Abstract
We have used quantitative epifluorescence microscopy of fluorescent ATP to measure single-nucleotide turnover in skinned skeletal muscle fibers from mouse models of female aging and hormone treatment. Aging causes declines in muscle strength, often leading to frailty, disability, and loss of independence for the elderly. Female muscle is additionally affected by age due to reduction of ovarian hormone production with menopause. Estradiol (E2) is the key hormonal signal to skeletal muscle in females, and strength loss is attenuated by E2 treatment. To investigate E2 mechanisms on skeletal muscle, single fibers were isolated from sham-operated or ovariectomized (OVX) mice, with or without E2 treatment, and were incubated with mantATP. We measured decay of mantATP fluorescence in an ATP-chase experiment, as pioneered by Cooke and coworkers, who unveiled a novel regulated state of muscle myosin characterized by slow nucleotide turnover on the order of minutes, termed the super-relaxed state (SRX). We detected a slow phase of nucleotide turnover in approximately one-third of the myosin heads from sham fibers, consistent with SRX. Turnover was substantially faster in OVX fibers, with a turnover time constant for the slow phase of 65 ± 8 s as compared to 102 ± 7 s for sham fibers. 60-day E2 treatment in OVX mice substantially reversed this effect on SRX, while acute exposure of isolated muscles from OVX mice to E2 had no effect. We conclude that E2-mediated signaling reversibly regulates slow ATP turnover by myosin. Age- and hormone-related muscle functional losses may be targetable at the level of myosin structure/function for strategies to offset weakness and metabolic changes that occur with age.
Keywords: Regulatory light chain, phosphorylation, estrogen, aging, nucleotide turnover, ATP
1. Introduction
1.1 Muscle weakness, aging, and myosin
The loss of skeletal muscle strength is an undesirable consequence of aging, since it consistently predicts falls, mortality, and functional status such as mobility in elderly individuals [1]. Skeletal muscle of females is dually affected by age due to the simultaneous loss of ovarian hormone production. Declines in strength are accelerated around the time of menopause in women and following ovariectomy in rodents, and our meta-analyses showed that post-menopausal women on estrogen-based hormone therapy and ovariectomized rodents treated with estradiol are stronger than those that remain hormone deficient [2].
We have shown that in females, estradiol deficiency is detrimental to myosin and muscle functions [2,3,4,5,6,7]. Furthermore, we established that the strong-binding state of myosin during contraction is perturbed by aging, disease, and estradiol deficiency and is a major factor causing strength loss [4,5,6,8,9,10,11,12]. However, the molecular mechanism underlying estradiol’s impact on myosin’s molecular force-generating capacity is not known. Myosin consists of two heavy chains and two pairs of light chains and the structural arrangement on the thick filament is stabilized by myosin binding protein C (MyBP-C), the regulatory light chain (RLC), and titin. The light-chain-binding domain of myosin serves as a lever arm, transmitting the free energy of ATP hydrolysis in the actin-binding catalytic domain to produce force and movement during contraction [13]. Phosphorylation of RLC influences this molecular force generation in striated muscle [14] and is such a critical event that specific mutations disrupting RLC phosphorylation in cardiac myosin reduce force and cause cardiomyopathy [15,16]. In skeletal muscle, RLC phosphorylation modulates myosin structure, force, and power output [17,18]. Estradiol has recently been implicated in the regulation of RLC phosphorylation and contractility of cardiomyocytes [19,20]. In human skeletal muscle, a proteomic study showed age-dependent reduction of RLC phosphorylation [21], and it was recently shown that RLC phosphorylation is affected in muscle fibers of old women but not old men, relative to young counterparts [22]. Phosphorylation of RLC is also enhanced by estradiol in rodent skeletal muscle [23].
1.2. The super-relaxed state of myosin
A fascinating implication of myosin phosphorylation in aged female muscle relates to the newly discovered myosin super-relaxed state (SRX), which utilizes ATP much more slowly than in the conventional relaxed state, and is disrupted by phosphorylation of myosin RLC [24,25,26]. These insights have led us to test the hypothesis that estradiol deficiency disrupts the normal structure and function of myosin SRX in female muscle.
