Abstract
PURPOSE
To characterize the cardiorespiratory response to exercise before and after aerobic exercise training in patients with interstitial lung disease (ILD).
METHODS
We performed a clinical study, examining 13 patients (New York Heart Association/World Health Organization Functional Class II or III) before and after 10-weeks of supervised treadmill exercise walking, at 70–80% of heart rate reserve, 30–45 minutes per session, 3 times per week. Outcome variables included measures of cardiorespiratory function during a treadmill cardiopulmonary exercise test (tCPET), with additional near infrared spectroscopy measurements of peripheral oxygen extraction and bioimpedance cardiography measurements of cardiac output. 6-minute walk test distance (6MWD) was also measured.
RESULTS
All subjects participated in at least 24 of their 30, scheduled exercise sessions with no significant adverse events. After training, the mean 6MWD increased by 52±48 meters (P=.001), peak tCPET time increased by 163±130 seconds (P=.001), and time to achieve gas exchange threshold increased by 145±37 seconds (P<.001). Despite a negligible increase in peak oxygen uptake (VO2) with no changes to cardiac output, the overall work rate/VO2 relationship was enhanced after training. Muscle oxygen extraction increased by 16% (P=.049) after training.
CONCLUSION
Clinically significant improvements in cardiorespiratory function were observed after aerobic exercise training in this group of subjects with ILD. These improvements appear to have been mediated by increases in the peripheral extraction of oxygen rather than changes in oxygen delivery.
Key words or phrases: interstitial lung disease, pulmonary rehabilitation, aerobic exercise training, muscle oxygenation, 6 Minute Walk Test
Over 200 etiologies can result in interstitial lung disease (ILD), which is characterized by irreversible fibrotic reorganization of the lung parenchyma. Interstitial fibrosis creates a barrier to gas exchange at the alveolar-capillary interface, and reduces lung distensibility. As a result, hemoglobin oxygenation is impaired and the work of ventilation is increased, leading to diminished cardiorespiratory function,1,2 dyspnea,3,4 and poor exercise performance.5–7
In addition to low cardiorespiratory capacity, skeletal muscle weakness has also been associated with poor 6-minute walk test (6MWT) performance in patients with ILD,8,9 which is the most frequently used method of measuring exercise capacity in these patients.10–13 For patients with ILD, the minimal clinically important difference (MCID) for distance walked on the test (6MWD) is between 24 and 45 meters14,13 and a significant decline in 6MWD may warn of impending mortality, particularly in patients with idiopathic pulmonary fibrosis (IPF).14,15 Although increases in 6MWD beyond the MCID13,16–21 have been reported after aerobic exercise training (AET) in patients with ILD, the mechanism through which these increases were mediated was not identified.
AET is known to improve cardiorespiratory capacity in individuals who have oxygen (O2) delivery limitations,22–27 such as pulmonary arterial hypertension and congestive heart failure. Several studies have previously shown significant increases in 6MWD after AET in patients with ILD13,16–19,28,29 but only 2 of them attempted to measure cardiorespiratory capacity and only 129 of the 2 reported a small but significant increase. Those 2 studies aside, the overall cardiorespiratory adaptation to AET has not been characterized in patients with ILD.
A theoretical framework has been proposed in which AET-induced increases in peak oxygen uptake (VO2) and cardiorespiratory endurance might be mediated through different pathways.30–34 Empirical evidence suggests that increases in peak VO2 may primarily be mediated through increases in central circulatory O2 delivery, whereas increases in cardiorespiratory endurance appear more likely to be mediated through improved muscle O2 extraction. The limitation on hemoglobin oxygenation, characteristic of ILD, presents a unique paradigm for study within this framework, where training-induced increases in exercise capacity occur without an improvement in pulmonary respiration.17,19,35 If so, increases in O2 delivery may be prevented, even if peak cardiac output and muscle blood flow are improved. Adaptations in cardiorespiratory function would therefore be confined to the periphery and observable only as a function of improved muscle O2 extraction and its consequences.
We sought to examine this theoretical framework by comparing pulmonary gas exchange, O2 delivery and O2 extraction before and after a vigorous AET regimen in patients with ILD. We hypothesized that the cardiorespiratory response to treadmill cardiopulmonary exercise tests (tCEPT), particularly muscle O2 extraction capacity, would be improved after training, despite the irreversible limitations to pulmonary gas exchange that generally result from ILD.
METHODS
The institutional review boards of all collaborating authors approved the study prior to subject recruitment for this pre-intervention study. Subjects were recruited specifically for this research program from outpatient clinics in the national capital area between February 2009 and July 2012. After obtaining informed consent, a physician board certified in physical medicine and rehabilitation reviewed subjects’ medical histories and charts and performed physical examinations on all subjects to insure meeting of inclusion criteria and the absence of an exclusion criterion. Subjects’ most recent pulmonary function tests (PFT), transthoracic Doppler echocardiograms, and right heart catheterizations, typically performed during the past year for routine treatment, were included in the medical history assessment. Subjects with right ventricular systolic pressures ≥40 mmHg, estimated by Doppler echocardiography were further evaluated by right heart catheterization and those with a resting mean pulmonary arterial pressure of ≥25 mmHg were excluded. Subjects with right or left ventricular ejection fractions <40% by echocardiography, significant repolarization abnormalities or arrhythmias identified by resting 12-lead electrocardiogram (ECG) or untoward ECG changes and/or symptoms of ischemia during the baseline tCPET were also excluded. Also patients with forced expired ventilation/forced vital capacity (FEV1/FVC) ratio <65%, by PFT of participation were not included. The resting ECG, tCPET, and PFT were performed on the first day of participation.
