Abstract
Successful production of genetically modified mouse lines is dependent on germline transmission (GLT) of mutant alleles from chimeras. When natural mating fails to achieve GLT due to male infertility, sickness, or other problems, sperm can be harvested from chimeras and used for assisted reproductive technologies such as in vitro fertilization (IVF) to attempt to “rescue” GLT. However, a rational, evidence-based approach to determine if such extraordinary efforts should be attempted on a chimera has not been established. Therefore, in the present study we assessed the production, quality and genotype of epididymal sperm harvested from male chimeras generated by blastocyst or morula microinjection of gene targeted embryonic stem (ES) cell clones containing a LacZ expression cassette and that failed to achieve GLT. Results of this analysis enabled us to determine the cause of GLT failure, correlate coat color chimerism with the proportion of LacZ-positive sperm, and test the likelihood of achieving GLT by IVF. In 415 chimeras, 332 (80 %) produced no offspring by natural mating (“infertile”), while 83 (20 %) produced only wildtype offspring (“fertile”). Of the 332 infertile chimeras, 209 (63 %) failed to produce any sperm whatsoever, 48 (15 %) had extremely poor quality sperm, and 75 (23 %) had good quality sperm. These results indicate that most chimeras that do not achieve GLT by natural mating are infertile, and the primary cause of infertility is failed spermatogenesis. Genotyping of sperm from 519 chimeras revealed a significant positive linear correlation between coat color chimerism and mean percentage of LacZ-positive sperm (R2 = 0.95). Finally, IVF using good quality, LacZ-positive sperm from fertile and infertile chimeras “rescued” GLT for 19 out of 56 genes. We conclude that an assessment of coat color chimerism together with sperm quality and genotype can better inform the selection of chimeras for IVF to rescue GLT than coat color chimerism alone.
Keywords: Mouse, Chimera, Germline transmission, Rescue
Introduction
Germline transmission (GLT) is a critical milestone in the production of genetically modified mice derived from targeted embryonic stem (ES) cells. The gold-standard to test and confirm GLT of a mutant, gene-targeted allele is to genotype progeny produced from the mating of a male chimera to wildtype female mice. Several high-throughput mutagenesis programs use ES cell lines on a C57BL/6 genetic background (Seong et al. 2004; Poueymirou et al. 2006; Pettitt et al. 2009), but they are often cited as having lower germline competence than 129 derived lines (Ware et al. 2003; Hansen et al. 2008). Complicating matters is the observation that developmental defects, spermatogenesis malfunction, hermaphrodism, chromosome abnormalities and gene mutation often are associated with infertility in male chimeras (Shomer et al. 1997; Lin et al. 2000; Zhao et al. 2004; Shirley et al. 2004, Sugawara et al. 2005; Zheng et al. 2007; Fujihara et al. 2013). When male chimeras produce no or only wildtype pups, researchers have little choice but to either repeat the gene targeting experiment, conduct additional ES cell microinjections, or continue breeding chimeras in the hope that they might eventually achieve GLT. These approaches can be frustrating, increase experimental costs, inflict delay and prolong research, and can cause project failure.
Coat color chimerism is highly variable in male chimeras, and the GLT potential of C57BL/6N ES cell derived chimeras is not well correlated with coat color chimerism (Seong et al. 2004; Hansen et al. 2008). In contrast to qualitative assessment of somatic cell chimerism by coat color alone, it is reasonable to hypothesize that quantitative determination of sperm quality and germ cell chimerism are better predictors of GLT potential. If morphological, functional, and genotypic characteristics of chimeric sperm could be correlated with production of heterozygous mutant offspring, then one would be able to make more informed, evidence-based decisions for better management of male chimeras that fail to achieve GLT. For example, sperm from male chimeras could be harvested and tested to predict its suitability for using assisted reproductive technologies such as in vitro fertilization (IVF) to attempt to “rescue” GLT. Unfortunately, there are no published studies that correlate coat color chimerism with sperm quality and/or percent mutant sperm with which to predict the likelihood of producing mutant offspring by IVF. Without this knowledge, the value of attempting IVF to rescue GLT in infertile and/or nonproductive chimeras cannot be determined.
DNA extracted from copulatory plugs harvested from females after breeding to chimeric males has been used for quantitative polymerase chain reaction (qPCR) to genotype the proportion of gene-targeted (“mutant”) ES cell derived sperm in cells of the copulatory plug and to predict germline competent chimeras (Wilson and Sheardown 2011; Lee et al. 2013). Unfortunately, results from these studies do not support a strong correlation between GLT and genotype of the mutant allele (neo, lacZ). Therefore, in this study, we systematically examined the correlation between coat color chimerism and the percentage of mutant sperm in chimeras that failed to achieve GLT by natural mating. We investigated the cause of infertility, and tested the likelihood of achieving GLT by IVF using good quality sperm positive for LacZ in order to establish an effective, reliable and efficient decision-making algorithm for selecting chimeras for IVF to rescue GLT.
