Abstract
Objective:
Inadequate intake and preference for low-calorie foods are salient behavioral features of Anorexia Nervosa (AN). The neurocognitive mechanisms underlying pathological food choice have not been characterized. This study aimed to develop a new paradigm for experimentally modeling maladaptive food choice in AN.
Method:
Individuals with AN (n=22) and healthy controls (HC, n=20) participated in a computer-based Food Choice Task, adapted for individuals with eating disorders. Participants first rated 43 food images (including high-fat and low-fat items) for Healthiness and Tastiness; an item rated neutral on both blocks was then selected as the Reference item. On each of 42 subsequent trials participants were asked to choose between the food item presented and the Reference item.
Results:
The AN group was less likely to choose high-fat foods relative to HC, as evidenced both in multilevel logistic regression (z=2.59, p=0.009) and ANOVA (F(1,39)=7.80, p=0.008) analyses. Health ratings influenced choice significantly more in AN relative to HC (z=2.7, p=0.006), and were more related to Taste among AN (χ2=4.10, p=0.04). Additionally, Taste ratings declined with duration of illness(r=−0.50, p=0.02).
Conclusions:
The Food Choice Task captures the preference for low-fat foods among individuals with AN. The findings suggest that the experience of tastiness changes over time and may contribute to perpetuation of illness. By providing an experimental quantitative measure of food restriction, this task opens the door to new experimental investigations into the cognitive, affective and neural factors contributing to maladaptive food choices characteristic of AN.
INTRODUCTION
Anorexia nervosa (AN) is a severe disorder with a mortality rate among the highest of any psychiatric illness.1,2 Much of the high morbidity and mortality of AN can be related to sequelae of chronic starvation. These include the medical morbidities of the illness (50% of deaths are related to starvation),2 as well as the depressed mood and high anxiety that are exaggerated by the underweight state (up to 45% are from suicide3). In addition to the alarming mortality rate in AN, the severity of the illness is reflected in the high relapse rate, associated psychiatric morbidity, recurrent hospitalizations and the need for chronic treatment.4
Studies of eating behavior in AN have documented that individuals with AN eat fewer calories and consume a significantly lower percentage of calories from fat.5,6 Individuals with AN who eat more energy dense foods (e.g., higher fat content, fewer non-caloric beverages) and a more varied diet have better outcomes 1 year after hospitalization.7,8 A critical issue in improving our ability to treat AN is our limited understanding of the behavioral and neurobiological underpinnings of the persistent caloric restriction.9
Efforts to characterize eating in AN have focused, in large part, on identifying nutritional content,5,10 appetitive factors in eating,11-13 and/or the role of affect in nutritional patterns.14 The cognitive components driving food selection – or “food choice” – have not commonly been probed in eating disorders. Here, the word “choice” is used to convey that the illness is characterized by an individual’s persistent, active selection of a restrictive diet; we do not mean to suggest that AN itself is a choice (but rather that food behavior patterns can be operationalized as a series of choices). Food choice has been probed across healthy populations both as a window to better understand eating behavior15 and as a paradigm to probe the neural systems underlying decision making.16 Investigation of decision making in AN, on the other hand, has tended to focus on neuropsychological tasks not directly related to eating behavior.17-19 The connections between decision making and food selection have been recently examined in non-eating disorder populations by Hare et al,20 who found that dieters engage self-regulatory control centers in the prefrontal cortex in order to select less palatable but healthier foods. This approach to understanding decision making regarding food selection has yet to be applied to eating disorders.
We aimed to develop a paradigm (the Food Choice Task), based on the work of Hare et al,20 that could be used to investigate maladaptive food choice among individuals with eating disorders. In this study, we evaluated the task in individuals with AN, and compared their responses to those of healthy peers with no history of an eating disorder. We modified the task of Hare et al20 to include a range of foods appropriate for the study population. We hypothesized that, in this task, individuals with AN would make fewer selections of higher fat food compared with HC, consistent with existing knowledge about eating behavior in AN. In addition, we aimed to explore the subjective assessment of the “healthiness” and the “tastiness” of the food items, and the relationship between these assessments and food choice.