None of the subjects had a history of, or observable, ischemic heart disease, cardiomyopathy or left heart failure, right ventricular failure or cor pulmonale, pulmonary hypertension, hepatic or renal failure, metastatic cancer, disabling stroke, or severe psychiatric condition. Patients with uncontrolled diabetes mellitus, musculoskeletal or mitochondrial diseases that would limit tCPET performance, pregnancy, and those on antiretroviral therapy regimens were also excluded. Others excluded were active smokers and those admitting abuse of alcohol or other controlled or illegal substances. None of the subjects had participated in any form of regular AET, for at least 6 months prior to study enrollment.
Measurements
Before and after AET, subjects were weighed with their shoes on and hematocrit was measured from a venous blood sample. Skinfold measures were obtained from the site on the gastrocnemius muscle of the right leg at which light emitters and sensors for near infrared spectroscopy (NIRS) were to be attached. These data were collected because VO2 measures obtained from tCPET are generally normalized to body weight, and hematocrit and skinfold may influence NIRS muscle oxygenation measurements.
All of the tCPETs, and 6MWT were completed in the Cardiopulmonary and Applied Physiology Laboratory of the Rehabilitation Medicine Department at the NIH Clinical Center in Bethesda, MD. The 6MWT were conducted on an 80-meter circular track, according to the guidelines of the American Thoracic Society.36 tCPETs were conducted on a motorized treadmill, with computerized adjustments of belt speed and degree of incline. A modified-Naughton tCPET protocol27 was used. The target endpoint of the tCPET was the subject’s expressed desire to stop the test, despite strong verbal encouragement from the testing staff (volitional exhaustion). Tests were also stopped in the event of intolerable exertional dyspnea.
Pulmonary gas exchange was measured continuously using breath-by-breath open circuit spirometry (MedGraphics, Cardio 2 Ultima, Medical Graphics Corp, St Paul, MN). For subjects regularly using supplemental O2, medical air (21% O2 and 79% N2) was blended with respiratory grade O2 to raise the fraction of to 40% O2. The method for this procedure was previously described.27 The principal outcome measures for the tCPET were VO2, work rate (WR), gas exchange threshold (GET), time to attain GET (GET-time), and time to volitional exhaustion (tCPET-time). The GET was identified by the V-slope method. Peak VO2 was determined by an 8-breath average at the end of the test or at the end of the last completed stage, whichever was higher. WR was calculated using body weight, treadmill speed, and % grade of incline during tCPETs. A linear regression model was determined to fit the WR-VO2 iterations most closely and used to examine the work rate/VO2 relationship.
Heart rate was measured by ECG, and cardiac output and stroke volume were measured by transthoracic bioimpedance cardiography throughout the tCPET (PhysioFlow PF-05 Lab 1, Manatec Biomedical, France). Muscle O2 extraction measures included peak arteriovenous O2 difference (a-vO2) and muscle oxygenation indices, which were obtained by continuous wave near-infrared spectroscopy (NIRS; Oxymon MK-III, Artinis Medical Systems, The Netherlands). The a-vO2 was calculated as the quotient of VO2 and cardiac output. Muscle oxygenation was measured in the gastrocnemius muscle of the right leg and included amplitude changes in concentrations of deoxygenated ([deoxy-Hb/Mb]) and oxygenated ([oxy-Hb/Mb]) hemoglobin/myoglobin from rest to volitional exhaustion during tCPET. Total tissue hemoglobin ([tot-Hb/Mb]) concentration was calculated as the sum of [oxy-Hb/Mb] and [deoxy-Hb/Mb] whereas the hemoglobin concentration difference ([diff-Hb/Mb]) was determined by subtracting [deoxy-Hb/Mb] from [oxy-Hb/Mb].
Aerobic Exercise Training Regimen
AET comprised a 10-week, professionally supervised, program of treadmill walking for 30–45 minutes per session, 3 times per week in an outpatient pulmonary rehabilitation center. Completion of 24 of the 30 training sessions was required to sustain participation in the program. Each session began with a 5–10 minute warm-up not included in the 30–45 training period. The target intensity was 70–80% of subjects’ heart rate reserve determined from the initial tCPET.38 Treadmill speed and grade were continuously adjusted to keep the heart rate as close as possible to the target zone. All subjects requiring supplemental oxygen during training received a flow rate adequate to maintain SpO2 ≥85%.
Education
Subjects also received an ancillary series of lectures related to their disease process. These lectures were approximately 1 hour in length. They covered anatomy and physiology, lung disease processes, medication use, oxygen therapy, sleep disorders, preventing infection, airway clearance, interpreting pulmonary function tests, energy conservation, panic control, relaxation techniques, breathing retraining, community resources, advance directives, social well-being, nutrition, and benefits of exercise.
Statistical Analysis
Differences between the pre- and post-AET outcome measures were analyzed using one-tailed paired t-tests. A Related-Samples Wilcoxon Signed Rank Test was used for data that did not meet statistical assumptions of normalcy. Use of the 1-tailed analyses was justified by meeting both of the required underlying assumptions: 1) plausibility was substantiated in the background information that our results would be directional,18,29 and 2) our directional hypothesis was proposed prior to the analysis. Use of the 1-tailed analysis increased the statistical power of the study and minimized the probability of type-2 error while maintaining our a priori alpha level of 0.05. The significance of relationships between the independent variables was assessed by Pearson product moment correlation coefficients or Spearman ranked coefficients if the data did not meet the assumptions of normalcy. P-values ≤.05 were considered statistically significant. All statistical analyses were conducted using SPSS version 21 (IBM, Inc.). Data are presented as mean±1 standard deviation.
