Abstract
NMDA receptor antagonists such as ketamine have emerged as novel candidate treatments for major depressive disorder, but abuse potential of these agents is a concern. The NMDA antagonist phencyclidine has known abuse liability but undefined efficacy as an antidepressant. To further evaluate the relationship between antidepressant-like and abuse-related effects of NMDA antagonists, this study evaluated the effects of phencyclidine (1.0-10.0 mg/kg) in male Sprague-Dawley rats responding under two procedures that have been used to assess antidepressant-like effects [differential-reinforcement-of-low-rate (DRL) 72 s schedule of food reinforcement; N=9] and abuse-related drug effects [intracranial self-stimulation (ICSS); N=6]. Under the DRL 72 s schedule, phencyclidine (10.0 mg/kg) increased reinforcers and decreased responses without shifting the peak location of the interresponse time (IRT) distribution. Ketamine (10.0 mg/kg) also increased reinforcers and decreased responses, but unlike phencyclidine, it produced a rightward shift in the peak location of the IRT distribution. The 10.0 mg/kg phencyclidine dose that decreased DRL 72 s responding also decreased rates of ICSS for 50 min after its administration; however, abuse-related ICSS facilitation was observed at later times (100-300 min) or after a lower phencyclidine dose (3.2 mg/kg). These results suggest that phencyclidine produces weaker antidepressant-like effects, but stronger abuse-related effects than ketamine in these procedures.
Keywords: Phencyclidine, Ketamine, Differential-reinforcement-of-low-rate (DRL), Intracranial self-stimulation, Depression, Abuse liability
Introduction
Over the past decade, glutamatergic systems have emerged as a potential target for treating major depressive disorder (MDD). Specifically, drugs that target and antagonize the N-methyl-D-aspartate (NMDA) receptor complex can be a successful treatment for patients suffering from MDD [Berman et al. 2000; Murrough et al. 2013a,b; Preskorn et al. 2008; Zarate et al. 2006; Zarate et al. 2013]. For example, the noncompetitive NMDA receptor antagonist, ketamine, which has shown the most promise, produces rapid and sustained antidepressant effects following subanesthetic intravenous infusion in patients suffering from MDD [Berman et al. 2000; Murrough et al. 2013a,b; Zarate et al. 2006]. However, the abuse liability of NMDA antagonists, such as ketamine, is a concern [McCambridge et al. 2007; Shek, 2007; Substance Abuse and Mental Health Services Administration, 2013].
The relationship between the antidepressant and abuse-related effects of NMDA antagonists has not been defined. In preclinical studies, the high-affinity and selective noncompetitive NMDA receptor antagonist, MK-801 typically serves as a useful comparator to ketamine [Autry et al. 2011; Koike et al. 2011; Maeng et al. 2008]; however, we recently reported dissociable antidepressant-like and abuse-related effects of ketamine and MK-801. Specifically, ketamine produced an antidepressant-like profile in rats responding under a differential-reinforcement-of-low-rate (DRL) 72 s schedule of reinforcement; whereas, MK-801 produced a stimulant-like profile [Hillhouse and Porter, 2014]. Conversely, ketamine failed to produce an abuse-related facilitation of intracranial self-stimulation (ICSS) under a number of conditions; whereas, MK-801 produced a stimulant-like facilitation of ICSS [Hillhouse et al. 2014]. These results were interpreted to suggest stronger antidepressant-like effects, but weaker abuse-related effects for ketamine relative to MK-801.
Phencyclidine is another drug with NMDA antagonist effects [Bresink et al. 1995] and known abuse liability [Olthuis et al. 2013; Substance Abuse and Mental Health Services Administration, 2013], but possible antidepressant-like effects of phencyclidine have not been fully evaluated. Ketamine is a derivative of phencyclidine, and thus, it is possible that phencyclidine may produce antidepressant-like effects similarly to ketamine. While other NMDA antagonists like ketamine, MK-801 and memantine have been thoroughly evaluated in several preclinical procedures used to screen antidepressant drugs, only a single study has evaluated the effects of phencyclidine in the forced swim test in which treatment with 15.0 mg/kg did not display an antidepressant-like effect [Turgeon et al. 2007]. The limited preclinical and clinical literature on the possible antidepressant effects of phencyclidine warrant further preclinical evaluations. The DRL 72 s is an operant procedure that provides high predictive validity for screening novel antidepressant drugs. Under the DRL 72 s schedule, in which animals are reinforced only for responses that are emitted 72 s after the last lever press, clinically efficacious antidepressant drugs will increase reinforcers, decrease responses, and produce a rightward shift in the peak location of the interresponse time (IRT) distribution (for full review see [O’Donnell et al. 2004]). The DRL 72 s procedure has been used since the 1980s as a tool to reliably screen antidepressant. For example, antidepressant drugs such as monoamine oxidase inhibitors, tricyclic antidepressants and selective serotonin reuptake inhibitors (SSRI) produce antidepressant effects in the DRL 72 s task, while drugs from other pharmacological classes such as morphine and alcohol do not produce antidepressant-like effects [O’Donnell and Seiden, 1982; O’Donnell and Seiden, 1983; Sokolowski & Seiden, 1999].
