Abstract
Background
Decisions to proceed with surgical versus percutaneous revascularization for multivessel coronary artery disease are often based on subtle clinical information that may not be captured in contemporary registries. The present study sought to evaluate the association between surgical ineligibility documented in the medical record and long-term mortality among patients with unprotected left main or multivessel coronary artery disease undergoing percutaneous coronary intervention (PCI).
Methods and Results
All subjects undergoing non-emergent PCI for unprotected left main or multivessel coronary artery disease were identified at two academic medical centers from 2009 – 2012. Documentation of surgical ineligibility was assessed through review of the electronic medical record. Cox proportional hazard models adjusted for known mortality risk factors were created to assess long-term mortality in patients with and without documentation of surgical ineligibility. Among 1013 subjects with multivessel coronary artery disease, 218 (22 %) were deemed ineligible for coronary artery bypass graft surgery. The most common explicitly cited reasons for surgical ineligibility in the medical record were poor surgical targets (24 %), advanced age (16 %) and renal insufficiency (16 %). After adjustment for known risk factors, documentation of surgical ineligibility remained independently associated with an increased risk of in-hospital (OR: 6.26, 95% CI: 2.16 – 18.15, P<0.001) and long-term mortality (HR: 2.98, 95% CI: 1.88 – 4.72, P<0.001) after PCI.
Conclusions
Documented surgical ineligibility is common and associated with significantly increased long-term mortality among patients undergoing PCI with unprotected left main or multivessel coronary disease, even after adjustment for known risk factors for adverse events during percutaneous revascularization.
Keywords: Percutaneous coronary intervention, risk-adjustment, surgical eligibility
Background
The optimal revascularization strategy for patients with left main or multivessel coronary artery disease has been an important focus for comparative effectiveness research.1–5 Several cohort studies and meta-analyses have suggested that coronary artery bypass graft surgery (CABG) is associated with reduced adverse events when compared to percutaneous coronary intervention (PCI) for revascularization of these patients.1,3,6,7 Additional randomized trials have confirmed that diabetic patients with this coronary anatomy may particularly benefit from surgical revascularization, though outcomes among non-diabetic patients are similar.5,8 With this in mind, clinical guidelines and appropriate use criteria have favored surgical revascularization among patients with left main or multivessel coronary artery disease when there are no other extenuating circumstances.9–11 In clinical practice, however, physicians commonly encounter patients that would have been excluded from clinical trials due to significant medical comorbidities and thus may not be subject to their findings.
The most compelling reason clinicians may choose one revascularization strategy over another occurs when a patient is deemed eligible for only one of the potential options.12 The determination of eligibility for surgical revascularization is inherently subjective and may be due to factors such as the perceived frailty of the patient or the quality of the distal arteries to accept bypass grafts. Several of these characteristics are not measured or too subtle to be captured in procedural registries and thus not incorporated into commonly used risk models for percutaneous revascularization.12 Because of this, these risk models may inadequately characterize procedural risk in several situations where they are commonly employed: 1) comparative effectiveness research, 2) assessment of hospital quality13 and 3) clinical decision making.14 Previous examination of the prevalence and impact of documented ineligibility for surgical revascularization has been limited to a single study of patients with unprotected left main disease.12 There are limited data examining surgical ineligibility among the broader population of patients with “surgical anatomy,” including patients with multivessel coronary artery disease.
With this in mind, the present study sought to 1) examine the frequency of documented surgical ineligibility among patients with known unprotected left main or multivessel coronary artery disease undergoing PCI and 2) assess the association between surgical ineligibility and mortality after adjustment for risk factors routinely used to predict mortality in clinical registries for PCI.
Methods
Population
All patients presenting to two academic medical centers within an integrated health system (Brigham and Women’s Hospital and Massachusetts General Hospital) that undergo percutaneous or surgical coronary revascularization are included in an ongoing institutionally sponsored registry, the Partners Long-Term Outcomes Database. This registry includes data fields for the National Cardiovascular Data Registry (NCDR) CathPCI registry as well as the Society of Thoracic Surgeons (STS) dataset and relies on linkage to the National Death Index to assess long-term mortality using direct identifiers. The present project focused on patients with coronary anatomy suitable for surgical revascularization that subsequently underwent PCI. Surgical anatomy was defined as one of the following: 1) unprotected left main coronary artery disease (>50 %), 2) three vessel coronary artery disease (>70 %) or 3) two vessel coronary artery disease with stenosis (>70 %) in the proximal left anterior descending artery as defined in the appropriate use criteria for PCI.9,11 To ensure that only non-emergent cases were included, subjects that underwent emergent revascularization for cardiac arrest, cardiogenic shock or ST-elevation myocardial infarction were excluded from the analysis. Patients with a history of prior CABG were also excluded as they represent a subgroup of patients for whom the underlying coronary anatomy and risks of repeat surgical revascularization are unique. The present project has been reviewed and approved with a waiver of informed consent from the institutional review board at Partners Healthcare.
