Abstract
Background and Purpose
Migraine with aura is an established stroke risk factor, and excitatory mechanisms such as spreading depression are implicated in the pathogenesis of both migraine and stroke. Spontaneous spreading depression waves originate within the peri-infarct tissue and exacerbate the metabolic mismatch during focal cerebral ischemia. Genetically enhanced spreading depression susceptibility facilitates anoxic depolarizations and peri-infarct spreading depressions and accelerates infarct growth, suggesting that susceptibility to spreading depression is a critical determinant of vulnerability to ischemic injury. Because chronic treatment with migraine prophylactic drugs suppresses spreading depression susceptibility, we tested whether migraine prophylaxis can also suppress ischemic depolarizations and improve stroke outcome.
Methods
We measured the cortical susceptibility to spreading depression and ischemic depolarizations, and determined tissue and neurological outcome after middle cerebral artery occlusion in wild type and familial hemiplegic migraine type 1 knock-in mice treated with vehicle, topiramate or lamotrigine daily for 7 weeks or as a single dose shortly before testing.
Results
Chronic treatment with topiramate or lamotrigine reduces the susceptibility to KCl- or electrical stimulation-induced spreading depressions as well as ischemic depolarizations in both wild-type and familial hemiplegic migraine type 1 mutant mice. Consequently, both tissue and neurological outcomes are improved. Notably, treatment with a single dose of either drug is ineffective.
Conclusions
These data underscore the importance of hyperexcitability as a mechanism for increased stroke risk in migraineurs, and suggest that migraine prophylaxis may not only prevent migraine attacks but also protect migraineurs against ischemic injury.
Keywords: migraine prophylaxis, topiramate, lamotrigine, peri-infarct depolarization, anoxic depolarization, middle cerebral artery occlusion
INTRODUCTION
Migraine is the most common neurological condition, affecting 10-20% of the population1. Stroke is a major cause of death and disability worldwide. An intriguing association between migraine and stroke is well established. Epidemiological studies identified migraine with aura as an independent factor increasing stroke risk by more than 2-fold2. The relative risk is particularly high in otherwise healthy young adults without cardiovascular risk factors. The prevalence of migraine is on par with that of other known stroke risk factors.
Spreading depression (SD), an intense depolarization that underlies migraine aura, also occurs in peri-infarct tissue as an overlapping mechanism between migraine and stroke. Although SD does not cause injury in the healthy brain, recurrent peri-infarct SDs and depolarizations (PIDs) worsen the metabolic mismatch in ischemic tissue and promote infarct growth during hyperacute stroke in both experimental animals3-5 and in humans6, 7.
Indirect evidence implicates enhanced cerebral excitability in common migraine8, 9, as well as in familial hemiplegic migraine (FHM). FHM1 mutations enhance CaV2.1 channel open probability, presynaptic calcium influx and cortical glutamate release, and render the brain hyperexcitable10. As a result, FHM1 mutations markedly enhance SD susceptibility11, 12. Underscoring the importance of SD in migraine and stroke, transgenic mice expressing FHM1 mutations exhibit faster onset of anoxic depolarization (AD) and rapid infarct growth linked to higher frequency of PIDs during experimentally induced focal cerebral ischemia13.
Chronic treatment with widely prescribed migraine prophylactic drugs of various pharmacological classes dose-dependently suppresses SD susceptibility in rats as a possible mechanism of action14. The majority of these drugs, however, are ineffective after a single dose, reminiscent of the delayed onset of action requiring chronic treatment in migraine prophylaxis. We, therefore, examined the efficacy of migraine prophylactic drugs on stroke outcome and its mechanisms in relation to ischemic depolarizations. We chose topiramate as a prophylactic drug because it is efficacious in migraine prophylaxis15, and inhibits experimental SD upon chronic treatment in rats14. We tested lamotrigine because it also inhibits SD upon chronic treatment in rats16, although its efficacy in migraine is not proven17. Both drugs have been studied previously in experimental focal ischemia models without consistent efficacy, albeit as a single dose or as short-term post-ischemic dosing18-22. Therefore, neither drug has been tested as a prophylactic intervention in stroke.
We therefore tested these drugs in commonly employed experimental models of focal cerebral ischemia, and did this not only in wild-type (WT) but also in FHM1 mutant mice to test drug efficacy on a background of cerebral hyperexcitability modeling migraine. Here, we show that chronic daily treatment for 7 weeks with the migraine prophylactic drugs topiramate or lamotrigine delays AD, inhibits PID occurrence and improves tissue and neurological outcome after filament occlusion of the middle cerebral artery in both WT and FHM1 mutant mice. In contrast, single doses of each drug are ineffective, suggesting that the efficacy of migraine prophylactic drugs in stroke corresponds to their efficacy on SD, and that SD susceptibility is a critical but modifiable determinant of vulnerability to ischemic injury.
METHODS
Experimental animals
All experimental procedures were carried out in accordance with the Guide for Care and Use of Laboratory Animals (NIH Publication No. 85-23, 1996), and were approved by the institutional review board (MGH Subcommittee on Research Animal Care, SRAC). In addition to C57BL/6J WT mice, transgenic knock-in Cacna1a migraine mouse models homozygous for the R192Q FHM1 mutation were used, generated by a gene targeting approach11, 23, and backcrossed on C57BL/6J background for more than 10 generations. We studied mice between 2-6 months of age (23-30g), because stroke risk is highest in young adult migraineurs. We studied male mice in stroke experiments to avoid the confounding effects of female hormones on outcome24, 25, and female mice in SD experiments due to their higher SD susceptibility compared to males12, and because migraine is more prevalent in women.
Treatment paradigm
In the chronic treatment group, we treated mice for 7 weeks with once a day orogastric gavage doses of migraine prophylactic drugs topiramate (80 mg/kg/d) or lamotrigine (30 mg/kg/d), and compared these with vehicle (ORA plus/ORA sweet); the last daily dose was administered 2 hours before the experiment. In a separate cohort, we tested the efficacy of a single dose of these drugs administered 2 hours before the experiment. We selected the doses based on previously reported efficacy in other experimental models in mice26, 27. All experiments were carried out with the investigators blinded, and confirmatory genotyping was done in mutant cohorts.
