Abstract
Background
Glucagon-like peptide-1 (GLP-1) agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors (GLP-1 agents) may be protective in heart failure (HF). We set out to determine whether GLP-1 agent use is associated with HF risk in diabetics.
Methods and Results
Retrospective cohort study of members of a large health system. We identified >19,000 adult diabetics from January 1, 2000–July 1, 2012. GLP-1 agent users were matched 1:2 to controls using propensity matching based on age, race, gender, coronary disease, HF, diabetes duration, and number of anti-diabetic medications. The association of GLP-1 agents with time to HF hospitalization was tested with multivariable Cox regression. All-cause hospitalization and mortality were secondary endpoints. We identified 1,426 users of GLP-1 agents and 2,798 controls. Both were similar except for angiotensin-converting enzyme inhibitors/angiotensin receptor blocker (ACEi/ARB) use, number of anti-diabetic medications and age. There were 199 hospitalizations, of which 128 were for HF, and 114 deaths. GLP-1 agents were associated with reduced risk of HF hospitalization (adjusted hazard ratio [aHR] 0.51; 95% confidence interval [CI] 0.34–0.77, p=0.002), all-cause hospitalization (aHR 0.54; 95% CI 0.38–0.74, p=0.001), and death (aHR 0.31; 95% CI 0.18–0.53, p=0.001).
Conclusions
GLP-1 agents may reduce the risk of HF events in diabetics.
Keywords: GLP-1 agonist, DPP-4 inhibitor, heart failure, outcomes
INTRODUCTION
Heart failure (HF) continues to be an enormous public health problem in the U.S. with a prevalence of 5.7 million individuals affected, an incidence of over 500,000 new cases annually (1) and an estimated 5-year mortality rate of 50% (2). Insulin resistance and diabetes mellitus (DM) have long been recognized as important risk factors for the development of HF. Data from the Framingham Heart Study indicate that men and women with DM are 4–5 times more likely to develop HF, even after controlling for other risk factors such as coronary artery disease (CAD) and valvular heart disease (3). Even early manifestations of insulin resistance, such as the metabolic syndrome, have been associated with increased risk of incident HF (4, 5). Additionally, DM worsens functional capacity and clinical outcomes in patients with established HF (6–8). With this important association in mind, attention has been given to examining the cardiovascular (CV) effects of anti-diabetic medications on outcomes in patients with HF.
Agents that target the GLP-1 pathway, including GLP-1 agonists and dipeptidyl-peptidase-4 (DPP-4) inhibitors, have recently received much attention. GLP-1 is an incretin hormone that leads to a rapid rise in circulating insulin levels after nutrient intake, and it is quickly inactivated in circulating blood by the enzyme DPP-4 (9). Studies in animal models and small human trials have shown that these agents appear to have favorable CV effects relevant to HF, including improved hemodynamics and exercise capacity (10–13). However, the actual clinical relevance to HF patients remains largely unknown.
Recently completed randomized controlled trials have shown that DPP-4 inhibitors saxagliptin and alogliptin do not have a significant impact on major adverse cardiac events related to atherosclerotic CV disease (14, 15). However, the trials did not primarily address the effect of these agents on heart failure outcomes in diabetics. To inquire into this knowledge gap, we performed a retrospective, propensity-matched analysis to determine whether exposure to agents affecting the GLP-1 pathway (GLP-1 agonists and DPP-4 inhibitors, collectively referred to here as ‘GLP-1 agents’) is associated with time to first hospitalization for HF in patients with DM.
METHODS
Study Population
We performed a retrospective cohort study of subjects receiving care through Henry Ford Health System, a vertically integrated health system serving the primary and specialty health care needs of individuals in southeast Michigan. The system includes several hospitals, a multi-specialty physician group of approximately 1000 physicians, and an affiliated health maintenance organization (HMO). The system maintains a central repository of administrative data, which we queried for this study. For the subset of patients enrolled in the HMO, data included insurance claims information, as well as enrollment and disenrollment dates. The study population was limited to individuals who were continuously enrolled in the HMO for at least 1 year before the DM diagnosis and who received care through system physicians. Therefore, we had information available for health care visits and prescription fills, both within and outside the health system. Using electronic data sources, we identified patients ≥ 18 years of age with a primary diagnosis of DM, an oral anti-diabetic drug fill, and no prior diagnosis of HF between January 1, 2000 and July 1, 2012. Patients were followed until the first of the following events: a HF hospitalization, death, disenrollment from the health plan, or the end of follow-up on July 1, 2012. The study was approved by the Institutional Review Board at Henry Ford Hospital.