In the present study we have detected the SRX state in permeabilized mouse muscle fibers, using fluorescent ATP (mantATP) and fluorescence microscopy to detect the kinetics of ATP turnover, as developed by Cooke and coworkers [26]. Skeletal muscle fibers were prepared from adult ovary-intact and ovariectomized mice treated with placebo or estradiol. We hypothesize that estradiol deficiency, as occurs in aged females, stabilizes the SRX state, decreasing isometric force, thus leading to muscle weakness. Here we provide the first report of SRX ATP turnover in a mouse model for human pathophysiological mechanisms using isolated single skeletal muscle fibers and confocal epifluorescence. Furthermore, we have identified a regulatory mechanism that is highly relevant to biological aging and the problem of muscle weakness that occurs due to age in females.
2. Materials and Methods
2.1. Animals
Female C57BL/6 mice aged 3–4 months (National Institute on Aging) underwent bilateral ovariectomy surgeries (OVX) or sham operations (Sham), which consisted of the same procedures as the OVX except that the ovaries were not removed. For some experiments, subgroups of OVX mice immediately received a treatment of 17β-estradiol (OVX+E2) via 60-day time release pellets as described previously [5]. Mice resumed normal cage activities for 6–8 weeks. On the day of sacrifice, mice were anesthetized by an i.p. injection of pentobarbital sodium (100 mg/kg body mass), and psoas and extensor digitorum longus (EDL) muscles were harvested. Psoas muscles were immediately prepared for SRX experiments, and EDL muscles were flash frozen in liquid nitrogen. Uteri were dissected and weighed as an indicator of successful ovariectomy surgery and E2 treatment (average uterine mass for Sham, OVX, OVX+E2: 105.9 mg, 14.0 mg, and 136.3 mg, respectively). While still under anesthesia, mice were euthanized by exsanguination. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Minnesota and complied with the American Physiological Society guidelines.
2.2. SRX Experiments
Freshly dissected mouse psoas fibers were dissected into ~1 mm bundles and chemically skinned as described previously [26,27]. Single muscle fibers were mounted in 35 mm glass bottom culture dishes (Bioptechs, Butler, PA) to be used with the confocal microscope. In the SRX experiment, fibers were first incubated in a solution containing 250 μM mantATP. After 5 min, this solution was exchanged for one containing 4 mM ATP (“chase”). The solution also contained 120 mM KAc, 5 mM K-phosphate, 5 mM magnesium acetate, 4 mM EGTA, and 50 mM MOPS pH 6.8 [26]. Experiments were performed at 23 ± 1°C with an Olympus FV1000 IX2 confocal microscope and 60X UPlanApo N oil immersion objective lens (1.42 NA) for differential interference contrast and epifluorescence imaging. Fluorescence was acquired with 405 nm excitation and a 460/50-nm emission filter. Images were scanned in a 512x512 pixel grid with a total exposure time of 1.1 s and an effective pixel size of 414 nm. Chase experiments were performed during time-lapse imaging for 400–600 s. Fiber fluorescence was acquired and analyzed as described previously [26]. Images were imported into ImageJ and fluorescence intensities within a circular region (20 μm diameter) were recorded. Fluorescence signal was analyzed as the average of three separate regions in each fiber minus the average of three regions outside the fiber in the same image. The data were fit to a two-exponential decay using a nonlinear least-squares algorithm in Origin 9.0 (OriginLab Corp.). Two exponentials were sufficient to achieve r2 > 0.99; a third exponential did not substantially improve the fit.