ICSS is a family of procedures used to reliably assess and compare the abuse liability of drugs in which animals are trained to lever press for brain stimulation [Carlezon and Chartoff, 2007; Kornetsky et al. 1979; Negus and Miller, 2014]. Specifically, the “frequency-rate” ICSS procedure manipulates the frequency of brain stimulation while maintaining a constant stimulation intensity. The frequency-rate procedure engenders a broad range of baseline ICSS rates, and is sensitive to drug effects on both low and high ICSS rates. Most drugs of abuse will increase low ICSS rates maintained at low stimulation frequencies, and this is typically interpreted as an abuse-related “facilitation” of ICSS [Negus and Miller, 2014; Wise et al. 1992]. Moreover, drugs can decrease high ICSS rates maintained at high stimulation frequencies, and this is typically interpreted as abuse-limiting depression of ICSS [Negus and Miller, 2014]. Phencyclidine has been previously tested twice under frequency-rate procedure; however, the dose range (i.e. 2.5-5.0 mg/kg) and pretreatment time was limited [Carlezon Jr and Wise, 1993; Wise et al. 1992].
To address these limitations, the present study compared the antidepressant-like effects of the noncompetitive NMDA receptor antagonists phencyclidine and ketamine using the DRL 72 s procedure. Additionally, the frequency-rate ICSS procedure was used to evaluate the dose- and time-dependent abuse-related effects of phencyclidine because this procedure is sensitive to both the abuse-related and abuse-limiting effects of drugs.
Materials and Methods
Animals
Fifteen adult male Sprague-Dawley rats (Harlan Laboratories Inc, Frederick, MD) were used in the DRL 72 s and ICSS experiments, and weighed between 300 and 350 grams at the start of experiments. All rats were housed individually in plastic rodent cages in the vivarium on a 12-h light/dark cycle (lights on 0600 h). After acclimation to the vivarium, the nine rats in the DRL experiment were given restricted access to food (Harlan Teklad Lab Diets, Teklad LM-485) in order to maintain them at 85% of their free feeding body weights. The six rats in the ICSS experiment had free access to food. All rats had free access to water while in their home cage. Procedures complied with the Guide for the Care and Use of Laboratory Animals [Institute of Laboratory Animal Resources, 2011] and were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Differential-Reinforcement-of-low-Rate
DRL training and test sessions were identical to those described previously for studies with ketamine and MK-801 (Hillhouse and Porter, 2014). The DRL experiment was conducted in four operant conditioning chambers (Model ENV-008-VP, Med Associates, St Albans, Vermont, USA) equipped with a retractable response lever, house light, and pellet dispenser for delivery of 45 mg purified diet pellets (Product #F0021, Bio-Serv, Frenchtown, NJ, USA). The house light was on for the duration of all training and testing sessions, which lasted 60 min. During initial training sessions, rats were trained to lever press for food reinforcement under a fixed-ratio 1 (FR 1) and these training sessions concluded after 50 reinforcers were earned or 60 min. Following completion of lever press training, rats started DRL training in which a reinforcer (i.e. pellet) was delivered if the rat withheld a response for the specified interresponse interval. If a response was emitted prior to the termination of the interresponse interval, the interresponse timer was reset, and no pellet was delivered. The interresponse interval was increased over 28 sessions starting with 4.5 s and ending with the final interresponse interval of 72 s. Rats met training criteria when the number of responses per session for each rat varied by ≤10% of the mean for five of six consecutive sessions. Test sessions with phencyclidine (1.0-10.0 mg/kg, i.p.) were conducted twice a week (typically Tuesday and Friday) with one training session before each test session. Ketamine (1.0-10.0 mg/kg, i.p.) was also studied as a positive control. A saline baseline was determined for each drug. Drug and dose order were administered by Latin-square design.
For the DRL experiment, there were three dependent variables: (1) total number of reinforcers earned, (2) total number of responses emitted, and (3) interresponse times (IRT) for all responses. Reinforcer, response, and IRT data were analyzed using one-way repeated-measures analysis of variance (ANOVA) with drug dose treated as the within-subjects factor. IRT distributions were obtained by recording responses in 25 6-s bins, with the first 6 sec bin representing “burst responding.” A peak location analysis was performed to assess significant shifts in the IRT distributions (Hillhouse et al. 2014; Richards et al. 1993). Specifically, the median of the IRT distribution for each individual rat was determined after eliminating burst responses from the total number of responses, and the medians (peak location) were then analyzed using one-way repeated-measures ANOVA. Holm–Sidak post-hoc test were conducted after all significant ANOVAs. ICSS and DRL data were analyzed using GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA, USA) and the criterion for significance was set at P < 0.05.
Intracranial Self-Stimulation
ICSS surgeries, training, and test sessions were identical to those described previously for studies with ketamine and MK-801 (Hillhouse et al. 2014). Briefly, rats were anesthetized with isoflurane gas (2.5–3% in oxygen; Webster Veterinary, Phoenix, Arizona, USA) for implantation of stainless-steel electrodes. The cathode of each electrode (Plastics One, Roanoke, Virginia, USA) was 0.25 mm in diameter and covered with polyamide insulation, except at the flattened tip. The anode was 0.124 mm in diameter and uninsulated. The cathode was implanted in the left medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior and 1.7 mm lateral from the bregma, and 8.8 mm below the skull) (Pereira DO Carmo et al. 2009). The anode was wrapped around one of three skull screws to serve as the ground, and the skull screws and electrode assembly were secured with orthodontic resin. Rats received ketoprofen (5 mg/kg i.p. for 2 days) as a postoperative analgesic and were allowed to recover for at least 7 days before commencing ICSS training.