RESULTS
Thirteen patients with New York Heart Association/World Health Organization (NYHA/WHO) Class II or III ILD (Table 1) were studied. 69% of the patients required supplemental O2. At baseline, total lung capacity was 57.0 ± 17.5%, FVC was 52.9 ±18.0%, and lung diffusion (DLCO, available in 12 of 13 subjects) was 39.4 ± 15.0% of predicted values. While FEV1 was only 57.0 + 23.3% of predicted values, the FEV1/FVC ratio was 83.4 ± 3.6% of predicted. Subjects were deconditioned as indicated by an attained peak VO2 of only 73 ± 32% of the predicted values for sedentary normal individuals of similar age and gender at baseline. However, when considering only patients that performed the tCPET without supplemental oxygen, this percentage was much lower (61 ± 22 %). Diminished resting cardiac output was not apparent in these subjects but a slight resting tachycardia with a normal stroke volume was observed at baseline (Table 3). Neither cardiac output nor stoke volume appeared to be diminished at peak exercise. End-tidal partial pressure of oxygen (PETO2) and end-tidal partial pressure of carbon dioxide PETCO2 were substantially higher than expected at rest as well as at peak exercise. Arteriovenous O2 difference was slightly higher than expected at rest and was severely diminished at peak exercise. Results of the baseline pulmonary and cardiorespiratory assessments were consistent with a restrictive breathing pattern accompanied by normal central circulatory O2 delivery, impaired pulmonary gas exchange, and diminished muscle O2 extraction capacity.
Table 1.
Baseline Characteristics of Study Patients
| Characteristics | All Patients (n=13) |
|---|---|
| Age, y, mean (SD) | 57.0 (9.1) |
| Female, n (%) | 9 (69) |
| Race/ethnicity, n (%) | |
| White | 11 (85) |
| African American | 2 (15) |
| BMI, kg/m2, mean (SD) | 28.6 (4.8) |
| Supplemental O2 use, n (%) | 5 (38) |
| Diagnosis, n (%) | |
| Nonspecific interstitial pneumonitis | 6 (46) |
| Idiopathic pulmonary fibrosis | 3 (23) |
| Systemic sclerosis | 2 (15) |
| Desquamative interstitial pneumonia | 1 (8) |
| Sjögren's syndrome | 1 (8) |
| NYHA/WHO Functional Classification, n (%) | |
| Class II | 6 (46) |
| Class III | 7 (54) |
Abbreviations: BMI, body mass index; NYHA/WHO, New York Heart Association/World Health Organization
Table 3.
Cardiorespiratory Measures
| Outcome Measures | Pre-training | Post-training | Difference | P value |
|---|---|---|---|---|
| Resting | ||||
| VO2, mL·kg−1·min−1 | 5.4 (2.4) | 5.2 (2.3) | − 0.2 | .384 |
| VCO2, mL·kg−1·min−1 | 4.3 (1.3) | 4.0 (1.6) | − 0.3 | .234 |
| f, breaths/min | 31 (8.4) | 31 (9.5) | 0 | .463 |
| Ve, l/min | 15.3 (4.0) | 14.7 (5.2) | − 0.6 | .323 |
| Vt, ml/min | 2.1 (0.6) | 2.3 (0.8) | + 0.2 | .172 |
| RER | 0.88 (0.21) | 0.80 (0.23) | − 0.08 | .463 |
| a-vO2, vol% | 6.1 (2.7) | 6.6 (2.8) | + 0.5 | .091 |
| PETO2, mmHg | 154.8 (61.7) | 149.4 (54.6) | − 5.4 | .197 |
| PETCO2, mmHg | 38.0 (5.7) | 38.9 (6.7) | + 0.9 | .125 |
| Heart rate, beats/min | 103 (13.1) | 95 (11.3) | − 8 | .024 |
| Cardiac stroke volume, ml | 72.0 (13.8) | 67.9 (16.0) | − 4.1 | .136 |
| Cardiac output, l/min | 7.3 (1.7) | 6.4 (1.6) | − 0.9 | .027 |
| Peak | ||||
| VO2, mL·kg−1·min−1 | 17.4 (5.5) | 18.2 (5.0) | + 0.8 | .048 |
| VCO2, mL·kg−1·min−1 | 19.5 (4.7) | 19.8 (4.7) | + 0.3 | .293 |
| f, breaths/min | 53 (11.0) | 52 (9.3) | − 1 | .359 |
| Ve, l/min | 55.1 (15.13) | 54.0 (16.1) | − 1.1 | .192 |
| Vt, ml/min | 1029 (361.2) | 1055 (419.4) | + 26 | .242 |
| RER | 1.17 (0.23) | 1.11 (0.17) | − 0.06 | .037 |
| a-vO2 difference, % | 9.2 (2.2) | 10.7 (2.9) | + 1.5 | .049a |
| PETO2, mmHg | 160 (58.4) | 158 (56.1) | − 2 | .08a |
| PETCO2, mmHg | 41 (9.6) | 43 (9.7) | + 2 | .055 |
| Heart rate, beats/min | 148 (18.0) | 144 (14.2) | − 4 | .086 |
| Cardiac stroke volume, ml | 105 (20.1) | 99 (21.8) | − 6 | .179 |
| Cardiac output, l/min | 15.7 (3.8) | 14.5 (4.0) | − 1.2 | .177 |
Wilcoxon Signed Ranks Test.