METHODS
Participants
Participants were twenty-two individuals with AN presenting to the New York State Psychiatric Institute/Columbia University Medical Center Eating Disorders Research Clinic, and twenty group-matched healthy controls (HC). Eligible patients met DSM-521 criteria for AN22 and were receiving inpatient or outpatient treatment through the Eating Disorders Research Clinic. HC called the clinic in response to advertisements. Individuals were excluded if they had a known history of a neurological disorder or injury, or reported drug or alcohol abuse in the last 6 months. HC were included if they had no current or past psychiatric illness, including any history of an eating disorder, and had a BMI ≥ 18.5 kg/m2. Additional exclusion criteria for HC were significant medical illness, current psychotropic medication, or dietary restrictions (such as vegetarianism, or religious restrictions that would impact food choice in the task). This study was approved by the New York State Psychiatric Institute Institutional Review Board, and all participants provided written informed consent.
Procedures
Height and weight were measured on a beam balance scale (Detecto, Webb City, MO). Diagnosis was made by clinical interview with clinician (MD or PhD level) with expertise in eating disorders. Participants were administered a demographics questionnaire, the Eating Disorder Examination Questionnaire (EDE-Q),23 self-report measure of eating disorder psychopathology, Three Factor Restraint Questionnaire (TFEQ),24 and the Food Choice Task.
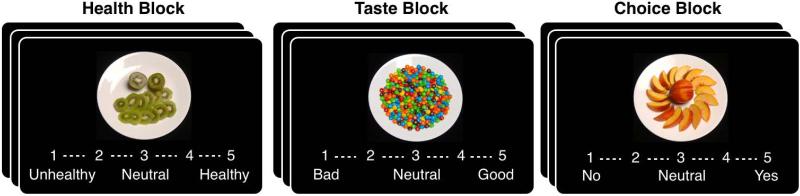
The Food Choice Task was adapted from the work of Hare et al20 to include images with a greater range of caloric density and macronutrient content. The food items were chosen to be representative of a range of dietary choices, instead of emphasizing “junk foods” and “healthy snack foods,” as in the original task. In each of two blocks, 43 food items were presented on a computer screen, of which 25 were low-fat and 18 were high-fat (images and list of food items available in Supplemental Materials, image files available upon request). Items with greater than 30% of calories derived from fat were categorized as “high-fat.” In the first block, participants provided ratings of how healthy each of a series of images of food were; in the second block, the participants rated how tasty each image was. There was no time limit for responding.
In the Health block, each image was rated by each participant on a 5 point scale, from “Healthy” to “Unhealthy” and in the Taste block each item was rated from “Good” to “Bad.” From these ratings, one food item that had been rated as “Neutral” (a score of 3) on both scales was selected as the Reference Item. If no item was rated 3 on both scales, the reference item was chosen from those Neutral on Health and “tasty” on the Taste scale. A neutral healthy but tasty item was selected because if would have greater overall value than an unhealthy item for a subject who made decisions based on health information (modified from the algorithm used by Hare et al).20 Then, in the Choice block, participants were instructed to imagine they would receive one of their selections at the end of the study as a snack. On each of 42 trials, the participant indicated whether they chose the food item presented or the Reference item, which did not change and was visible to them throughout the block (Reference image was presented next to the computer screen), with no time limit for responding.
Data Analysis
Demographic characteristics were compared between diagnostic groups (AN vs HC) using independent sample t tests for continuous variables and Chi square analysis for nominal variables.
Choices on the 5 point scale were converted to binary responses (yes or no preference for the Reference Item versus the trial-unique food item) and neutral responses were omitted. Choice and rating data were analyzed in two ways. Our primary analytic approach was to use multilevel regression models (lme4 linear mixed effects package for R,25 in order to account for random effects and unbalanced data (fewer higher fat than lower fat items) and to minimize the influence of outliers. Binomial choice data were modeled with multilevel logistic regression, in which participant choice (selection of the trial-unique food item over the constant neutral reference food) was the dependent variable. Where continuous rating data were entered as independent variables, ratings were z-scored. Continuous outcome rating data were modeled using multilevel linear regression. For these analyses, the significance of the partial correlation coefficients was assessed by χ2 statistics (and accompanying p values) which were derived for the estimates from type-III analysis of variance tables from the Anova function in the car package for R26 or from the esticon function in the doBy package when contrasting regression parameters. In these models, we additionally entered all within-subject effects as random by participant.27 Additionally we analyzed the data using ANOVA within the IBM SPSS Statistics 21 analysis package to confirm the pattern of results.