Study design
Study endpoints were defined a priori. Experiments were performed in three stages. First, efficacy of topiramate and lamotrigine was tested on SD susceptibility endpoints in WT and FHM1 mutant mice. Second, efficacy of both drugs on PID frequency and ischemic outcome was tested in two separate cohorts of WT mice. Lastly, efficacy of both drugs on ischemic outcome was tested in FHM1 mutant mice. Animals were randomly assigned to the treatment groups for each cohort. A different experimenter blinded to the treatment carried out each experimental stage. Experiments were carried out according to the intention-to-treat principle; therefore, data points were excluded only if technical failures prevented reliable data collection. Because focal cerebral ischemia experiments in WT and FHM1 mutant mouse cohorts were separated in time, and performed by different operators using different equipment and experimental setups, we could not perform comparisons of ischemic tissue and neurological outcome endpoints between WT and FHM1 mutant strains in this study.
Systemic physiological monitoring
Arterial pH, pO2, pCO2, and blood pressure were measured via a femoral artery catheter under isoflurane anesthesia (2.5% induction, 1.5% maintenance, in 70% N2O and 30% O2; Table 1) and maintained by endotracheal intubation and mechanical ventilation during electrophysiological recordings (i.e., SD susceptibility, PID frequency). In 24-hour survival experiments, these interventions were not performed to minimize morbidity and improve survival rates. Rectal temperature was controlled at 37°C.
Table 1.
Physiological parameters.
| Experiment | Treatment Duration | Genotype | Drug | BP | pH | pCO2 | O2 |
|---|---|---|---|---|---|---|---|
| SD | Chronic | WT | Control | 92±7 | 7.41±0.04 | 28±4 | 132±18 |
| WT | Topiramate | 98±9 | 7.34±0.03 | 30±4 | 140±12 | ||
| WT | Lamotrigine | 89±7 | 7.37±0.04 | 30±2 | 113±15 | ||
| R192Q | Control | 96±5 | 7.38±0.04 | 28±4 | 132±16 | ||
| R192Q | Topiramate | 88±7 | 7.33±0.04 | 27±3 | 142±11 | ||
| R192Q | Lamotrigine | 95±8 | 7.36±0.03 | 29±2 | 129±13 | ||
| Acute | WT | Control | 88±6 | 7.31±0.04 | 32±4 | 134±11 | |
| WT | Topiramate | 81±7 | 7.25±0.02 | 34±5 | 153±5 | ||
| WT | Lamotrigine | 82±4 | 7.33±0.04 | 34±5 | 137±16 | ||
| PID | Chronic | WT | Control | 95±16 | 7.38±0.03 | 38±5 | 122±30 |
| WT | Topiramate | 88±11 | 7.37±0.05 | 37±6 | 131±23 | ||
| WT | Lamotrigine | 94±10 | 7.40±0.05 | 33±6 | 136±25 | ||
| Acute | WT | Control | 92±13 | 7.40±0.04 | 36±3 | 116±13 | |
| WT | Topiramate | 82±11 | 7.35±0.07 | 37±6 | 129±12 | ||
| WT | Lamotrigine | 85±10 | 7.40±0.04 | 34±6 | 123±25 |
Data are displayed as mean ± standard deviation.
SD susceptibility
As described previously12, 3 burr holes were drilled under saline cooling at the following coordinates (mm from bregma): 3.5 posterior, 2 lateral (2 mm diameter for electrical stimulation and KCl application onto occipital cortex); 1.5 posterior, 2 lateral (1 mm diameter, recording site 1); 0.5 anterior, 2 lateral (1 mm diameter, recording site 2). The dura was kept intact to minimize trauma. Two glass capillary microelectrodes were placed to record extracellular steady (DC) potential and electrocorticogram. Electrical SD threshold was determined by escalating intensity cathodal square pulses (10-8000 µC) via a bipolar electrode placed on the occipital cortex, and then a 1-mm cotton ball soaked in 300 mM KCl was topically applied for 1 hour to record the frequency of evoked SDs. The protocol was then repeated on the opposite hemisphere. Data were averaged between the two hemispheres to yield a single data point per animal. SD frequency and threshold were taken as primary endpoints. The amplitude, propagation speed (distance/latency between the two recording electrodes), and duration at half-amplitude of the first SD in each hemisphere were also measured as secondary endpoints. There was no technical failure leading to exclusion in this cohort.
Transient filament occlusion of the middle cerebral artery (fMCAO)
A nylon monofilament was inserted into the internal via the external carotid artery followed by reperfusion after 60 minutes, under isoflurane anesthesia (2.5% induction, 1.5% maintenance, in 70% N2O and 30% O2) and laser Doppler monitoring (Perimed, Järfälla, Sweden), as described previously13.
PID occurrence
To record PIDs after fMCAO, mice were transferred on to a stereotaxic frame and two 0.5 mm diameter burr holes were carefully drilled under saline irrigation at the following coordinates (mm from bregma): 1.5 anterior, 0.5 lateral; 3.5 posterior, 0.5 lateral. These coordinates were chosen to be reliably outside the focal ischemic cortex to allow detection of PIDs. Two intracortical glass micropipettes were inserted at a depth of 250 µm, and extracellular slow potential changes were recorded for approximately two hours starting approximately 20 minutes after the onset of fMCAO. PID frequency was taken as a primary endpoint. Technical failures occurred in WT cohorts only, and led to the exclusion of 1 chronic and 1 single dose vehicle, 1 chronic and 1 single dose topiramate, and 3 single dose lamotrigine-treated mice for PID assessments. Extensive surgery, intubation, mechanical ventilation and arterial cannulation for PID monitoring precluded 24-hour survival. Therefore, infarct volumes were determined in a separate cohort.
Assessment of tissue and neurological outcome after fMCAO
After reperfusion, mice were transferred to a temperature-controlled incubator with access to food and water ad libitum. Neurological outcomes were scored as a primary endpoint 24 hours after reperfusion, using a five-point scale: 0, normal; 1, forepaw monoparesis; 2, circling to left; 3, falling to left; 4, no spontaneous walking and depressed consciousness; 5, death. Premature death after ischemia was incorporated in the neurological outcome scale because of the intention-to-treat design; however, infarct volume data from these mice were not measured due to postmortem confounders. Infarct volume was calculated by integrating the infarct area in ten 1-mm-thick 2,3,5-triphenyltetrazolium chloride (TTC)-stained coronal sections. Infarct volume was calculated as a primary endpoint by subtracting the volume of ipsilateral non-infarcted tissue from contralateral hemisphere. Ischemic swelling volume was also calculated as a secondary endpoint by subtracting the volume of contralateral hemisphere from the volume of ipsilateral hemisphere. Technical failures occurred in FHM1 cohorts only, and led to the exclusion of 2 chronic vehicle and 1 chronic topiramate-treated mice for tissue and neurological outcome assessments.