Data Sources
Data for this study was obtained from electronic administrative databases maintained by the health system and vital records from the Michigan Department of Community Health. The administrative data captured claims (i.e., coded diagnoses, procedures, and prescription fills) occurring both within and outside the health system. A master patient index contained demographic data (i.e., date of birth, sex and race). Laboratory results were available for tests performed within the health system. The Michigan State Division of Vital Records and Health Statistics was queried with the patient social security numbers to identify deaths.
Statistical Analysis
We identified patients with a diagnosis of DM with an oral medication fill and without a previous diagnosis of HF between January 1, 2000 and July 1, 2012. Those initiating treatment with a GLP-1 agonist of DPP-4 inhibitor (‘GLP-1 agents’) were matched 1:2 to controls using propensity score matching. Logistic regression was used to predict the use of a GLP-1 agent and then generate a propensity score. This regression model was adjusted for age, sex, race, DM duration, number of anti-diabetic drugs and CAD. Patients prescribed and not prescribed a GLP-1 agent were assigned in a 1:2 match where the propensity scores were matched to 0.001. Chi-squared tests for categorical responses and Student’s t-test for continuous variables were used to compare the groups on the propensity matching variables, duration of diabetes, number of anti-diabetic drugs, beta-blocker use, ACEi/ARB use, ejection fraction, myocardial infarction (MI), hypertension, kidney disease, peripheral arterial disease, stroke and chronic obstructive pulmonary disease.
Time to first hospitalization for HF was the primary endpoint for this study. Hospitalization for HF was the first inpatient admission with a primary discharge diagnosis of HF during the period of observation. A primary hospital discharge diagnosis of HF has been shown by our group and others to be a highly specific claim signature for HF (specificity 95–100%)(16, 17). Secondary endpoints included all-cause hospitalization and all-cause mortality. Secondary endpoints were also analyzed based on GLP-1 agonist or DPP-4 inhibitor use.
Multivariable Cox proportional hazards regression was used to model the association of treatment with a GLP-1 agent with time to first hospitalization for HF and other outcomes. Each model included the propensity score, number of anti-diabetic drugs, duration of diabetes, baseline beta-blocker use and ACEi/ARB use.
RESULTS
We identified more than 19,000 patients with a diagnosis of DM with an oral medication fill and without a previous diagnosis of HF between January 1, 2000 and July 1, 2012. A total of 1,426 new users of GLP-1 agents and 2,798 propensity score-matched (for age, race, gender, duration of diabetes, and number of anti-diabetic medications) controls were identified. The groups were well-matched overall, although there were statistically significant differences in age, number of anti-diabetic drugs taken and ACEi/ARB use. Patients in the GLP-1 group were slightly younger (GLP-1 group 60 ± 11.4 vs. control group 61.2 ± 12.4 years, p=0.003) and took less concomitant anti-diabetic drugs when compared with controls (GLP-1 group, 1.35 medications ± 0.87 vs. control group 1.53 ± 0.60 medications, p=0.001). Hypertension was the most common comorbidity, and there were similar prevalences of other comorbid conditions between groups. Patients in both groups had a very low rate of established CV disease including CAD, prior MI, stroke and peripheral arterial disease. Use of beta-blockers was similar between the groups, while the control group showed greater ACEi/ARB use (GLP-1 group 62.1% vs. control group 66.7%, p=0.003) (Table 1). With regards to concurrent anti-diabetic drugs, approximately half of the patients in each group were treated with a biguanide (GLP-1 group 48.4% vs. control group 41.1%, p=0.09) and close to 30% were treated with a sulfonylurea (GLP-1 group 27.6% vs. control group 29%, p=0.344). A higher proportion of patients in the control group were treated with insulin (GLP-1 group 5.8% vs. control group 13.1%, p=0.001), whereas a higher proportion in the GLP-1 group were treated with a thiazolidinedione (GLP-1 group 10.8% vs. control group 6.2%, p=0.001) (Table 1).
Table 1.