Phosphorylation of RLC, measured for intact muscle fibers from the same mice, showed a similar pattern to the SRX data. OVX-treated fibers showed a 35 ± 4% decrease (compared to Sham) in the fraction of phosphorylated RLC, and E2 treatment restored the phosphorylation level to within experimental error of the Sham value, as we reported previously using Western immunoblots [23]. In the present study, the SRX experiments were performed on skinned fibers with uniformly low RLC phosphorylation due to endogenous phosphatases, thus these results reflect the effects of estradiol on SRX without confounding effects of RLC phosphorylation.
2.3. Statistical Analysis
Data are expressed as mean ± SEM of independent experiments (one fiber per experiment.) Data were analyzed by one-way ANOVA using Origin 9.0. Holm-Sidak post hoc tests were performed with significance accepted at p<0.05.
3. Results
3.1 Measuring single-nucleotide turnovers in permeable mouse muscle fibers
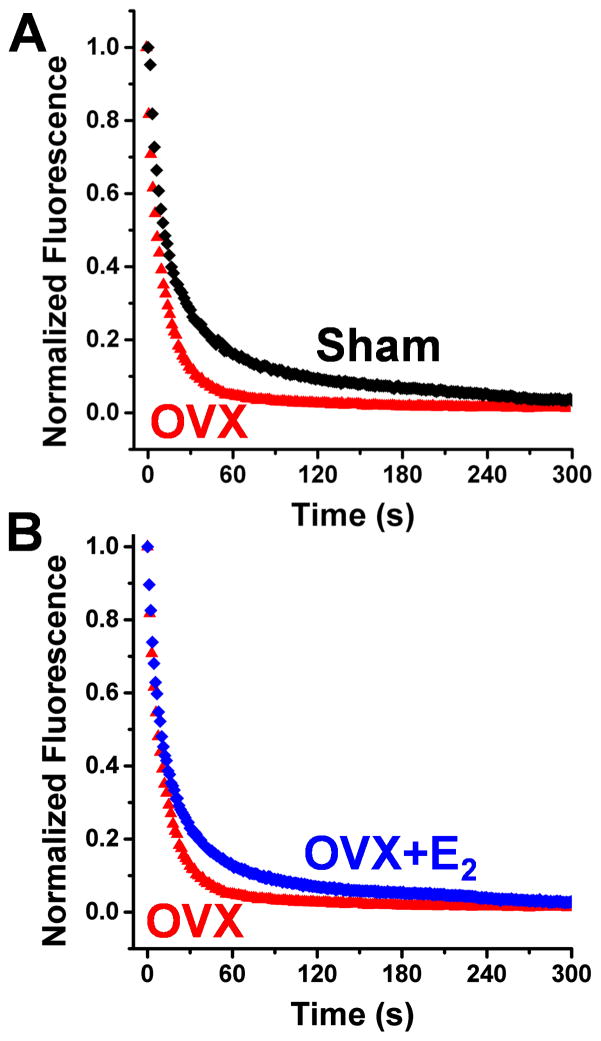
Quantitative epifluorescence microscopy was used to measure ATP turnover kinetics in permeable skeletal muscle from mice (Fig. 1, Fig. 2). During the chase experiment, the fluorescence intensity decreases as bound mant nucleotides (mantATP hydrolyzed into mantADP and Pi) are released from myosin and replaced by ATP (Fig. 2). Thus, the fluorescence intensity decays as the fluorescence from mantATP is eliminated by competition from ATP. We first validated our assay with rabbit fibers obtaining results similar to those established in the literature (data not shown) [26]. We proceeded to measure turnover in mouse fibers and found that ATP turnover in mouse is similarly biphasic (Fig. 2), with a slow time constant (T2, see Table 1) associated with super-relaxed myosin heads (SRX). Although the T2 in mouse was about half as long as observed in rabbit, the mouse T2 was decreased by ADP chase or low temperature, effects demonstrated previously for the SRX in rabbit [26].