We assessed the relationship between questionnaire-based measures of restraint (TFEQ and EDE-Q) and the proportion of high-fat food choices made in the Choice block of the task with Spearman rank-order correlations.
RESULTS
Twenty-two individuals with AN (n=10 binge-purge subtype, n=12 restricting subtype; 16 were hospitalized) and 20 HC participated. Data from one individual with AN were excluded from analyses due to failure to understand the task instructions. The groups were well matched for age, gender, and ethnicity; clinical characteristics and related statistics are shown in Table 1.
Table 1.
Participant Clinical Characteristics
| HC n=20 |
AN n=21 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | t | df | p | |||
| Age (years) | 26.3 | 5.8 | 18-37 | 29.4 | 11.2 | 16-64 | −1.11 | 39 | 0.27 |
| BMI (kg/m2) | 21.0 | 1.7 | 18.5-25.9 | 17.5 | 1.9 | 13-20.4 | 6.3 | 39 | <0.001 |
| EDE-Q, global | 0.2 | 0.4 | 0.0-1.6 | 3.8 | 1.5 | 0.6-5.7 | −10.2 | 32 | <0.001 |
| EDE-Q, restraint | 0.2 | 0.5 | 0-2.4 | 3.5 | 2.0 | 0-6.0 | −7.3 | 33 | <0.001 |
| TFEQ, restraint scale | 4.8 | 3.6 | 1-14 | 15.6 | 6.1 | 0-21 | −6.6 | 34 | <0.001 |
| Caucasian | N | (%) | N | (%) | |||||
| 16 | 80.0 | 17 | 81.0 | ||||||
| Female | 19 | 95.0 | 20 | 95.2 | |||||
| Restricting subtype | -- | 11 | 52.4 | ||||||
EDE-Q and TFEQ data were not available for all patients. For EDE-Q, n=14 AN and for TFEQ n=16 AN.
Task Outcome
Food Choice:
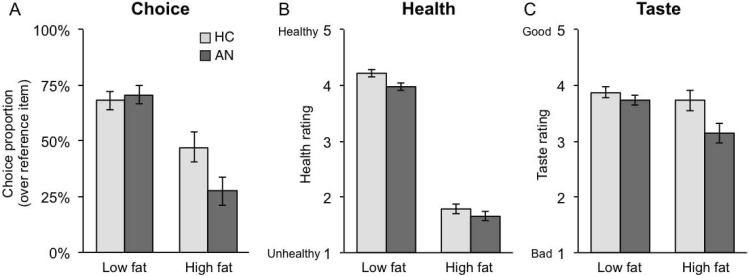
As hypothesized, individuals with AN demonstrated a preference for the low-fat foods. We assessed food choices entering Food type (low/high fat coded 0/1) and Group (HC/AN coded −1/+1) as independent variables in a multilevel logistic regression examining food choices, which has the advantage of accounting for the unequal numbers of low and high-fat food items. There was a significant effect of Food type (high vs. low-fat), such that high-fat foods were less likely to be chosen relative to low-fat foods (z=− 6.8, p < 0.0001). There was no main effect of Group (z=0.48, p=0.63); there was a Group × Food type interaction such that the AN group was less likely to choose high-fat foods relative to HC (z=−2.59, p=0.009, see Figure 2A). Results from the ANOVA were consistent, showing a significant main effect of Food type (F(1,39)= 65.27, p<0.001), no effect of Group (F(1,39)= 1.69, p=0.201), and a significant Food type × Group interaction (F(1,39)= 7.80, p=0.008). A comparison of AN subtypes revealed a main effect of Food type (z= −3.13, p=0.0004), but no effect of subtype or interaction with subtype (p’s >0.4).
Figure 2. Food Choice Task Outcomes.
A) Choice describes selection of the high or low-fat item vs. a neutral Reference Item. While both groups were less likely to choose the high-fat item, AN were significantly less likely than HC to choose high-fat foods. B) When rating items for Health, both groups rate the High-fat items lower. C) High-fat food items were rated less tasty overall and there was a trend towards an interaction between group and taste such that AN rated high-fat foods as less tasty compared to controls.
