.24 | 0.04 |
| Heart Rate (bpm) | 0.11 | 0.36 |
ASF: American-style football
Univariate P-values <0.10 were included in the multivariate analysis
P <0.05 in multivariate analysis
DISCUSSION
In this analysis of vascular function among freshman collegiate ASF participants and non-athletic controls, we observed two principal findings. First, participation in a single ASF season leads to increases in CPP, an index of central arterial pressure load that is also a validated marker of cardiovascular disease risk.15–17 Second, ASF participants demonstrated relative increases in arterial stiffness, as measured by PWV, when compared to non-athletic undergraduate students after adjustment for numerous potential confounding factors. The PWV values observed in our ASF cohort approximate upper limits of normal for age and height indexed reference values derived from the general population.23 These data suggest that collegiate ASF participation is associated with the development of early sub-clinical vascular dysfunction, which may predispose to the observed increased prevalence of hypertension observed in youthful ASF participants.
The limited data on arterial stiffness in the context of chronic exercise exposure suggest a sport-specific phenomenon.24–27 Kakiyama et al demonstrated significant reductions in PWV (5.8 ± 0.2 vs. 5.5 ± 0.2, P <0.01) following 8 weeks of cycle ergometer training among sedentary men.24 In contrast, Miyachi et al observed a 21% reduction in arterial compliance after 4 months of resistance training among healthy men.25 Prior work has also shown that increases in arterial stiffness attributable to resistance training are associated with the development left ventricular hypertrophy suggesting a mechanistic link between vascular function and end-organ response to exercise.27 Importantly, the impact of gender and ethnicity on the vascular response to specific forms of exercise remain uncertain and prior to this effort, ASF athletes have not been studied.
Previous cross-sectional work has established a high prevalence of pre-hypertension and hypertension among professional ASF athletes.3 These data may provide pathophysiologic insight to the reported increased incidence of non-genetic cardiomyopathy and attendant mortality among retired ASF athletes and increased cardiovascular disease mortality among certain retired ASF athletes.28,29 In the general population, arterial stiffness precedes the onset of overt hypertension,6 and increased arterial stiffness predicts adverse cardiovascular events additively to, and independently of traditional cardiovascular risk factors.15–20 This study was therefore designed to determine if ASF athletes demonstrate evidence of vascular dysfunction and if so, at which phase of an ASF career this pathologic process begins to develop.
Findings from the current study further advance our understanding of these issues in several ways. First, our data suggest relative arterial stiffening is present in freshman collegiate ASF participants. Therefore, although confirmatory data are required, our study provides compelling rationale for improved efforts aimed at identifying and treating ASF athletes that may be impacted by this condition. Future strategies designed to prevent or reverse this sub-clinical ASF-induced vasculopathy including reduction of dietary sodium intake, minimization of non-steroidal anti-inflammatory use, pharmacologic management of hypertension, and perhaps changes in ASF training regimens aimed at increasing the relative volume of isotonic/dynamic exercise are needed. Such work will require longitudinal and carefully controlled observational and interventional studies. Second, it is noteworthy that the relatively elevated PWV values observed in this study were present both before and after the first year of collegiate ASF exposure and independent of the rise in SBP. Future work, inclusive of high school ASF participants and older collegiate ASF participants, will be required to understand the exact temporal nature of this process and to clarify its progression and clinical impact among individuals that complete collegiate and professional ASF careers.
Several limitations of this study are noteworthy. First, mechanisms underlying the development of arterial stiffness in ASF participants were not addressed in our analysis. We could not adequately assess for changes in diet,30 caffeine intake, or undisclosed performance enhancing drug use that could have affected our data interpretation. Second, control subjects were recruited at only one institution, and not assessed longitudinally in parallel with ASF participants. However, we deliberately chose a control group that had been exposed to all aspects of high school and college life aside from ASF participation, and we felt undergraduates were similar at all three universities. Third, we recognize there may have been confounders present in newly matriculated collegiate ASF participants that could have affected the outcomes of our longitudinal analysis despite the controlled and similar training environment of most NCAA Division I collegiate ASF programs. Finally, we acknowledge the study cohort was limited to 32 ASF participants. However, we believe the repeated measures design of this study strengthened our analysis despite the limited number of ASF participants.
RESEARCH HIGHLIGHTS.