Materials and methods
Animals
All male chimeras were produced by microinjection of gene-targeted C57BL/6N ES cells (JM8.N4, JM8.F6, JM8A3.N1, JM8A1.N3, and VGB6) into mouse embryos at the University of California Davis Mouse Biology Program. Wild-type CD1 and C57BL/6N mice were purchased from Charles River Laboratories International, Inc. (Wilmington, MA), and wild-type C57BL/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were housed in a specific pathogen free vivarium with a 14 on/10 h off light cycle (7 am on and 9 pm off) and room environmental parameters meeting the ILAR guide recommendations. All mice were housed on Corncob bedding (Harlan 7097 m=1/4′′), fed with standard mouse diet (Harlan Teklad Global irradiated diet 2918) and provided with autoclaved water. Euthanasia was performed by CO2 asphyxiation according to the 2013 AVMA Guidelines on Euthanasia. The care, use, and disposition of all mice used in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the University of California Davis.
Reagents and media
BD DifcoTM skim milk was purchased from Voigt Global Distribution Inc. (Lawrence, KS), and equine chorionic gonadotropin (eCG) was purchased from the National Hormone and Peptide Program (Harbor-UCLA Medical Center, Torrance, CA). Human chorionic gonadotropin (hCG), D-(+)-raffinose pentahydrate, α-monothioglycerol (MTG), poly(vinyl alcohol) (PVA), methyl-β-cyclodextrin (MBCD), reduced L-glutathione (GSH), bovine serum albumin (BSA, embryo tested), embryo tested water, embryo-tested mineral oil and chemicals for preparation of Avertin were purchased from Sigma-Aldrich Corp. (St. Louis, MO). BSA-free M2 and KSOMaa media were purchased from Zenith Biotech (www.zenithbiotech.com/index.html) and 4 mg/ml BSA was added and filter-sterilized prior to use. Research Vitro Fert (RVF) medium was purchased from Cook Medical, Inc. (Bloomington, IN, USA).
Generation of male chimeras by microinjection
Embryos were collected in M2 medium from superovulated and naturally mated mice on 3.5 pdc (blastocysts from uteruses) or 2.5 pdc (8-cell morulae from oviducts), and then cultured in KSOMaa medium at 37 °C in5 % CO2 and humidified air prior to ES cell injection. Microinjection was performed in M2 medium using host blastocysts or morulae from CD1 or C57BL/6N strain. Each blastocyst was injected with 12-15 ES cells, and each morula was injected with 8-9 ES cells. Injected blastocysts or blastocysts developed from injected morulae after culture overnight in KSOMaa at 37 °C in 5 % CO2 and humidified air were transferred into the uterus of 2.5 dpc pseudo-pregnant CD1 recipients to produce chimeric mice. At 10–12 days of age male chimeras were assessed for coat color chimerism from 10, 30, 50, 70, 90, and 100 %.
Chimera breeding and GLT testing
For GLT testing, each male chimera with ≥10 % chimeric coat color at 7–8 weeks of age were bred with sexually mature wildtype female C57BL/6N mice (one male chimera and two wildtype females per cage). If no signs of pregnancy were observed after 30 days, females were removed and replaced with new females. If after 7 weeks there were no pups and still no signs of pregnancy, then the male was designated “infertile” and sperm harvested for quality analysis, cryopreservation and genotyping. If after 2–3 litters only wildtype pups were produced, then the male was designated “fertile” and its sperm were similarly processed as for infertile males.
Sperm cryopreservation and cold storage
Cryoprotective medium R18S3 + MTG (18 % raffinose, 3 % skim milk and 477 μM monothioglyercel) was used for sperm cryopreservation in liquid nitrogen (LN2) using cryovials as reported earlier (Ostermeier et al. 2008; Li et al. 2014). Briefly, cauda epididymal sperm were collected in 0.5 ml R18S3 + MTG per male, and incubated at 37 °C for 10 min for dispersion. Sperm suspension in R18S3 + MTG was pipetted to the bottom of each of four cryovials with 80 μl each cryovial for sperm cryopreservation, and the remaining sperm suspension was diluted with 1 ml Ca/Mg-free Dulbecco’s phosphate-buffered saline (DPBS) and filtered through a 70 μm sterile cell strainer (Fisher Scientific, catalog 22-363-548) to remove tissue fragments before genotyping. The filtered sperm suspension was transferred into a sterile 1.5 ml microcentrifuge tube, and centrifuged at 500×g for 5 min. The supernatant was removed and the sperm pellet was used for sperm genotyping.
For cold storage of sperm at 4 °C, two cauda epipidymides of a male were dissected out and placed into a sterile microcentrifuge tube containing 0.2 ml mineral oil, and then stored at 4 °C for 24 or 48 h before sperm collection for IVF and or sperm cryopreservation in liquid nitrogen. Sperm from vasa deferentia of a male were collected using the same method as for the cauda epipidymides, and then filtered through a 70 μm cell strainer before centrifugation as described above for sperm genotyping.