Abbreviations: tCPET, treadmill cardiopulmonary exercise test; VO2, minute oxygen uptake; VCO2, minute carbon dioxide expiration; f, breathing frequency; Ve, minute ventilation; Vt, tidal volume; RER, respiratory exchange ratio; a-vO2, arteriovenous oxygen difference; PETO2, end-tidal partial pressure of oxygen; PETCO2, end-tidal partial pressure of carbon dioxide.
Subjects participated in 90% (Table 2) of the training sessions without a serious adverse event. Five subjects spent less than 70% of the targeted 30–45 minutes in their training heart rate zone, while eight subjects spent 70% or more of the time in the training zone (Table 2). No significant changes in subjects’ body weight (81.5 ± 16.4 vs. 81.1 ± 17.1 kg, P=.200), gastrocnemius skin fold (23 ± 11 vs. 21 ± 11 mm, P=.239), or hematocrit (40.5 ± 2.8% vs. 40.7 ± 3.0%) were observed over the study period.
Table 2.
Aerobic Exercise Training Summary of Subjects Completing a Minimum of 24 Sessions
| Subject | Total Sessions |
Total Exercise Time (min) |
Time in Training Zone (% of total exercise time) |
Average HR (% HRR) |
Supplemental Oxygen Use (L/min) |
|---|---|---|---|---|---|
| 1 | 25 | 943 | 97 | 72 | - |
| 2 | 30 | 1159 | 97 | 71 | 6 |
| 3 | 27 | 1065 | 44 | 68 | 6 |
| 4 | 26 | 1020 | 73 | 70 | 6–15 |
| 5 | 25 | 976 | 85 | 72 | - |
| 6 | 25 | 975 | 96 | 71 | 3–6 |
| 7 | 28 | 1074 | 68 | 70 | 10–15 |
| 8 | 25 | 975 | 44 | 68 | 2–4 |
| 9 | 27 | 1027 | 11 | 67 | 6–15 |
| 10 | 27 | 1065 | 89 | 71 | - |
| 11 | 29 | 1036 | 82 | 70 | - |
| 12 | 28 | 1095 | 92 | 72 | 3–5 |
| 13 | 26 | 1042 | 38 | 69 | 2–12 |
| Mean | 27 | 1035 | 70 | 70 | 7 |