Baseline characteristics.
| Variable | GLP-1 group (n=1426) | Control group (n=2798) | p value |
|---|---|---|---|
| Age (years), mean ± sd | 60.0 ± 11.4 | 61.2 ± 12.4 | 0.003 |
| Sex (female), n (%) | 778 (54.6) | 1487 (53.2) | 0.384 |
| Race (white), n (%) | 542 (38) | 1055 (37.7) | 0.113 |
| Diabetes duration (days), mean ± sd | 901.8 ± 605.8 | 894.8 ± 602.9 | 0.724 |
| Number of anti-diabetic drugs, mean ± sd | 1.35 ± 0.87 | 1.53 ± 0.60 | 0.001 |
| Hemoglobin A1c (%), mean ± sd | 7.98 ± 1.77 | 8.06 ± 1.90 | 0.181 |
| Beta-blocker use, n (%) | 460 (32.3) | 945 (33.8) | 0.323 |
| ACEi/ARB use, n (%) | 885 (62.1) | 1865 (66.7) | 0.003 |
| LVEF >50%, n (%) | 30/54 (55.6) | 99/177 (55.4) | 0.961 |
| Past Medical History | |||
| Coronary artery disease, n (%) | 23 (1.6) | 87 (1.8) | 0.623 |
| Myocardial infarction, n (%) | 3 (0.2) | 8 (0.3) | 0.760 |
| Hypertension, n (%) | 641 (45) | 1328 (47.5) | 0.122 |
| Chronic kidney disease, n (%) | 0 (0) | 0 (0) | 0 |
| Peripheral arterial disease, n (%) | 6 (0.4) | 16 (0.6) | 0.519 |
| Stroke, n (%) | 0 (0) | 3 (0.1) | 0.556 |
| COPD, n (%) | 43 (3) | 86 (3.1) | 0.917 |
| Anti-diabetic medications | |||
| Alpha-glucosidase inhibitor, n (%) | 1 (0.1) | 4 (0.1) | 0.669 |
| Biguanides, n (%) | 690 (48.4) | 1431 (51.1) | 0.09 |
| Meglitinides, n (%) | 4 (0.3) | 6 (0.2) | 0.741 |
| Sulfonylureas, n (%) | 394 (27.6) | 812 (29) | 0.344 |
| Thiazolidinediones, n (%) | 154 (10.8) | 173 (6.2) | 0.001 |
| Insulin, n (%) | 83 (6) | 366 (13.1) | 0.001 |
GLP-1: Glucagon-like peptide-1; COPD: Chronic obstructive pulmonary disease; LVEF: Left ventricular ejection fraction; ACEi: Angiotensin-converting enzyme inhibitor; ARB: Angiotensin receptor blocker; sd: Standard deviation.
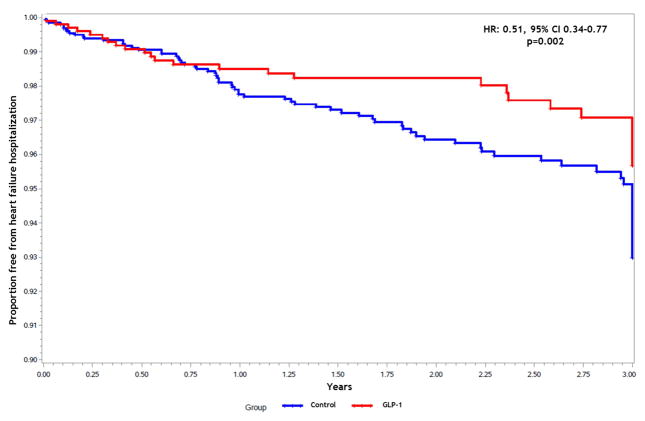
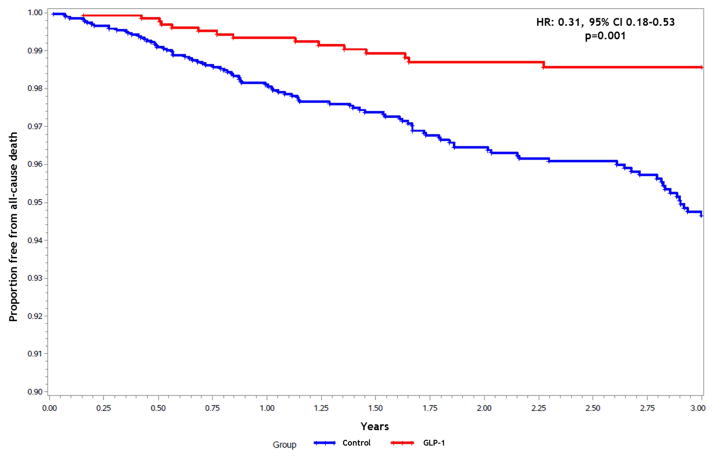
The median observation period was 742 days. During the observation period, there were 199 all-cause hospitalizations of which 128 (64.3%) for HF, and there were 114 deaths. Treatment with GLP-1 agents was associated with a significant reduction in the likelihood of hospitalization for HF when compared with controls (adjusted HR [aHR]: 0.51; 95% confidence interval [CI] 0.34–0.77, p=0.002). Use of a GLP-1 agent remained a significant predictor of reduced HF hospitalization risk after adjusting for the propensity score and baseline beta-blocker and ACEi/ARB use (Figure 1). Among patients that had a HF hospitalization, the proportion with preserved ejection fraction (≥50%) was similar in each group (GLP-1 group 64.6% vs. Control group 61.1%, p=0.6). Secondary endpoint results are summarized in Table 2. Exposure to GLP-1 agents was associated with a significantly lower risk of all-cause mortality (aHR: 0.31; 95% CI 0.18–0.53, p=0.001) (Figure 2), as well as a lower risk of all-cause hospitalization compared to the control group (aHR: 0.54; 95% CI 0.38–0.74, p=0.001). When causes of death were analyzed, there were no differences in the likelihood of cardiac death or heart failure-related death between groups.
