Abstract
Purpose
To analyze intraocular pressure (IOP) response after 20-mg decanted intravitreal triamcinolone acetonide (IVTA) followed by early prophylactic IOP-lowering therapy.
Methods
We retrospectively reviewed IOP results of 120 high-dose decanted IVTA injections from 58 non-glaucomatous patients with macular edema, with antiglaucoma therapy prescribed from week 1 regardless of baseline IOP.
Results
In cases of consistent compliance with IOP-lowering drugs (79.2%), IOP increased by 2 mmHg at 4 months (p=0.300) and returned to baseline at 6 months. In cases of non-compliance (20.8%), IOP increased by 7 mmHg at 1 month (p<0.001) and returned to baseline after starting treatment. Multivariate regression analysis showed that non-vitrectomized eyes and non-compliance with IOP-lowering drugs were independent predictors for IOP rise over 21 mmHg (p=0.0098 and p=0.0019, respectively). Non-vitrectomized eyes had a 46% greater chance to experience IOP rise compared to vitrectomized. Poor compliance with IOP-lowering drugs lead to a 45% greater likelihood of experiencing IOP rise compared to compliant patients. Multiple injections were not associated with increased risk for IOP rise over 21 mmHg (p=0.273). Out of 120 cases, 2 eyes (1.7%) developed uncontrolled IOP and required glaucoma surgery by 4 months, with good final IOP outcome.
Conclusion
20-mg decanted IVTA can be safely used to treat macular edema in nonglaucomatous patients; IOP elevation can be adequately controlled with prophylactic antiglaucoma drugs. Non-compliance with prophylactic therapy creates an early spike in IOP, and vitreous status can significantly impact IOP rise. Compliance with IOP-lowering drugs should be stressed to patients receiving high-dose IVTA especially if non-vitrectomized.
Keywords: triamcinolone acetonide, Kenalog, macular edema, intraocular pressure, glaucoma
Introduction
Intravitreal (IV) triamcinolone acetonide (TA) has been utilized as a treatment option for a variety of intraocular inflammatory, vascular, edematous and proliferative processes. Even with the advent of anti-vascular endothelium grow factor (VEGF) agents, IVTA remains an effective and low-cost treatment modality when used alone or in combination with other treatment options. However, it is well known that many side effects may occur after multiple IVTA, including intraocular pressure (IOP) elevation, cataract formation and progression, and pseudo or true (infectious) endophthalmitis.1 These adverse effects, especially elevated IOP, can pose significant long-term consequences including the development of glaucoma, potentially necessitating intraocular surgery.
Kenalog-40 (Brystol-Meyers-Squibb, Princeton, NJ), initially developed for intraarticular or intramuscular applications, is the most used TA for IV injection in the US despite not being recommended for intraocular use by its manufacturer. Most groups give 4-mg injections of Kenalog, which includes the benzyl alcohol preservative that is possibly toxic.2 This is prepared by injecting 0.1 ml of the mixed commercial preparation. On the contrary, the decanted formulation of Kenalog (obtained after manually removing the supernatant from the vial) provides up to 40 mg/mL of TA without carrying the toxicity of the preservative.3 Indeed, we have previously shown that decanting decreases the dose of benzyl alcohol going into the eye by 80%.2
The rationale for using high concentrations of IVTA for macular edema arises from previous studies that demonstrated that the duration of the effect of TA in human eyes increases significantly with the dose.4 This means that the higher the dose, the longer the activity of IVTA. While TA concentration in human aqueous remains above therapeutic concentration for 90 days after a 4-mg IV injection5, our group previously demonstrated that a 20-mg IV injection provides a longer therapeutic concentration, up to 150 days in human aqueous, suggesting an even longer therapeutic concentration of TA in vitreous.6 The long-acting activity of TA in the vitreous chamber has the important ability to inhibit the inflammatory response and to reduce edema formation1; therefore, it’s not surprising that the visual gain after high-dose IVTA is greater than low-dose IVTA as previously reported.4 This finding translates into a lower treatment burden for macular edema.