| SD | 1.6 | 59.0 | 27.7 | 1.7 | 1.5 |
Abbreviations: HRR, heart rate reserve
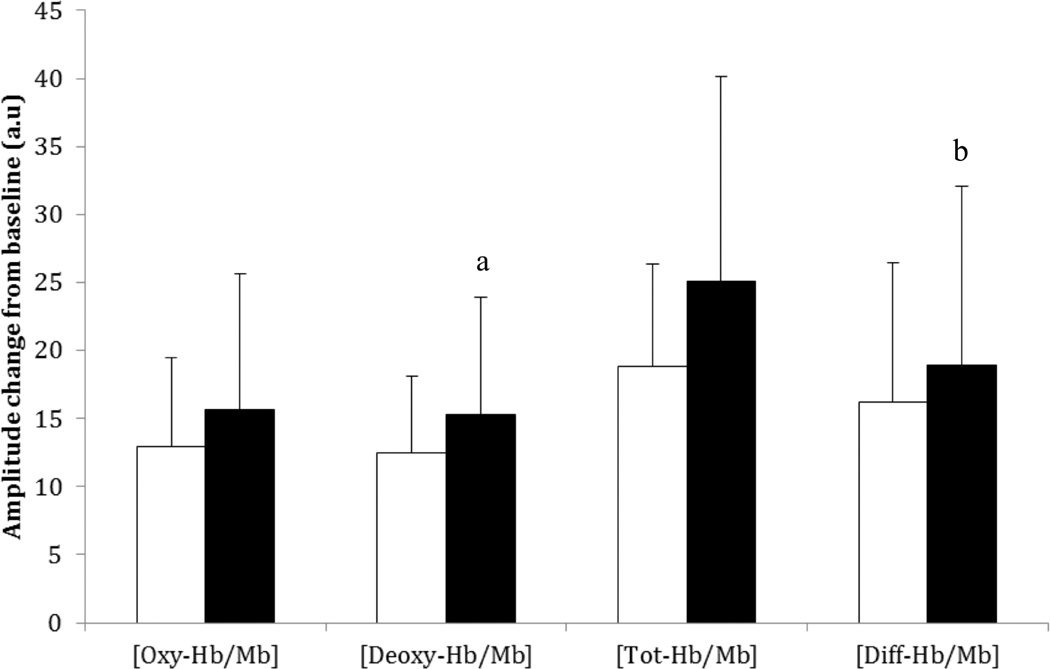
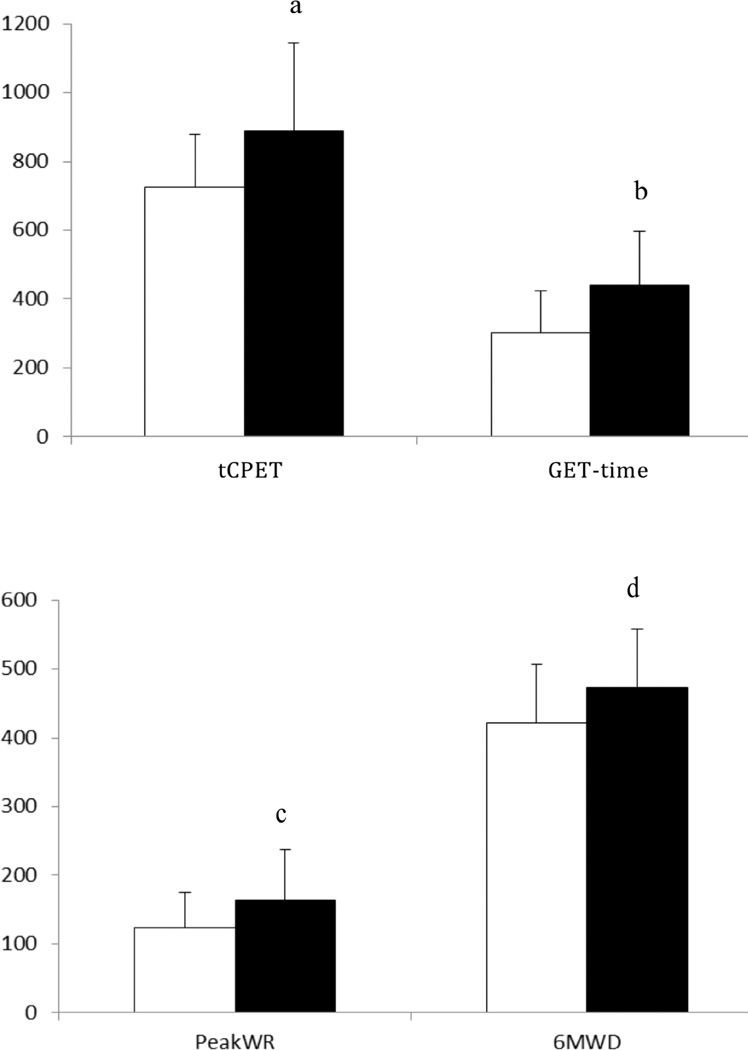
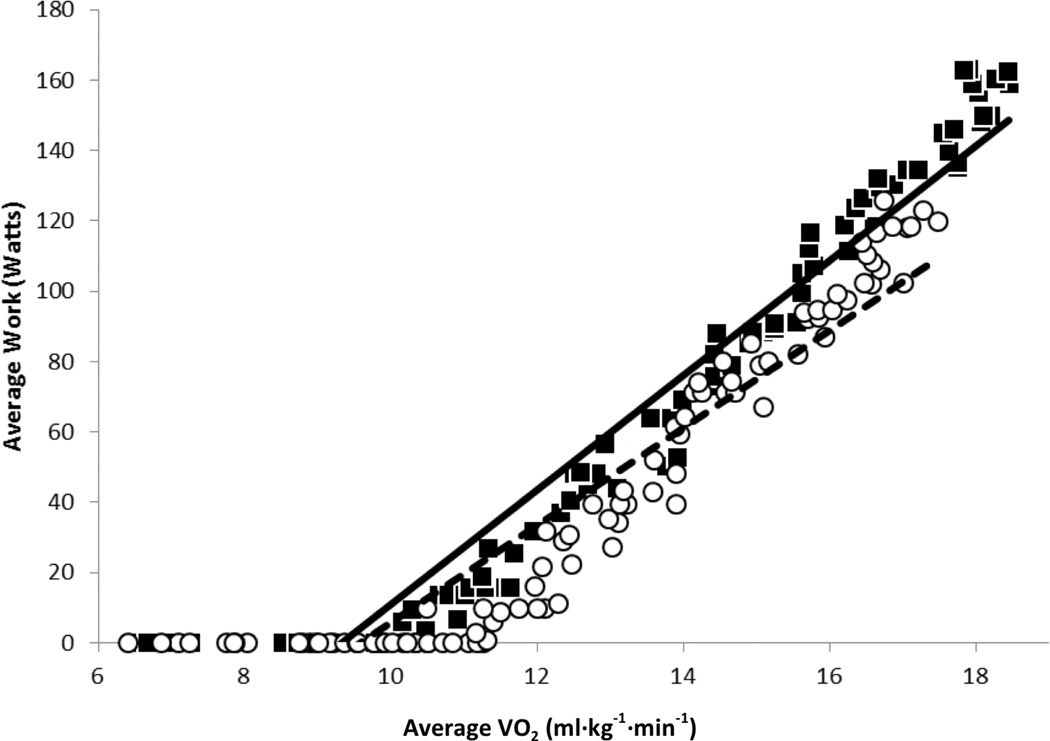
After training, resting heart rate and cardiac output were significantly reduced but still uncompromised (Table 3). While a significant increase in peak cardiac output, and stroke volume were not observed, peak a-vO2 was significantly higher after training. Additionally, [deoxy-Hb/Mb] and [diff-Hb/Mb] amplitudes were increased without a significant increase in [tot-Hb/Mb] after training (Figure 3) and the increases in a-vO2 and [deoxy-Hb/Mb] correlated significantly (r=0.511; P=.045). Increases of 12% (~52 meters) in 6MWD and 32% (~39 watts) in peak WR were observed after AET. Significant increases in GET-time and tCPET-time were also observed (Figure 1). A statistically significant increase in peak VO2 was observed after training but this increase was too small to be of clinical relevance.39 Conversely, WR was higher at any given level of VO2 after AET (Figure 2).
Figure 3.
Muscle oxygenation capacity in the untrained and trained conditions. Bars represent concentration changes for oxygenated ([oxy-Hb/Mb]), deoxygenated ([deoxy-Hb/Mb]), total ([Tot-Hb/Mb]), and difference (diff-Hb/Mb) in hemoglobin/myoglobin. White bars represent the untrained condition and black bars represent the trained condition. Error bars represent 1 standard deviation unit.
a[deoxy-Hb/Mb] was significantly greater in the trained versus the untrained state (P=.037).
b[diff-Hb/Mb] was significantly greater in the trained versus the untrained state (P=.027).
Figure 1.