Measurements
Clinical and procedural information was abstracted from the electronic medical record and included in the institutional registry. The entire electronic medical record, including admission notes, consult notes, nursing notes, outpatient notes, catheterization reports and discharge summaries, was then queried to identify the presence of a cardiothoracic surgery consult note or explicit documentation of surgical ineligibility at any time prior to percutaneous revascularization. Surgical ineligibility was defined by the treating clinicians using terms such as “ineligible for surgery” or “too high risk for surgery” and independent of the views of the physician chart abstractors. Because of this, subjects that did not have clear and explicit documentation of surgical ineligibility were considered eligible for bypass surgery. Similarly, subjects with a documented patient preference for percutaneous revascularization were also deemed eligible for surgery and noted to have explicit documentation of a discussion regarding treatment options. For those that were deemed ineligible, the explicitly documented reasons for surgical ineligibility in the medical record were recorded and further categorized according to a previously published taxonomy.12 To assess interobserver variability, two independent blinded physicians reviewed the electronic medical record for a random 10 % of the cohort and the results were compared. Long-term mortality was assessed through a review of the National Death Index and subsequent linkage with the institutional registry, as described previously.15
Statistical Analysis
Summary statistics were reported as means with standard deviations (SD) for continuous variables or medians and interquartile ranges (IQR) for non-normally distributed continuous data. T-tests and Mann-Whitney U tests were used to compare normally and non-normally distributed continuous variables respectively. Chi-squared tests were used to evaluate differences in proportions. Interobserver variability was assessed with the Kappa statistic. Kaplan-Meier survival curves were generated stratified by the presence or absence of surgical ineligibility documentation and log-rank tests were used to compare the curves. Using a previously validated model for procedural risk from the NCDR CathPCI dataset, logistical regression models were created with and without the addition of documented surgical ineligibility as a covariate to assess in-hospital mortality.14,16 Similar cox proportional hazards models with and without documentation of surgical ineligibility were also created to assess long-term mortality. The variables incorporated into both of these models included demographic characteristics (age, body mass index), past medical history (cerebrovascular disease, heart failure, peripheral vascular disease, diabetes mellitus, renal disease requiring hemodialysis, prior PCI), cardiac function (ejection fraction), presentation (cardiogenic shock, ST-elevation myocardial infarction) and angiographic characteristics (in-stent thrombosis, chronic total occlusion, disease in the left main coronary artery, disease in the proximal left anterior descending coronary artery, multivessel coronary artery disease).14,16 These models were then used to calculated predicted in-hospital and long-term mortality, respectively. To quantify the extent to which surgical ineligibility improved mortality prediction over the NCDR risk score, we calculated the adjusted hazard ratio and integrated discrimination index (IDI) for surgical ineligibility as described previously.17 C-statistics were also computed for the model with and without the addition of surgical ineligibility as a covariate. These c-statistics were subsequently compared using the method of Delong, the standard method to compare correlated or nested c-statistics.18 All statistical analyses were performed using SAS 9.3 (Cary, NC). A p-value < 0.05 was considered statistically significant.
Results
Population
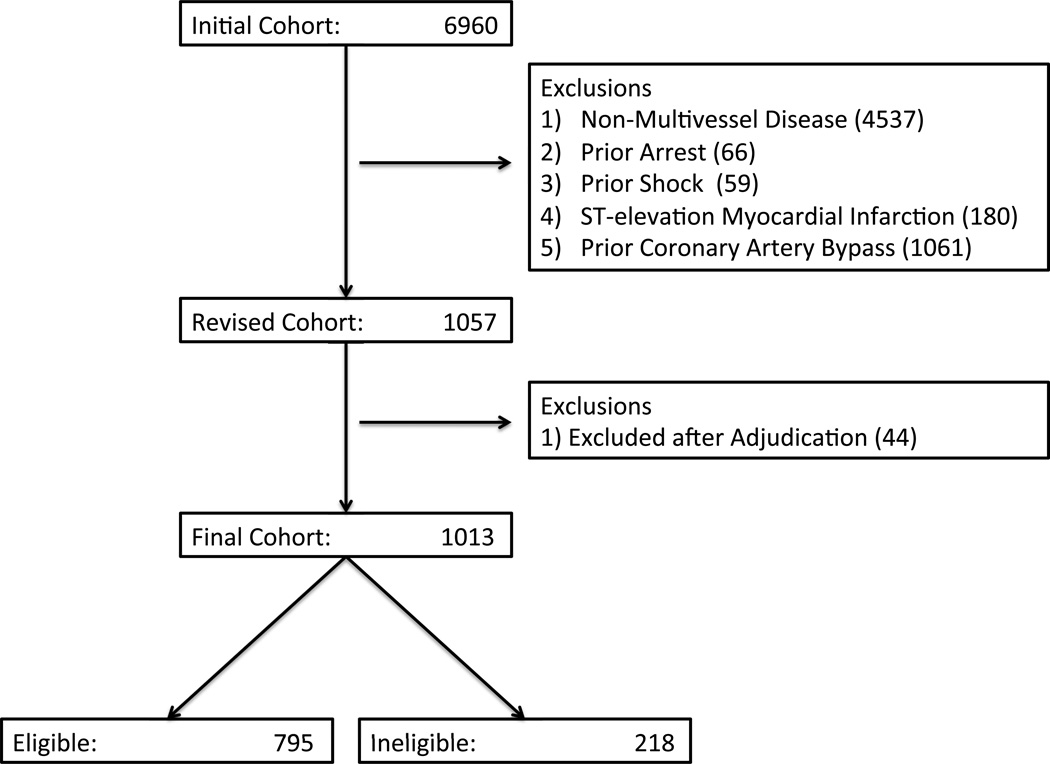
Among 6960 subjects undergoing non-emergent PCI from 2009 – 2012, 1013 (15 %) had unprotected left main or multivessel coronary artery disease. Using all available documents in the electronic medical record, 218 (22 %) subjects were deemed ineligible for surgical revascularization by the treating clinicians (Figure 1). The interobserver agreement of assessing documentation of surgical ineligibility within the electronic medical record was high (Κ: 0.923, 95% CI: 0.837 – 1.000).