Measurement of AD latency
The latency between fMCAO and AD onset was measured as a secondary endpoint using the characteristic secondary hypoperfusion caused by AD on laser Doppler tracings, as described in detail previously13. We measured this parameter in all WT mice undergoing fMCAO either for PID frequency determination or infarct and neurological outcome assessment. Absence of a detectable secondary hypoperfusion due to technical reasons was taken as an a priori exclusion criterion for this dataset. Although this occurred more commonly, it resulted in the exclusion of only 16 out of 112 animals in which this secondary endpoint was studied, distributed relatively evenly among experimental groups.
Statistical analysis
Data were analyzed using SPSS (v11.0) and GraphPad Prism 6, and presented as whisker-box plot (whiskers, full range; box, 25-75% range; line, median; cross, mean) in the figures and mean ± standard deviation in the table. Statistical tests used to analyze each dataset, group sizes (n) and details of statistical outcomes are provided in the figure legends. P values are two-tailed, and P<0.05 was considered statistically significant.
RESULTS
Suppression of KCl-induced or electrically triggered cortical SD
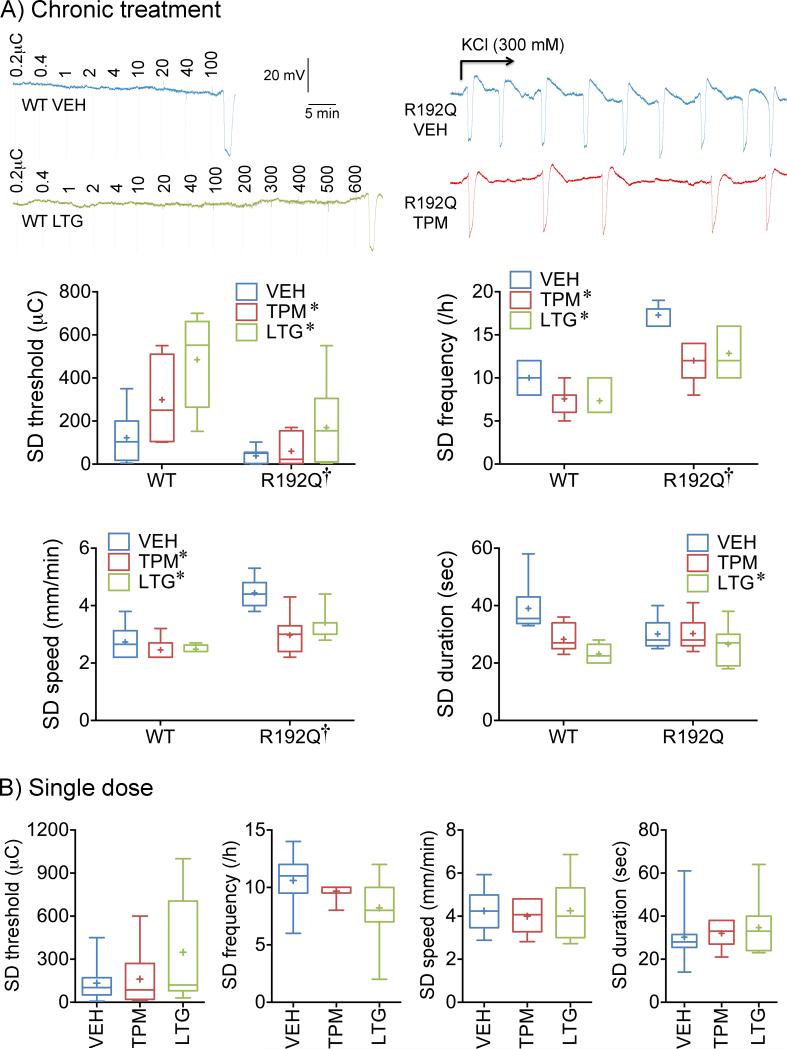
We have previously shown in rats that migraine prophylactic drugs suppress SD susceptibility14. To first test whether migraine prophylactic drugs are also efficacious in mice, we treated WT and FHM1 knock-in mice with chronic daily injections of topiramate or lamotrigine for 7 weeks. Chronic treatment with topiramate or lamotrigine elevated the electrical threshold for SD induction, and reduced the frequency of KCl-induced SDs (Figure 1A). Both drugs also reduced the SD propagation speed by approximately 30%, albeit only in the FHM1 mutant. In addition, lamotrigine decreased SD duration, and tended to be more efficacious on all SD endpoints compared with topiramate. A single dose of either drug administered 2 hours before SD, tested in WT mice only, did not affect any of the SD attributes although a trend for lamotrigine to elevate the electrical threshold and reduce KCl-induced SD frequency was noted (Figure 1B).
Figure 1. Chronic topiramate and lamotrigine treatment suppresses SD susceptibility.
A) Representative electrophysiological tracings show SD triggered upon stepwise escalating cortical cathodal stimulation at intensities indicated above each tracing to determine the SD threshold (left), and repetitive SDs triggered by continuous topical KCl application for 1h onto the cortex to determine SD frequency (right), in wild-type (WT) or FHM1 (R192Q) mutant mice after 7 weeks of daily treatment with vehicle (VEH, blue), topiramate (TPM, red) or lamotrigine (LTG, green). Whisker-box plots summarize the effects of chronic treatment on SD threshold, frequency, speed and duration. n=6, 7 and 6 WT mice in vehicle, topiramate and lamotrigine groups, respectively; n=7 R192Q mice in vehicle, topiramate, and lamotrigine groups each. Twoway ANOVA followed by Sidak's and Tukey's multiple comparisons. SD threshold: genotype effect F(1,34)=18.8, p=0.0001; treatment effect F(2,34)=8.4, p=0.0011; interaction F(2,34)=1.9, p=0.1674. SD frequency: genotype effect F(1,34)=83.8, p<0.0001; treatment effect F(2,34)=15.4, p<0.0001; interaction F(2,34)=1.8, p=0.1857. SD speed: genotype effect F(1,34)=42.8, p<0.0001; treatment effect F(2,34)=10.7, p=0.0002; interaction F(2,34)=4.8, p=0.0142. SD duration: genotype effect F(1,34)=0.3, p=0.5647; treatment effect F(2,34)=7.8, p=0.0016; interaction F(2,34)=3.8, p=0.0332. Post-hoc comparisons: *p<0.05 vs vehicle; †p<0.05 vs. WT.