Sperm DNA extraction and quantitative PCR
Sperm pellet DNA was extracted using an alkaline method by re-suspending the pellet with 400 μl of 50 mM NaOH and heated at 95 °C for 20 min followed by neutralization with 200 μl of 500 mM Tris and stored at −20 °C until use. Genotypes were assayed using Taqman® quantitative PCR via the multiplexed relative cycle threshold method (Lee et al., 2013). Briefly, 1 μL of each DNA sample was tested in a total reaction volume of 10 μl in quadruplicate utilizing Qiagen QuantiTect PCR kit with ROX chemistry. Plates with 384 wells each were processed on an AB7900HT system with the following cycles: 95 °C for 15 min and then 40 cycles of 95 °C for 30 s, 60 °C for 1 min. Primers and probe for detection of mutant sequence (LacZ) in the final concentration of each reaction were forward primer ATCAGGATATG TGGCGGATGA (0.84 μM), reverse primer TGATT TGTGTAGTCGGTTTATGCA (0.84 μM) and probe 6FAM-CGGCATTTTCCGTG-MGB/NFQ (0.28 μM). Primers/probe for endogenous reference sequences were in the final concentration of each reaction: forward primer GTCATCAAGTGAGAAAGACATC CT (0.84 μM), reverse primer CATCATGAATTTTG ATAAGCCCATT (0.84 μM), and probe VIC-CTCC TGGCTGCCTG-MGB/NFQ (0.28 μM). Each sperm DNA sample average ΔCt was compared against the averaged ΔCt of two heterozygous mutant animal sperm DNA controls (ΔΔCt). Percent mutation of sperm cells was calculated as % = (2−(ΔCtSperm-ΔCtHet))*50 where % is expressed as a whole number (ABI PRISM 7700 Sequence Detection System User Bulletin #2, p.11–17. PE Applied Biosystems, 1997).
Sperm analysis and assessment
For sperm quality assessment, 10 μl fresh sperm was mixed with 190 μl warm M2 medium. Sperm concentration, sperm count per male (2 cauda epididymides), and progressive motility (% of motile sperm with average path velocity ≥50 μm/s and straightness ratio ≥50 %) were assessed using an IVOS computerized sperm analyzer (Hamilton Thorne, Beverly, MA) at 37 °C. Sperm morphology was assessed under a phase contrast microscope, and at least 100 sperm were scored for percentages of sperm with normal sperm head and sperm tail. Abnormal sperm head shapes included triangular, olive, pin, banana, amorphous, collapsed, and abnormal hook, etc., and abnormal sperm tail shapes included bent, coiled and crinkled, etc.
Sperm recovery by IVF
Cryopreserved sperm were thawed in 37 °C water bath for 10 min, and then 25 μl of frozen-thawed sperm was added to each of three 90 μl drops of MBCD medium (BSA-free TYH medium containing 1 mg/ml PVA and 0.75 mM MBCD) for pre-incubation 30 min at 37 °C in 5.5 % CO2 and humidified air prior to insemination as described earlier (Takeo and Nakagata 2011; Li et al. 2014). Cumulus-oocyte complexes (COCs) collected from 5 to 8 superovulated females (75–160 oocytes) were added into an IVF drop of 250 μl RVF medium containing 5.14 mM CaCl2 and 1 mM GSH (Li et al. 2014). Twenty-five μl motile sperm were slowly collected from the peripheral part of a MBCD medium drop, and then expelled gently and directly onto each of the COCs under a dissecting microscope with minimum medium. Oocytes were collected from superovulated C57BL/6N females 14–15 h post hCG injection. Superovulation was induced by IP injection of 7.5 IU eCG followed by IP injection of 7.5 IU hCG 47 h later. Fertilization rate was calculated as the 2-cell embryo percent of the oocytes used.
Embryo transfer (ET)
Two-cell stage embryos derived by IVF were transferred into the oviducts (10–13 for each oviduct, 20–25 per recipient) of 0.5 days post coitum (dpc) pseudopregnant CD-1 recipient mice anesthetized with 1.25 % Avertin by intraperitoneal injection. Microinjected blastocysts or blastocysts developed from injected 8-cell embryos after culture overnight in KSOMaa were transferred into the uteruses of 2.5–dpc pseudopregnant CD1 recipients (6–8 embryos per uterine horn). After 45–60 min anesthesia, 0.1 ml Buprenex (0.03 mg/ml; Western Medical Supply, Inc., Arcadia, CA, USA) was administered subcutaneously in the flank of each mouse postoperatively. Recipients were kept warm on a heating pad until fully recovered from anesthesia. All pregnant recipients were allowed to go to term and give birth to litters for health and genotyping analysis. At 10 days of age, toe clips from pups were taken for genotyping. The health and number of pups born per litter were determined at 21 days after birth.
Statistical analysis
GraphPad Prism five software (GraphPad Software, Inc., San Diego, CA, USA) was used for statistical analysis. To detect group differences, sperm fertilization rates, percentages of mutant sperm, progressive motility, percentages of normal sperm heads and tails, and pup birth rates were arcsine transformed, and then analyzed by one-way ANOVA followed by Tukey HSD tests or two-tailed t test, and P < 0.05 was chosen as an indication of statistical significance. Data are expressed as mean ± standard deviation (SD). The correlation between percentage of coat color chimerism and percentage of mutant sperm was analyzed by linear regression.