Figure 1.
Freedom from heart failure hospitalization by treatment group.
HR: Hazard ratio; CI: Confidence interval; GLP-1: Glucagon-like peptide-1 agents.
Table 2.
Risk of heart failure hospitalization, all-cause hospitalization and mortality.
| Endpoint | Adjusted hazard ratio* (95% confidence interval) | p value |
|---|---|---|
| Heart failure hospitalization | 0.51 (0.34–0.77) | 0.002 |
| All-cause hospitalization | 0.54 (0.38–0.74) | 0.001 |
| Mortality | 0.31 (0.18–0.53) | 0.001 |
Analysis adjusted for propensity score, number of anti-diabetic drugs, duration of diabetes, baseline beta-blocker use and ACEi/ARB use.
Figure 2.
Freedom from all-cause mortality by treatment group.
HR: Hazard ratio; CI: Confidence interval; GLP-1: Glucagon-like peptide-1 agents.
To assess whether the above associations were drug or class-specific, we stratified our analysis for those using GLP-1 agonists or DPP-4 inhibitors exclusively, and we reassessed the likelihood of primary and secondary endpoints as compared with control individuals (Table 3). A total of 1,189 patients were treated exclusively with a DPP-4 inhibitor and 205 were treated exclusively with a GLP-1 agonist. Subjects treated with a DPP-4 inhibitor had a significantly lower risk of HF hospitalization (aHR: 0.58; 95% CI 0.38, 0.88, p=0.011), all-cause mortality (aHR: 0.34; 95% CI 0.19, 0.58, p=0.001), and all-cause hospitalization (aHR: 0.60, 95% CI 0.43, 0.84, p=0.003). Only 1 subject treated with GLP-1 agonists was hospitalized with HF, so a comparison with controls was not possible. Individuals treated with GLP-1 agonists had a lower risk of all-cause hospitalization (aHR: 0.17; 95% CI 0.02, 1.23, p=0.079) and all-cause death (aHR: 0.17; 95% CI 0.02, 1.22, p=0.078), but these estimates did not reach statistical significance.
Table 3.
Primary and secondary endpoints based on DPP-4 inhibitor or GLP-1 agonist use.
| DPP-4 inhibitor (n=1189) | GLP-1 agonist (n=205) | |||
|---|---|---|---|---|
|
| ||||
| Endpoint | Adjusted hazard ratio* (95% confidence interval) | p value | Adjusted hazard ratio* (95% confidence interval) | p value |
| Heart failure hospitalization | 0.58 (0.38–0.88) | 0.011 | - | - |
| All-cause hospitalization | 0.60 (0.43–0.84) | 0.003 | 0.17 (0.02–1.23) | 0.079 |
| Mortality | 0.34 (0.19–0.58) | 0.001 | 0.17 (0.02–1.22) | 0.078 |
GLP-1: Glucagon-like peptide-1, DPP-4: dipeptidyl peptidase-4.
Analysis adjusted for propensity score, number of anti-diabetic drugs, duration of diabetes, baseline beta-blocker use and ACEi/ARB use
DISCUSSION
The relationship between DM and HF is an area of intense interest at both the clinical and basic science levels. Patients with DM are at high risk of developing HF, and among those who suffer from HF, the presence of DM is associated with worse clinical outcomes (6, 7). The mechanisms by which DM contributes to HF, and vice versa, remain incompletely understood, as is the impact of differing anti-diabetic strategies on the development of HF or HF-related outcomes. The findings of our study suggest that exposure to agents that affect the GLP-1 pathway (i.e., both GLP-1 agonist and DPP-4 inhibitors) may reduce the likelihood of developing HF in patients with diabetes. In addition, treatment with these drugs may reduce the risk of all-cause hospitalization and mortality. These positive effects were observed in spite of higher use of ACEi/ARB and lower use of TZDs in the control group. When comparing the two drug classes studied, these benefits were observed in subjects taking both DPP4-inhibitors and GLP-1 agonists.. Subjects in whom GLP-1 agonists were used showed reduced risk of HF and all-cause hospitalization without a statistically-significant improvement in mortality, although the total number of GLP-1 agonist users in our study was low, obscuring whether any true difference may exist.