B) Whisker-box plots summarize the effect of a single dose of each drug on SD frequency, threshold, speed and duration in WT mice. n=10, 6 and 9 mice in vehicle, topiramate and lamotrigine groups, respectively. One-way ANOVA followed by Holm-Sidak's multiple comparisons test. Treatment effects were not statistically significant.
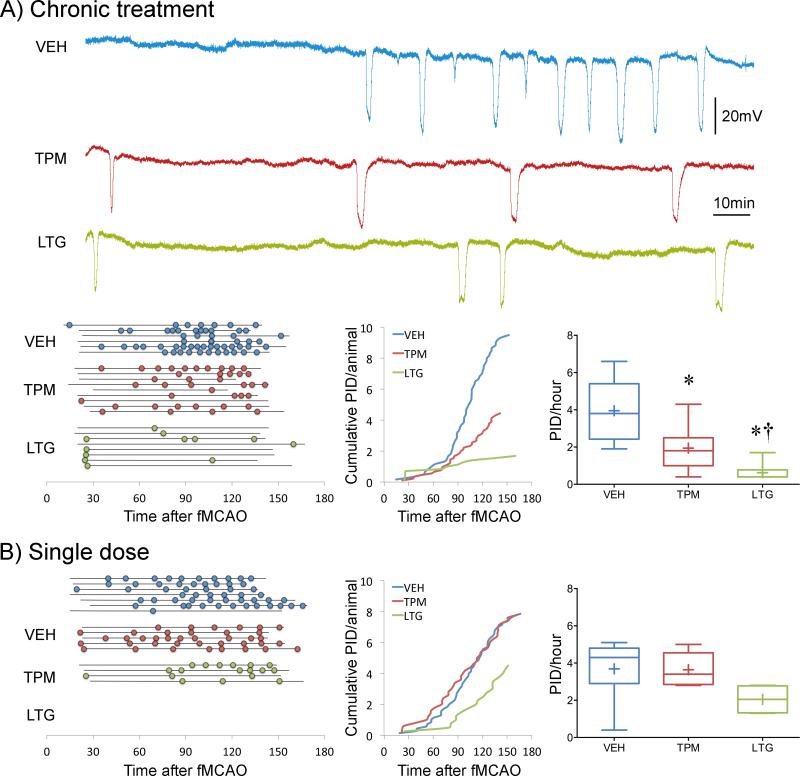
Suppression of cortical PIDs during middle cerebral artery occlusion
We next tested whether migraine prophylactic drugs also suppress PIDs, akin to SD. Intracortical microelectrode recordings during fMCAO showed that chronic treatment with topiramate or lamotrigine reduced PID occurrence by 50% and 80%, respectively (Figure 2A). A single dose of topiramate 2 hours before ischemia onset was ineffective, whereas lamotrigine showed a strong trend (Figure 2B). In a separate cohort of mice, we also found that chronic treatment with valproate (200 mg/kg, i.p. for 6 weeks) also reduced the number of PIDs (3.1±0.6 PIDs/h) compared with vehicle (5.7±0.5 PIDs/h; p<0.001, n=5 each), consistent with its inhibitory effect on KCl- or electrically-induced SDs previously shown in rats14, and suggesting a class effect for migraine prophylactic drugs on PIDs.
Figure 2. Chronic topiramate and lamotrigine treatment suppresses PIDs.
A) Upper panel shows representative electrophysiological tracings of repetitive PIDs that spontaneously arise around focal ischemic tissue during filament middle cerebral artery occlusion (fMCAO) after 7 weeks of daily treatment with vehicle (VEH, blue), topiramate (TPM, red) or lamotrigine (LTG, green) in WT mice. Lower left panel summarizes all experiments. Horizontal lines indicate the time of onset and end of electrophysiological recordings with respect to fMCAO onset in each mouse, and circles indicate PIDs. Line graph shows average cumulative PID occurrence per mouse as a function of time. When calculating the cumulative PID occurrence over time, differences in group sizes and recording durations were taken into account. Whisker-box plots show average overall PID frequency. n=6, 9 and 8 mice in vehicle, topiramate and lamotrigine groups, respectively. One-way ANOVA followed by Holm-Sidak's multiple comparisons test. Treatment effect F(2,23)=18.1, p<0.0001. Post-hoc comparisons: *p<0.05 vs. VEH; †p<0.05 vs. TPM.
B) Left panel summarizes all experiments where horizontal lines indicate the time of onset and end of electrophysiological recordings with respect to fMCAO onset in each mouse, and circles indicate PIDs. Line graph shows average cumulative PID occurrence per mouse as a function of time. When calculating the cumulative PID occurrence over time, differences in group sizes and recording durations were taken into account and corrected for. Whisker-box plots show average overall PID frequency. n=7, 5 and 4 mice in vehicle, topiramate and lamotrigine groups, respectively. One-way ANOVA followed by Holm-Sidak's multiple comparisons test. Treatment effects were not statistically significant.