ICSS experiments were conducted in six sound-attenuating chambers that contained operant conditioning chambers (Model ENV-007-VP-CT, Med Associates) equipped with a single response lever, stimulus lights, house light, and an ICSS stimulator (Model SG-510, Med Associates) for delivery of brain stimulation. The house light was illuminated during behavioral sessions, and lever-press responding under a FR 1 schedule produced a 0.5-s train of 100-μs square-wave cathodal pulses and concurrent illumination of stimulus lights over the response lever. The terminal schedule consisted of sequential 10-min components. During each component, a descending series of 10 current frequencies was presented, with a 60-s trial at each frequency, and the available frequency decreased from 158 to 56 Hz in 0.05 log unit steps during the 10 trial components. Each frequency trial consisted of a 10-s “timeout” followed by a 50-s “response” period. During the “time out,” five noncontingent electrical brain stimulations were delivered to the rat at the frequency available during that trial, and lever-press responding had no consequence. During this “response” period responding under the FR 1 schedule produced electrical brain stimulation at the same frequency that was delivered during the 10-s “timeout.” Training continued until rates exceeded 50% maximum control rates for the first three to six trials for three sequential components for at least three consecutive days. Training concluded with habituation to saline injections until there were no significant injection effects on ICSS frequency-rate curves.
ICSS test sessions for dose-effect testing consisted of five sequential components. The first component of each test session was considered an acclimation component, and data from this component were discarded. Data from the second and third components were considered “baseline components” and were used to calculate control parameters of frequency-rate curves for that test session in that rat (see below). Immediately after completion of the baseline components, a dose of phencyclidine (1.0-10.0 mg/kg) was administered intraperitoneally, and after the designated pretreatment time of 30 min, ICSS was evaluated during two test components (10 min each, 20 min total). Testing was conducted twice per week (typically Tuesday and Friday). Dose order was administered using a Latin-square design. Additional studies were conducted to investigate the time-course of effects produced by 10.0 mg/kg phencyclidine. For time-course studies, baseline components were conducted as above. Rats were then immediately injected with phencyclidine, and pairs of ICSS test components were initiated 10, 30, 100, and 300 min after the injection. Saline has been tested previously using this time-course procedure (e.g. [Altarifi et al. 2012]), and ICSS rates were stable across all time points.
For ICSS experiments, the primary dependent variable was reinforcement rate (i.e. stimulations per minute during each frequency trial). To normalize these data, raw reinforcement rates from each trial in each rat were converted to percent maximum control rate (%MCR), with MCR defined as the mean of the maximal rates observed during the second and third baseline components for any given rat in any given session. Thus, %MCR values for each trial were calculated as %MCR = (reinforcement rate during a frequency trial ÷ maximum control rate) × 100. For each experimental manipulation, data from all test components were averaged first within each rat and then across rats to yield mean test curves for each manipulation. Data were analyzed using two-way repeated measures ANOVA with drug dose or time as one factor and ICSS frequency as the other factor. Holm–Sidak post-hoc test were conducted after all significant ANOVAs (see [Hillhouse et al. 2014] for complete description of data analysis). The total number of stimulations delivered across all 10 frequency trials was determined for each component was used to provide a summary of ICSS performance. The average number of total stimulations per test component was expressed as a percentage of the average number of total stimulations per component during the second and third baseline components (% baseline).
Drugs
Phencyclidine HCl (Sigma Aldrich, St. Louis, MO, USA) and (±) ketamine HCl (Sigma) were dissolved in 0.9% physiological saline. Drugs were administered intraperitoneally at a volume of 1.0 ml/kg. Doses and pretreatment times for phencyclidine (1.0-10.0 mg/kg, 30 min) and ketamine (1.0-10.0 mg/kg, 5 min) were based on preliminary and previous studies [Carlezon and Wise, 1993; Hillhouse and Porter, 2014; Schaefer and Michael, 1990; Wise et al. 1992].
Results
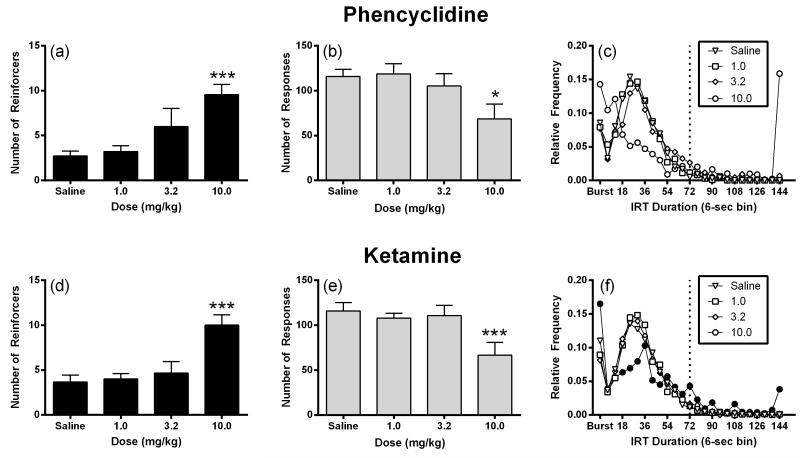
Figure 1 compares the effects of phencyclidine and ketamine on DRL 72 s performance. The 1.0 and 3.2 mg/kg dose of phencyclidine did not significantly alter the number of reinforcers earned or number of responses emitted; whereas, the dose of 10.0 mg/kg phencyclidine significantly increased reinforcers (F 3,24 = 7.77, P < 0.001; Figure 1a) and significantly decreased the number of responses (F3,24 = 4.64, P < 0.05; Figure 1b). Phencyclidine (1.0-10.0 mg/kg) did not produce a significant shift in the peak location of the IRT distribution (F 3,24 = 2.44, P > 0.05; Figure 1c). For comparison, treatment with 1.0 and 3.2 mg/kg ketamine did not significantly affect the number of reinforcers earned or the number of responses emitted; whereas, treatment with 10.0 mg/kg ketamine significantly increased the number of reinforcers earned (F 3,24 = 9.90, P < 0.001; Figure 1d), decreased the number of responses emitted (F3,24 = 13.87, P < 0.001; Fig 1e). In contrast to phencyclidine, the 10.0 mg/kg dose of ketamine produced a significant rightward shift in the peak location of the IRT distribution (F 3,24 = 3.57, P < 0.05; Figure 1f).