Figure 1.
Study Population. Flow-diagram depicting inclusion and exclusion criteria for analysis.
Demographics
The demographic characteristics for those with documentation of surgical ineligibility and those without are reproduced in Table 1. Ineligible subjects were older (72 vs. 67 years, P<0.001) and more likely to be female (42 % vs. 66 %, P=0.039). A greater proportion of patients deemed ineligible for surgery had concomitant cerebrovascular disease (27 % vs. 13 %, P<0.001), chronic lung disease (30 % vs. 14 %, P<0.001), congestive heart failure (38 % vs. 12 %, P<0.001), diabetes mellitus (45 % vs. 38 %, P=0.039), hypertension (92 % vs. 86 %, P=0.015), peripheral artery disease (36 % vs. 15 %, P<0.001) and a prior myocardial infarction (52 % vs. 31 %, P<0.001). The predicted in-hospital mortality for those that were surgically ineligible was also increased (0.023 vs. 0.009, P<0.01).
Table 1.
Demographic characteristics.
| Ineligible (n = 218) |
Eligible (n = 793) |
P-Value | |
|---|---|---|---|
| Age (years) | 72 ± 12 | 67 ± 12 | <0.001 |
| Male, n (%) | 187 (58) | 522 (66) | 0.039 |
| Race, n (%) | 0.117 | ||
| Asian | 9 (4) | 22 (3) | |
| Black | 13 (6) | 39 (5) | |
| Hispanic | 10 (5) | 35 (4) | |
| Native American | 1 (1) | 0 (0) | |
| White | 184 (84) | 676 (85) | |
| Other | 1 (1) | 21 (3) | |
| Insurance Payer, n (%) | <0.001 | ||
| Commercial | 61 (28) | 338 (43) | |
| Government | 154 (71) | 445 (56) | |
| International | 0 (0) | 3 (1) | |
| None | 3 (1) | 7 (1) | |
| Presenting Symptoms, n (%) | <0.001 | ||
| No Angina | 76 (35) | 146 (18) | |
| Stable Angina | 23 (11) | 190 (24) | |
| Unstable Angina | 65 (30) | 215 (27) | |
| Non-ST Elevation Myocardial Infarction | 49 (22) | 223 (28) | |
| Medical Comorbidities, n (%) | |||
| Cerebrovascular Disease | 58 (27) | 106 (13) | <0.001 |
| Chronic Lung Disease | 65 (30) | 110 (14) | <0.001 |
| Congestive Heart Failure | 82 (38) | 92 (12) | <0.001 |
| Diabetes Mellitus | 99 (45) | 299 (38) | 0.039 |
| Dyslipidemia | 205 (94) | 741 (93) | 0.751 |
| Hypertension | 201 (92) | 682 (86) | 0.015 |
| Peripheral Arterial Disease | 78 (36) | 120 (15) | <0.001 |
| Prior Myocardial Infarction | 114 (52) | 244 (31) | <0.001 |
| Prior Percutaneous Intervention | 64 (29) | 272 (34) | 0.170 |
| Valvular Heart Disease | 5 (3) | 11 (1) | 0.342 |
| Laboratory Values | |||
| Glomerular Filtration Rate (ml / min) | 62 ± 35 | 74 ± 28 | <0.001 |
| In-Hospital Mortality | |||
| Predicted Mortality (NCDR CathPCI) | 0.023 ± 0.034 | 0.009 ± 0.032 | <0.001 |
All data presented as mean ± standard deviation for continuous variables and number (percentage) for categorical variables
Angiography
The angiographic characteristics for those with and without documentation of surgical ineligibility are shown in Table 2. Subjects ineligible for surgical revascularization were more likely to undergo procedures through the femoral approach (81 % vs. 63 %, P<0.001). A larger proportion of patients deemed ineligible for surgery had left main disease (33 % vs. 10 %, P<0.001) and had high complexity lesions (51 % vs. 34 %, P<0.001). The number of lesions (P<0.001), stents placed (P<0.001) and length of stents (P<0.001) were all significantly greater among surgically ineligible patients undergoing percutaneous revascularization as well. Subjects that were deemed eligible for surgical revascularization, however, had a greater number of vessels revascularized (P<0.001).