Health and Taste Ratings:
Multilevel linear regression analyses were also used to examine patterns in Health and Taste ratings. Health or Taste ratings were dependent variables and Food type (low/high fat coded 0/1) and Group (HC/AN coded −1/+1) were entered as independent variables. Both groups rated high-fat foods as less healthy (Estimate: =−2.38, χ2 = 2062, p<0.0001), demonstrating that all participants had a similar assessment of the health value of food items. Additionally, the AN group rated food items as less healthy overall (Estimate= −0.12, χ2 = 5.78, p<0.016). There was no interaction between Group and Food type on health ratings (Estimate=0.06, χ2 = 1.13, p=0.29). Health rating results from the ANOVA followed the same pattern: a main effect of Food type (F(1,39)= 2062, p<0.001), a marginal effect of Group (F(1,39)=3.82, p=0.058), and no interaction between Group and Food type (F(1,39)=1.14, p=0.293). For the Taste ratings, multilevel linear regression showed a main effect of Food type (Estimate=−0.38, χ2 = 8.80, p<0.003) with high-fat foods rated as less tasty. We did not find a main effect of Group (Estimate=−0.07, χ2 = 1.01, p=0.3), but there was a trend towards a Group × Food type interaction (Estimate=−0.23, χ2 = 3.15, p=0.076) such that the AN group rated high-fat foods as less tasty than the HC group. Again, results from the ANOVA were similar. There was a main effect of Food type (F(1,39)= 8.80, p=0.005), a main effect of Group (F(1,39)=5.34, p=0.026), and a marginally significant interaction between Group and Food type (F(1,39)= 3.16, p=0.083). There were no differences in ratings between AN subtypes on Health or Taste ratings (p’s>0.4).
Associations between food choice and clinical parameters
Among individuals with AN, there were significant inverse correlations between the proportion of high-fat foods chosen and both EDE-Q (Spearman’s rho=−0.58, p=0.03) and TFEQ (Spearman’s rho=−0.53, p=0.04) restraint scores. Among HC (where the range of restraint was narrow), there was no significant association between these measures and proportion of high fat foods chosen.
Due to the inclusion of one individual whose BMI was 25.9 kg/m2, slightly above the normal range, we assessed the relationship between weight and outcome and found no significant association between BMI and selection of high fat food, in the whole group or in either diagnostic group. While mean age was well matched, the age range differed between HC and AN (see Table 1). There was no significant association between age and proportion of high-fat foods chosen.
In the AN group, all Reference items were low-fat food items; in the HC group, four individuals had reference items that were high-fat (avocado, burger, grilled cheese, and pizza). Exclusion of data from these four individuals did not significantly alter the results.
Associations between health, taste and choice
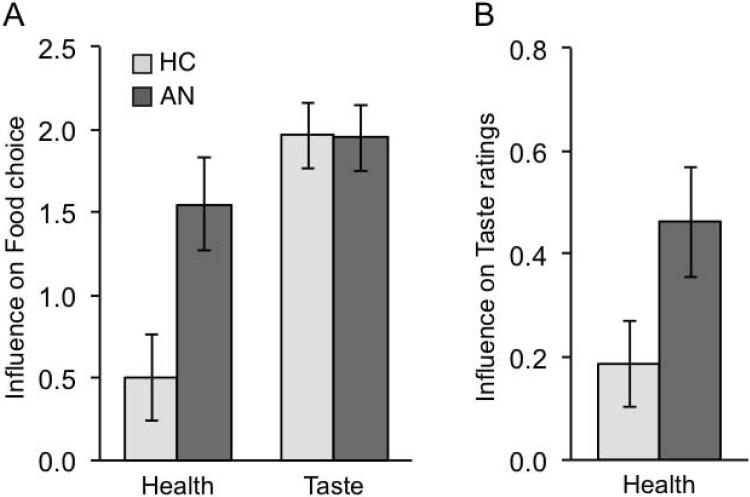
To examine the influence of Health vs. Taste valuations on food decisions in the subsequent Choice block, we entered ratings (z-scored Health and Taste ratings) and group (HC/AN coded as −1/+1) as independent variables in a multilevel logistic regression. As seen in Figure 3A, both Health (z=5.4, p<0.0001) and Taste (z=13.8, p<0.0001) ratings influenced food choices, but Health ratings influenced choice significantly more in AN relative to HC (z=2.7, p=0.006). To assess how Health and Taste ratings affected food choice among HC and AN respectively, we ran two additional models. We entered ratings (z-scored Health and Taste ratings) and group (HC/AN coded as 0/1) to assess effects on food choice among HC and found that Taste ratings more strongly influenced choice than did Health ratings (Contrast between Health and Taste Estimate=1.46, χ2=14.2, p=0.0002). Next, we entered ratings (z-scored Health and Taste ratings) and group (HC/AN coded as 1/0) to assess effects on food choice among AN and found, in contrast to HC, that both Taste and Health ratings significantly influenced choice and did not differ significantly (Contrast between Health and Taste Estimate=0.40, χ2=1.05, p=0.3).