Results
Ineffective spermatogenesis is the primary cause of male infertility
We examined 415 male chimeras that failed to achieve GLT by natural mating, including 332 (80 %) infertile chimeras that produced no offspring and 83 (20 %) fertile chimeras that produced only wildtype offspring. Of the 332 infertile chimeras, 209 males (63 %) failed to produce any sperm whatsoever (Fig. 1). At necropsy these mice all had small testes and clear epididymides containing no sperm. Additionally, 48 males (15 %) had extremely poor sperm quality, as indicated by low sperm counts (number of cauda epididymal sperm per male), low sperm motility, and high percentage of sperm with abnormal morphology of heads and tails (Table 1). In comparison, 75 males (23 %) produced good quality sperm with significantly better characteristics than the chimeras with poor quality sperm (Table 1, P < 0.05). There were no differences in sperm count, % abnormal sperm head or sperm tail morphology (P > 0.05) between infertile chimeras with good quality sperm and fertile chimeras, except for a significantly (P < 0.05) lower progressive motility of sperm from the infertile chimeras. These results indicate that the primary reason male chimeras do not achieve GLT by natural mating is infertility, and the principle cause for infertility is failed spermatogenesis.
Fig. 1.
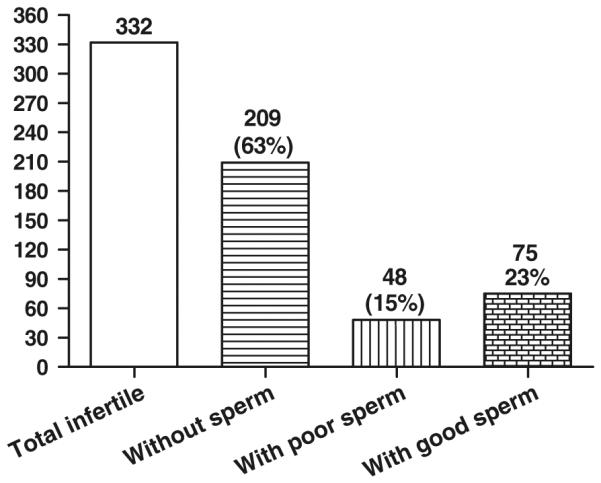
Infertile chimeric mice produced no sperm (209 males), poor quality sperm (48 males), or good quality sperm (75 males). Total 332 infertile male chimeras examined
Table 1.
Comparison of cauda epididymal sperm counts, sperm progressive motility and sperm morphology between infertile chimeras with poor or good sperm quality and fertile chimeras
| Sperm counts (×106/male) |
Progressive motility (%) |
Sperm with abnormal heads (%) |
Sperm with abnormal tails (%) |
|
|---|---|---|---|---|
| Poor sperm group (n = 48) | 5.4 ± 4.4a | 8.9 ± 8.8a | 65.9 ± 20.2a | 70.5 ± 28.5a |
| Good sperm group (n = 75) | 21.1 ± 11.2b | 21.4 ± 11.0b | 29.0 ± 21.9b | 27.4 ± 15.5b |
| Fertile chimeras (n = 83) | 35.9 ± 12.6b | 44.1 ± 11.3c | 20.2 ± 15.6b | 24.5 ± 13.1b |
Values with different superscripts a, b and c within the same column are significantly different (P < 0.05)
GLT-rescue by IVF using cryopreserved and genotype-positive sperm
After assessing sperm quality, we then examined the extent to which sperm genotyping could be used to further refine the selection of chimeras for IVF. For these experiments, we performed a generic LacZ qPCR to quantify the proportion of gene targeted mutant sperm relative to wildtype (non-targeted) sperm from 158 male chimeras with good quality sperm, including the 75 infertile males and 83 fertile males mentioned above. Of these mice, 71 males (45 %) produced only wildtype or a very low proportion (0–5 %) of LacZ-positive sperm. IVF was not attempted using sperm from these males. On the other hand, 87 males (55 %) produced a higher proportion (6–50 %) of LacZ-positive sperm. From this group, we chose 1 chimera with the highest proportion of LacZ-positive sperm for each unique gene. Therefore, IVF was attempted using sperm from 49 males.
IVF-derived embryos were surgically transferred at the 2-cell stage to pseudopregnant recipient female mice. From all offspring, the presence of the mutant allele was tested by PCR genotyping of DNA extracted from toe tissue. Of the 49 chimeras (49 genes) selected for GLT rescue by IVF, GLT was confirmed in litters for 16 chimeras (16 genes) but failed in litters for 33 chimeras (33 genes). In litters with at least 1 mutant offspring, the gene targeted allele was genotype confirmed in 92 (38.7 %) of 238 pups born.
There was a significantly higher percentage of mutant sperm from chimeras in the GLT-rescued group than that of chimeras in the GLT-rescue failed group (37.8 ± 10.9 % vs. 20.0 ± 8.6 %, P < 0.001, Table 2). Further, within the GLT-rescued group, the mutant pup rate (40.7 ± 19.2 %) was not significantly different (P > 0.05) from the mutant sperm rate (37.8 ± 10.9 %), indicating the utility of cauda epididymal sperm genotype for predicting the likelihood of achieving GLT by IVF.
Table 2.