Table 2.
Procedural characteristics.
| Ineligible (n = 218) |
Eligible (n = 793) |
P-Value | |
|---|---|---|---|
| Procedural Access, n (%) | <0.001 | ||
| Femoral | 177 (81) | 496 (63) | |
| Radial | 34 (16) | 263 (33) | |
| Coronary Anatomy, n (%) | <0.001 | ||
| Left Main Disease | 71 (33) | 78 (10) | |
| Three Vessel Disease | 89 (41) | 315 (40) | |
| Two Vessel Disease and proximal LAD | 58 (27) | 400 (50) | |
| Coronary Anatomy Complexity, n (%) | |||
| Bifurcation Lesions | 8 (4) | 48 (6) | 0.173 |
| Chronic Total Occlusion | 3 (1) | 15 (2) | 0.610 |
| High Lesion Complexity (Type C)* | 110 (51) | 272 (34) | <0.001 |
| Coronary Intervention | |||
| Number of Vessels Treated | 1.42 ± 0.57 | 1.57 ± 0.61 | <0.001 |
| Number of Lesions Treated | 2.00 ± 1.00 | 1.71 ± 0.87 | <0.001 |
| Number of Stents Placed | 2.31 ± 1.62 | 1.87 ± 1.17 | <0.001 |
| Total Stent Length (mm) | 38 (18 – 64) | 30 (18 – 46) | <0.001 |
| Coronary Intervention Vessels, n (%) | |||
| Left Main | 47 (22) | 39 (5) | <0.001 |
| Left Anterior Descending | 135 (62) | 523 (66) | 0.289 |
| Left Circumflex Coronary Artery | 94 (43) | 275 (34) | 0.022 |
| Right Coronary Artery | 62 (28) | 278 (35) | 0.067 |
| Intra-Aortic Balloon Pump, n (%) | 30 (14) | 9 (1) | <0.001 |
All data presented as mean ± standard deviation or median (intraquartile range) for continuous variables and number (percentage) for categorical variables
High lesion complexity is defined as a lesion with at least one of the following characteristics: diffuse (length > 2 cm), excessive tortuosity of the proximal segment, extremely angulated segments (> 90 degrees), total occlusions (> 3 months) or inability to protect major side branches.
Ineligibility
The sources of documentation for surgical ineligibility are included in Table 3. As shown, a documented evaluation by the cardiothoracic surgery service was present in 95 (9%) of the 1013 patients undergoing percutaneous revascularization with surgical anatomy. In the 218 patients deemed ineligible, formal surgical evaluation and documentation was identified in 63 (29%) of the 218 patients. For those deemed ineligible for surgery, the majority of documentation addressing surgical candidacy was obtained from a cardiology consult note (37 %), the discharge summary (24 %) or the cardiac catheterization report (21 %) detailing the revascularization procedure. The majority of patients considered eligible for surgery did not have explicit documentation discussing surgical candidacy in the electronic medical record (81 %). As shown in Table 4, poor surgical targets (24 %), advanced age (16 %) and renal insufficiency (16 %) were the most commonly cited characteristics that were deemed to significantly increase the surgical risk.
Table 3.
Documentation source of surgical ineligibility.
| Ineligible (n = 218) |
Eligible (n = 793) |
P-Value | |
|---|---|---|---|
| Surgical Consult Documentation, n (%) | 63 (29) | 32 (5) | <0.001 |
| Eligibility Documentation Source, n (%) | <0.001 | ||
| Cardiology Catheterization Report | 43 (21) | 40 (5) | |
| Cardiology Consult Note | 77 (37) | 50 (6) | |
| Discharge Summary | 50 (24) | 35 (4) | |
| Surgical Consult Note | 36 (17) | 24 (3) | |
| None | 0 (0) | 644 (81) | |
Table 4.
Criteria associated with surgical ineligibility.
| Prevalence | |
|---|---|
| Poor Targets / Conduits, n (%) | 52 (24) |
| Advanced Age, n (%) | 35 (16) |
| Renal Insufficiency, n (%) | 35 (16) |
| Severe Lung Disease, n (%) | 32 (15) |
| Severe Systolic Dysfunction, n (%) | 31 (14) |
| Malignancy, n (%) | 24 (11) |
| Severe Peripheral Arterial Disease, n (%) | 17 (8) |
| Extensive Nonviable Myocardium, n (%) | 14 (6) |
| Severe Aortic Calcification, n (%) | 13 (6) |
| Cachexia, n (%) | 9 (4) |
| Hematologic Abnormality, n (%) | 9 (4) |
| End-Stage Liver Disease, n (%) | 8 (4) |
| Morbid Obesity, n (%) | 7 (3) |
| Severe Cerebrovascular Disease, n (%) | 7 (3) |
| Cognitive Dysfunction, n (%) | 6 (3) |
| Gastrointestinal Bleeding, n (%) | 6 (3) |
| Systemic Infection, n (%) | 5 (2) |
| Chest Wall Abnormality, n (%) | 2 (1) |
| Immunosuppressed, n (%) | 2 (1) |
| Pulmonary Hypertension, n (%) | 1 (1) |
All data presented as number (percentage) with the total number of patients deemed surgically ineligible (218) used as the denominator.