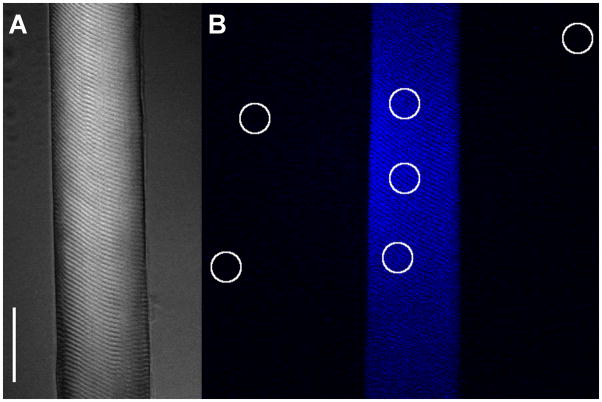
Fig. 1.
(A) Single mouse skeletal muscle fiber DIC image (see Materials and Methods). Scale bar is 40 μm. (B) Fluorescence image of mouse fiber loaded with mantATP prior to start of ATP chase. 3 circular regions of interest (ROI) within the fiber monitor the time course of mantATP fluorescence intensity decay during the ATP chase. Circular ROI’s outside of fiber are used for background subtraction, a method adapted from studies in rabbit skeletal muscle fibers [26].
Fig. 2.
ATP turnover in skinned mouse psoas muscle fibers. (A) Fibers incubated in fluorescent mantATP were chased with ATP and turnover was observed as loss of fluorescence. OVX treatment (red) eliminates the slow phase of ATP turnover in Sham (black). (B) Chronic E2 treatment (blue) reverses the OVX effect, restoring the slow phase of ATP turnover. Results are quantitated in Table 1.
Table 1. Kinetics of ATP turnover in a fluorescent ATP chase assay.
Two-exponential fits to fluorescence intensity from mouse psoas fibers incubated with mantATP (250 μM) prior to ATP chase (4 mM) at time 0 s. The long phase T2 is interpreted as slow nucleotide turnover associated with the super-relaxed state.
| Treatment | n | P1 % | T1 (s) | P2 % | T2 (s) |
|---|---|---|---|---|---|
| Sham | 7 | 83 ± 2 | 12 ± 1 | 17 ± 2 | 102 ± 7 |
| OVX | 8 | 76 ± 3 | 10 ± 2 | 24 ± 3 | 65 ± 8 * |
| OVX+E2 | 7 | 74 ± 5 | 13 ± 2 | 26 ± 5 | 89 ± 13 # |
T2 differs from Sham (p = 0.011);
T2 does not differ significantly from Sham (p = 0.36) or OVX (p = 0.079), Holm-Sidak post hoc test.
3.2 SRX Experiments in Sham and OVX mice
The ATP turnover kinetics in single fibers from Sham and OVX mice was determined by ATP chase (representative data in Fig. 2A, all data analyzed in Table 1). T1, the exponential lifetime associated with release of nucleotide by relaxed myosin heads, was not different between Sham and OVX fibers with turnover times of 12 ± 1 s and 10 ± 2 s respectively. In contrast, the lifetime T2 for the much slower phase, corresponding to SRX heads, was significantly different between Sham and OVX fibers, with turnover times of 102 ± 7 s and 65 ± 8 s, respectively (Table 1, Fig. 3). Thus, chronic absence of circulating ovarian hormone affects the myosin heads that release nucleotide slowly, as these super-relaxed (SRX) heads exhibit ATP turnover that is nearly twice as fast as in control (Sham) fibers. The fraction of heads P2 in the SRX state was not significantly affected by the OVX treatment (Table 1).
Fig. 3.