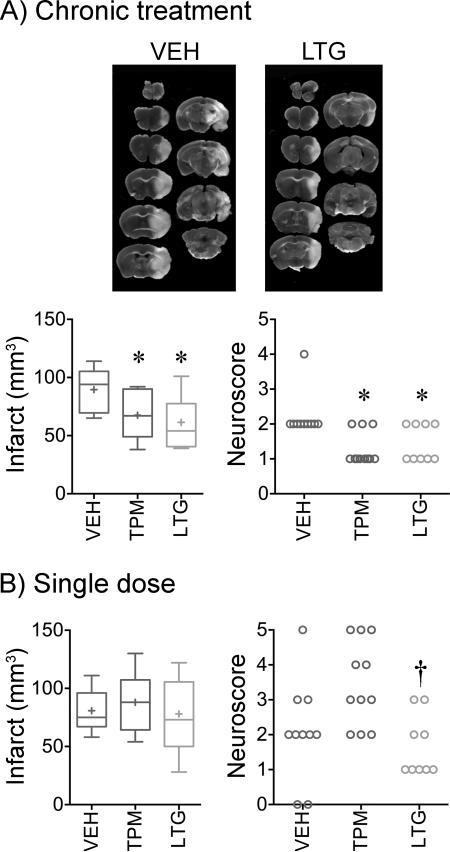
Improved stroke outcomes after chronic treatment
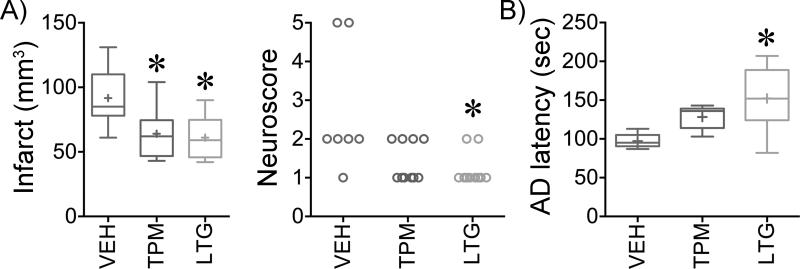
We next tested whether suppression of PIDs translated into improved stroke outcomes in WT mice. Chronic treatment with either drug reduced the infarct size after transient fMCAO by approximately 30%, and improved neurological outcomes (Figure 3A). Smaller infarcts predominantly reflected less severe cortical involvement (71±10, 50±11 and 48±9 mm3 in vehicle, topiramate and lamotrigine groups, respectively; p<0.05). Ischemic brain swelling, calculated by subtracting the contralateral from ipsilateral hemispheric volume, was also reduced by chronic topiramate or lamotrigine treatment compared with vehicle (8±2, 8±2 and 16±2 mm3, respectively; p<0.05), possibly linked to less frequent PIDs. Neurological outcomes assessed using a combined death and disability score as a clinically relevant endpoint13 were improved after chronic treatment with topiramate or lamotrigine compared with vehicle (Figure 3A). In contrast to chronic treatment, single doses of either drug did not affect any of the outcome endpoints compared with vehicle after transient fMCAO (Figure 3B).
Figure 3. Chronic topiramate and lamotrigine treatment improves stroke outcomes.
A) Representative TTC-stained 1-mm-thick coronal sections show the infarct 24 hours after 1-hour transient filament middle cerebral artery occlusion. Whisker-box plot summarizes the indirect infarct volumes after 7 weeks of daily treatment with vehicle (VEH, blue), topiramate (TPM, red) or lamotrigine (LTG, green) in WT mice. Neurological deficit scores are also shown in individual animals. n=10, 11 and 9 mice in vehicle, topiramate and lamotrigine groups, respectively. One-way ANOVA followed by Holm-Sidak's multiple comparisons test for infarct volume, or Kruskal-Wallis followed by Dunn's multiple comparisons test for neurological deficit score. Infarct volume: treatment effect F(2,27)=5.5, p=0.01. Neuroscore: treatment effect Kruskal-Wallis statistic 12.3, p=0.0021. Post-hoc comparisons: *p<0.05 vs. vehicle.
B) Whisker-box plot summarizes the indirect infarct volumes after a single dose of vehicle, topiramate or lamotrigine in WT mice. n=10, 11 and 9 mice in vehicle, topiramate and lamotrigine groups, respectively. One-way ANOVA followed by Holm-Sidak's multiple comparisons test. Neuroscore: treatment effect Kruskal-Wallis statistic 9.4, p=0.009. Post-hoc comparisons: †p<0.05 vs. topiramate
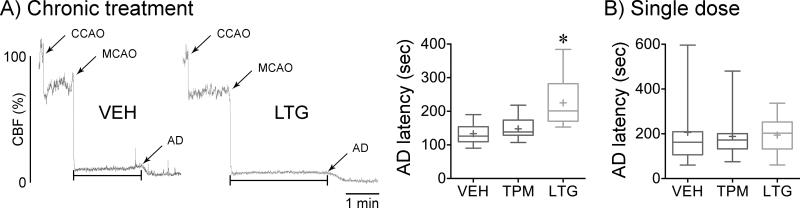
Delayed anoxic depolarization onset
Anoxic depolarization (AD) represents loss of membrane ionic gradients upon ischemic failure of Na+/K+-ATPase function. We have previously shown that migraine mutations hasten AD after focal ischemia and this correlated well with SD susceptibility and tissue outcome13. Therefore, we assessed whether decreased SD susceptibility after administrating migraine prophylactic drugs was associated with delayed AD onset in WT mice, detected by its cerebral vasoconstrictive effect as previously described4, 13. Chronic treatment with lamotrigine, but not topiramate, delayed the onset of AD by approximately 25% (Figure 4A). The magnitude of CBF reduction in the ischemic core did not differ among groups (residual CBF 12±5%, 13±5% and 12±3% of baseline for vehicle, topiramate and lamotrigine, respectively), eliminating the possibility that slower AD onset was due to milder ischemia. A single dose of either drug did not affect the latency to AD (Figure 4B).
Figure 4. Chronic topiramate and lamotrigine treatment shortens AD latency after ischemia onset.
A) Left panel shows representative laser Doppler cerebral blood flow (CBF) reductions induced by occlusion of the common carotid artery (CCAO) and the middle cerebral artery (MCAO), and the subsequent drop in CBF that marks the onset of AD. AD latency is measured as shown by the horizontal line. This secondary endpoint was measured in all fMCAO experiments performed for PID frequency and tissue and neurological outcome assessments. Whisker-box plot summarizes AD latency after 7 weeks of daily treatment with vehicle (VEH, blue), topiramate (TPM, red) or lamotrigine (LTG, green) in WT mice. n=17, 14 and 13 mice in vehicle, topiramate and lamotrigine groups, respectively. One-way ANOVA followed by Holm-Sidak's multiple comparisons test. Treatment effect F(2,41)=16.0, p<0.0001. Post-hoc comparisons: *p<0.05 vs. vehicle and topiramate.