Comparisons of mutant sperm rates and mutant pup rates for chimeras that produced heterozygous mutant offspring (“GLT-rescued”) and wildtype-only offspring (“GLT-rescue-failed”) by IVF
| No. Genes | Mutant sperm (%) |
No. embryos transferred |
No. pups born (%) |
No. mut pups (%) |
Mutant pup rate (%) |
|
|---|---|---|---|---|---|---|
| GLT rescued | 16 | 37.8 ± 10.9a | 679 | 238 (35.1) | 92 (38.7) | 40.7 ± 19.2a |
| GLT rescue-failed | 33 | 20.0 ± 8.6b | 1,568 | 501 (32.0) | 0 | 0 |
Values with different superscripts a, b are significantly different (P < 0.001), and values with the same superscript a are not significantly different (P > 0.05)
GLT rescue by IVF using cold stored and genotype-positive sperm
Because sperm genotyping can take some time after harvesting sperm, we sought to determine if sperm from chimeric males can be stored at 4 °C before IVF while awaiting genotyping results. We collected sperm for genotyping from the vasa deferentia of two infertile male chimeras for two genes, and stored the cauda epididymides in mineral oil at 4 °C until epididymal sperm collection for IVF the next morning. We also collected sperm for genotyping from the vasa deferentia of five infertile chimeras for five genes and stored the cauda epididymides in mineral oil at 4 °C for 24 h before sperm were collected for cryopreservation in LN2. A few weeks later the sperm were thawed for IVF. As shown in Table 3, sperm after storage at 4 °C for 24 h or after 24 h cold storage followed by cryopreservation in LN2 were fertile in vitro, and GLT of 3 genes was achieved for 3 of 7 chimeras.
Table 3.
GLT rescue by IVF using sperm stored at 4 °C for 24 h or after 24 h cold storage followed by cryopreservation in LN2
| Strain | LacZ positive sperm |
No. oocytes used |
No. 2-cell embryos produced |
Fert rate (%) |
No. embryos transferred |
No. pups born (mut) |
Epi storage time at 4 °C |
Sperm cryo in LN2 before IVF? |
|---|---|---|---|---|---|---|---|---|
| C57BL/6 J | – | 132 | 116 | 87.9 | – | – | 48 h | No |
| BL3129 | 36 % | 319 | 74 | 23.2 | 24 | 6 (3) | 24 h | No |
| BL2779 | 12 % | 399 | 84 | 21.1 | 25 | 6 (4) | 24 h | Yes |
| BL3184 | 40 % | 479 | 52 | 10.9 | 51 | 25 (7) | 24 h | Yes |
IVF using sperm from Epi of a wildtype C57BL6J male after 48 h cold storage was performed as a control Fert fertilization, Epi cauda epididymis, mut mutant
Correlation between coat color chimerism and mutant sperm
The correlation between % coat color chimerism and % mutant sperm in male chimeras was analyzed using 519 chimeras for 246 genes (Fig. 2; Table 4). These mice include the 48 infertile males (for 36 genes) that produced poor quality sperm, the 158 infertile and fertile males (for 79 genes) that produced good quality sperm, and an additional 313 male chimeras (for 131 genes) that produced good quality sperm (data not shown) and were used for sperm genotyping analysis only. Although there was a large variation in percentage of mutant sperm at each percent of chimeric coat color (10, 30, 50, 70, 90, and 100 %), there was significant linear correlation between percentages of chimeric coat color and mean percentages of mutant sperm (R2 = 0.95; Fig. 2). The higher the percentage of chimeric coat color, the higher the mean percentage of mutant sperm produced. The maximum percentage of mutant sperm was 50 %.
Fig. 2.
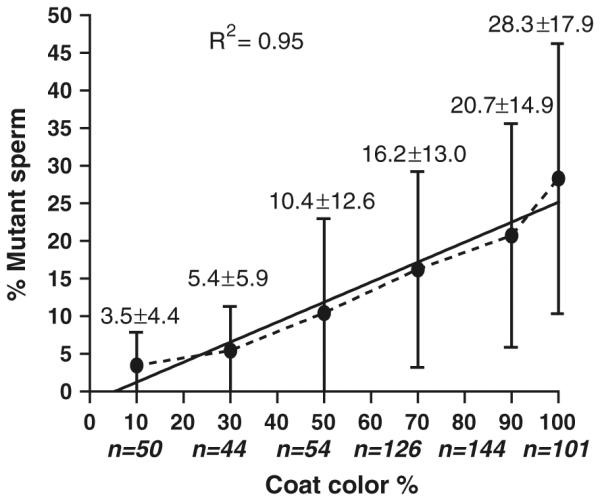
Linear regression between % of coat color and % of mutant sperm (Mean ± SD). R2, the coefficient of determination; n, the number of male chimeras
Table 4.
Statistics results of mutant sperm percentages under each chimera coat color grade
| Coat color | 10 % | 30 % | 50 % | 70 % | 90 % | 100 % |
|---|---|---|---|---|---|---|
| Number of values (males) | 50 | 44 | 54 | 126 | 144 | 101 |
| Minimum | 0 | 0 | 0 | 0 | 0 | 0 |
| 25 % Percentile | 0 | 1 | 2 | 7 | 9 | 13 |
| Median | 1 | 3 | 7 | 12 | 19 | 27 |
| 75 % Percentile | 6 | 7 | 13 | 22 | 29 | 50 |
| Maximum | 18 | 23 | 50 | 50 | 50 | 50 |
| Mean ± SD | 4 ± 4 | 5 ± 6 | 10 ± 13 | 16 ± 13 | 21 ± 15 | 28 ± 18 |
Choosing a chimera for GLT-rescue by IVF
As described above, chimeras in the GLT-rescued group produced significantly higher percentages of mutant sperm than that in the GLT-rescue failed group. Here we combined the mutant sperm data used in Tables 2 and 3 (16 + 3 = 19 GLT-rescued genes and 33 + 4 = 37 GLT-rescue-failed genes) in order to determine if there is a reasonable minimum percent of mutant and good quality sperm for choosing a chimera for GLT-rescue by IVF. As shown in Table 5, the mean ± SD of percentage of mutant sperm was 36 ± 12 in the combined GLT rescued group, and 20 ± 8 in the combined GLT-rescue-failed group. The lowest percentage of mutant sperm was 12 % in the GLT-rescued group. Further, the median and mean of the % mutant sperm in the GLT-rescue-failed group were 19 and 20, respectively. Therefore, 20 % mutant sperm is a reasonable “cutoff” for selecting good quality sperm for IVF.