Test duration (tCPET), time to gas exchange threshold (GET-time), peak work rate (WR) and 6-minute walk test distance (6MWD) in the untrained (white bars) and trained (black bars) patients with ILD. Error bars equal 1 standard deviation unit.
atCPET was 163±130 seconds longer in the trained than in the untrained state (P=.001).
bGET-time was 145±15 seconds longer in the trained than in the untrained state (P<.001).
cPeak work rate (WR) was 43±37 watts greater (P=.002).
d6MWD was 52±48 meters farther in the trained versus the untrained state (P=.001)
Figure 2.
Work rate-oxygen uptake (VO2) slope in the untrained (white circles) and trained (black squares) conditions. Regression equation was y=13.838x−132.54; R2 = 08813 for the untrained condition (dotted line) and y=16.274x−151.86; R2=09343 for the trained (solid line) condition. Slope was significantly higher (P=.002) in the trained (14.89±4.94) than in the untrained (11.86±4.87) condition.
DISCUSSION
The cardiorespiratory adaptation to vigorous AET was characterized in patients with ILD. The cardiorespiratory response to tCEPT, particularly muscle O2 extraction, was improved after AET. Before AET, cardiorespiratory capacity was found to be limited by diminished muscle O2 extraction, as well as the pulmonary consequences of ILD. A significant increase in muscle O2 extraction was observed after AET without an increase in either central O2 delivery or muscle O2 availability. We therefore suspect that the increase in O2 extraction may have resulted from improved mitochondrial function. Concomitant with the increase in muscle O2 extraction, was an improvement in cardiorespiratory endurance, as measured by 6MWD, peak WR, GET-time, and tCPET-time. Together, these findings lend support to a more general construct suggesting that cardiorespiratory endurance may be mediated primarily through improved muscle O2 extraction rather than by increases in cardiac output or peak VO2.
The overall pattern of adaptation observed in this study was different than expected. Resulting from increases in both central circulatory O2 delivery to the muscles and muscle O2 extraction,39 peak VO2 is generally expected to increase by 12% to 21%, in healthy normal subjects, following AET.40 At submaximal work rates, VO2 tends to remain similar in the pre- and post-training conditions.31 Therefore, any given level of submaximal work becomes more economical in a post-training state because the work is sustained at a lower percentage of maximum cardiorespiratory capacity.31 Conversely, peak VO2 increased by only 4.4% in these patients with ILD. This was similar in magnitude to that observed in a previous report (6.2%)29 but its clinical relevance could not be determined.26,27,39–42 Patients in the current study adapted to AET primarily through a mechanism that permitted a higher work rate to be achieved at any given VO2, while prolonging the occurrence of the anaerobic threshold and the attainment of peak VO2. While, peak VO2 may be influenced by non-physiological factors such as motivation, the leftward shift in the WR-VO2 slope and increased GET-time are markers that are not affected by nonphysiological influences.
O2 delivery to the tissues is dependent on adequate oxygenation of hemoglobin at the alveoli. In ILD, the irreversible fibrotic parenchyma creates a diffusion barrier that impedes hemoglobin oxygenation. A restricted ventilatory pattern can exacerbate the impediment by creating alveolar hypoxia, decreasing ventilation to perfusion ratio, and increasing the work of breathing. In the current study, peak cardiac output, stroke volume and heart rate did not increase after AET in patients with ILD. However, a 16% improvement in peak a-vO2, as well as a significant increase in unloading of O2 from the hemoglobin in the muscle microvasculature, was observed after training. Thus, the observed improvements in cardiorespiratory function appeared to be mediated only by increased muscle O2 extraction capacity.
Clinical Implications
In the current study, the increase in 6MWD after AET exceeded the increases observed in most of the previous studies,13,16–20,28 as well as the current ILD-specific MCID estimates.14,13 The training regimen used in this study was more vigorous than those used in previous studies yet high levels of participation were observed without any significant adverse events. After training a given work rate was met by a lower VO2, thus reducing multisystemic stress at any given, intensity of activity. Moreover, the post-training increase in muscle O2 extraction did not adversely affect SpO2 during exercise. The current study demonstrated that even very vigorous aerobic exercise training might be safe, quite well tolerated, and beneficial for improving muscle O2 extraction, work rate to VO2 ratio, and overall exercise and physical activity performance in patients with ILD.
Study Limitations
First, the small sample size and absence of a non-training control group preclude the ability to generalize our results. Further validation of the findings in a larger, broader group of patients with ILD is required. Despite this, a significant cardiorespiratory adaptation to aerobic exercise training was observed in the subjects of this study. For variables not achieving statistical significance, the numerical differences in pre- versus post-training scores were typically small and generally did not trend toward a clinically important improvement.
Second, the clinical course of ILD often includes a rapid decline in systemic function that could further diminish cardiorespiratory capacity over a short period of time. If so, the effect of training may have been underestimated in this study. Alternatively, the rapid decline in systemic function in ILD could potentially preclude acquiring the physiologic benefits of exercise training noted in this study, particularly at later stages of the disease. It should also be recognized that patients with ILD and pulmonary hypertension, who were excluded from this study, may adapt differently to aerobic exercise training.
Third, 6MWD is a performance measure that, like volitional exhaustion, can be influenced by acclimation. We could not account for the influence of possible Hawthorne or practice effects. However, no significant increases in 6MWD were observed for non-exercise training control groups in previous studies.17,18
Lastly, the sample was heterogeneous with respect to inclusion of patients with various ILD etiologies. The size of the sample was insufficient for subgroup analyses based on etiology. This again may impede the ability to generalize information over the entire ILD classification. However, a degree of homogeneity in the sample was established because a restrictive breathing pattern, impaired gas exchange, and poor exercise performance, all of which are cardinal manifestations of all ILD etiologies, were present in each of the subjects included.