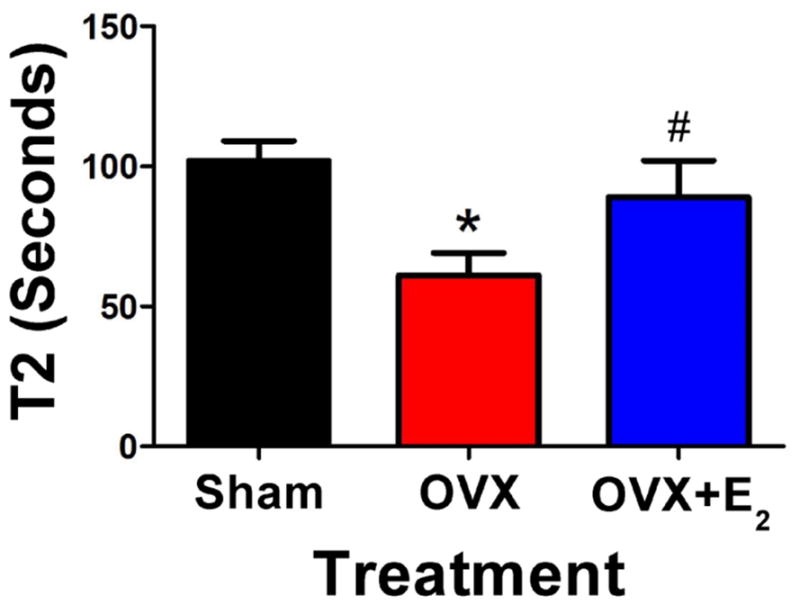
Disruption of the super-relaxed state in OVX mice. The exponential time constant T2, corresponding to the SRX in skinned muscle fibers, varied with ovariectomy (OVX) and estradiol treatment (OVX+E2) in mice. See Table 1 for statistics (*,#).
3.3 SRX Experiments in OVX mice supplemented with E2
OVX mice with 60 days of E2 time-release capsule treatment, from the time of ovariectomy through tissue harvest (OVX+E2) showed substantial reversal of the OVX effect; increasing T2 (Fig. 2B, Table 1). No parameters of nucleotide turnover in fibers from OVX+E2 mice were significantly different from the Sham control. These results indicate that E2 signalling plays a decisive role in regulating the SRX state of myosin in skeletal muscle. Acute exposure of permeabilized muscle fibers to 2 μM E2 for 30 min prior to chase experiments had no effect (data not shown).
4. Discussion
We have measured single-nucleotide turnover in resting skeletal muscle fibers from mouse models of human aging and women’s health, with the goal of gaining insight into mechanisms by which aging and loss of ovarian hormone may lead to altered metabolic rate and exacerbated muscle weakness in post-menopausal women. The slow phase of ATP turnover, attributed to the super-relaxed state (SRX) of myosin, exhibits a turnover time in mouse skeletal fibers (T2 in Table 1 and Fig. 3) that is ten times longer than the fast phase (T1) and about half of the T2 value reported previously for rabbit muscle [26]. It is likely that T2 is even longer (slower) in human muscle, making human muscle even more sensitive to the level of circulating estradiol. Our main finding is that the slow rate of ATP turnover (1/T2) is reversibly sensitive to the level of circulating E2 in the female mouse (Fig. 3). The loss of E2 in OVX mice leads to modified myosin ATPase kinetics, accelerating the rate of SRX ATP turnover, and this rate is restored to control levels by a 60-day E2 treatment (Fig. 3, Table 1). Thus E2 is a key modulator of SRX.
The disruption of SRX perturbation by OVX and reversal by E2 treatment correlates with the effects on RLC phosphorylation in intact muscle, suggesting that estradiol signaling regulates RLC phosphorylation, as suggested by previous studies on cardiac muscle [19,20], skeletal muscle in aging women [22], and our own studies in skeletal muscle of female mice [23]. However, in the present study, we eliminated the acute effects of RLC phosphorylation by performing SRX measurements on skinned fibers in which RLC phosphorylation was uniformly low, so the present effects are attributable directly to the chronic effects of estradiol treatment.