Mortality
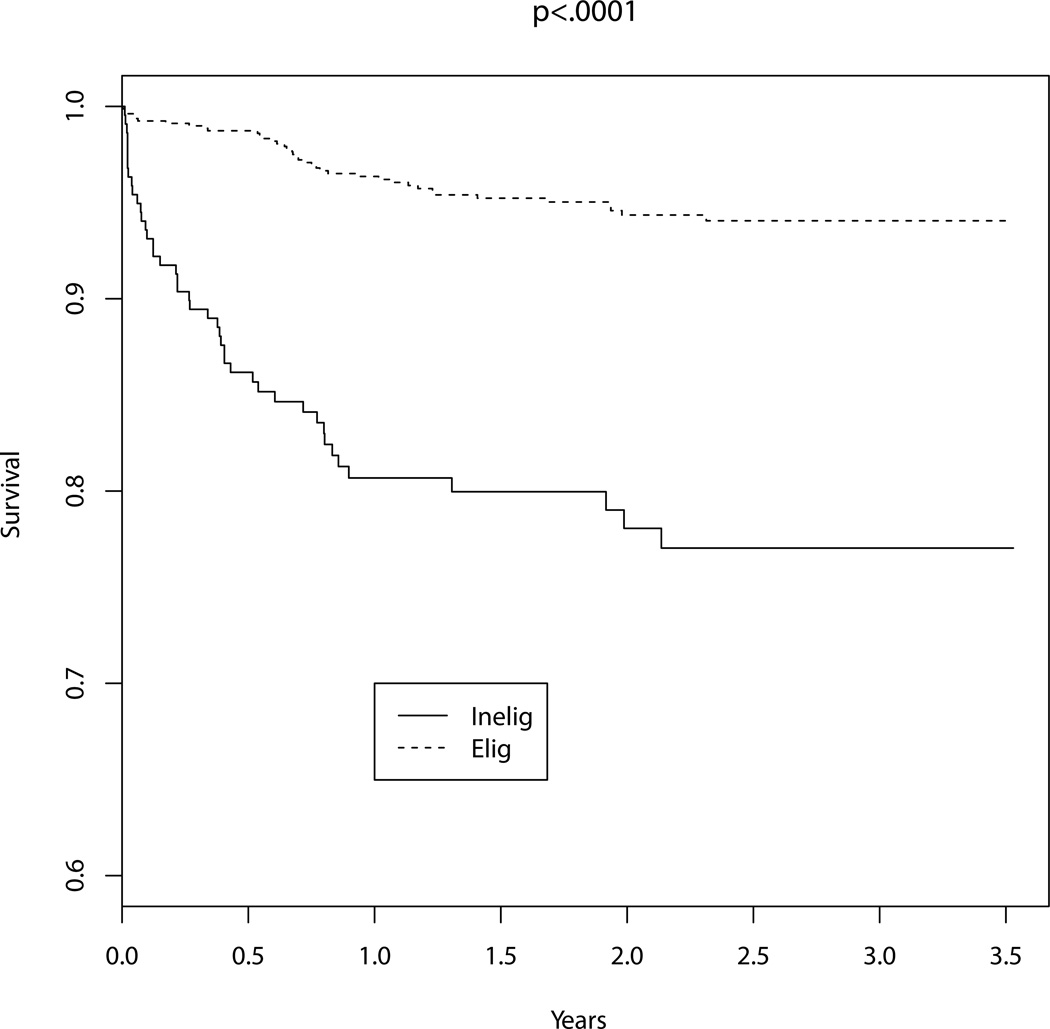
The unadjusted in-hospital mortality among patients undergoing percutaneous revascularization was greater for those deemed ineligible for cardiac surgery (15 / 218, 7 %) compared to those that were eligible for the procedure (5 / 793, 1 %, P<0.001). Unadjusted long-term mortality was also significantly greater in subjects deemed ineligible for surgery compared with those that were considered surgical candidates, as shown in Figure 2 (HR: 4.81, 95% CI: 3.12 – 7.40). After adjustment for predicted mortality risk, surgical ineligibility remained independently associated with increased odds of in-hospital death (OR: 6.26, 95% CI: 2.16 – 18.15, P<0.001) and long-term mortality (HR: 2.98, 95 % CI: 1.88 – 4.72, P<0.001). The addition of surgical ineligibility to the previously validated risk-adjustment model significantly improved the predictive capability of the model (c-statistic 0.753 of NCDR risk score vs. 0.792 including surgical ineligibility variable, P<0.01). The integrated discrimination improvement after the addition of surgical ineligibility to the model was 0.04 (95% CI: 0.02 – 0.05) and the relative integrated discrimination improvement was 0.40 (95% CI: 0.21 – 0.60), suggesting marked improvement in risk discrimination with addition of surgical ineligibility to the risk model.