As the efficacy and duration of IVTA increases with higher doses, we could also expect an increase in the side effects compared to low-dose IVTA, especially elevated and uncontrolled IOP. Using the standard low-dose TA concentration of 4-mg, mild to moderate IOP elevation has been reported in 28 - 42% of patients, typically within the first 3 months following injection, but usually this condition is controlled with topical agents alone if IOP rises.1 Some authors suggest that a premedication with topical steroids may be useful to identify possible steroid responders; excluding these patients from IVTA treatment may lower the incidence of IOP elevation.7, 8 Previous studies have demonstrated that there is a similar incidence of IOP elevation using 20-mg decanted IVTA or 4-mg IVTA.3, 4 However, IOP-lowering drugs were started only in cases of IOP elevation during follow-up. We believe that a prophylactic IOP-lowering treatment started a few weeks after IVTA may be another reasonable option to avoid uncontrolled IOP spikes. Therefore, the aim of the present study was to analyze the IOP response after 20-mg decanted Kenalog over a 6-month period, and to determine if early prophylactic IOP-lowering therapy resulted in lower IOP-related complications.
Methods
Starting from 2008, a standardized IOP-lowering treatment protocol was adopted at the Jacobs Retina Center at Shiley Eye Center (University of California San Diego) for all patients scheduled for 20-mg decanted IVTA injection (Kenalog, Brystol-Meyers-Squibb, Princeton, NJ) for macular edema. One week after IVTA injection, patients were instructed to start IOP-lowering treatment with Dorzolamide/Timolol or Brimonidine Tartrate 0.1% (in cases of contraindication of Dorzolamide/Timolol), regardless of baseline IOP. With the approval of the Institutional Review Board at UCSD, we retrospectively reviewed the charts of all patients who underwent high-dose decanted IVTA injection between 2008 and 2012 for macular edema secondary to retinal vein occlusion, diabetic retinopathy, uveitis or post-surgical macular edema. We included only eyes that had a minimum follow-up of 6 months after IVTA. We excluded patients who were treated with topical and/or systemic steroids within 3 months prior to injection in order to assess the TA effect. Since high-dose decanted TA was not performed in eyes with documented history of glaucoma or ongoing use of IOP-lowering medications, this study did not analyze the effect of IVTA on such eyes.
The preparation of the 20-mg decanted TA was performed as described in a previous report.3 Briefly, Kenalog vials (40 mg/mL, Bristol-Myers-Squibb, Princeton, NJ) were permanently kept in a dedicated holding box that was designed to maintain the vials at a 45-degree angle with the top up. Two hours are generally needed to make the drug precipitate. After removing the supernatant (0.8 mL), 0.2 mL of the remaining slurry was then loaded into a syringe and a total of 0.1 mL (20 mg) was injected using a 27 G needle via pars plana. IOP-lowering drops were prescribed as above. Patients had IOP checked by applanation tonometry on the day of injection and at each subsequent follow-up visit, generally at 1, 3, and 6 months.
Chart review included collection of multiple data points including age, gender, diagnosis, IOP, vitreous status (natural or vitrectomized), and lens status (phakic or pseudophakic) at the date of IVTA injection (considered as the baseline visit). IOP measurements for all the subsequent visits were collected, as well as the need for any laser or surgical treatment for glaucoma. Patient compliance with IOP-lowering drug was implied from visit notes and patients were considered compliant if they described consistent use of antiglaucoma medications at each follow-up visit.
Statistical analyses were performed with JMP statistical discovery software (version 11) with Mixed Models in which subjects were assigned as Random Effect. The models with random effects were fitted with restricted maximum likelihood method. A p-value <0.05 was considered to be statistically significant.
Results
120 IV injections of 20-mg decanted Kenalog from 65 eyes of 58 patients were reviewed and included in the study. Out of 58 patients, 29 received more than one IVTA (median = 2), with at least 3 months between injections. The baseline characteristics of the patients and the indications for IVTA injection are summarized in Table 1.
Table 1.