CONCLUSION
Patients in this study adapted to aerobic exercise training through a mechanism that increased cardiorespiratory endurance. Muscle O2 extraction was enhanced in the post-training condition, without a change in cardiopulmonary O2 delivery or muscle O2 availability. From a clinical perspective, the results of this study provide evidence that exercise training of even vigorous intensities may be safe and well tolerated by patients with ILD and that participation in aerobic exercise may improve muscle O2 extraction, and exercise performance in these patients.
Acknowledgments
Funding: NIH/IRP 1 Z01 CL060068-02 CC
Drs. Randall Keyser and Lisa Chin were supported by interinstitutional personnel agreements awarded to George Mason University by the National Institutes of Health Clinical Center, Department of Rehabilitation Medicine and both of these authors and Dr. Joshua Woolstenhulme have received travel support to conferences to present research data by the NIH. Dr. Keyser is also an Associate Editor for Medicine and Science in Sports and Exercise.
Footnotes
Conflicts of Interest:
None of the other authors have potential conflicts of interest
References
- 1.Donner CF, Carone M, Patessio A, Appendini L. Cardiopulmonary exercise testing in interstitial lung disease. Z Kardiol. 1994;83(SUPPL 3):159–162. [PubMed] [Google Scholar]
- 2.Chung F, Dean E. Pathophysiology and cardiorespiratory consequences of interstitial lung disease - Review and clinical implications: A special communication. Phys Ther. 1989;69:956–966. doi: 10.1093/ptj/69.11.956. [DOI] [PubMed] [Google Scholar]
- 3.Collard HR, Pantilat SZ. Dyspnea in interstitial lung disease. Curr Opin Support Palliat Care. 2008;2:100–104. doi: 10.1097/SPC.0b013e3282ff6336. [DOI] [PubMed] [Google Scholar]
- 4.Madison JM, Irwin RS. Chronic cough in adults with interstitial lung disease. Curr Opin Pulm Med. 2005;11:412–416. doi: 10.1097/01.mcp.0000174249.07762.37. [DOI] [PubMed] [Google Scholar]
- 5.Vogiatzis I, Zakynthinos S. Factors limiting exercise tolerance in chronic lung diseases. Compr Physiol. 2012;2:1779–1817. doi: 10.1002/cphy.c110015. [DOI] [PubMed] [Google Scholar]
- 6.Markovitz GH, Cooper CB. Exercise and interstitial lung disease. Curr Opin Pulm Med. 1998;4:272–280. doi: 10.1097/00063198-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Bye PTP, Anderson SD, Woolcock AJ, Young IH, Alison JA. Bicycle endurance performance of patients with interstitial lung disease breathing air and oxygen. Am Rev Respir Dis. 1982;126:1005–1012. doi: 10.1164/arrd.1982.126.6.1005. [DOI] [PubMed] [Google Scholar]
- 8.Marciniuk DD, Sridhar G, Clemens RE, Zintel TA, Gallagher CG. Lung volumes and expiratory flow limitation during exercise in interstitial lung disease. J Appl Physiol. 1994;77:963–973. doi: 10.1152/jappl.1994.77.2.963. [DOI] [PubMed] [Google Scholar]
- 9.Nishiyama O, Taniguchi H, Kondoh Y, et al. Quadriceps weakness is related to exercise capacity in idiopathic pulmonary fibrosis. Chest. 2005;127:2028–2033. doi: 10.1378/chest.127.6.2028. [DOI] [PubMed] [Google Scholar]
- 10.Chetta A, Aiello M, Foresi A, et al. Relationship between outcome measures of six-minute walk test and baseline lung function in patients with interstitial lung disease. Sarcoidosis Vasc Diffuse Lung Dis. 2001;18:170–175. [PubMed] [Google Scholar]
- 11.King TE, Jr, Behr J, Brown KK, et al. BUILD-1: A randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:75–81. doi: 10.1164/rccm.200705-732OC. [DOI] [PubMed] [Google Scholar]
- 12.Baughman RP, Sparkman BK, Lower EE. Six-minute walk test and health status assessment in sarcoidosis. Chest. 2007;132:207–213. doi: 10.1378/chest.06-2822. [DOI] [PubMed] [Google Scholar]
- 13.Holland AE, Hill CJ, Conron M, Munro P, McDonald CF. Small changes in six-minute walk distance are important in diffuse parenchymal lung disease. Respir Med. 2009;103:1430–1435. doi: 10.1016/j.rmed.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 14.Du Bois RM, Weycker D, Albera C, et al. Six-minute-walk test in idiopathic pulmonary fibrosis: Test validation and minimal clinically important difference. Am J Respir Crit Care Med. 2011;183:1231–1237. doi: 10.1164/rccm.201007-1179OC. [DOI] [PubMed] [Google Scholar]
- 15.Swigris JJ, Wamboldt FS, Behr J, et al. The 6 minute walk in idiopathic pulmonary fibrosis: Longitudinal changes and minimum important difference. Thorax. 2010;65:173–177. doi: 10.1136/thx.2009.113498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira G, Feuerman M, Spiegler P. Results of an 8-week, outpatient pulmonary rehabilitation program on patients with and without chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2006;26:54–60. doi: 10.1097/00008483-200601000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Nishiyama O, Kondoh Y, Kimura T, et al. Effects of pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis. Respirology. 2008;13:394–399. doi: 10.1111/j.1440-1843.2007.01205.x. [DOI] [PubMed] [Google Scholar]