These results are in contrast with recently-reported data from a large randomized-controlled trial of saxagliptin in patients with type 2 diabetes and high CV risk (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus [SAVOR-TIMI 53]) (14). This trial failed to show any improvement in cardiovascular outcomes (cardiovascular death, non-fatal MI or non-fatal ischemic stroke) when saxagliptin was compared to placebo. Additionally, SAVOR-TIMI 53 reported increased risk of HF hospitalization with saxagliptin use (289 [3.5%] vs. 228 [2.8%] with placebo, p=0.007) (14). In contrast with the population studied in SAVOR-TIMI 53, the patients in our study had a very low rate of established CAD (2% vs. 78%) and MI (<1% vs. 38%), and overall CV risk was comparatively low. While our study was not specifically designed to look at HF outcomes in patients with diabetes without HF, CAD or MI, such a substantial difference in baseline CV risk compared to the SAVOR-TIMI 53 population could explain the favorable findings in our diabetic cohort. It is possible that GLP-1 agents may be effective in decreasing HF risk in patients with diabetes without a previous history of CAD or MI by altering the natural history of cardiovascular disease before the development of significant structural abnormalities. Also, the findings of SAVOR-TIMI 53 must be interpreted with caution in the context of a trial with a non-significant primary endpoint and in which the overall number of HF hospitalizations was relatively low. Additionally, it is possible that patients enrolled in the randomized controlled trial may be not be representative of a real world cohort as has been shown in other instances (32).
There are a number of possible mechanisms that could help explain the positive observations of our study. GLP-1 is an incretin hormone produced by intestinal cells in response to nutrient intake. Once GLP-1 binds to its receptor, there is a rapid rise in circulating insulin levels proportional to glucose levels. GLP-1 is thought to exert most of its effects via the GLP-1 receptor, a G-protein coupled receptor acting through cyclic adenosine monophosphate as second messenger, with subsequent activation of protein kinase A (9). Receptor-independent effects have also been described (18). The receptor is expressed throughout the gastrointestinal tract, but it has also been identified in the heart. Levels of circulating GLP-1 decrease rapidly (half-life <2 minutes) due to inactivation by DPP-4. The cardiovascular effects of GLP-1 have been observed in multiple animal models of myocardial infarction (MI) and HF. These studies show that GLP-1 improves myocardial glucose uptake, reduces oxidative stress, has anti-apoptotic properties, reduces infarct size (19), and can reverse ventricular remodeling. GLP-1 also appears to increase cardiac contractility and reduce systemic vascular resistance (10, 20–22). These beneficial effects of GLP-1 activation may explain the association of GLP-1-targeted therapy with improved clinical outcomes in patients with DM and HF.
The human effects of GLP-1 agents have been studied extensively in the context of atherosclerotic CV disease. In a randomized study of 20 patients with CAD and preserved left ventricular (LV) systolic function undergoing coronary artery bypass graft surgery, a 72-hour infusion of GLP-1 was associated with reduced requirements for inotropic support and with a reduction in the frequency of ventricular arrhythmias (23). In another study of 20 patients, infusions of GLP-1 limited the transient impairment in LV contractility following coronary artery occlusion in patients undergoing percutaneous coronary interventions (PCI) for stenoses of their left anterior descending coronary artery (24). More recently, in a controlled trial of patients being treated with primary PCI for ST-elevation MI, individuals were randomized to either intravenous exenatide or placebo for 6 hours. Those treated with exenatide showed significant improvement in myocardial salvage and reduced infarct size (25). Observations from another recent analysis (26) showed favorable cardiovascular effects of GLP-1 agents in patients without a recent history of major cardiovascular events treated with exenatide. In this study, treated patients showed a 19% reduction in the likelihood of myocardial infarction, ischemic stroke or coronary revascularization procedure compared with patients treated with non-exenatide glucose-lowering agents. In addition, exenatide-treated patients demonstrated a 12% reduction in CV hospitalization and 6% reduction in hospitalization from any cause. These data are supported by findings from two meta-analyses showing decreased risk of adverse CV outcomes with GLP-1 agents (27, 28). However, other meta-analyses aimed at assessing the safety of GLP-1 agents (29–31) did not find any difference in the likelihood of major CV events with these drugs. Randomized controlled trials in progress are expected to provide further data regarding the CV effects of GLP-1 agonists (Exenatide Study of Cardiovascular Event Lowering Trial (EXSCEL): A Trial To Evaluate Cardiovascular Outcomes After Treatment With Exenatide Once Weekly In Patients With Type 2 Diabetes Mellitus, ClinicalTrials.gov identifier NCT01144338) and DPP-4 inhibitors (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results - A Long Term Evaluation (LEADER), ClinicalTrails.gov identifier NCT01179048) (33). Of note, these trials are not expected to assess the potential effect of GLP-1 agents on HF risk in diabetics as a primary endpoint (33).