Further study will be needed to understand this mechanism in more detail and to answer new questions that arise. For example, if estradiol affects primarily the fraction of phosphorylated myosin RLC, why do we see a change in the time constant of one population, rather than a change in the mole fractions themselves (Fig. 3, Table 1)? Is it possible that estradiol acts by influencing other phosphorylation targets involved in myosin-based contractility, such as myosin-binding protein C [28], titin, or troponin [29]? What about other post-translational protein modifications, such as oxidation [12,30]? What are the other components in the estradiol signaling pathway that eventually lead to regulation of SRX [31,32]? In addition to the established direct effects of RLC phosphorylation on SRX [23,26], do the observed effects, observed in the absence of phosphorylation, also arise from a compensatory response? Of most interest to us as structural biophysicists, what changes in protein structure and dynamics stabilize or destabilize SRX? Site-directed spectroscopic probe studies on myosin and other myofilament proteins [33,34] in SRX fibers will be needed to approach this question directly.
5. Summary and Conclusion
Direct measurement of nucleotide turnover in single permeabilized muscle fibers has enabled us to uniquely test novel concepts of estrogenic mechanisms at the molecular level of actin-myosin interactions. Further pursuit of the biochemical and structural mechanisms of this effect on the super-relaxed state of muscle is likely to have a substantial impact on understanding important signaling pathways in aging and women’s health.
HIGHLIGHTS.
Skeletal myosin nucleotide turnover is perturbed by ovariectomy treatment in mice.
This alteration of muscle function is reversed by chronic estradiol treatment.
The slow nucleotide turnover is regulated through hormone-mediated pathways.
Reduced estradiol production may link age-related weakness to myosin activity.
The thick filament structure is a potential target for muscle strength modulation.
Acknowledgments
We thank Simon Gruber for helpful discussions and Octavian Cornea for helping to prepare the manuscript. Fluorescence microscopy was performed at the University Of Minnesota Imaging Centers with assistance from Guillermo Marqués. This work was supported primarily by NIH grants to DDT (R37 AG26160 and R37 AG26160-09S2). Support was also provided by NIH grants to DL (R01 AG31743) and BC (K99 HL122397). KJP was supported by NIH grant T32 AR7612.
Abbreviations
- E2
estradiol
- KAc
potassium acetate
- mantATP
2′-(or-3′)-O-(N-Methylanthraniloyl) Adenosine 5′-Triphosphate
- MOPS
3-(N-morpholino) propanesulfonic acid
- MyBP-C
myosin binding protein C
- RLC
regulatory light chain
- P1
population one
- P2
population two
- pRLC
phosphorylated RLC
- Sham
sham-operated
- s
seconds
- SRX
super-relaxed state
- T1
turnover time constant one
- T2
turnover time constant two
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Visser M, Schaap LA. Consequences of sarcopenia. Clin Geriatr Med. 2011;27:387–399. doi: 10.1016/j.cger.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Greising SM, Baltgalvis KA, Lowe DA, Warren GL. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64:1071–1081. doi: 10.1093/gerona/glp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greising SM, Carey RS, Blackford JE, Dalton LE, Kosir AM, Lowe DA. Estradiol treatment, physical activity, and muscle function in ovarian-senescent mice. Exp Gerontol. 2011;46:685–693. doi: 10.1016/j.exger.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowe DA, Baltgalvis KA, Greising SM. Mechanisms Behind Estrogen’s Beneficial Effect on Muscle Strength in Females. Exercise and Sport Sciences Reviews. 2010;38:61–67. doi: 10.1097/JES.0b013e3181d496bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moran AL, Nelson SA, Landisch RM, Warren GL, Lowe DA. Estradiol replacement reverses ovariectomy-induced muscle contractile and myosin dysfunction in mature female mice. J Appl Physiol. 2007;102:1387–1393. doi: 10.1152/japplphysiol.01305.2006. [DOI] [PubMed] [Google Scholar]