Baseline Characteristics of the Sample
| Number of IVTA Injections | 120 |
| Number of Eyes | 65 |
| Number of Patients | 58 |
| Sex | |
| Male | 30 (51.7%) |
| Female | 28 (48.3%) |
| Age (years) | |
| Range | 33-89 |
| Mean ± SD | 70.2 ± 12.9 |
| IOP (mmHg) | |
| Range | 1-21 |
| Mean ± SD | 14.6 ± 4.2 |
| Vitreous Status | |
| Natural | 15 (12.5%) |
| Vitrectomized | 105 (87.5%) |
| Lens Status | |
| Natural | 21 (17.5%) |
| Implant | 99 (82.5%) |
| Diagnosis | |
| Post-surgical Macular Edema | 60 (50%) |
| Retinal Vein Occlusions | 24 (20%) |
| Diabetic Retinopathy | 14 (11.7%) |
| Uveitis | 8 (6.7%) |
| Wet AMD | 7 (5.8%) |
| Radiation Retinopathy | 7 (5.8%) |
IVTA, intravitreal triamcinolone acetonide; SD, standard deviation; IOP, intraocular pressure; AMD, age-related macular degeneration
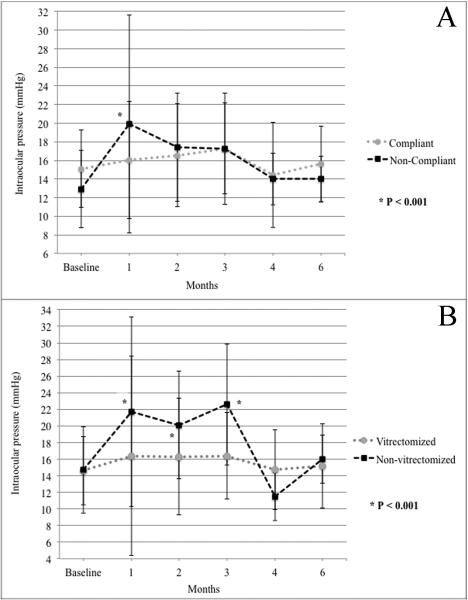
In 95 of 120 injections (79.2%), patients reported consistent compliance with IOP-lowering drugs, while in 25 of 120 injections (20.8%) patients forgot to start antiglaucoma therapy at 1-week time point. In cases of compliance with the prophylactic therapy, mean IOP increased by 2 mmHg at 3-4 months (p=0.300) and returned to baseline at 6 months (Figure 1, A). In cases of non-compliance, mean IOP increased by 7 mmHg at month 1 (p<0.001) but returned to baseline after starting IOP-lowering treatment at the following visit (Figure 1, A). Maximum IOP was greater than 21 mmHg after 16 of 95 injections in patients compliant with the therapy (16.8%) and after 13 of 25 injections of non-compliant patients (52.0%, p=0.001). Each group had one case with IOP between 31 and 40 mmHg, and another single case with IOP greater than 40 mmHg during follow-up period. None of the injected eyes had an IOP lower than 5 mmHg during follow-up. Combinations of antiglaucomatous topical drugs were sufficient to lower the IOP to normal levels in all but 2 cases in the compliant group.
Fig 1.
Changes in intraocular pressure (IOP) after 20-mg decanted Kenalog intravitreal injection from baseline to month 6. (A) In cases of non-compliance with prophylactic IOP-lowering therapy, mean IOP increased by 7 mmHg at month 1 (p<0.001) but returned to baseline after starting IOP-lowering treatment at the following visit. Compliant patients did not experience the early IOP spike; IOP was maintained stable overtime. (B) In cases of non-vitrectomized eyes, significantly higher IOP measurements were noted during the first 3 months after Kenalog injection when compared to vitrectomized eyes (p<0.001). In cases of vitrectomized eye, IOP was maintained stable overtime.
In cases of non-vitrectomized eyes, significantly higher IOP measurements were noted during the first 3 months after IVTA when compared to vitrectomized eyes (p<0.001; Figure 1, B). Maximum IOP was greater than 21 mmHg in 20 of 105 injections of vitrectomized eyes (19.0%) and in 9 out of 15 non-vitrectomized eyes (60.0%, p=0.002). Each group had one case with IOP between 31 and 40 mmHg, and one case with IOP greater than 40 mmHg during follow-up period.