Table 5.
Statistics results of mutant sperm percentages in the rescued gene group vs. rescue-failed gene group
| Mutant sperm %, GLT rescued |
Mutant sperm %, rescue-failed |
|
|---|---|---|
| Number of values | 19 | 37 |
| Minimum | 12 | 7 |
| 25 % Percentile | 28 | 13 |
| Median | 36 | 19 |
| 75 % Percentile | 50 | 25 |
| Maximum | 50 | 41 |
| Mean ± SD | 36 ± 12 | 20 ± 8 |
Since there was significant linear correlation between percentages of chimeric coat color and mean percentages of mutant sperm as described above (Fig. 2 and Table 4), the data summarized in Table 6 can be used to determine a reasonable cutoff coat color percentage for choosing a chimera for GLT rescue by IVF. These data were obtained by analysing the mutant sperm percentages of the 519 chimeric males representing 246 genes that were used in above Fig. 2 and Table 4. The data in Table 6 indicate that very few chimeric males with 10 or 30 % coat color produced C10 % mutant sperm, and therefore are generally not useful for GLT rescue. On the other hand, 39 % of tested chimeras with 50 % coat color produced C10 % mutant sperm, but only 11 % of the tested males at this coat color level produced C20 % mutant sperm. Therefore, compared to coat color chimerism alone, chimeras with greater than 50 % coat color chimerism and genotype-positive good quality sperm is a better cutoff for selecting chimeras for GLT rescue by IVF. Chimeric males with C70 % coat color chimerism were most effective for GLT rescue. In general, the higher the coat color percentage, the higher proportion of mutant sperm.
Table 6.
Numbers and % of chimeric males with different % of mutant sperm under each chimera coat color grade
| Coat color % | 10 % | 30 % | 50 % | 70 % | 90 % | 100 % |
|---|---|---|---|---|---|---|
| No. males (%) with ≥10 % mut sperm | 8 (16 %) | 8 (18 %) | 21(39 %) | 75 (60 %) | 99 (69 %) | 81 (80 %) |
| No. males (%) with ≥20 % mut sperm | 0 | 2 (5 %) | 6 (11 %) | 42 (33 %) | 70 (49 %) | 63 (62 %) |
| No. males (%) with ≥30 % mut sperm | 0 | 0 | 5 (9 %) | 19 (15 %) | 33 (23 %) | 46 (46 %) |
| No. males (%) with ≥40 % mut sperm | 0 | 0 | 4 (7 %) | 9 (7 %) | 21 (15 %) | 36 (36 %) |
| No. males (%) with 50 % mut sperm | 0 | 0 | 2 (4 %) | 4 (3 %) | 15 (10 %) | 27 (27 %) |
Discussion
Despite many significant technical advances in genome targeting, ES cell cloning, and embryo manipulation over the last several years, the ability to rapidly and reliably achieve GLT of mutant alleles in an efficient manner by natural mating of mouse chimeras is a significant and sometimes challenging milestone on the way to the derivation of genetically-altered mice for biomedical research. Male chimeras that either fail to produce any offspring or that generate only wildtype offspring after prolonged breeding present a quandary to the researcher, and a stark choice to either continue mating indefinitely, re-inject ES cell clones, or re-target and re-inject de novo. All of these approaches are costly in time and expense without guarantee of success. Depending on one’s expertise and experience, an alternative strategy is to harvest sperm from chimeras in the hopes of “rescuing” GLT by IVF. However, even this approach can be problematic, as infertile chimeras may have little to no sperm, or sperm that are abnormal or poor quality, making it unsuitable for IVF. In contrast, fertile chimeras that produce only wildtype offspring by definition have normal and motile sperm but can still fail GLT because proportionately fewer sperm harboring the targeted allele reduces the chance to achieve vertical transmission. Therefore, evidence-based options for better management of poor-performing chimeras are needed.
One way to increase the likelihood of a successful IVF “rescue” attempt is to select mice with high percentage coat color chimerism. However, especially with chimeras on the C57BL/6 genetic background, even this option is variably effective (Seong et al. 2004; Hansen et al. 2008). Attempting to select chimeras based on genotyping of sperm “plugs” is of limited predictive value (Wilson and Sheardown 2011; Lee et al. 2013). Further, sperm analysis, IVF, and embryo transfer are themselves costly procedures and warrant careful and thoughtful consideration of the likelihood for success before proceeding with this course of action.