The present study has limitations that should be considered. First, the study was retrospective and therefore, despite propensity matching and multivariable adjustment, there may be residual confounding. Prospective randomized evaluation of the effect of GLP-1 agents on HF risk in diabetics is required to further clarify the findings of our study. Our data originated from a single health system, it could be argued that our findings may not be generalizable, yet our study population was demographically similar to that of southeast Michigan and the Detroit metropolitan statistical area (34). Finally, the information used in this study was primarily sourced from administrative and claims data. Although these sources have some potential for misclassification, we have previously shown that hospital discharge diagnoses for HF are highly specific (16) and that pharmacy claims produce consistently predictive measures of drug exposure (35).
CONCLUSION
In conclusion, we show that drugs that enhance GLP-1 activity including DPP-4 inhibitors and GLP-1 agonists appear to produce favorable cardiovascular effects among individuals with DM. These apparent benefits were manifest through a reduction in HF hospitalizations, all-cause hospitalizations, and all-cause death. These benefits were observed in a diabetic cohort with a low prevalence of CAD and prior MI. Additional prospective randomized controlled trials are required to support or refute these findings.
Acknowledgments
FUNDING SOURCES: This research was supported in part by the National Heart, Lung, and Blood Institute (Lanfear K23HL085124, R01HL103871; Williams R01HL079055, R01HL118267), the National Institute of Allergy and Infectious Diseases (Williams R01AI079139, R01AI061774) and the National Institute of Diabetes and Digestive and Kidney Diseases (Williams R01DK064695).
ABBREVIATIONS
- HF
heart failure
- DM
diabetes mellitus
- GLP-1
glucagon-like peptide-1
- DPP-4
dipeptidyl-peptidase-4
- ACEi
angiotensin-converting enzyme inhibitor
- ARB
angiotensin receptor blocker
- CI
confidence interval
- aHR
adjusted hazard ratio
- HMO
health maintenance organization
- LV
left ventricular
- PCI
percutaneous coronary intervention
- MI
myocardial infarction
- NYHA
New York Heart Association
Footnotes
DISCLOSURES
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012 Jan 3;125(1):188–97. doi: 10.1161/CIR.0b013e3182456d46. Epub 2012/01/05. eng. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004 Jul 21;292(3):344–50. doi: 10.1001/jama.292.3.344. Epub 2004/07/22. eng. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974 Jul;34(1):29–34. doi: 10.1016/0002-9149(74)90089-7. eng. [DOI] [PubMed] [Google Scholar]
- 4.Azevedo A, Bettencourt P, Almeida PB, Santos AC, Abreu-Lima C, Hense HW, et al. Increasing number of components of the metabolic syndrome and cardiac structural and functional abnormalities--cross-sectional study of the general population. BMC cardiovascular disorders. 2007;7:17. doi: 10.1186/1471-2261-7-17. Epub 2007/06/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ingelsson E, Arnlöv J, Lind L, Sundström J. Metabolic syndrome and risk for heart failure in middle-aged men. Heart. 2006 Oct;92(10):1409–13. doi: 10.1136/hrt.2006.089011. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domanski M, Krause-Steinrauf H, Deedwania P, Follmann D, Ghali JK, Gilbert E, et al. The effect of diabetes on outcomes of patients with advanced heart failure in the BEST trial. J Am Coll Cardiol. 2003 Sep 3;42(5):914–22. doi: 10.1016/s0735-1097(03)00856-8. Epub 2003/09/06. eng. [DOI] [PubMed] [Google Scholar]
- 7.Dries DL, Sweitzer NK, Drazner MH, Stevenson LW, Gersh BJ. Prognostic impact of diabetes mellitus in patients with heart failure according to the etiology of left ventricular systolic dysfunction. J Am Coll Cardiol. 2001 Aug;38(2):421–8. doi: 10.1016/s0735-1097(01)01408-5. Epub 2001/08/14. eng. [DOI] [PubMed] [Google Scholar]