In cases of phakic eyes, significantly higher IOP measurements were noted during follow-up after IVTA when compared to pseudophakic eyes (p=0.003). Maximum IOP was greater than 21 mmHg in 14 of 99 injections of pseudophakic eyes (14.1%) and in 4 of 21 injections in phakic eyes (19.0%, p=0.123). Two cases in the group of phakic patients had an IOP between 31 and 40 mmHg, and 2 had an IOP greater than 40 mmHg during follow-up period.
Multivariate regression analysis showed that age, gender, diagnosis, and also lens status were not predictors for IOP rise, while non-vitrectomized eyes and non-compliance with IOP-lowering drugs were independent predictors for IOP rise over 21 mmHg (p=0.0098 and p=0.0019, respectively; Table 2). Non-vitrectomized patients had a 46% greater chance of experiencing an IOP greater than 21 mmHg compared to vitrectomized patients. Cases of poor compliance with IOP-lowering drugs had a 45% greater chance of experiencing an IOP greater than 21 mmHg compared to compliant patients. In addition, patients who underwent multiple injections were not at increased risk for IOP rise over 21 mmHg (p=0.273).
Table 2.
Association between intraocular pressure rise over 21 mmHg, vitreous status, and compliance with prophylactic antiglaucoma therapy.
| Level 1 | Level 2 | Odds Ratio* | Prob>Chisq | 95% CI | |
|---|---|---|---|---|---|
| Vitreous status | Vitrectomized | Non-Vitrectomized | 4.707 | 0.0098 | 1.462 - 15.659 |
| Non-Vitrectomized | Vitrectomized | 0.212 | 0.0098 | 0.064 - 0.684 | |
|
| |||||
|
Compliance with
prophylactic IOP-lowering therapy |
Compliant | Non-Compliant | 4.847 | 0.0019 | 1.804 - 13.301 |
| Non-Compliant | Compliant | 0.206 | 0.0019 | 0.075 - 0.554 | |
CI, confidence interval; IOP, intraocular pressure.
Tests and confidence intervals on Odds Ratios are likelihood ratio based.
Out of 120 cases, 8 developed IOP greater than 21 mmHg during follow-up, of which only two (1.7%) were uncontrolled despite maximal medical therapy. Both eyes with uncontrolled IOP were non-vitrectomized, and patients were compliant with IOP-lowering drugs. IOP rose to 40 mmHg between month 1 and 2. One patient required canaloplasty by month 4, the other required trabeculectomy with mitomycin-C by month 4; both patients had good final IOP outcome but some loss of peripapillary neuro-fiber layer thickness. No complications occurred after glaucoma surgery.
Discussion
The present study demonstrated a low incidence of elevated and uncontrolled IOP in the setting of prophylactic IOP-lowering therapy when using high-dose decanted IVTA for macular edema. When this group of non-glaucomatous patients was uniformly prophylaxed, irrespective of baseline IOP, the rate of uncontrolled IOP was low (1.7%). We confirmed the importance of compliance with IOP-lowering therapy, as the chances of recording an IOP greater than 21 mmHg were 45% higher in non-compliant cases. Also, we confirmed that non-vitrectomized eyes had a 46% greater risk of IOP over 21 mmHg; however, using prophylactic IOP-lowering medications the risk of IOP elevation above 31 or 41 mmHg was not increased. And finally, repeated IVTA injections did not affect the IOP.