Central aortic pressure increases after one season of American-style football (ASF)
Collegiate ASF athletes exhibit arterial stiffening compared to collegiate controls
ASF induced sub-clinical vascular dysfunction may begin prior to college matriculation
Acknowledgments
Sources of Funding: This study was funded, in part, by the T32 NIH Ruth L. Kirschstein National Research Service Award Institutional Research Training Grant (J.H.K.) and the American Heart Association FTF2220328 (A.L.B).
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could a3ect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.National Federation of State High School Associations. [Accessed July 25, 2014];Participation statistics. http://www.nfhs.org/participation.
- 2.National Collegiate Athletic Association. [Accessed July 25, 2014];National Collegiate Athletic Association Web site. http://www.ncaa.org.
- 3.Tucker AM, Vogel RA, Lincoln AE, Dunn RE, Ahrensfield DC, Allen TW, Castle LW, Heyer RA, Pellman EJ, Strollo PJ, Jr, Wilson PW, Yates AP. Prevalence of cardiovascular disease risk factors among National Football League Players. JAMA. 2009;301:2111–2119. doi: 10.1001/jama.2009.716. [DOI] [PubMed] [Google Scholar]
- 4.Weiner RB, Wang F, Isaacs SK, Malhotra R, Berkstresser B, Kim JH, Hutter AM, Jr, Picard MH, Wang TJ, Baggish AL. Blood pressure and left ventricular hypertrophy during American-style football participation. Circulation. 2013;128:524–531. doi: 10.1161/CIRCULATIONAHA.113.003522. [DOI] [PubMed] [Google Scholar]
- 5.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC, Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT, Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 6.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. doi: 10.1001/2012.jama.10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santhanam L1, Tuday EC, Webb AK, Dowzicky P, Kim JH, Oh YJ, Sikka G, Kuo M, Halushka MK, Macgregor AM, Dunn J, Gutbrod S, Yin D, Shoukas A, Nyhan D, Flavahan NA, Belkin AM, Berkowitz DE. Decreased S-nitrosylation of tissue transglutaminase contributes to age-related increases in vascular stiffness. Circ Res. 2010;107:117–125. doi: 10.1161/CIRCRESAHA.109.215228. [DOI] [PubMed] [Google Scholar]
- 8.Kraemer WJ, Torine JC, Silvestre R, French DN, Ratamess NA, Spiering BA, Hatfield DL, Vingren JL, Volek JS. Body size and composition of National Football League players. J Strength Cond Res. 2005;19:485–489. doi: 10.1519/18175.1. [DOI] [PubMed] [Google Scholar]
- 9.Karpinos AR, Roumie CL, Nian H, Diamond AB, Rothman RL. High prevalence of hypertension among collegiate football athletes. Circ Cardiovasc Qual Outcomes. 2013;6:716–723. doi: 10.1161/CIRCOUTCOMES.113.000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baggish AL, Wang F, Weiner RB, Elinoff JM, Tournoux F, Boland A, Picard MH, Hutter AM, Jr, Wood MJ. Training-specific changes in cardiac structure and function: a prospective and longitudinal assessment of competitive athletes. J Appl Physiol. 2008;104:1121–1128. doi: 10.1152/japplphysiol.01170.2007. [DOI] [PubMed] [Google Scholar]
- 11.Baggish AL, Hutter AM, Jr, Wang F, Yared K, Weiner RB, Kupperman E, Picard MH, Wood MJ. Cardiovascular screening in college athletes with and without electrocardiography: A cross-sectional study. Ann Intern Med. 2010;152:269–275. doi: 10.7326/0003-4819-152-5-201003020-00004. [DOI] [PubMed] [Google Scholar]
- 12.Baggish AL, Weiner RB, Yared K, Wang F, Kupperman E, Hutter AM, Jr, Picard MH, Wood MJ. Impact of family hypertension history on exercise-induced cardiac remodeling. Am J Cardiol. 2009;104:101–106. doi: 10.1016/j.amjcard.2009.02.052. [DOI] [PubMed] [Google Scholar]