Figure 3. Influence of Health vs. Taste valuations on food decisions.
Regression coefficients for Health and Taste rating influence on food choices and association between Health and Taste ratings. A) Taste ratings strongly influenced choices in both HC and AN, but Health ratings influenced food choices significantly more in AN relative to HC. B) Health ratings were more strongly correlated with Taste ratings in AN relative to HC.
Additionally, we examined the relationship between Health and Taste ratings across groups. Taste ratings were now the dependent variable and Health ratings (z-scored) and group (HC/AN coded as 0/1) were entered as independent variables in a multilevel linear regression. Health ratings more strongly related to Taste ratings in AN relative to HC (Estimate=0.28, χ2=4.10, p=0.04, Fig 3B). In the AN group, mean Taste ratings across all foods was negatively associated with duration of illness (r(19)=−0.50, p=0.02).
DISCUSSION
This study presents data supporting the use of a computer task as a model for actual food choice among individuals with AN. Consistent with clinical impressions and objective data regarding food intake, individuals with AN chose high-fat foods significantly less frequently than did HC. Furthermore, the tendency to choose low-fat foods was associated with scores on measures of the specific psychopathology of AN (EDE-Q and TFEQ), supporting the validity of the task and its sensitivity to the severity of illness. Therefore, this task has potential value in the study of decisions regarding what to eat and what to avoid eating, the salient behavioral symptoms of AN.6,8,28
Food Choice Task Adaptation for AN
AN is a challenging illness to understand and to treat.29 The Food Choice Task paradigm described here frames dietary restriction as choice behavior, wherein individuals with AN show a preference for low-fat food items. Previous work using choice paradigms have often been designed to address the pursuit of healthy foods in a population that is prone to select highly palatable snack foods.20,30,31 The adaptations implemented for this study created a set of appealing food items that minimize the representation of “junk” and “diet” foods and therefore are more appropriate for use in eating disorder populations. Other paradigms that address food choice have included food auctions that assess the individualized salience of food items by measuring willingness to pay to obtain food items for consumption.16,30 Such measures may be less suitable in eating disorder populations as the outcome also involves other aspects of reward processing, which may be abnormal in AN.32,33 One study aimed to measure food choice in AN using a paradigm to assess “liking” and “wanting.” In a task showing 16 food images, Cowdrey et al34 found that individuals with AN expressed less “wanting” of high calorie foods and more “wanting” for low calorie foods. Reaction time was reported as a measure of choice preference. Our findings are consistent, and the Food Choice Task more directly examines which foods are chosen.
Health and Taste Influence Food Choice Differentially
In addition to the main outcome of significantly decreased choice of high-fat foods and increased choice of low-fat foods, the current study provides information about valuation processes that inform food choice. The difference between Health ratings for high-fat versus low-fat foods demonstrated that all participants had a clear understanding of the health value of food items. However, knowledge of the fat content of the food items more strongly influenced the choice of what to eat among individuals with AN, compared with HC. Furthermore, the influence of Health and Taste ratings on food choices among individuals with AN differed from those of HC. Whereas Taste ratings influenced choices significantly more than Health ratings in these non-dieting HC, both Health and Taste ratings influenced food choices in AN. Healthiness and tastiness are thought to exert separate influences on food choices such that decisions require integration of these factors.31 This idea is supported by the lack of correlation between Health and Taste ratings in healthy individuals in previous work with this task, even among dieting individuals.20 Although Taste and Health ratings were modestly correlated among HC in the present study, there was a significantly increased correlation among individuals with AN between these two ostensibly distinct scales, suggesting differences in the process of valuation of food items distinct from the choice process. Additionally, average taste-ratings across all foods declined significantly with duration of illness. Since taste preference is known to be learned,35,36 these data suggest that, over time, the hedonic value of food declines in AN, presumably facilitating persistent reduction of caloric intake.