B) Whisker-box plot summarizes AD latency after a single dose of vehicle, topiramate or lamotrigine in WT mice. n=13, 16 and 13 mice in vehicle, topiramate and lamotrigine groups, respectively. One-way ANOVA followed by Holm-Sidak's multiple comparisons test. Treatment effects were not statistically significant.
Improved stroke outcomes after chronic treatment in FHM1 mice
After showing that migraine prophylaxis with topiramate and lamotrigine improves stroke outcomes in WT mice, we also tested whether efficacy is sustained in migraine-susceptible FHM1 brains. Chronic treatment with either topiramate or lamotrigine reduced infarct size after transient fMCAO in FHM1 mutants by 30-35% (Figure 5); however, improved neurological function and the delay in the onset of AD reached statistical significance only in the lamotrigine group.
Figure 5. Chronic topiramate and lamotrigine treatment improves stroke outcomes in FHM1 mutant mice.
A) Whisker-box plot summarizes the indirect infarct volumes after 7 weeks of daily treatment with vehicle (VEH, blue), topiramate (TPM, red) or lamotrigine (LTG, green) in R192Q mutant mice. Neurological deficit scores are also shown in individual animals. n=7, 10 and 10 mice in vehicle, topiramate and lamotrigine groups, respectively. *p<0.05 vs. vehicle. One-way ANOVA followed by Holm-Sidak's multiple comparisons test for infarct volume, and Kruskal-Wallis followed by Dunn's multiple comparisons test for neurological deficit score. Infarct volume: treatment effect F(2,24)=6.0, p=0.0075. Neuroscore: treatment effect Kruskal-Wallis statistic 8.6, p=0.0136. Post-hoc comparisons: *p<0.05 vs. vehicle.
B) Whisker-box plot summarizes AD latency after a single dose of vehicle, topiramate or lamotrigine (LTG, green) in R192Q mutant mice. n=5, 11 and 10 mice in vehicle, topiramate and lamotrigine groups, respectively. One-way ANOVA followed by Holm-Sidak's multiple comparisons test. Treatment effect F(2,23)=6.8, p=0.0048. Post-hoc comparisons: *p<0.05 vs. vehicle and topiramate.
DISCUSSION
Migraine is an established risk factor for ischemic stroke. We have recently shown that genetically enhanced SD susceptibility worsens the impact of cerebral ischemia on the brain by facilitating ischemic depolarization events13, as one mechanism to explain the increased risk of stroke in migraineurs, Conversely, we here show that pharmacological suppression of SD susceptibility by migraine prophylactic drugs inhibit AD and PIDs and improve stroke evolution in both WT and FHM1 mutant mice. The magnitude of SD suppression by each drug corresponded well with the magnitude of AD and PID suppression, and stroke outcome. Consistent with this, genetically reduced susceptibility to SD as observed in rolling Nagoya and leaner mice, which have spontaneously arisen mutations in the Cacna1a gene leading to loss of CaV2.1 function, was associated with smaller infarcts, compared with WT upon experimental stroke28. These data strongly support intrinsic SD susceptibility of brain tissue (i.e., the tissue factor) as an important determinant of stroke outcome.
Although in vitro studies of topiramate and lamotrigine have suggested a neuroprotective effect29, in vivo studies were generally negative in various models of focal cerebral ischemia18-22. All studies, however, have tested single doses or short-term treatment administered before or after ischemia onset. Our data suggest that chronic treatment is required for efficacy, as has been the case for SD suppression in rats14, 16 and for the prophylactic effect on migraine in patients. Both topiramate and lamotrigine have been shown to acutely inhibit various voltage-gated ion channels as well as glutamatergic neurotransmission30, 31. However, whether chronic treatment simply enhances these effects by achieving higher tissue levels, or induces structural or gene expression changes, remains to be determined.
Although PIDs are generally thought to enlarge infarcts by worsening the supply demand mismatch, an alternative and possibly complementary mechanism is a further increase in cerebral excitability by SD shown in neocortical slices32, 33; PID inhibition by migraine prophylaxis may prevent this delayed hyperexcitability and improve outcome. Of course, glial cells critically modulate SD susceptibility, and glial protective effects of topiramate and lamotrigine34-36 may also contribute to PID suppression and infarct reduction.
It is well established that PIDs worsen stroke outcomes6, 37, and that drugs acutely inhibiting PIDs after a single dose (e.g., NMDA receptor antagonists) are protective in focal cerebral ischemia both in experimental animals and in stroke patients4, 38-40. However, clinical translation of this neuroprotective target has been difficult due to the cognitive and sedative side effects of such potent drugs41-43. In this respect, migraine prophylaxis may provide a better-tolerated anti-excitatory treatment alternative targeting to suppress SD and PIDs in stroke prophylaxis. Consistent with this notion, chronic treatment with lamotrigine was reported to diminish stroke-like episodes in a migraineur with mitochondrial encephalopathy, lactic acidosis and stroke-like episodes44, suggesting that the approach may be even more efficacious in hyperexcitable subsets.
SUMMARY AND CONCLUSIONS
In summary, our data suggest that pharmacological suppression of SD susceptibility may protect against ischemic injury in patients at high risk for stroke, migraineurs and non-migraineurs alike. Whether migraine prophylaxis clinically improves stroke outcomes or reduces the stroke risk remains to be tested in large population-based studies. Although chronic treatment purely as a form of stroke prophylaxis may not be justified at this time due to potential side effects, migraine patients who are already on a migraine prophylactic regimen may indeed see a reduction in their stroke risk as an additional benefit.
Acknowledgements
None.
Funding sources: This work was supported by the American Heart Association (10SDG2610275), the Claflin Distinguished Award from the Massachusetts General Hospital, National Institutes of Health (NS061505, NS055104, NS35611); Fondation Leducq; The Heitman Foundation; The Ellison Foundation; Netherlands Organization for Scientific Research (903-52-291 and Vici 918.56.602; Spinoza 2009); EU BRAINPATH (612360); and the Centre for Medical Systems Biology in the framework of the Netherlands Genomics Initiative.
Footnotes
Disclosures: Authors declare no financial conflict of interest.