- 13.Croft LB, Belanger A, Miller MA, Roberts A, Goldman ME. Comparison of National Football League linemen versus nonlinemen of left ventricular mass and left atrial size. Am J Cardiol. 2008;102:343–347. doi: 10.1016/j.amjcard.2008.03.065. [DOI] [PubMed] [Google Scholar]
- 14.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 15.McEniery CM, Yasmin, McDonnell B, Munnery M, Wallace SM, Rowe CV, Cockcroft JR, Wilkinson IB. Central pressure: variability and impact of cardiovascular risk factors: the Anglo-Cardiff Collaborative Trial II. Hypertension. 2008;51:1476–1482. doi: 10.1161/HYPERTENSIONAHA.107.105445. [DOI] [PubMed] [Google Scholar]
- 16.Wang KL, Cheng HM, Chuang SY, Spurgeon HA, Ting CT, Lakatta EG, Yin FC, Chou P, Chen CH. Central or peripheral systolic pulse pressure: which best relates to target-organs and future mortality? J Hypertens. 2009;27:461–467. doi: 10.1097/hjh.0b013e3283220ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agabiti-Rosei E, Mancia G, O’Rourke MF, Roman MJ, Safar ME, Smulyan H, Wang JG, Wilkinson IB, Williams B, Vlachopoulos C. Central blood pressure measurements and antihypertensive therapy: a consensus document. Hypertension. 2007;50:154–160. doi: 10.1161/HYPERTENSIONAHA.107.090068. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton-Tyrell K, Verbeke F, Wang KL, Webb DJ, Willum Hansen T, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yasmin, Brown MJ. Similarities and differences between augmentation index and pulse wave velocity in the assessment of arterial stiffness. QJM. 1999;92:595–600. doi: 10.1093/qjmed/92.10.595. [DOI] [PubMed] [Google Scholar]
- 22.Patel RS, AL Mheid I, Morris AA, Ahmed Y, Kavtaradze N, Ali S, Dabhadkar K, Brigham K, Hooper WC, Alexander RW, Jones DP, Quyyumi AA. Oxidative stress is associated with impaired arterial elasticity. Atherosclerosis. 2011;218:90–95. doi: 10.1016/j.atherosclerosis.2011.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reusz GS, Cseprekal O, Temmar M, Kis E, Cherif AB, Thaleb A, Fekete A, Szabó AJ, Benetos A, Salvi P. Reference values of pulse wave velocity in healthy children and teenagers. Hypertension. 2010;56:217–224. doi: 10.1161/HYPERTENSIONAHA.110.152686. [DOI] [PubMed] [Google Scholar]
- 24.Kakiyama T, Sugawara J, Murakami H, Maeda S, Kuno S, Matsuda M. Effects of short-term endurance training on aortic distensibility in young males. Med Sci Sports Exerc. 2005;37:267–271. doi: 10.1249/01.mss.0000152733.12578.5a. [DOI] [PubMed] [Google Scholar]
- 25.Miyachi M, Kawano H, Sugawara J, Takahashi K, Hayashi K, Yamazaki K, Tabata I, Tanaka H. Unfavorable effects of resistance training on central arterial compliance. A randomized intervention study. Circulation. 2004;110:2858–2863. doi: 10.1161/01.CIR.0000146380.08401.99. [DOI] [PubMed] [Google Scholar]
- 26.Choi Y, Maeda S, Otsuki T, Miyaki A, Shimojo N, Yoshizawa M, Shiraki H, Ajisaka R. Oxidative stress and arterial stiffness in strength and endurance trained athletes. Artery Research. 2010;4:52–58. [Google Scholar]
- 27.Miyachi M, Donato AJ, Yamamoto K, Takahashi K, Gates PE, Moreau KL, Tanaka H. Greater age-related reductions in central arterial compliance in resistance-trained men. Hypertension. 2003;41:130–135. doi: 10.1161/01.hyp.0000047649.62181.88. [DOI] [PubMed] [Google Scholar]
- 28.Baron S, Rinsky R National Institute of Occupational Safety and Health, Department of Health and Human Services. Letter. Jan 10, 1994. National Football League causes of death. [Google Scholar]
- 29.Baron SL, Hein MJ, Lehman E, Gersic CM. Body mass index, playing position, race, and the cardiovascular mortality of retired professional football players. Am J Cardiol. 2012;109:889–896. doi: 10.1016/j.amjcard.2011.10.050. [DOI] [PubMed] [Google Scholar]
- 30.Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension. 2004;44:35–41. doi: 10.1161/01.HYP.0000132767.74476.64. [DOI] [PubMed] [Google Scholar]