Study Strengths and Limitations
This study included the use of multilevel regression analyses, which takes into consideration individual ratings on each trial and allows for integration of all of the information from a participant’s responses. This statistical approach allowed us to overcome the potential limitation that the number of high-fat and low-fat items was not evenly matched. As this task continues to be adapted for use in AN, it may benefit from increasing the number of high-fat items relative to low-fat. Of note, the Taste ratings indicate that, in the current task, the low-fat food items were generally rated more highly for tastiness than the high-fat items. In post-participation interviews participants consistently reported that the images of fruit were highly appealing (in the Taste ratings, the 5 most highly rated items by both groups were all fruit), which may explain this counterintuitive finding. In this study, we did not assess hunger prior to participation. Future iterations of the task might benefit from increasing the number of images of appealing high-fat items, and standardizing timing of administration in relation to meals.
Food Choice Data Contribute to Neural Model Development
The neural mechanisms underlying food choice have begun to be characterized in healthy individuals, with a particular focus on mechanisms that may facilitate avoidance of high-fat (highly palatable) foods. Among dieters, neural systems related to self-control have been suggested to facilitate healthy choices.20 Related to this work, others have shown that, among dieters, go/no-go or stop-signal tasks can be used to influence actual food choice.37,38 These data, which focus on the role of motor impulsivity in food choice, further support the role of self-control mechanisms in food choice among dieters. Schonberg et al30 also employed a go/no-go task to study food choices and the subjective value assigned to food items. Their work investigated the role of attentional processes (rather than motor impulsivity), and showed that subjective value of food increased somewhat automatically, without association with reward or reinforcements, when the food item was associated with cues, possibly by increasing attention towards certain items. Taken together, these studies begin to suggest cognitive and neural mechanisms that are relevant to food choice. For example, the work of Schonberg et al suggest that it may be that among AN the act of repeatedly choosing one food increases attention to that food, and thereby enhances its value, which then serves to facilitate the choice of low-fat items. Similarly, it may be that the self-control mechanisms required for “healthy” food choice among dieters are less relevant among individuals with AN in whom the hedonic value of food may decline with persistence of illness.
In summary, this study presents data supporting a computer based paradigm that captures the food choices made by individuals with AN. The relevance of the Food Choice Task in this study to actual behavior in AN suggests that it can be used in experimental and neuroimaging studies to develop an understanding of the neural mechanisms underlying food choice in AN, and ultimately to develop interventions to influence food choice. The task can be easily adapted for use in fMRI studies, and can be used to characterize the neural mechanisms that drive food choice. As the relationship between task performance and caloric intake continue to be established, the Food Choice Task may be a useful proximal measure of treatment success. As clinical research increasingly emphasizes the significance of treatment targets, this approach warrants continued development.
Supplementary Material
Figure 1. Food Choice Task.
Participants provide ratings of 43 foods in three sequential blocks. In the Health and Taste Blocks they rated the image on a 5-point Likert scale, with 3 as a neutral rating. In the Choice block, they used the rating scale to indicate strength of preference for the food item, as compared with a neutral rated reference item, such that “No” indicated selection of the Reference item and “Yes” indicated selection of the item on that trial. The Reference image was visible next to the computer screen throughout the block.
Acknowledgements
This work was supported by NIMH K23 MH076195 (PI: Steinglass), NIMH R01 MH079397 (PI: Walsh), and the Global Foundation for Eating Disorders (PI: Steinglass).
The authors wish to acknowledge Bradley B. Doll, PhD for his guidance in statistical analyses, and Janet Schebendach, PhD and Eve Vagg for their expertise in food photography.