Intravitreal steroids have known adverse effects including IOP elevation, cataract formation, infectious or non-infectious endophthalmitis; IOP elevation is the most common side effect among these. From previous studies we have learned that high doses of intravitreal steroids (up to 20-mg) can be used safely with better visual outcome4 and with similar safety profile3 when compared to standard doses (4-mg) that are used in everyday clinic practice. In a large meta-analysis of previously reported data and case series studies, Jonas et al. reported that intravitreal injections of approximately 20 mg of TA can increase IOP beyond 21 mmHg in up to 40% of patients, if not treated with IOP-lowering drugs.9 Usually investigators start IOP-lowering treatment if IOP exceeds approximately 25 mmHg, shifting to combination of drugs if IOP continues to be elevated. After starting topical antiglaucoma treatment, Jonas demonstrated that IOP reduces to baseline or lower levels approximately 8 to 9 months after the injection in all but 1% of the eyes.9 The decision to delay starting IOP-lowering treatment until IOP is greater than 25 mmHg may be clinically questionable, especially because we know that 40% of the cases develop excessively high IOP starting during the first week after 20-mg IVTA.9 Moreover, if patients skip a scheduled follow-up visit, an early IOP rise may be missed with possible subsequent optic nerve damage. Therefore, we believe that prophylactic treatment with IOP-lowering drugs may be a reasonable alternative to prevent glaucomatous damage after high-dose IVTA injection. Although prophylactic antiglaucomatous treatment could potentially induce hypotony, the lowest IOP level detected in our population during follow-up was 5 mmHg and we found no hypotony-related complications in our series. In cases of consistent compliance with IOP-lowering drugs, we found that IOP increased by only 2 mmHg at month 4, while in non-compliant cases IOP increased more robustly and at a more rapid rate (7 mmHg at month 1). The importance of compliance with prophylactic medications was highlighted with univariate regression analysis, with a 45% greater risk of experiencing IOP over 21 mmHg in cases of poor compliance. The effectiveness of prophylactic IOP lowering therapy and the highlighted risk of increased IOP in noncompliant patients accentuates the importance of careful patient selection in the use of high-dose IVTA. In addition, our data support the finding that IOP over 21 mmHg does not occur more frequently after a repeat 20-mg IVTA than after the first injection, as previously described by other investigators.10 However, this finding was likely due to ascertainment bias; eyes with elevated IOP were not felt to be candidate for a second IVTA injection.
In agreement with the literature3, 11, we also found that control of IOP was better in cases of previously vitrectomized eyes compared to non-vitrectomized eyes. The latter had a 46% greater risk of experiencing IOP over 21 mmHg compared to the former. Therefore, the vitreous status plays an important role in predicting IOP response to decanted IVTA. Previous investigators suggested that the reason for rapid clearance of TA in vitrectomized eye might be related largely to the condition and state of the vitreous.12 The vitreous is made of gel-like highly viscous materials, and highly viscous vitreous would have very slow circulation; therefore, it is likely that these properties of the normal vitreous would confine intravitreal corticosteroid to a small space. Furthermore, TA is largely water insoluble, and this would further slow down its clearance in normal vitreous. However, in vitrectomized eyes, less-viscous liquid fills the eye, increasing intravitreal circulation, promoting a diffuse distribution of TA throughout the vitreal cavity,12 and hastening its dissolution.
Despite a significant reduction in IOP elevation when using prophylactic antiglaucoma medications, 2 cases in our series (1.7%) developed uncontrolled glaucoma on maximal medical treatment. Both the eyes were non-vitrectomized, and both patients claimed to be compliant with the prophylactic antiglaucomatous therapy. This finding is in line with previous studies where penetrating surgery was necessary in a minority of patients on maximal medical treatment after standard or high dose intravitreal triamcinolone.9