Figure 2.
Mortality. Kaplan-Meier estimates comparing long-term mortality among those with surgical anatomy undergoing percutaneous revascularization stratified by documentation of surgical ineligibility. Surgical inelibility (Inelig) was associated with a significant increase in mortality when compared to those that were surgically eligible (Elig, Log-Rank < 0.001).
Discussion
The present study evaluated over 1000 consecutive patients with left main or multivessel coronary artery disease undergoing percutaneous revascularization in an integrated health system. Within this population, we found that documented surgical ineligibility was common and associated with significantly greater in-hospital and long-term mortality even after accounting for risk factors employed in a contemporary and widely used risk adjustment model for percutaneous revascularization. In fact, the addition of surgical ineligibility as a covariate to this model significantly improved its ability to predict mortality. These findings have important implications for comparative effectiveness research, evaluating hospital quality and procedural appropriateness, and the application of risk-prediction estimates to guide clinical decision making.
Comparative Effectiveness Research
The optimal revascularization strategy for patients with multivessel coronary artery disease has long been a subject of interest in comparative effectiveness research. Over the last three decades, numerous randomized trials have been performed to evaluate clinical outcomes among those treated with surgical or percutaneous revascularization.5,8 Observational data, however, has not always remained consistent with these findings.19,20 Previous research has demonstrated that surgical ineligibility, a characteristic often not measured in contemporary observational datasets, was associated with a 5 – 6 fold increase in mortality among 101 patients undergoing unprotected left main PCI.12 Our results expand upon these findings by including all patients with coronary anatomy that would favor surgical revascularization according to current professional society guidelines and appropriate use criteria – those with three vessel coronary artery disease or two vessel disease with a severe stenosis in the proximal left anterior descending artery. The data demonstrate that documentation of surgical ineligibility confers additional risk in these populations as well, even after adjusting for contemporary risk factors. It is possible that surgical ineligibility in itself may represent a variety of other clinical characteristics that are poorly captured in administrative or clinical datasets, including general frailty or poor psychosocial support. Due to both their high prevalence and large effect size, these unmeasured characteristics have the potential to undermine the results of large observational studies comparing revascularization strategies, even those employing rigorous statistical methods to limit confounding.21
Hospital Quality Assessment and Appropriateness
Risk-adjusted mortality is a commonly employed benchmark to assess hospital PCI quality. The accuracy of such reports hinges, in part, on the inclusion of prognostically important variables in risk-adjustment models as well as their distribution among hospitals.22 In Massachusetts, where these data are used to publicly profile hospital performance, the importance of documenting PCI cases done for “compassionate use” was found to significantly improve mortality risk-prediction and attenuate the decline in procedures performed in the setting of cardiogenic shock.23 Similarly, our findings support the idea that documented surgical ineligibility may be an important variable to consider in risk-adjustment models used for hospital quality assessment, given its significant association with PCI outcomes and the likelihood that these patients would be concentrated at institutions that performed cardiac surgery. Many of the surgically ineligible patients that received percutaneous revascularization may have been treated as salvage cases or in the setting of compassionate use, two situations in which the inclusion of surgical ineligibility data could have significant impact on published mortality data and thus clinical practice in states with public reporting of outcomes. The increased anatomical complexity of the surgical ineligible patients supports this notion. In contrast, the surgically eligible patients were found to have lower anatomical complexity suggesting greater clinical equipoise between surgical and percutaneous revascularization thus leading to a large number of eligible patients pursuing PCI. Perhaps this should be considered when evaluating hospital quality and procedural appropriateness.
Clinical Decision Making
Clinical guidelines and appropriate use criteria have favored surgical revascularization for patients with left main or multivessel coronary artery disease when there is no compelling indication for one treatment modality over the other.9–11 When a compelling indication may exist, however, the guidelines advocate for a heart team approach with input from cardiac surgeons and interventional cardiologists. Interestingly, the present study suggests that formal consultation and electronic documentation from a cardiac surgeon was uncommon in patients with left main or multivessel coronary artery disease undergoing PCI. Perhaps cardiologists treating these patients employed risk prediction instruments such as the STS score or Euroscore to determine the potential morbidity of undergoing surgical revascularization.24,25 As previously described, these scores aid clinicians in identifying patients that may be high risk for surgical revascularization and thus benefit from a less invasive approach. The data from the present study suggests that increased surgical risk that leads to operative ineligibility does not automatically imply that percutaneous revascularization is a safer option. In fact, addition of surgical ineligibility to similar risk scores developed for percutaneous revascularization suggests increased procedural risk. Further, our data suggest that percutaneous revascularization in these patients results in fewer vessels treated and perhaps greater residual ischemia.