References
- 1.Arcelus J, Mitchell AJ, Wales J, Nielsen S. Mortality Rates in Patients With Anorexia Nervosa and Other Eating Disorders: A Meta-analysis of 36 Studies. Archives of General Psychiatry. 2011 Jul;68(7):724–731. doi: 10.1001/archgenpsychiatry.2011.74. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan PF. Mortality in anorexia nervosa. American Journal of Psychiatry. 1995;152(7):1073–1075. doi: 10.1176/ajp.152.7.1073. [DOI] [PubMed] [Google Scholar]
- 3.Suokas JT, Suvisaari JM, Grainger M, Raevuori A, Gissler M, Haukka J. Suicide attempts and mortality in eating disorders: a follow-up study of eating disorder patients. Gen Hosp Psychiatry. 2014 May-Jun;36(3):355–357. doi: 10.1016/j.genhosppsych.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Steinhausen HC. The outcome of anorexia nervosa in the 20th century. American Journal of Psychiatry. 2002;159(8):1284–1293. doi: 10.1176/appi.ajp.159.8.1284. [DOI] [PubMed] [Google Scholar]
- 5.Hadigan CM, Anderson EJ, Miller KK, et al. Assessment of macronutrient and micronutrient intake in women with anorexia nervosa. Int J Eat Disord. 2000 Nov;28(3):284–292. doi: 10.1002/1098-108x(200011)28:3<284::aid-eat5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 6.Mayer LE, Schebendach J, Bodell LP, Shingleton RM, Walsh BT. Eating behavior in anorexia nervosa: before and after treatment. Int J Eat Disord. 2012 Mar;45(2):290–293. doi: 10.1002/eat.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schebendach J, Mayer LE, Devlin MJ, Attia E, Walsh BT. Dietary energy density and diet variety as risk factors for relapse in anorexia nervosa: a replication. Int J Eat Disord. 2012 Jan;45(1):79–84. doi: 10.1002/eat.20922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schebendach JE, Mayer LE, Devlin MJ, et al. Dietary energy density and diet variety as predictors of outcome in anorexia nervosa. Am J Clin Nutr. 2008 Apr;87(4):810–816. doi: 10.1093/ajcn/87.4.810. [DOI] [PubMed] [Google Scholar]
- 9.Walsh BT. The enigmatic persistence of anorexia nervosa. Am J Psychiatry. 2013 May 1;170(5):477–484. doi: 10.1176/appi.ajp.2012.12081074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jauregui Lobera I, Bolanos Rios P. Choice of diet in patients with anorexia nervosa. Nutricion hospitalaria. 2009 Nov-Dec;24(6):682–687. [PubMed] [Google Scholar]
- 11.Stoner SA, Fedoroff IC, Andersen AE, Rolls BJ. Food preferences and desire to eat in anorexia and bulimia nervosa. Int J Eat Disord. 1996 Jan;19(1):13–22. doi: 10.1002/(SICI)1098-108X(199601)19:1<13::AID-EAT3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 12.Hetherington M, Rolls BJ. Sensory-specific satiety in anorexia and bulimia nervosa. Ann N Y Acad Sci. 1989;575:387–398. doi: 10.1111/j.1749-6632.1989.tb53259.x. [DOI] [PubMed] [Google Scholar]
- 13.Rolls BJ, Andersen AE, Moran TH, McNelis AL, Baier HC, Fedoroff IC. Food intake, hunger, and satiety after preloads in women with eating disorders. Am J Clin Nutr. 1992 Jun;55(6):1093–1103. doi: 10.1093/ajcn/55.6.1093. [DOI] [PubMed] [Google Scholar]
- 14.Lavender JM, De Young KP, Wonderlich SA, et al. Daily Patterns of Anxiety in Anorexia Nervosa: Associations With Eating Disorder Behaviors in the Natural Environment. J Abnorm Psychol. 2013 May 6; doi: 10.1037/a0031823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGowan L, Cooke LJ, Gardner B, Beeken RJ, Croker H, Wardle J. Healthy feeding habits: efficacy results from a cluster-randomized, controlled exploratory trial of a novel, habit-based intervention with parents. Am J Clin Nutr. 2013 Sep;98(3):769–777. doi: 10.3945/ajcn.112.052159. [DOI] [PubMed] [Google Scholar]
- 16.Schonberg T, Bakkour A, Hover AM, Mumford JA, Poldrack RA. Influencing food choices by training: evidence for modulation of frontoparietal control signals. J Cogn Neurosci. 2014 Feb;26(2):247–268. doi: 10.1162/jocn_a_00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tchanturia K, Liao PC, Uher R, Lawrence N, Treasure J, Campbell IC. An investigation of decision making in anorexia nervosa using the Iowa Gambling Task and skin conductance measurements. J Int Neuropsychol Soc. 2007 Jul;13(4):635–641. doi: 10.1017/S1355617707070798. [DOI] [PubMed] [Google Scholar]
- 18.Lopez C, Tchanturia K, Stahl D, Treasure J. Central coherence in eating disorders: a systematic review. Psychol Med. 2008 Oct;38(10):1393–1404. doi: 10.1017/S0033291708003486. [DOI] [PubMed] [Google Scholar]
- 19.Galimberti E, Fadda E, Cavallini MC, Martoni RM, Erzegovesi S, Bellodi L. Executive functioning in anorexia nervosa patients and their unaffected relatives. Psychiatry Res. 2013 Aug 15;208(3):238–244. doi: 10.1016/j.psychres.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009 May 1;324(5927):646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. American Psychiatric Association; Arlington,VA: 2013. [Google Scholar]
- 22.Sysko R, Roberto CA, Barnes RD, Grilo CM, Attia E, Walsh BT. Test-retest reliability of the proposed DSM-5 eating disorder diagnostic criteria. Psychiatry Res. 2012 Apr 30;196(2-3):302–308. doi: 10.1016/j.psychres.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luce KH, Crowther JH. The reliability of the Eating Disorder Examination-Self-Report Questionnaire Version (EDE-Q) Int J Eat Disord. 1999 Apr;25(3):349–351. doi: 10.1002/(sici)1098-108x(199904)25:3<349::aid-eat15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 24.Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29(1):71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 25.Bates D, Maechler M, Bolker B. Ime4:Linear mixed-effects models using S4 classes. Vol Version: 0.999999-22011.