To our knowledge, a rational, evidence-based approach to determine if extraordinary efforts should be attempted on fertile or infertile chimeras that fail GLT has not been established. Therefore, in the present study we assessed the production, quality and genotype of epididymal sperm harvested from male chimeras generated by blastocyst or morula microinjection of gene targeted ES cell clones containing a LacZ marker which failed to achieve GLT. We sought to understand the characteristics and features of male chimeras that fail to achieve GLT by natural mating and to determine criteria for selecting chimeras for IVF “rescue”. After breeding chimeric male mice to wildtype females for several weeks, we examined the incidence and cause of infertility in chimeras that failed to achieve GLT. We also analyzed the correlation between coat color chimerism and percentage of LacZ-positive sperm.
Our results demonstrate that the primary cause of failure of chimeras to achieve GLT by natural mating is infertility. Further, the main reason for infertility in male chimeras is failed spermatogenesis. These findings are not entirely surprising, as developmental defects, spermatogenesis malfunction, hermaphrodism, chromosome abnormalities and gene mutation have been associated previously with male infertility of chimeras (Shomer et al. 1997; Lin et al. 2000; Zhao et al. 2004; Shirley et al. 2004; Sugawara et al. 2005; Zheng et al. 2007; Fujihara et al. 2013). Nevertheless, our study appears to implicate problems associated with the development of mature sperm as the most likely culprit for causing chimeras to fail GLT.
In many of the infertile chimeras that did have sperm, the morphological and functional characteristics of sperm were poor. Ultimately, only about a quarter of infertile chimeras have sufficiently good quality sperm suitable for attempting IVF. Therefore, our results support a recommendation to conduct a basic analysis of sperm in order to select the relatively small number of infertile chimeras producing sperm with the highest likelihood for generating either wildtype or mutant offspring by IVF. From this group, to facilitate the selection of chimeras with sperm that have the greatest chance for producing mutant offspring, genotyping sperm for the targeted allele provides additional valuable data for making an informed decision. Using a generic qPCR reaction to detect the selectable marker LacZ present in all the targeted alleles reported in this study, not surprisingly we again found that the proportion of genotype-positive sperm was highly linearly correlated with coat color chimerism. Generally speaking, the higher the percent coat color, the higher the mean percent mutant sperm. However, because of the high variability in the percent of mutant sperm at each level of coat color chimerism, and the observation that even a 100 % coat color chimera can have 0 % mutant sperm, our data indicates that quantitative determination of sperm chimerism by genotyping is a better predictor of GLT potential than qualitative assessment of somatic cell chimerism by coat color alone.
Because genotyping mutant sperm takes time, it is usually necessary to cryopreserve sperm after harvesting from chimeras before using for IVF. For that reason, all of our experiments were performed on cauda epididymal sperm cryopreserved in liquid nitrogen using cryovials and recovered for use in IVF. In addition, we also demonstrated that, when necessary, sperm from vasa deferentia can be used for sperm genotyping while cauda epididymides can be stored in mineral oil at 4 °C for 1–2 days awaiting sperm genotyping before IVF. Previous studies reported that Lifor preservation medium (Lifeblood Medical Inc, NJ, USA), although expensive, is better for cold storage of C57BL/6J sperm in epididymis (Takeo et al. 2012, 2014). Also, we used cell strainers with mesh size 70 μm to filter sperm suspension to remove tissue fragments and prepare a relatively pure sperm population for genotyping. This method is simple and cost-effective, but it does not remove all somatic cells. The presence of somatic cells might be contributing to the variability in percent of mutant sperm in chimeras at all levels of coat color chimerism.
In summary, our results indicate that, in addition to the extent of coat color chimerism, assessment of sperm quality and genotype are together highly informative for selecting fertile and infertile chimeras to harvest sperm for attempting IVF rescue of GLT that has not been achieved by natural mating. It is not foolproof, however, as some chimeras with sperm harboring relatively high percentages of the mutant allele also failed GLT by IVF. Although not specifically investigated, we hypothesize a number of reasons why in some cases IVF using sperm samples with LacZ positive cells fail to produce mutant pups, including 1) contamination of sperm samples with somatic cells containing LacZ and 2) infertility caused by the targeted mutation. Nonetheless, our data suggests that after prolonged breeding, infertile male chimeras and chimeras that produce only wildtype offspring with >50 % coat color chimerism, good quality sperm, and >20 % genotype-positive sperm are likely to be better candidates for selection to attempt rescue of GLT by IVF than chimeras chosen at random. Future studies are needed to enhance the population of mutant sperm from chimeras so as to facilitate an even higher GLT rescue rate.
Acknowledgments
This research was funded in part by NIH grant 5U42OD011175 KOMP Phase II Mouse Production and Cryopreservation, NIH grant 5U42OD012210 Mutant Mouse Regional Resource Center, and by the UC Davis Mouse Biology Program.