- 18.Holland AE, Hill CJ, Conron M, Munro P, McDonald CF. Short term improvement in exercise capacity and symptoms following exercise training in interstitial lung disease. Thorax. 2008;63:549–554. doi: 10.1136/thx.2007.088070. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira A, Garvey C, Connors GL, et al. Pulmonary rehabilitation in interstitial lung disease: Benefits and predictors of response. Chest. 2009;135:442–447. doi: 10.1378/chest.08-1458. [DOI] [PubMed] [Google Scholar]
- 20.Holland AE, Hill CJ, Glaspole I, Goh N, McDonald CF. Predictors of benefit following pulmonary rehabilitation for interstitial lung disease. Respir Med. 2012;106:429–435. doi: 10.1016/j.rmed.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Holland A, Hill C. Physical training for interstitial lung disease. Cochrane Database Syst Rev. 2008;(4) doi: 10.1002/14651858.CD006322.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Piepoli MF, Conraads V, Corrà U, et al. Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail. 2011;13:347–357. doi: 10.1093/eurjhf/hfr017. [DOI] [PubMed] [Google Scholar]
- 23.Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the diagnosis and management of heart failure in adults. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary rehabilitation: Joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131(5 Suppl):4S–42S. doi: 10.1378/chest.06-2418. [DOI] [PubMed] [Google Scholar]
- 25.Cindy Ng LW, Mackney J, Jenkins S, Hill K. Does exercise training change physical activity in people with COPD? A systematic review and meta-analysis. Chron Respir Dis. 2012;9:17–26. doi: 10.1177/1479972311430335. [DOI] [PubMed] [Google Scholar]
- 26.Mereles D, Ehlken N, Kreuscher S, et al. Exercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension. Circulation. 2006;114:1482–1489. doi: 10.1161/CIRCULATIONAHA.106.618397. [DOI] [PubMed] [Google Scholar]
- 27.Chan L, Chin LMK, Kennedy M, et al. Benefits of intensive treadmill exercise training on cardiorespiratory function and quality of life in patients with pulmonary hypertension. Chest. 2013;143:333–343. doi: 10.1378/chest.12-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foster S, Thomas HM., III Pulmonary rehabilitation in lung disease other than chronic obstructive pulmonary disease. Am Rev Respir Dis. 1990;141(3 I):601–604. doi: 10.1164/ajrccm/141.3.601. [DOI] [PubMed] [Google Scholar]
- 29.Salhi B, Troosters T, Behaegel M, Joos G, Derom E. Effects of pulmonary rehabilitation in patients with restrictive lung diseases. Chest. 2010;137:273–279. doi: 10.1378/chest.09-0241. [DOI] [PubMed] [Google Scholar]
- 30.Brooks GA, Fahey TD, Baldwin KM. Exercise Physiology: Human Bioenergetics and Its Applications. Boston: McGraw Hill; 2005. [Google Scholar]
- 31.Bassett DR, Jr, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 2000;32:70–84. doi: 10.1097/00005768-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Davies KJA, Packer L, Brooks GA. Biochemical adaptation of mitochondria, muscle, and whole-animal respiration to endurance training. Arch Biochem Biophys. 1981;209:539–554. doi: 10.1016/0003-9861(81)90312-x. [DOI] [PubMed] [Google Scholar]
- 33.Davies KJA, Packer L, Brooks GA. Exercise bioenergetics following sprint training. Arch Biochem Biophys. 1982;215:260–265. doi: 10.1016/0003-9861(82)90303-4. [DOI] [PubMed] [Google Scholar]
- 34.Davies KJA, Maguire JJ, Brooks GA. Muscle mitochondrial bioenergetics, oxygen supply, and work capacity during dietary iron deficiency and repletion. Am J Physiol Endocrinol Metab. 1982;5:E418–E427. doi: 10.1152/ajpendo.1982.242.6.E418. [DOI] [PubMed] [Google Scholar]
- 35.Naji NA, Connor MC, Donnelly SC, McDonnell TJ. Effectiveness of pulmonary rehabilitation in restrictive lung disease. J Cardiopulm Rehabil. 2006;26:237–243. doi: 10.1097/00008483-200607000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Crapo RO, Casaburi R, Coates AL, et al. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 37.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. 1986 Jun;60:2020–2027. doi: 10.1152/jappl.1986.60.6.2020. [DOI] [PubMed] [Google Scholar]
- 38.Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957;35:307–315. [PubMed] [Google Scholar]
- 39.Jones AM, Carter H. The effect of endurance training on parameters of aerobic fitness. Sports Med. 2000;29:373–386. doi: 10.2165/00007256-200029060-00001. [DOI] [PubMed] [Google Scholar]
- 40.Wenger HA, Bell GJ. The interactions of intensity, frequency and duration of exercise training in altering cardiorespiratory fitness. Sports Med. 1986;3:346–356. doi: 10.2165/00007256-198603050-00004. [DOI] [PubMed] [Google Scholar]
- 41.Mentz RJ, Schulte PJ, Fleg JL, et al. Clinical characteristics, response to exercise training, and outcomes in patients with heart failure and chronic obstructive pulmonary disease: Findings from Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION) Am Heart J. 2013;165:193–199. doi: 10.1016/j.ahj.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swank AM, Horton J, Fleg JL, et al. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: Results from Heart Failure and a Controlled Trial to Investigate Outcomes of Exercise Training. Circ Heart Fail. 2012;5:579–585. doi: 10.1161/CIRCHEARTFAILURE.111.965186. [DOI] [PMC free article] [PubMed] [Google Scholar]