- 26.Fox J, Weisberg S. An R Companion to Applied Regression. Sage; 2011. [Google Scholar]
- 27.Schielzeth H, Forstmeier W. Conclusions beyond support: overconfident estimates in mixed models. Behavioral ecology : official journal of the International Society for Behavioral Ecology. 2009 Mar;20(2):416–420. doi: 10.1093/beheco/arn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hetherington MM, Rolls BJ. Eating behavior in eating disorders: response to preloads. Physiol Behav. 1991 Jul;50(1):101–108. doi: 10.1016/0031-9384(91)90505-i. [DOI] [PubMed] [Google Scholar]
- 29.Steinhausen HC. The outcome of anorexia nervosa in the 20th century. Am J Psychiatry. 2002 Aug;159(8):1284–1293. doi: 10.1176/appi.ajp.159.8.1284. [DOI] [PubMed] [Google Scholar]
- 30.Schonberg T, Bakkour A, Hover AM, et al. Changing value through cued approach: an automatic mechanism of behavior change. Nat Neurosci. 2014 Apr;17(4):625–630. doi: 10.1038/nn.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rangel A. Regulation of dietary choice by the decision-making circuitry. Nat Neurosci. 2013 Dec;16(12):1717–1724. doi: 10.1038/nn.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner A, Aizenstein H, Venkatraman VK, et al. Altered reward processing in women recovered from anorexia nervosa. American Journal of Psychiatry. 2007 Dec;164(12):1842–1849. doi: 10.1176/appi.ajp.2007.07040575. [DOI] [PubMed] [Google Scholar]
- 33.Kaye WH, Wagner A, Fudge JL, Paulus M. Neurocircuity of eating disorders. Curr Top Behav Neurosci. 2011;6:37–57. doi: 10.1007/7854_2010_85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowdrey FA, Finlayson G, Park RJ. Liking compared with wanting for high- and low-calorie foods in anorexia nervosa: aberrant food reward even after weight restoration. Am J Clin Nutr. 2013 Mar;97(3):463–470. doi: 10.3945/ajcn.112.046011. [DOI] [PubMed] [Google Scholar]
- 35.Wardle J, Cooke LJ, Gibson EL, Sapochnik M, Sheiham A, Lawson M. Increasing children's acceptance of vegetables; a randomized trial of parent-led exposure. Appetite. 2003 Apr;40(2):155–162. doi: 10.1016/s0195-6663(02)00135-6. [DOI] [PubMed] [Google Scholar]
- 36.Ventura AK, Worobey J. Early influences on the development of food preferences. Current biology : CB. 2013 May 6;23(9):R401–408. doi: 10.1016/j.cub.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 37.Veling H, Aarts H, Papies EK. Using stop signals to inhibit chronic dieters' responses toward palatable foods. Behav Res Ther. 2011 Nov;49(11):771–780. doi: 10.1016/j.brat.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Veling H, Aarts H, Stroebe W. Using stop signals to reduce impulsive choices for palatable unhealthy foods. British journal of health psychology. 2013 May;18(2):354–368. doi: 10.1111/j.2044-8287.2012.02092.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.