Limitations
The present study should be interpreted in the context of several limitations. Ascertainment of surgical ineligibility was based upon documentation in the electronic medical record. Because of this, discussions regarding surgical ineligibility that took place during the course of patient care but were not explicitly documented could lead to the misclassification of patients as eligible for surgical revascularization. It is important to note that inclusion of these patients as surgically ineligible would only serve to increase the measured mortality difference between the two populations, rather than demonstrating improved mortality in the ineligible group. Residual confounding between surgical ineligibility and mortality may also exist outside of the collected data. Further, the present analysis does not evaluate differences in outcomes among surgically ineligible patients that are treated medically and those that receive percutaneous revascularization in the setting of disease salvage or compassionate use. The mortality rates for similar patients treated conservatively may be even higher than those observed with PCI. Finally, the population in this study was gathered from subjects undergoing treatment at two academic tertiary care medical centers and may not be generalizable to other settings. Additional prospective studies including diverse patient populations could be designed to address these limitations.
Conclusions
In conclusion, documented surgical ineligibility is common and is strongly associated with increased mortality after percutaneous intervention for patients with unprotected left main and multivessel coronary disease, even above and beyond commonly employed risk-adjustment models for percutaneous revascularization.
Supplementary Material
Acknowledgments
Funding Sources: Dr. Yeh is supported by a career development award from the National Heart, Lung, and Blood Institute. The study was supported, in part, by the Hassenfeld Scholars Program.
Footnotes
Disclosures: None.
References
- 1.Hlatky MA, Boothroyd DB, Bravata DM, Boersma E, Booth J, Brooks MM, Carrié D, Clayton TC, Danchin N, Flather M, Hamm CW, Hueb WA, Kähler J, Kelsey SF, King SB, Kosinski AS, Lopes N, McDonald KM, Rodriguez A, Serruys P, Sigwart U, Stables RH, Owens DK, Pocock SJ. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet. 2009;373:1190–1197. doi: 10.1016/S0140-6736(09)60552-3. [DOI] [PubMed] [Google Scholar]
- 2.Serruys PW, Morice M-C, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Ståhle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW SYNTAX Investigators. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 3.Shahian DM, O’Brien SM, Sheng S, Grover FL, Mayer JE, Jacobs JP, Weiss JM, Delong ER, Peterson ED, Weintraub WS, Grau-Sepulveda MV, Klein LW, Shaw RE, Garratt KN, Moussa ID, Shewan CM, Dangas GD, Edwards FH. Predictors of long-term survival after coronary artery bypass grafting surgery: results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database (the ASCERT study) Circulation. 2012;125:1491–1500. doi: 10.1161/CIRCULATIONAHA.111.066902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hlatky MA, Boothroyd DB, Baker L, Kazi DS, Solomon MD, Chang TI, Shilane D, Go AS. Comparative effectiveness of multivessel coronary bypass surgery and multivessel percutaneous coronary intervention: a cohort study. Ann Intern Med. 2013;158:727–734. doi: 10.7326/0003-4819-158-10-201305210-00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, Desai AS, Gersh BJ, Magnuson EA, Lansky A, Boineau R, Weinberger J, Ramanathan K, Sousa JE, Rankin J, Bhargava B, Buse J, Hueb W, Smith CR, Muratov V, Bansilal S, King S, 3rd, Bertrand M, Fuster V FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375–2384. doi: 10.1056/NEJMoa1211585. [DOI] [PubMed] [Google Scholar]
- 6.Hannan EL, Wu C, Walford G, Culliford AT, Gold JP, Smith CR, Higgins RSD, Carlson RE, Jones RH. Drug-eluting stents vs. coronary-artery bypass grafting in multivessel coronary disease. N Engl J Med. 2008;358:331–341. doi: 10.1056/NEJMoa071804. [DOI] [PubMed] [Google Scholar]
- 7.Wu C, Camacho FT, Zhao S, Wechsler AS, Culliford AT, Lahey SJ, King SB, Walford G, Gold JP, Smith CR, Jordan D, Higgins RSD, Hannan EL. Long-term mortality of coronary artery bypass graft surgery and stenting with drug-eluting stents. Ann Thorac Surg. 2013;95:1297–1305. doi: 10.1016/j.athoracsur.2012.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. N Engl J Med. 1996;335:217–225. doi: 10.1056/NEJM199607253350401. [DOI] [PubMed] [Google Scholar]