- 6.Moran AL, Warren GL, Lowe DA. Removal of ovarian hormones from mature mice detrimentally affects muscle contractile function and myosin structural distribution. J Appl Physiol. 2006;100:548–559. doi: 10.1152/japplphysiol.01029.2005. [DOI] [PubMed] [Google Scholar]
- 7.Moran AL, Warren GL, Lowe DA. Soleus and EDL muscle contractility across the lifespan of female C57BL/6 mice. Exp Gerontol. 2005;40:966–975. doi: 10.1016/j.exger.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Lowe DA, Warren GL, Snow LM, Thompson LV, Thomas DD. Muscle activity and aging affect myosin structural distribution and force generation in rat fibers. J Appl Physiol. 2004;96:498–506. doi: 10.1152/japplphysiol.00842.2003. [DOI] [PubMed] [Google Scholar]
- 9.Baltgalvis KA, Call JA, Nikas JB, Lowe DA. Effects of prednisolone on skeletal muscle contractility in mdx mice. Muscle Nerve. 2009;40:443–454. doi: 10.1002/mus.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowe DA, Surek JT, Thomas DD, Thompson LV. Electron paramagnetic resonance reveals age-related myosin structural changes in rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2001;280:C540–547. doi: 10.1152/ajpcell.2001.280.3.C540. [DOI] [PubMed] [Google Scholar]
- 11.Lowe DA, Thomas DD, Thompson LV. Force generation, but not myosin ATPase activity, declines with age in rat muscle fibers. Am J Physiol Cell Physiol. 2002;283:C187–192. doi: 10.1152/ajpcell.00008.2002. [DOI] [PubMed] [Google Scholar]
- 12.Prochniewicz E, Lowe DA, Spakowicz DJ, Higgins L, O’Conor K, Thompson LV, Ferrington DA, Thomas DD. Functional, structural, and chemical changes in myosin associated with hydrogen peroxide treatment of skeletal muscle fibers. Am J Physiol Cell Physiol. 2008;294:C613–626. doi: 10.1152/ajpcell.00232.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rayment I, Holden HM, Whittaker M, Yohn CB, Lorenz M, Holmes KC, Milligan RA. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993;261:58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- 14.Stull JT, Kamm KE, Vandenboom R. Myosin light chain kinase and the role of myosin light chain phosphorylation in skeletal muscle. Arch Biochem Biophys. 2011;510:120–128. doi: 10.1016/j.abb.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenberg MJ, Kazmierczak K, Szczesna-Cordary D, Moore JR. Cardiomyopathy-linked myosin regulatory light chain mutations disrupt myosin strain-dependent biochemistry. Proc Natl Acad Sci U S A. 2010;107:17403–17408. doi: 10.1073/pnas.1009619107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muthu P, Kazmierczak K, Jones M, Szczesna-Cordary D. The effect of myosin RLC phosphorylation in normal and cardiomyopathic mouse hearts. J Cell Mol Med. 2012;16:911–919. doi: 10.1111/j.1582-4934.2011.01371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colson BA, Locher MR, Bekyarova T, Patel JR, Fitzsimons DP, Irving TC, Moss RL. Differential roles of regulatory light chain and myosin binding protein-C phosphorylations in the modulation of cardiac force development. J Physiol. 2010;588:981–993. doi: 10.1113/jphysiol.2009.183897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xeni J, Gittings WB, Caterini D, Huang J, Houston ME, Grange RW, Vandenboom R. Myosin light-chain phosphorylation and potentiation of dynamic function in mouse fast muscle. Pflugers Arch. 2011;462:349–358. doi: 10.1007/s00424-011-0965-y. [DOI] [PubMed] [Google Scholar]
- 19.Kararigas G, Bito V, Sipido KR, Regitz-Zagrosek V. Estrogen induces cardiomyocyte contractility via an MLCK/MRLC-dependent mechanism. The FASEB Journal. 2012;26:1054. [Google Scholar]