References
- Fujihara Y, Kaseda K, Inoue N, Ikawa M, Okabe M. Production of mouse pups from germline transmission-failed knockout chimeras. Transgenic Res. 2013;22:195–200. doi: 10.1007/s11248-012-9635-x. [DOI] [PubMed] [Google Scholar]
- Hansen GM, Markesich DC, Burnett MB, Zhu Q, Dionne KM, Richter LJ, Finnell RH, Sands AT, Zambrowicz BP, Abuin A. Large-scale gene trapping in C57BL/6 N mouse embryonic stem cells. Genome Res. 2008;18:1670–1679. doi: 10.1101/gr.078352.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AY, Evans K, Willis B, Lloyd KC. Combining sperm plug genotyping and coat color chimerism predicts germline transmission. Transgenic Res. 2013;22:1265–1272. doi: 10.1007/s11248-013-9731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MW, Vallelunga JM, Kinchen KL, Rink KL, Zarrabi J, Shamamian AO, Lloyd KCK. IVF recovery of mutant mouse lines using sperm cryopreserved with MTG in cryovials. Cryo Lett. 2014;35:145–153. [PMC free article] [PubMed] [Google Scholar]
- Lin SR, Yu IS, Huang PH, Tsai CW, Lin SW. Chimeric mice with disruption of the gene coding for phosphatidylinositol glycan class A (Pig-a) were defective in embryogenesis and spermatogenesis. Br J Haematol. 2000;110:682–693. doi: 10.1046/j.1365-2141.2000.02209.x. [DOI] [PubMed] [Google Scholar]
- Ostermeier GC, Wiles MV, Farley JS, Taft RA. Conserving, distributing and managing genetically modified mouse lines by sperm cryopreservation. PLoS One. 2008;3:e2792. doi: 10.1371/journal.pone.0002792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettitt SJ, Liang Q, Rairdan XY, et al. Agouti C57BL/6 N embryonic stem cells for mouse genetic resources. Nat Methods. 2009;7:493–495. doi: 10.1038/nmeth.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poueymirou WT, Auerbach W, Frendewey D, et al. F0 generation mice fully derived from gene-targeted embryonic stem cells allowing immediate phenotypic analyses. Nat Biotechnol. 2006;25:91–99. doi: 10.1038/nbt1263. [DOI] [PubMed] [Google Scholar]
- Seong E, Saunders TL, Stewart CL, Burmeister M. To knockout in 129 or in C57BL/6: that is the question. Trends Genet. 2004;20:59–62. doi: 10.1016/j.tig.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Shirley CR, Hayashi S, Mounsey S, Yanagimachi R, Meistrich ML. Abnormalities and reduced reproductive potential of sperm from Tnp1- and Tnp2-null double mutant mice. Biol Reprod. 2004;71:1220–1229. doi: 10.1095/biolreprod.104.029363. [DOI] [PubMed] [Google Scholar]
- Shomer NH, Foltz CJ, Li X, Fox JG. Diagnostic exercise: infertility in two chimeric mice. Lab Anim Sci. 1997;47:321–323. [PubMed] [Google Scholar]
- Sugawara N, Tokunaga Y, Maeda M, Komaba R, Araki Y. A successful pregnancy outcome using frozen testicular sperm from a chimeric infertile male with a 46, XX/46, XY karyotype: case report. Hum Reprod. 2005;20:147–148. doi: 10.1093/humrep/deh587. [DOI] [PubMed] [Google Scholar]
- Takeo T, Nakagata N. Reduced glutathione enhances fertility of frozen/thawed C57BL/6 mouse sperm after exposure to methyl-beta-cyclodextrin. Biol Reprod. 2011;85:1066–1072. doi: 10.1095/biolreprod.111.092536. [DOI] [PubMed] [Google Scholar]
- Takeo T, Tsutsumi A, Omaru T, Fukumoto K, Haruguchi Y, Kondo T, Nakamuta Y, Takeshita Y, Matsunaga H, Tsuchiyama S, Sakoh K, Nakao S, Yoshimoto H, Shimizu N, Nakagata N. Establishment of a transport system for mouse epididymal sperm at refrigerated temperatures. Cryobiology. 2012;65:163–168. doi: 10.1016/j.cryobiol.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Takeo T, Fukumoto K, Kondo T, Haruguchi Y, Takeshita Y, Nakamuta Y, Tsuchiyama S, Yoshimoto H, Shimizu N, Li MW, Kinchen K, Vallelunga J, Lloyd KCK, Nakagata N. Investigations of motility and fertilization potential in thawed cryopreserved mouse sperm from cold-stored epididymides. Cryobiology. 2014;68:12–17. doi: 10.1016/j.cryobiol.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware CB, Siverts LN, Nelson AM, Morton JF, Ladiges WC. Utility of a C57BL/6 ES line versus 129 ES lines for targeted mutations in mice. Transgenic Res. 2003;12:743–746. doi: 10.1023/b:trag.0000005246.35812.c8. [DOI] [PubMed] [Google Scholar]
- Wilson S, Sheardown SA. Identification of germline competent chimaeras by copulatory plug genotyping. Transgenic Res. 2011;20:429–433. doi: 10.1007/s11248-010-9413-6. [DOI] [PubMed] [Google Scholar]
- Zhao M, Shirley CR, Hayashi S, Marcon L, Mohapatra B, Suganuma R, Behringer RR, Boissonneault G, Yanagimachi R, Meistrich ML. Transition nuclear proteins are required for normal chromatin condensation and functional sperm development. Genesis. 2004;38:200–213. doi: 10.1002/gene.20019. [DOI] [PubMed] [Google Scholar]
- Zheng H, Stratton CJ, Morozumi K, Jin J, Yanagimachi R, Yan W. Lack of Spem1 causes aberrant cytoplasm removal, sperm deformation, and male infertility. Proc Natl Acad Sci USA. 2007;104:6852–6857. doi: 10.1073/pnas.0701669104. [DOI] [PMC free article] [PubMed] [Google Scholar]