Figure 1.
Effects of phencyclidine and ketamine on DRL 72 s. Left panels (a) show drug effects on number of reinforcers earned. Abscissae indicate drug dose. Ordinates indicate number of reinforcers. Center panel (b) show drug effects on number of responses emitted. Abscissae indicate drug dose. Ordinates indicate number of responses. Right panels (c) show drug effects on interresponse time (IRT) distribution. Abscissae indicate interresponse time. Ordinates indicate the relative frequency of responses. Filled points represent significant shifts in peak location after drug treatment as determined by peak location analysis followed by a Holm-Sidak post hoc test, P < 0.05. Reinforcers and responses are expressed as means ± S.E.M for nine rats. IRT data are expressed as means for nine rats. *P < 0.05, ***P < 0.001 versus saline.
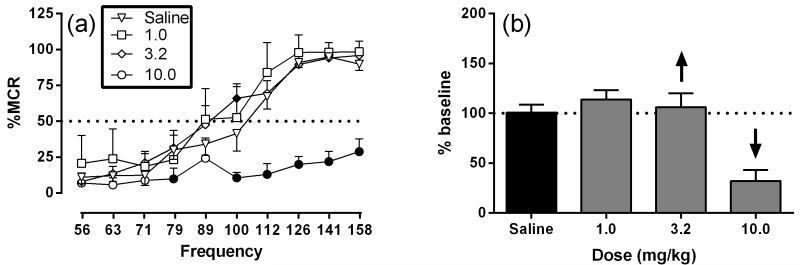
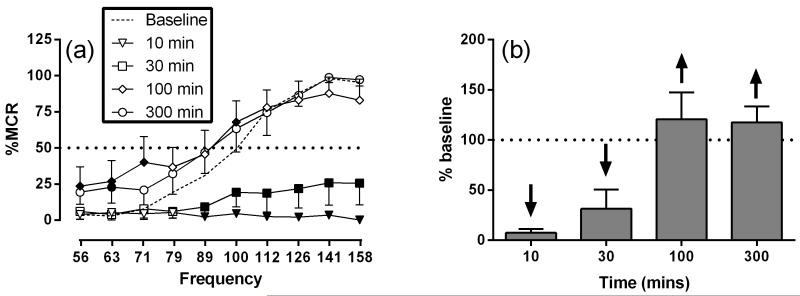
Figure 2 shows the effects of phencyclidine effects on ICSS. The mean ± standard error of the mean (SEM) maximum control rate (MCR) was 57.73 ± 2.62 stimulation per trial, and the mean total stimulations per component delivered across all frequencies was 279.5 ± 24.71. Following saline treatment, brain stimulation maintained a frequency-dependent increase in ICSS rates. Phencyclidine produced mixed rate-increasing and rate-decreasing effects that depended on both dose and frequency of brain stimulation. Phencyclidine produced a significant main effect of frequency (F 9,45 = 70.74, P < 0.001), dose (F 3,15 = 24.91, P < 0.001) and significant interaction (F27,135 = 6.64, P < 0.001). Treatment with 1.0 mg/kg phencyclidine did not significantly alter ICSS rates. The intermediate dose of phencyclidine, 3.2 mg/kg, significantly increased ICSS at one frequency (100 Hz); whereas, the high dose of phencyclidine, 10.0 mg/kg, significantly decreased ICSS at six of the seven highest frequencies (79-158 Hz) (Figure 2 a,b). Figure 3 shows the time-course effects of phencyclidine effects on ICSS. Phencyclidine (10.0 mg/kg) produced significant main effect of frequency (F 9,45 = 54.25, P < 0.001), time (F 4,20 = 12.20, P < 0.001) and significant interaction (F36,180 = 9.83, P < 0.001). Treatment with 10 mg/kg phencyclidine produced a significant decrease in ICSS rates from 10-30 and 30-50 across a range of intermediate and high frequencies (89-158 Hz) but increased ICSS rates at 100-120 min (56-71 and 100 Hz) and 300-320 min (63 Hz) (Figure 3a,b).
Figure 2.
Dose-effects for phencyclidine on ICSS. Left panel (a) shows dose-effects phencyclidine on full ICSS frequency-rate curve. Doses are indicated in legends. Abscissa indicates frequency of electrical brain stimulation (Hz) (log scale). Ordinate indicates percent maximum control reinforcement rate (%MCR). Filled points represent frequencies at which ICSS rates after drug treatment were significantly different from vehicle rates as determined by a two-way ANOVA followed by a Holm-Sidak post hoc test, P < 0.05. Right panel (b) show summary ICSS data expressed as percent baseline total stimulations delivered across all frequencies of brain stimulation per test component. Abscissa indicates drug dose (mg/kg). Ordinate indicates percent baseline stimulations per test component. Upward/downward arrows indicate significant drug-induced increase/decrease in ICSS relative to saline for at least one brain stimulation frequency as determined by analysis of full frequency-rate curves in the left panels. All data show mean ± SEM for six rats.
Figure 3.