Bibliography
- 1.IHS The international classification of headache disorders. Cephalalgia. (2nd edition.) 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 2.Kurth T, Schurks M, Logroscino G, Gaziano JM, Buring JE. Migraine, vascular risk, and cardiovascular events in women: Prospective cohort study. Bmj. 2008;337:a636. doi: 10.1136/bmj.a636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hopwood SE, Parkin MC, Bezzina EL, Boutelle MG, Strong AJ. Transient changes in cortical glucose and lactate levels associated with peri-infarct depolarisations, studied with rapid-sampling microdialysis. J Cereb Blood Flow Metab. 2005;25:391–401. doi: 10.1038/sj.jcbfm.9600050. [DOI] [PubMed] [Google Scholar]
- 4.Shin HK, Dunn AK, Jones PB, Boas DA, Moskowitz MA, Ayata C. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26:1018–1030. doi: 10.1038/sj.jcbfm.9600252. [DOI] [PubMed] [Google Scholar]
- 5.Strong AJ, Anderson PJ, Watts HR, Virley DJ, Lloyd A, Irving EA, et al. Peri-infarct depolarizations lead to loss of perfusion in ischaemic gyrencephalic cerebral cortex. Brain : a journal of neurology. 2007;130:995–1008. doi: 10.1093/brain/awl392. [DOI] [PubMed] [Google Scholar]
- 6.Dreier JP, Woitzik J, Fabricius M, Bhatia R, Major S, Drenckhahn C, et al. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129:3224–3237. doi: 10.1093/brain/awl297. [DOI] [PubMed] [Google Scholar]
- 7.Dohmen C, Sakowitz OW, Fabricius M, Bosche B, Reithmeier T, Ernestus RI, et al. Spreading depolarizations occur in human ischemic stroke with high incidence. Annals of neurology. 2008;63:720–728. doi: 10.1002/ana.21390. [DOI] [PubMed] [Google Scholar]
- 8.Anttila V, Winsvold BS, Gormley P, Kurth T, Bettella F, McMahon G, et al. Genome-wide meta-analysis identifies new susceptibility loci for migraine. Nat Genet. 2013;45:912–917. doi: 10.1038/ng.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anttila V, Stefansson H, Kallela M, Todt U, Terwindt GM, Calafato MS, et al. Genome-wide association study of migraine implicates a common susceptibility variant on 8q22.1. Nat Genet. 2010;42:869–873. doi: 10.1038/ng.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tottene A, Conti R, Fabbro A, Vecchia D, Shapovalova M, Santello M, et al. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in ca(v)2.1 knockin migraine mice. Neuron. 2009;61:762–773. doi: 10.1016/j.neuron.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 11.van den Maagdenberg AM, Pietrobon D, Pizzorusso T, Kaja S, Broos LA, Cesetti T, et al. A cacna1a knockin migraine mouse model with increased susceptibility to cortical spreading depression. Neuron. 2004;41:701–710. doi: 10.1016/s0896-6273(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 12.Eikermann-Haerter K, Dilekoz E, Kudo C, Savitz SI, Waeber C, Baum MJ, et al. Genetic and hormonal factors modulate spreading depression and transient hemiparesis in mouse models of familial hemiplegic migraine type 1. The Journal of clinical investigation. 2009;119:99–109. doi: 10.1172/JCI36059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eikermann-Haerter K, Lee JH, Yuzawa I, Liu CH, Zhou Z, Shin HK, et al. Migraine mutations increase stroke vulnerability by facilitating ischemic depolarizations. Circulation. 2012;125:335–345. doi: 10.1161/CIRCULATIONAHA.111.045096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz MA. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol. 2006;59:652–661. doi: 10.1002/ana.20778. [DOI] [PubMed] [Google Scholar]
- 15.Brandes JL, Saper JR, Diamond M, Couch JR, Lewis DW, Schmitt J, et al. Topiramate for migraine prevention: A randomized controlled trial. JAMA. 2004;291:965–973. doi: 10.1001/jama.291.8.965. [DOI] [PubMed] [Google Scholar]
- 16.Bogdanov VB, Multon S, Chauvel V, Bogdanova OV, Prodanov D, Makarchuk MY, et al. Migraine preventive drugs differentially affect cortical spreading depression in rat. Neurobiol Dis. 2011;41:430–435. doi: 10.1016/j.nbd.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Linde M, Mulleners WM, Chronicle EP, McCrory DC. Antiepileptics other than gabapentin, pregabalin, topiramate, and valproate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;6:CD010608. doi: 10.1002/14651858.CD010608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madden K, Clark W, Lessov N. Failure of ischemic neuroprotection by potentiators of gamma-aminobutyric acid. Clinical medicine & research. 2003;1:119–124. doi: 10.3121/cmr.1.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, Shuaib A, Li Q, Siddiqui MM. Neuroprotection by delayed administration of topiramate in a rat model of middle cerebral artery embolization. Brain Res. 1998;804:169–176. doi: 10.1016/s0006-8993(98)00410-7. [DOI] [PubMed] [Google Scholar]
- 20.Ataus SA, Onal MZ, Ozdem SS, Locke KW, Balkan S. The effects of citicoline and lamotrigine alone and in combination following permanent middle cerebral artery occlusion in rats. Int J Neurosci. 2004;114:183–196. doi: 10.1080/00207450490249329. [DOI] [PubMed] [Google Scholar]
- 21.Traystman RJ, Klaus JA, DeVries AC, Shaivitz AB, Hurn PD. Anticonvulsant lamotrigine administered on reperfusion fails to improve experimental stroke outcomes. Stroke. 2001;32:783–787. doi: 10.1161/01.str.32.3.783. [DOI] [PubMed] [Google Scholar]
- 22.Smith SE, Meldrum BS. Cerebroprotective effect of lamotrigine after focal ischemia in rats. Stroke. 1995;26:117–121. doi: 10.1161/01.str.26.1.117. discussion 121-112. [DOI] [PubMed] [Google Scholar]