Synthetic preservative-free steroid compounds are available for intravitreal use in the US.However, clinicians should consider the additional cost of preservative-free forms when compared to traditional Kenalog. Moreover, decanting or other methods of purifying commercial Kenalog permits the clinician to choose from a variety of concentrations for IV injections, depending on single-patient characteristic. The Ozurdex (Allergan, Inc., Irvine, CA) dexamethasone intraocular implant has been proven effective in the treatment of non-infectious uveitis13 and macular edema from a variety of retinal diseases including vein occlusions and diabetes.14-16 Dexamethasone is 5 times more potent and is more hydrophilic than TA, allowing for higher vitreous concentrations17; however it has shorter half-life in the vitreous cavity. The biodegradable implant releases the drug by diffusion in a biphasic fashion, with higher doses up to 60 days, followed by a rapid decline between day 60 and 90, and then it achieves lower steady levels.17 This pattern of drug release translated into a similar pattern of visual improvement after treatment with dexamethasone implant as shown by large clinical trials; the peak in visual gain was at day 60 after each injection, followed by a rapid decline.14-16 Therapeutic concentration of high-dose decanted TA has the benefit of lasting greater than 150 days in human aqueous, suggesting an even longer therapeutic concentration of TA in vitreous.6 Despite different clearance in human eyes between 0.7 mg Ozurdex and 20-mg decanted Kenalog, the drugs seem to have similar IOP safety profile: 1.7% of cases needed glaucoma surgery in our study, and 1.2% of patients in the Geneva study.15
Fluocinolone acetonide is a corticosteroid approved for the treatment of non-infectious uveitis with 1/24th the solubility of dexamethasone in an aqueous solution, allowing steroid release over a much longer time period.18 The Retisert (Bausch and Lomb, Rochester, NY) is a non-biodegradable, 0.59 mg sustained-release intravitreal implant, designed to continuously release fluocinolone acetonide in a linear fashion over a period of approximately 2.5 years. Although this implant can provide long-term control of uveitis, the significant adverse ocular effects including virtually a 100% cataract formation rate along with a high rate of progression to glaucoma,19 making it an option for use only when patients are recalcitrant to other interventions.
Triesence (Alcon Laboratories, Inc., Ft. Worth, TX) and Trivaris (Allergan, Inc, Irvine, CA) are also new synthetic preservative-free TA compounds for intraocular use. Both drugs provide high concentrations of TA, that is 40 mg/mL for Triesence, and 80 mg/mL for Trivaris.20 Although these preservative-free TA formulations have not been compared to decanted Kenalog in clinical trials, recent literature has shown that they do not have equal pharmacokinetics or pharmacodynamics. More precisely, Kenalog lasts longer than these other TA formulations in an in vivo model20, likely because Kenalog has larger particle sizes. This may translate into longer therapeutic duration and lower rate of drug-associated complications; however, further studies are necessary to answer this question.
In conclusion, our retrospective study in non-glaucomatous eyes confirmed that decanted high-dose IVTA plus prophylactic antiglaucoma treatment is a relatively safe choice when considering intravitreal steroids to treat macular edema. After IVTA injection, clinicians should cautiously follow patients whose compliance with eye drops is questionable, especially if non-vitrectomized, as these patients are at high risk of developing uncontrolled IOP. However, compliance with prophylactic IOP-lowering therapy should be stressed to all patients. A larger prospective study is indicated.
Summary Statement.
Using prophylactic IOP-lowering therapy after 20-mg decanted intravitreal Kenalog, IOP was well-compensated and glaucoma surgery was needed in 1.7% of the cases. Non-vitrectomized eyes had a 46% greater chance to experience IOP over 21 mmHg. Poor compliance with IOP-lowering drugs lead to a 45% greater likelihood of experiencing IOP over 21 mmHg.
Acknowledgments
Financial support: This study was supported in part by NIH grants R01EY007366 and R01EY018589 (WRF), R01EY020617 (LC), and an unrestricted fund from Research to Prevent Blindness to the Department of Ophthalmology, University of California San Diego.
Footnotes
Financial disclosure: None of the authors have any financial interests to disclose. No conflicting relationship exists for any author.