- 20.Kararigas G, Bito V, Tinel H, Becher E, Baczko I, Knosalla C, Albrecht-Kupper B, Sipido KR, Regitz-Zagrosek V. Transcriptome characterization of estrogen-treated human myocardium identifies myosin regulatory light chain interacting protein as a sex-specific element influencing contractile function. Journal of the American College of Cardiology. 2012;59:410–417. doi: 10.1016/j.jacc.2011.09.054. [DOI] [PubMed] [Google Scholar]
- 21.Gelfi C, Vigano A, Ripamonti M, Pontoglio A, Begum S, Pellegrino MA, Grassi B, Bottinelli R, Wait R, Cerretelli P. The human muscle proteome in aging. J Proteome Res. 2006;5:1344–1353. doi: 10.1021/pr050414x. [DOI] [PubMed] [Google Scholar]
- 22.Miller MS, Bedrin NG, Callahan DM, Previs MJ, Jennings ME, 2nd, Ades PA, Maughan DW, Palmer BM, Toth MJ. Age-Related Slowing of Myosin-Actin Cross-Bridge Kinetics Is Sex-Specific and Predicts Decrements in Whole Skeletal Muscle Performance in Humans. J Appl Physiol. 1985 doi: 10.1152/japplphysiol.00563.2013. (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai S, Lowe D. Regulation of skeletal muscle strength by estradiol: neuronal nitric oxide synthase and myosin regulatory light chain. The FASEB Journal. 2013;27:14. [Google Scholar]
- 24.Hooijman P, Stewart MA, Cooke R. A new state of cardiac myosin with very slow ATP turnover: a potential cardioprotective mechanism in the heart. Biophys J. 2011;100:1969–1976. doi: 10.1016/j.bpj.2011.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naber N, Cooke R, Pate E. Slow myosin ATP turnover in the super-relaxed state in tarantula muscle. J Mol Biol. 2011;411:943–950. doi: 10.1016/j.jmb.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stewart MA, Franks-Skiba K, Chen S, Cooke R. Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc Natl Acad Sci U S A. 2010;107:430–435. doi: 10.1073/pnas.0909468107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karatzaferi C, Franks-Skiba K, Cooke R. Inhibition of shortening velocity of skinned skeletal muscle fibers in conditions that mimic fatigue. American journal of physiology. Regulatory integrative and comparative physiology. 2008;294:R948–955. doi: 10.1152/ajpregu.00541.2007. [DOI] [PubMed] [Google Scholar]
- 28.Colson BA, Rybakova IN, Prochniewicz E, Moss RL, thomas DD. Cardiac myosin binding protein-C restricts intrafilament torsional dynamics of actin in a phosphorylation-dependent manner. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1213027109. Accepted, with minor revisions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colson BA, Gruber SJ, Thomas DD. Structural dynamics of muscle protein phosphorylation. J Muscle Res Cell Motil. 2012 doi: 10.1007/s10974-012-9317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moen RJ, Cornea S, Oseid DE, Binder BP, Klein JC, Thomas DD. Redox-sensitive residue in the actin-binding interface of myosin. Biochemical and biophysical research communications. 2014 doi: 10.1016/j.bbrc.2014.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogers NH, Witczak CA, Hirshman MF, Goodyear LJ, Greenberg AS. Estradiol stimulates Akt, AMP-activated protein kinase (AMPK) and TBC1D1/4, but not glucose uptake in rat soleus. Biochem Biophys Res Commun. 2009;382:646–650. doi: 10.1016/j.bbrc.2009.02.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wohlers LM, Sweeney SM, Ward CW, Lovering RM, Spangenburg EE. Changes in contraction-induced phosphorylation of AMP-activated protein kinase and mitogen-activated protein kinases in skeletal muscle after ovariectomy. J Cell Biochem. 2009;107:171–178. doi: 10.1002/jcb.22113. [DOI] [PubMed] [Google Scholar]
- 33.Thomas DD, Muretta JM, Colson BA, Mello RN, Kast D. 4.12 Spectroscopic Probes of Muscle Proteins. In: Edward HE Editor-in-Chief, editor. Comprehensive Biophysics. Elsevier; Amsterdam: 2012. pp. 226–250. [Google Scholar]
- 34.Mello RN, Thomas DD. Three distinct actin-attached structural states of myosin in muscle fibers. Biophys J. 2012;102:1088–1096. doi: 10.1016/j.bpj.2011.11.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]