Time-course of effects produced by 10.0 mg/kg phencyclidine. Left panel (a) shows phencyclidine effects on full ICSS frequency-rate curves. Time points are indicated in legends Filled points represent frequencies at which ICSS rates after drug treatment were significantly different from baseline rates as determined by a two-way ANOVA followed by a Holm-Sidak post hoc test, P < 0.05. Right panel (b) shows summary ICSS data expressed as percent baseline total stimulations delivered across all frequencies of brain stimulation per test component. Abscissae indicate Time (min). Ordinates indicate percent baseline stimulations per test component. Other details as in Figure 2. All data show mean ± SEM for six rats.
Discussion
The present study is the first to report on the antidepressant-like effects of phencyclidine using the DRL 72 s procedure, and only the second study to evaluate the preclinical antidepressant-like effects of phencyclidine. While the 10.0 mg/kg dose of phencyclidine increased reinforcers and decreased responses, it did not produce a rightward shift in the peak location of the IRT distribution. These phencyclidine effects are similar to effects of some SSRIs, which increase reinforcement rate and decrease response rate, but tend to not significantly shift the peak location of the IRT distributions [O’Donnell et al. 2005; Sokolowski and Seiden, 1999]; these effects are interpreted as a partial antidepressant-like effect. Ketamine produced effects similar to other antidepressants, such as tricyclic antidepressants and monoamine oxidase inhibitors, which consistently increase reinforcement rate, decrease response rate, and shift the peak location of the IRT distributions to the right [Hillhouse and Porter, 2014; O’Donnell et al. 2005; O’Donnell and Seiden, 1983; van Hest et al. 1992]; these behavioral effects are interpreted as an antidepressant-like effect. The DRL 72 s behavioral effects produced by phencyclidine and ketamine differ from effects of the other noncompetitive NMDA antagonist MK-801. MK-801 produces a stimulant-like behavioral profile in DRL 72 s, which is characterized by a decrease in reinforcers, increase in responses, and a leftward shift in the peak location of the IRT distribution [Ardayfio et al. 2008; Hillhouse and Porter, 2014]. Thus, in the DRL 72 s procedure, the effects of phencyclidine were more similar to those of ketamine than to MK-801, although phencyclidine failed to produce the antidepressant-like rightward shift in the peak location of the IRT distribution.
The antidepressant-like effects of phencyclidine and ketamine in the DRL 72 s procedure are consistent with other preclinical studies evaluating the antidepressant-like effects of NMDA antagonists like ketamine and memantine. For example, acute administration of ketamine and memantine produce an antidepressant-like reduction in time spent immobile in the forced swim test in rats [Engin et al. 2009; Gigliucci et al. 2013; Moryl et al. 1993; Reus et al. 2010] and mice [Autry et al. 2011; Maeng et al. 2008]. Additionally, a single acute injection of ketamine produces antidepressant-like effects in the learned helplessness procedure by reducing the number of escape failures in rats [Koike et al. 2011] and mice [Maeng et al. 2008], which is a procedure that typically requires repeated administration of monoamine based antidepressant drugs. Furthermore, ketamine produced sustained (>24 hours pretreatment time) antidepressant-like effects in the forced swim test and chronic unpredictable stress procedure [Autry et al. 2011; Gigliucci et al. 2013; Li et al. 2010; Maeng et al. 2008]. In contrast to the antidepressant-like effects of phencyclidine in the present study and the sustained antidepressant-like effects of ketamine, a higher phencyclidine dose (15.0 mg/kg) did not alter time spent immobile in the forced swim test in rats tested with a 24 hour pretreatment time, suggesting that phencyclidine does not produce a sustained antidepressant-like effect in the forced swim test; however, it is important to note a single dose was tested and the primary aim of that study was to use a high dose phencyclidine to assess the negative symptoms of schizophrenia [Turgeon et al. 2007].
The ICSS results from the present study are consistent with previous studies showing that phencyclidine increased rates and/or decreased threshold of ICSS maintained under FR 1 schedule of reinforcement in ICSS procedures that manipulate frequency of brain stimulation [Carlezon Jr and Wise, 1993; Wise et al. 1992] or intensity of brain stimulation [Kornetsky et al. 1979; Spielewoy and Markou, 2003], respectively; however, the 10.0 mg/kg dose of phencyclidine, which had not been previously tested in frequency-rate ICSS, decreased rates in the present study. The present study extended previous findings by testing a 10-fold dose range of phencyclidine and providing the time-course of phencyclidine effects on ICSS. Specifically, an intermediate phencyclidine dose (3.2 mg/kg) produced weak but exclusive facilitation of ICSS in the dose-effect study. The higher phencyclidine dose (10.0 mg/kg) initially depressed ICSS, which was consistent with reduced response rates seen in the DRL 72 s procedure; however, ICSS facilitation was apparent at later time points (100-320 min) after the behavioral disruption subsided. These phencyclidine effects are similar to the dose- and time-dependent facilitation of ICSS by MK-801, but contrast with the finding that ketamine only depressed ICSS [Hillhouse et al. 2014]. Thus, in the ICSS procedure, phencyclidine effects were more similar to those of MK-801 than to ketamine.