- 9.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography, Heart Failure Society of America, Society of Cardiovascular Computed Tomography. ACCF/SCAI/STS/AATS/AHA/ASNC 2009 Appropriateness Criteria for Coronary Revascularization: a report by the American College of Cardiology Foundation Appropriateness Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, and the American Society of Nuclear Cardiology Endorsed by the American Society of Echocardiography, the Heart Failure Society of America, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2009;53:530–553. doi: 10.1016/j.jacc.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–e651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 11.Patel MR, Dehmer GJ, Hirshfeld JW, Smith PK, Spertus JA ACCF/SCAI/STS/AATS/AHA/ASNC/HFSA/SCCT 2012. Appropriate use criteria for coronary revascularization focused update: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, Society for Cardiovascular Angiography and Interventions, Society of Thoracic Surgeons, American Association for Thoracic Surgery, American Heart Association, American Society of Nuclear Cardiology, and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2012;59:857–881. doi: 10.1016/j.jacc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 12.McNulty EJ, Ng W, Spertus JA, Zaroff JG, Yeh RW, Ren XM, Lundstrom RJ. Surgical candidacy and selection biases in nonemergent left main stenting: implications for observational studies. JACC Cardiovasc Interv. 2011;4:1020–1027. doi: 10.1016/j.jcin.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Wu C, Hannan EL, Walford G, Ambrose JA, Holmes DR, Jr, King SB, 3rd, Clark LT, Katz S, Sharma S, Jones RH. A risk score to predict in-hospital mortality for percutaneous coronary interventions. J Am Coll Cardiol. 2006;47:654–660. doi: 10.1016/j.jacc.2005.09.071. [DOI] [PubMed] [Google Scholar]
- 14.Peterson ED, Dai D, DeLong ER, Brennan JM, Singh M, Rao SV, Shaw RE, Roe MT, Ho KKL, Klein LW, Krone RJ, Weintraub WS, Brindis RG, Rumsfeld JS, Spertus JA NCDR Registry Participants. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J Am Coll Cardiol. 2010;55:1923–1932. doi: 10.1016/j.jacc.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCabe JM, Kennedy KF, Eisenhauer AC, Waldman HM, Mort EA, Pomerantsev E, Resnic FS, Yeh RW. Reporting trends and outcomes in ST-segment-elevation myocardial infarction national hospital quality assessment programs. Circulation. 2014;129:194–202. doi: 10.1161/CIRCULATIONAHA.113.006165. [DOI] [PubMed] [Google Scholar]
- 16.Brennan JM, Curtis JP, Dai D, Fitzgerald S, Khandelwal AK, Spertus JA, Rao SV, Singh M, Shaw RE, Ho KKL, Krone RJ, Weintraub WS, Weaver WD, Peterson ED National Cardiovascular Data Registry. Enhanced mortality risk prediction with a focus on high-risk percutaneous coronary intervention: results from 1,208,137 procedures in the NCDR (National Cardiovascular Data Registry) JACC Cardiovasc Interv. 2013;6:790–799. doi: 10.1016/j.jcin.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Kerr KF, McClelland RL, Brown ER, Lumley T. Evaluating the incremental value of new biomarkers with integrated discrimination improvement. Am J Epidemiol. 2011;174:364–374. doi: 10.1093/aje/kwr086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 19.Feit F, Brooks MM, Sopko G, Keller NM, Rosen A, Krone R, Berger PB, Shemin R, Attubato MJ, Williams DO, Frye R, Detre KM. Long-term clinical outcome in the Bypass Angioplasty Revascularization Investigation Registry: comparison with the randomized trial. BARI Investigators. Circulation. 2000;101:2795–2802. doi: 10.1161/01.cir.101.24.2795. [DOI] [PubMed] [Google Scholar]
- 20.Javaid A, Steinberg DH, Buch AN, Corso PJ, Boyce SW, Pinto Slottow TL, Roy PK, Hill P, Okabe T, Torguson R, Smith KA, Xue Z, Gevorkian N, Suddath WO, Kent KM, Satler LF, Pichard AD, Waksman R. Outcomes of coronary artery bypass grafting versus percutaneous coronary intervention with drug-eluting stents for patients with multivessel coronary artery disease. Circulation. 2007;116:I200–I206. doi: 10.1161/CIRCULATIONAHA.106.681148. [DOI] [PubMed] [Google Scholar]
- 21.Kirtane AJ, Pinto DS, Moses JW. Comparative effectiveness of revascularization strategies. N Engl J Med. 2012;367:476. doi: 10.1056/NEJMc1206011. author reply 477. [DOI] [PubMed] [Google Scholar]
- 22.Measuring and improving quality of care: a report from the American Heart Association/American College of Cardiology First Scientific Forum on Assessment of Healthcare Quality in Cardiovascular Disease and Stroke. Circulation. 2000;101:1483–1493. doi: 10.1161/01.cir.101.12.1483. [DOI] [PubMed] [Google Scholar]
- 23.Resnic FS, Normand S-LT, Piemonte TC, Shubrooks SJ, Zelevinsky K, Lovett A, Ho KKL. Improvement in mortality risk prediction after percutaneous coronary intervention through the addition of a “compassionate use” variable to the National Cardiovascular Data Registry CathPCI dataset: a study from the Massachusetts Angioplasty Registry. J Am Coll Cardiol. 2011;57:904–911. doi: 10.1016/j.jacc.2010.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michel P, Roques F, Nashef SAM EuroSCORE Project Group. Logistic or additive EuroSCORE for high-risk patients? Eur J Cardiothorac Surg. 2003;23:684–687. doi: 10.1016/s1010-7940(03)00074-5. discussion 687. [DOI] [PubMed] [Google Scholar]
- 25.Shahian DM, O’Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand S-LT, DeLong ER, Shewan CM, Dokholyan RS, Peterson ED, Edwards FH, Anderson RP Society of Thoracic Surgeons Quality Measurement Task Force. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1--coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88:S2–S22. doi: 10.1016/j.athoracsur.2009.05.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.