- 23.van den Maagdenberg AM, Pizzorusso T, Kaja S, Terpolilli N, Shapovalova M, Hoebeek FE, et al. High cortical spreading depression susceptibility and migraine-associated symptoms in ca(v)2.1 s218l mice. Ann Neurol. 2010;67:85–98. doi: 10.1002/ana.21815. [DOI] [PubMed] [Google Scholar]
- 24.Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab. 2000;20:631–652. doi: 10.1097/00004647-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Krause DN, Duckles SP, Pelligrino DA. Influence of sex steroid hormones on cerebrovascular function. Journal of applied physiology. 2006;101:1252–1261. doi: 10.1152/japplphysiol.01095.2005. [DOI] [PubMed] [Google Scholar]
- 26.Kaminski RM, Banerjee M, Rogawski MA. Topiramate selectively protects against seizures induced by atpa, a glur5 kainate receptor agonist. Neuropharmacology. 2004;46:1097–1104. doi: 10.1016/j.neuropharm.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Cuadrado A, Bravo J, Armijo JA. Synergistic interaction between felbamate and lamotrigine against seizures induced by 4-aminopyridine and pentylenetetrazole in mice. Eur J Pharmacol. 2003;465:43–52. doi: 10.1016/s0014-2999(03)01460-2. [DOI] [PubMed] [Google Scholar]
- 28.Tian X, Zhou Y, Gao L, He G, Jiang W, Li W, et al. Analysis of ischemic neuronal injury in cav2.1 channel alpha1 subunit mutant mice. Biochem Biophys Res Commun. 2013;434:60–64. doi: 10.1016/j.bbrc.2013.03.066. [DOI] [PubMed] [Google Scholar]
- 29.Costa C, Martella G, Picconi B, Prosperetti C, Pisani A, Di Filippo M, et al. Multiple mechanisms underlying the neuroprotective effects of antiepileptic drugs against in vitro ischemia. Stroke. 2006;37:1319–1326. doi: 10.1161/01.STR.0000217303.22856.38. [DOI] [PubMed] [Google Scholar]
- 30.Martella G, Costa C, Pisani A, Cupini LM, Bernardi G, Calabresi P. Antiepileptic drugs on calcium currents recorded from cortical and pag neurons: Therapeutic implications for migraine. Cephalalgia. 2008;28:1315–1326. doi: 10.1111/j.1468-2982.2008.01682.x. [DOI] [PubMed] [Google Scholar]
- 31.Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. 2004;5:553–564. doi: 10.1038/nrn1430. [DOI] [PubMed] [Google Scholar]
- 32.Berger M, Speckmann EJ, Pape HC, Gorji A. Spreading depression enhances human neocortical excitability in vitro. Cephalalgia. 2008;28:558–562. doi: 10.1111/j.1468-2982.2008.01556.x. [DOI] [PubMed] [Google Scholar]
- 33.Ghadiri MK, Kozian M, Ghaffarian N, Stummer W, Kazemi H, Speckmann EJ, et al. Sequential changes in neuronal activity in single neocortical neurons after spreading depression. Cephalalgia. 2012;32:116–124. doi: 10.1177/0333102411431308. [DOI] [PubMed] [Google Scholar]
- 34.Angehagen M, Ronnback L, Hansson E, Ben-Menachem E. Topiramate reduces ampa-induced ca(2+) transients and inhibits glur1 subunit phosphorylation in astrocytes from primary cultures. J Neurochem. 2005;94:1124–1130. doi: 10.1111/j.1471-4159.2005.03259.x. [DOI] [PubMed] [Google Scholar]
- 35.Angehagen M, Ben-Menachem E, Ronnback L, Hansson E. Topiramate protects against glutamate- and kainate-induced neurotoxicity in primary neuronal-astroglial cultures. Epilepsy Res. 2003;54:63–71. doi: 10.1016/s0920-1211(03)00039-1. [DOI] [PubMed] [Google Scholar]
- 36.Pavone A, Cardile V. An in vitro study of new antiepileptic drugs and astrocytes. Epilepsia. 2003;44(Suppl 10):34–39. doi: 10.1046/j.1528-1157.44.s10.5.x. [DOI] [PubMed] [Google Scholar]
- 37.Hartings JA, Watanabe T, Bullock MR, Okonkwo DO, Fabricius M, Woitzik J, et al. Spreading depolarizations have prolonged direct current shifts and are associated with poor outcome in brain trauma. Brain. 2011;134:1529–1540. doi: 10.1093/brain/awr048. [DOI] [PubMed] [Google Scholar]
- 38.Sakowitz OW, Kiening KL, Krajewski KL, Sarrafzadeh AS, Fabricius M, Strong AJ, et al. Preliminary evidence that ketamine inhibits spreading depolarizations in acute human brain injury. Stroke. 2009;40:e519–522. doi: 10.1161/STROKEAHA.109.549303. [DOI] [PubMed] [Google Scholar]
- 39.Taghibiglou C, Martin HG, Lai TW, Cho T, Prasad S, Kojic L, et al. Role of nmda receptor-dependent activation of srebp1 in excitotoxic and ischemic neuronal injuries. Nat Med. 2009;15:1399–1406. doi: 10.1038/nm.2064. [DOI] [PubMed] [Google Scholar]
- 40.Tu W, Xu X, Peng L, Zhong X, Zhang W, Soundarapandian MM, et al. Dapk1 interaction with nmda receptor nr2b subunits mediates brain damage in stroke. Cell. 2010;140:222–234. doi: 10.1016/j.cell.2009.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ikonomidou C, Turski L. Why did nmda receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 42.Lees KR, Asplund K, Carolei A, Davis SM, Diener HC, Kaste M, et al. Glycine antagonist (gavestinel) in neuroprotection (gain international) in patients with acute stroke: A randomised controlled trial. Gain international investigators. Lancet. 2000;355:1949–1954. doi: 10.1016/s0140-6736(00)02326-6. [DOI] [PubMed] [Google Scholar]
- 43.Dyker AG, Edwards KR, Fayad PB, Hormes JT, Lees KR. Safety and tolerability study of aptiganel hydrochloride in patients with an acute ischemic stroke. Stroke; a journal of cerebral circulation. 1999;30:2038–2042. doi: 10.1161/01.str.30.10.2038. [DOI] [PubMed] [Google Scholar]
- 44.Finsterer J, Barton P. Regression of stroke-like lesions in melas-syndrome after seizure control. Epileptic Disord. 2010;12:330–334. doi: 10.1684/epd.2010.0338. [DOI] [PubMed] [Google Scholar]