Fixed-interval (FI) schedules of reinforcement also engender a wide range of response rates with low rates of behavior occurring during the early portion of the interval and high rates occurring toward the end of the interval, which is similar to the baseline behavior produced by frequency-rate ICSS, and provide a baseline rate of behavior that is a useful comparison for frequency-rate ICSS [Bauer et al. 2013a]. The ICSS results from the present study are consistent with the effects of phencyclidine on behavior maintained under FI schedules of reinforcement. Low doses of phencyclidine consistently increase low rates of responding maintained under FI schedules of reinforcement in nonhuman primates [Brady et al. 1980; Byrd, 1982], rats [Segal et al. 1981; Wagner et al. 1984], and mice [Wenger and Dews, 1976]; whereas, high doses of phencyclidine consistently decrease rates under FI schedules of reinforcement [Brady et al. 1980; Byrd, 1982; Segal et al. 1981; Wagner et al. 1984; Wenger and Dews, 1976]. For example, in rhesus monkeys responding under a fixed-interval 5 min schedule, phencyclidine produced a modest increase in low rates of behavior, shown with rate-dependency plots, at low doses of phencyclidine. The time-course of effects for phencyclidine in rhesus monkeys responding under the FI 5 min schedule are consistent with those in the present study where the high dose of phencyclidine depressed responding initially (5-60 min) and recovered to control rates by 100 min [Brady et al. 1980]. It has also been shown that low and intermediate doses of phencyclidine produce a modest increase of low rates in rats responding under a FI 90 s schedule [Wagner et al. 1984].
ICSS is typically used as behavioral procedure to assess the abuse liability of drugs, and specifically, the frequency-rate procedure used in the present study provides an evaluation of both the abuse-related and abuse-limiting effects of drugs (Negus and Miller, 2014; Carlezon and Chartoff, 2007; Kornetsky et al. 1979; Wise, 1996). In the present study phencyclidine produced a dose- and time-dependent abuse-related facilitation of ICSS that are consistent with other preclinical procedures used to assess the abuse liability of drugs. For example, phencyclidine maintains self-administration in nonhuman primates [Beardsley et al. 1990; Carroll et al. 1994; Carroll et al. 2000; Marquis and Moreton, 1987; Newman et al. 2007; Newman et al. 2008; Nicholson et al. 2007], rats [Carlezon and Wise, 1996; Collins et al. 1984; Marquis et al. 1989], and dogs [Risner, 1982]. While phencyclidine is known to possess abuse liability, the illicit use of the drug is relatively low compared to other drugs of abuse (e.g. marijuana and stimulants) [Olthuis et al. 2013; Substance Abuse and Mental Health Services Administration, 2013]. In addition to producing abuse-related facilitation of ICSS in the present study, phencyclidine also produced abuse-limited depression of ICSS, which is consistent with the relatively low illicit use of phencyclidine.
In conclusion, the results from the present study add to an emerging body of literature evaluating antidepressant-like and abuse-related effects of NMDA receptor antagonists in preclinical behavioral models. Specifically, our findings provide further evidence that expression of these effects may be related to NMDA receptor affinity. For example, the low-affinity antagonists ketamine (Ki = 1,190 nM) and produce antidepressant-like effects in the DRL 72 s procedure with little evidence from ICSS procedures for abuse potential, whereas the high-affinity antagonist MK-801 (Ki = 2.5 nM) fails to produce antidepressant-effects in the DRL 72 s procedure, but does produce abuse-related facilitation of ICSS [Bresink et al., 1995; Hillhouse and Porter, 2014; Hillhouse et al. 2014]. In comparison to ketamine and MK-801, phencyclidine has intermediate affinity at NMDA receptors (Ki = 42 nM) [Bresink et al. 1995] and it produced a mixed-profile of behavioral effects that included both partial antidepressant-like effects in the DRL 72 s procedure and abuse-related facilitation of ICSS. While the present preclinical results provide evidence that phencyclidine may produce antidepressant-like effects, it is unlikely that phencyclidine will be tested in clinical settings because of abuse liability concerns.
Acknowledgments
Funding Source: This research was supported by National Institutes of Health grant R01-NS070715 (SSN).
Footnotes
Conflicts of Interest: None declared
References
- Ardayfio PA, Benvenga MJ, Chaney SF, Love PL, Catlow J, Swanson SP, Marek GJ. The 5-Hydroxytryptamine2A receptor antagonist R-(+)-α-(2,3-Dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl-4-piperidinemethanol (M100907) attenuates impulsivity after both drug-induced disruption (dizocilpine) and ehancement (antidepressant drugs) of differential-reinforcement-of-low-rate 72-s behavior in the rat. J Pharmacol Exp Therap. 2008;327:891–897. doi: 10.1124/jpet.108.143370. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Peng-fei C, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. Rate-dependent effects of monoamine releasers on intracranial self-stimulation in rats: implications for abuse liability assessment. Behav pharmacol. 2013a;24:448–458. doi: 10.1097/FBP.0b013e328363d1a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. Br J Pharmacol. 2013b;168:850–862. doi: 10.1111/j.1476-5381.2012.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Hayes BA, Balster RL. The self-administration of MK-801 can depend upon drug-reinforcement history, and its discriminative stimulus properties are phencyclidine-like in rhesus monkeys. J Pharmacol Exp Therap. 1990;252:953–959. [PubMed] [Google Scholar]