References
- 1.Veritti D, Di Giulio A, Sarao V, Lanzetta P. Drug safety evaluation of intravitreal triamcinolone acetonide. Expert opinion on drug safety. 2012;11:331–40. doi: 10.1517/14740338.2012.635141. [DOI] [PubMed] [Google Scholar]
- 2.Morrison VL, Koh HJ, Cheng L, Bessho K, Davidson MC, Freeman WR. Intravitreal toxicity of the kenalog vehicle (benzyl alcohol) in rabbits. Retina. 2006;26:339–44. doi: 10.1097/00006982-200603000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Tammewar AM, Cheng L, Kayikcioglu OR, et al. Comparison of 4 mg versus 20 mg intravitreal triamcinolone acetonide injections. Br J Ophthalmol. 2008;92:810–3. doi: 10.1136/bjo.2007.126227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spandau UH, Derse M, Schmitz-Valckenberg P, Papoulis C, Jonas JB. Dosage dependency of intravitreal triamcinolone acetonide as treatment for diabetic macular oedema. Br J Ophthalmol. 2005;89:999–1003. doi: 10.1136/bjo.2004.062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beer PM, Bakri SJ, Singh RJ, Liu W, Peters GB, 3rd, Miller M. Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology. 2003;110:681–6. doi: 10.1016/S0161-6420(02)01969-3. [DOI] [PubMed] [Google Scholar]
- 6.Cheng L, Banker AS, Martin M, Kozak I, Freeman WR. Triamcinolone acetonide concentration of aqueous humor after decanted 20-mg intravitreal injection. Ophthalmology. 2009;116:1356–9. doi: 10.1016/j.ophtha.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 7.Singh IP, Ahmad SI, Yeh D, et al. Early rapid rise in intraocular pressure after intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2004;138:286–7. doi: 10.1016/j.ajo.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Smithen LM, Ober MD, Maranan L, Spaide RF. Intravitreal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol. 2004;138:740–3. doi: 10.1016/j.ajo.2004.06.067. [DOI] [PubMed] [Google Scholar]
- 9.Jonas JB, Degenring RF, Kreissig I, Akkoyun I, Kamppeter BA. Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Ophthalmology. 2005;112:593–8. doi: 10.1016/j.ophtha.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 10.Jonas JB, Degenring R, Kreissig I, Akkoyun I. Safety of intravitreal high-dose reinjections of triamcinolone acetonide. Am J Ophthalmol. 2004;138:1054–5. doi: 10.1016/j.ajo.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 11.Chin HS, Park TS, Moon YS, Oh JH. Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and nonvitrectomized eyes. Retina. 2005;25:556–60. doi: 10.1097/00006982-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Schindler RH, Chandler D, Thresher R, Machemer R. The clearance of intravitreal triamcinolone acetonide. Am J Ophthalmol. 1982;93:415–7. doi: 10.1016/0002-9394(82)90130-1. [DOI] [PubMed] [Google Scholar]
- 13.Lowder C, Belfort R, Jr., Lightman S, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. Arch Ophthalmol. 2011;129:545–53. doi: 10.1001/archophthalmol.2010.339. [DOI] [PubMed] [Google Scholar]
- 14.Haller JA, Bandello F, Belfort R, Jr., et al. Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal vein occlusion twelve-month study results. Ophthalmology. 2011;118:2453–60. doi: 10.1016/j.ophtha.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Haller JA, Bandello F, Belfort R, Jr., et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134–1146. doi: 10.1016/j.ophtha.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 16.Callanan DG, Gupta S, Boyer DS, et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology. 2013;120:1843–51. doi: 10.1016/j.ophtha.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 17.London NJ, Chiang A, Haller JA. The dexamethasone drug delivery system: indications and evidence. Advances in therapy. 2011;28:351–66. doi: 10.1007/s12325-011-0019-z. [DOI] [PubMed] [Google Scholar]
- 18.Jaffe GJ, Yang CH, Guo H, Denny JP, Lima C, Ashton P. Safety and pharmacokinetics of an intraocular fluocinolone acetonide sustained delivery device. Invest Ophthalmol Vis Sci. 2000;41:3569–75. [PubMed] [Google Scholar]
- 19.Arcinue CA, Ceron OM, Foster CS. A comparison between the fluocinolone acetonide (Retisert) and dexamethasone (Ozurdex) intravitreal implants in uveitis. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2013;29:501–7. doi: 10.1089/jop.2012.0180. [DOI] [PubMed] [Google Scholar]
- 20.Zacharias LC, Lin T, Migon R, et al. Assessment of the differences in pharmacokinetics and pharmacodynamics between four distinct formulations of triamcinolone acetonide. Retina. 2013;33:522–31. doi: 10.1097/IAE.0b013e3182647f69. [DOI] [PubMed] [Google Scholar]