- 8.Guazzi M, Brambilla R, Pontone G, Agostoni P, Guazzi MD. Effect of non-insulin-dependent diabetes mellitus on pulmonary function and exercise tolerance in chronic congestive heart failure. Am J Cardiol. 2002 Jan 1;89(2):191–7. doi: 10.1016/s0002-9149(01)02199-3. eng. [DOI] [PubMed] [Google Scholar]
- 9.Davidson MH. Cardiovascular effects of glucagonlike peptide-1 agonists. Am J Cardiol. 2011 Aug 2;108(3 Suppl):33B–41B. doi: 10.1016/j.amjcard.2011.03.046. Epub 2011/08/10. eng. [DOI] [PubMed] [Google Scholar]
- 10.Liu Q, Anderson C, Broyde A, Polizzi C, Fernandez R, Baron A, et al. Glucagon-like peptide-1 and the exenatide analogue AC3174 improve cardiac function, cardiac remodeling, and survival in rats with chronic heart failure. Cardiovasc Diabetol. 2010;9:76. doi: 10.1186/1475-2840-9-76. Epub 2010/11/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004 Mar 2;109(8):962–5. doi: 10.1161/01.CIR.0000120505.91348.58. Epub 2004/02/26. eng. [DOI] [PubMed] [Google Scholar]
- 12.Nathanson D, Ullman B, Löfström U, Hedman A, Frick M, Sjöholm Å, et al. Effects of intravenous exenatide in type 2 diabetic patients with congestive heart failure: a double-blind, randomised controlled clinical trial of efficacy and safety. Diabetologia 2012. 2012 Apr 01;55(4):926–35. doi: 10.1007/s00125-011-2440-x. English. [DOI] [PubMed] [Google Scholar]
- 13.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-Like Peptide-1 Infusion Improves Left Ventricular Ejection Fraction and Functional Status in Patients With Chronic Heart Failure. Journal of Cardiac Failure. 2006;12(9):694–9. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 14.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. The New England journal of medicine. 2013 Oct 3;369(14):1317–26. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 15.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. The New England journal of medicine. 2013 Oct 3;369(14):1327–35. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 16.Alqaisi F, Williams LK, Peterson EL, Lanfear DE. Comparing methods for identifying patients with heart failure using electronic data sources. BMC Health Serv Res. 2009;9:237. doi: 10.1186/1472-6963-9-237. Epub 2009/12/22. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee WY, Capra AM, Jensvold NG, Gurwitz JH, Go AS. Gender and risk of adverse outcomes in heart failure. Am J Cardiol. 2004 Nov 1;94(9):1147–52. doi: 10.1016/j.amjcard.2004.07.081. Epub 2004/11/03. eng. [DOI] [PubMed] [Google Scholar]
- 18.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz S-S, Drucker DJ, Husain M. Cardioprotective and Vasodilatory Actions of Glucagon-Like Peptide 1 Receptor Are Mediated Through Both Glucagon-Like Peptide 1 Receptor-Dependent and -Independent Pathways. Circulation. 2008 May 6;117(18):2340–50. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 19.Ravassa S, Zudaire A, Diez J. GLP-1 and cardioprotection: from bench to bedside. Cardiovascular research. 2012 May 1;94(2):316–23. doi: 10.1093/cvr/cvs123. Epub 2012/03/16. eng. [DOI] [PubMed] [Google Scholar]
- 20.Nikolaidis LA, Elahi D, Hentosz T, Doverspike A, Huerbin R, Zourelias L, et al. Recombinant Glucagon-Like Peptide-1 Increases Myocardial Glucose Uptake and Improves Left Ventricular Performance in Conscious Dogs With Pacing-Induced Dilated Cardiomyopathy. Circulation. 2004 Aug 24;110(8):955–61. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 21.Poornima I, Brown SB, Bhashyam S, Parikh P, Bolukoglu H, Shannon RP. Chronic glucagon-like peptide-1 infusion sustains left ventricular systolic function and prolongs survival in the spontaneously hypertensive, heart failure-prone rat. Circulation Heart failure. 2008 Sep;1(3):153–60. doi: 10.1161/CIRCHEARTFAILURE.108.766402. Epub 2009/09/04. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vyas AK, Yang KC, Woo D, Tzekov A, Kovacs A, Jay PY, et al. Exenatide improves glucose homeostasis and prolongs survival in a murine model of dilated cardiomyopathy. PloS one. 2011;6(2):e17178. doi: 10.1371/journal.pone.0017178. Epub 2011/03/02. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sokos GG, Bolukoglu H, German J, Hentosz T, Magovern GJ, Jr, Maher TD, et al. Effect of glucagon-like peptide-1 (GLP-1) on glycemic control and left ventricular function in patients undergoing coronary artery bypass grafting. Am J Cardiol. 2007 Sep 1;100(5):824–9. doi: 10.1016/j.amjcard.2007.05.022. Epub 2007/08/28. eng. [DOI] [PubMed] [Google Scholar]