- Brady KT, Balster RL, Meltzer LT, Schwertz D. Comparison of phencyclidine and three analogues on fixed-interval performance in rhesus monkeys. Pharmacol Biochem Behav. 1980;12:67–71. doi: 10.1016/0091-3057(80)90417-7. [DOI] [PubMed] [Google Scholar]
- Bresink I, Danysz W, Parsons CG, Mutschler E. Different binding affinities of NMDA receptor channel blockers in various brain regions—Indication of NMDA receptor heterogeneity. Neuropharmacol. 1995;34:533–540. doi: 10.1016/0028-3908(95)00017-z. [DOI] [PubMed] [Google Scholar]
- Byrd LD. Comparison of the behavioral effects of phencyclidine, ketamine, d-amphetamine and morphine in the squirrel monkey. J Pharmacol Exp Therap. 1982;220:139–144. [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protocols. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. Phencyclidine-induced potentiation of brain stimulation reward: acute effects are not altered by repeated administration. Psychopharmacol (Berl) 1993;111:402–408. doi: 10.1007/BF02253528. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Wise RA. Rewarding Actions of Phencyclidine and Related Drugs in Nucleus Accumbens Shell and Frontal Cortex. J Neurosci. 1996;16:3112–3122. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Carmona GN, Rodefer JS. Phencyclidine (PCP) self-administration and withdrawal in rhesus monkeys: Effects of buprenorphine and dizocilpine (MK-801) pretreatment. Pharmacol Biochem Behav. 1994;48:723–732. doi: 10.1016/0091-3057(94)90339-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Cosgrove KP, Campbell UC, Morgan AD, Mickelberg JL. Reductions in ethanol, phencyclidine, and food-maintained behavior by naltrexone pretreatment in monkeys is enhanced by open economic conditions. Psychopharmacol (Berl) 2000;148:412–422. doi: 10.1007/s002130050071. [DOI] [PubMed] [Google Scholar]
- Collins RJ, Weeks J, Cooper M, Good P, Russell R. Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacol (Berl) 1983;82:6–13. doi: 10.1007/BF00426372. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D, Dickson CT. Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models. Neurosci. 2009;161:359–369. doi: 10.1016/j.neuroscience.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Gigliucci V, O’Dowd G, Casey S, Egan D, Gibney S, Harkin A. Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacol (Berl) 2013;228:157–166. doi: 10.1007/s00213-013-3024-x. [DOI] [PubMed] [Google Scholar]
- Hillhouse TM, Porter JH. Ketamine, but not MK-801, produces antidepressant-like effects in rats responding on a differential-reinforcement-of-low-rate operant schedule. Behav pharmacol. 2014;25:80–91. doi: 10.1097/FBP.0000000000000014. [DOI] [PubMed] [Google Scholar]
- Hillhouse TM, Porter JH, Negus SS. Dissociable effects of the noncompetitive NMDA receptor antagonists ketamine and MK-801 on intracranial self-stimulation in rats. Psychopharmacol (Berl) 2014;231:2705–2716. doi: 10.1007/s00213-014-3451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources . Guide for the Care and Use of Laboratory Animals. 8th ed Institute of Laboratory Animals Resources, Commission of Life Sciences, National Research Council; Washington DC: 2011. [Google Scholar]
- Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224:107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU, McLean S, Jacobson JO. Intracranial self-stimulation thresholds: A model for the hedonic effects of drugs of abuse. Arch General Psychiat. 1979;36:289–292. doi: 10.1001/archpsyc.1979.01780030055004. [DOI] [PubMed] [Google Scholar]
- Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, Li X-Y, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of α-Amino-3-Hydroxy-5-Methylisoxazole-4-Propionic acid receptors. Biol Psychiatr. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Marquis KL, Moreton JE. Animal models of intravenous phencyclinoid self-administration. Pharmacol Biochem Behav. 1987;27:385–389. doi: 10.1016/0091-3057(87)90587-9. [DOI] [PubMed] [Google Scholar]
- Marquis K, Webb M, Moreton JE. Effects of fixed ratio size and dose on phencyclidine self-administration by rats. Psychopharmacol (Berl) 1989;97:179–182. doi: 10.1007/BF00442246. [DOI] [PubMed] [Google Scholar]
- McCambridge J, Winstock A, Hunt N, Mitcheson L. 5-Year trends in use of hallucinogens and other adjunct drugs among UK dance drug users. Europ Addition Res. 2007;13:57–64. doi: 10.1159/000095816. [DOI] [PubMed] [Google Scholar]
- Moryl E, Danysz W, Quack G. Potential Antidepressive Properties of Amantadine, Memantine and Bifemelane. Pharmacol Toxicol. 1993;72:394–397. doi: 10.1111/j.1600-0773.1993.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, Iqbal S, Pillemer S, Foulkes A, Shah A, Charney DS, Mathew SJ. Antidepressant efficacy of ketamine in treatment-resistant major depression: A two-site randomized controlled trial. Am J Psychiat. 2013a;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrough JW, Perez AM, Pillemer S, Stern J, Parides MK, aan het Rot M, Collins KA, Mathew SJ, Charney DS, Iosifescu DV. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry. 2013b;74:250–256. doi: 10.1016/j.biopsych.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Miller LL. Intracranial Self-Stimulation to Evaluate Abuse Potential of Drugs. Pharmacol Rev. 2014;66:869–917. doi: 10.1124/pr.112.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JL, Perry JL, Carroll ME. Social stimuli enhance phencyclidine (PCP) self-administration in rhesus monkeys. Pharmacol Biochem Behav. 2007;87:280–288. doi: 10.1016/j.pbb.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JL, Perry JL, Carroll ME. Effects of altering reinforcer magnitude and reinforcement schedule on phencyclidine (PCP) self-administration in monkeys using an adjusting delay task. Pharmacol Biochem Behav. 2008;90:778–786. doi: 10.1016/j.pbb.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson KL, Mansbach RS, Menniti FS, Balster RL. The phencyclidine-like discriminative stimulus effects and reinforcing properties of the NR2B-selective N-methyl-D-aspartate antagonist CP-101 606 in rats and rhesus monkeys. Behav pharmaco. 2007;18:731–743. doi: 10.1097/FBP.0b013e3282f14ed6. [DOI] [PubMed] [Google Scholar]