- 24.Read PA, Hoole SP, White PA, Khan FZ, O’Sullivan M, West NE, et al. A pilot study to assess whether glucagon-like peptide-1 protects the heart from ischemic dysfunction and attenuates stunning after coronary balloon occlusion in humans. Circulation Cardiovascular interventions. 2011 Jun;4(3):266–72. doi: 10.1161/CIRCINTERVENTIONS.110.960476. Epub 2011/05/19. eng. [DOI] [PubMed] [Google Scholar]
- 25.Lonborg J, Vejlstrup N, Kelbaek H, Botker HE, Kim WY, Mathiasen AB, et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012 Jun;33(12):1491–9. doi: 10.1093/eurheartj/ehr309. Epub 2011/09/17. eng. [DOI] [PubMed] [Google Scholar]
- 26.Best JH, Hoogwerf BJ, Herman WH, Pelletier EM, Smith DB, Wenten M, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care. 2011 Jan;34(1):90–5. doi: 10.2337/dc10-1393. Epub 2010/10/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johansen OE, Neubacher D, von Eynatten M, Patel S, Woerle HJ. Cardiovascular safety with linagliptin in patients with type 2 diabetes mellitus: a pre-specified, prospective, and adjudicated meta-analysis of a phase 3 programme. Cardiovasc Diabetol. 2012;11:3. doi: 10.1186/1475-2840-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu D, Li L, Liu C. Efficacy and safety of dipeptidyl peptidase-4 inhibitors and metformin as initial combination therapy and as monotherapy in patients with type 2 diabetes mellitus: a meta-analysis. Diabetes, obesity & metabolism. 2013 Jun 26; doi: 10.1111/dom.12174. Epub 2013/06/28. Eng. [DOI] [PubMed] [Google Scholar]
- 29.Monami M, Cremasco F, Lamanna C, Colombi C, Desideri CM, Iacomelli I, et al. Glucagon-like peptide-1 receptor agonists and cardiovascular events: a meta-analysis of randomized clinical trials. Experimental diabetes research. 2011;2011:215764. doi: 10.1155/2011/215764. Epub 2011/05/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratner R, Han J, Nicewarner D, Yushmanova I, Hoogwerf BJ, Shen L. Cardiovascular safety of exenatide BID: an integrated analysis from controlled clinical trials in participants with type 2 diabetes. Cardiovasc Diabetol. 2011;10:22. doi: 10.1186/1475-2840-10-22. Epub 2011/03/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun F, Yu K, Wu S, Zhang Y, Yang Z, Shi L, et al. Cardiovascular safety and glycemic control of glucagon-like peptide-1 receptor agonists for type 2 diabetes mellitus: a pairwise and network meta-analysis. Diabetes research and clinical practice. 2012 Dec;98(3):386–95. doi: 10.1016/j.diabres.2012.09.004. Epub 2012/10/02. eng. [DOI] [PubMed] [Google Scholar]
- 32.Wang TS, Hellkamp AS, Patel CB, Ezekowitz JA, Fonarow GC, Hernandez AF. Representativeness of RELAX-AHF Clinical Trial Population in Acute Heart Failure. Circulation: Cardiovascular Quality and Outcomes. 2014 Mar 4;2014 doi: 10.1161/CIRCOUTCOMES.113.000418. [DOI] [PubMed] [Google Scholar]
- 33.Clinicaltrials.gov. 2013 Aug 23; Available from: http://clinicaltrials.gov/ct2/home.
- 34.Williams LK, Joseph CL, Peterson EL, Moon C, Xi H, Krajenta R, et al. Race-ethnicity, crime, and other factors associated with adherence to inhaled corticosteroids. The Journal of allergy and clinical immunology. 2007 Jan;119(1):168–75. doi: 10.1016/j.jaci.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 35.Habib ZA, Havstad SL, Wells K, Divine G, Pladevall M, Williams LK. Thiazolidinedione use and the longitudinal risk of fractures in patients with type 2 diabetes mellitus. The Journal of clinical endocrinology and metabolism. 2010 Feb;95(2):592–600. doi: 10.1210/jc.2009-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]