- O’Donnell JM, Marek GJ, Seiden LS. Antidepressant effects assessed using behavior maintained under a differential-reinforcement-of-low-rate (DRL) operant schedule. Neurosci Biobehav Rev. 2005;29:785–798. doi: 10.1016/j.neubiorev.2005.03.018. [DOI] [PubMed] [Google Scholar]
- O’Donnell J, Seiden L. Effects of monoamine oxidase inhibitors on performance during differential reinforcement of low response rate. Psychopharmacol (Berl) 1982;78:214–218. doi: 10.1007/BF00428153. [DOI] [PubMed] [Google Scholar]
- O’Donnell JM, Seiden LS. Differential-reinforcement-of-low-rate 72-second schedule: selective effects of antidepressant drugs. J Pharmacol Biochem Behav. 1983;224:80–88. [PubMed] [Google Scholar]
- Olthuis JV, Darredeau C, Barrett SP. Substance use initiation: The role of simultaneous polysubstance use. Drug Alcohol Rev. 2013;32:67–71. doi: 10.1111/j.1465-3362.2012.00470.x. [DOI] [PubMed] [Google Scholar]
- Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS. Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: Further studies on pain-depressed behavior. Pain. 2009;144:170–177. doi: 10.1016/j.pain.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-Methyl-D-Aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Stringari RB, Kirsch TR, Fries GR, Kapczinski F, Roesler R, Quevedo J. Neurochemical and behavioural effects of acute and chronic memantine administration in rats: Further support for NMDA as a new pharmacological target for the treatment of depression? Brain Res Bull. 2010;81:585–589. doi: 10.1016/j.brainresbull.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Réus G, Abelaira H, Stringari R, Fries G, Kapczinski F, Quevedo J. Memantine treatment reverses anhedonia, normalizes corticosterone levels and increases BDNF levels in the prefrontal cortex induced by chronic mild stress in rats. Metab Brain Dis. 2012;27:175–182. doi: 10.1007/s11011-012-9281-2. [DOI] [PubMed] [Google Scholar]
- Richards JB, Sabol KE, Seiden LS. DRL interresponse-time distributions: quantification by peak deviation analysis. J Exp Anal Behav. 1993;60:361–385. doi: 10.1901/jeab.1993.60-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risner ME. Intravenous self-administration of phencyclidine and related compounds in the dog. J Pharmacol Exp Therap. 1982;221:637–644. [PubMed] [Google Scholar]
- Schaefer G, Michael R. Interactions of naloxone with morphine, amphetamine and phencyclidine on fixed interval responding for intracranial self-stimulation in rats. Psychopharmacol (Berl) 1990;102:263–268. doi: 10.1007/BF02245931. [DOI] [PubMed] [Google Scholar]
- Segal SA, Moerschbaecher JM, Thompson DM. Effects of phencyclidine, d-amphetamine and pentobarbital on schedule-controlled behavior in rats. Pharmacol Biochem Behav. 1981;15:807–812. doi: 10.1016/0091-3057(81)90026-5. [DOI] [PubMed] [Google Scholar]
- Shek DTL. Tackling adolescent substance abuse in Hong Kong: Where we should and should not go. Sci World J. 2007;7:2021–2030. doi: 10.1100/tsw.2007.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski JD, Seiden LS. The behavioral effects of sertraline, fluoxetine, and paroxetine differ on the differential-reinforcement-of-low-rate 72-second operant schedule in the rat. Psychopharmacol (Berl) 1999;147:153–161. doi: 10.1007/s002130051155. [DOI] [PubMed] [Google Scholar]
- Spielewoy C, Markou A. Withdrawal from chronic phencyclidine treatment induces long-lasting depression in brain reward function. Neuropsychopharmacol. 2003;28:1106–1116. doi: 10.1038/sj.npp.1300124. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2012 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-46, HHS Publication No. (SMA) 13-4795. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2013. [Google Scholar]
- Turgeon SM, Lin T, Subramanian M. Subchronic phencyclidine exposure potentiates the behavioral and c-Fos response to stressful stimuli in rats. Pharmacol Biochem Behav. 2007;88:73–81. doi: 10.1016/j.pbb.2007.07.005. [DOI] [PubMed] [Google Scholar]
- van Hest A, van Drimmelen M, Olivier B. Flesinoxan shows antidepressant activity in a DRL 72-s screen. Psychopharmacol (Berl) 1992;107:474–479. doi: 10.1007/BF02245258. [DOI] [PubMed] [Google Scholar]
- Wagner G, Masters D, Tomie A. Effects of phencyclidine, haloperidol, and naloxone on fixed-interval performance in rats. Psychopharmacol (Berl) 1984;84:32–38. doi: 10.1007/BF00432020. [DOI] [PubMed] [Google Scholar]
- Wenger GR, Dews PB. The effects of phencyclidine, ketamine, delta-amphetamine and pentobarbital on schedule-controlled behavior in the mouse. J Pharmacol Exp Therap. 1976;196:616–624. [PubMed] [Google Scholar]
- Wise RA. Addictive Drugs and Brain Stimulation Reward. Ann Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bauco P, Carlezon WA, Jr, Trojniar W. Self-stimulation and drug reward mechanisms. Ann N Y Acad Sci. 1992;654:192–198. doi: 10.1111/j.1749-6632.1992.tb25967.x. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an n-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiat. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Mathews D, Ibrahim L, Chaves JF, Marquardt C, Ukoh I, Jolkovsky L, Brutsche NE, Smith MA, Luckenbaugh DA. A randomized trial of a low-trapping nonselective N-Methyl-D-Aspartate channel blocker in major depression. Biol Psychiatry. 2013;74:257–264. doi: 10.1016/j.biopsych.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]