Introduction
Heart failure (HF) afflicts nearly 6 million Americans, resulting in one million emergency department (ED) visits and over one million annual hospital discharges.1,2 An aging population and improved survival from cardiovascular diseases is expected to further increase HF prevalence.3 By 2030 an estimated 25% increase in HF prevalence will result in an additional 3 million affected individuals.1,4 Of the $39.2 billion spent on HF care in the US in 2010, inpatient admissions accounted for the single largest proportion. By 2030, the amount spent on hospital care for HF will be even greater as annual total costs are expected to be close to $70 billion.
Emergency providers play a significant role in the management of patients with acute heart failure (AHF). Therapeutic and disposition decisions made by emergency providers have direct impact on morbidity, mortality, and hospital length of stay, all of which affect health care costs.5–9 Over 80% of ED patients with AHF are admitted to the hospital, a proportion which has remained largely unchanged over the last 5 years.2 It is crucial that emergency physicians and other providers involved in early management understand the latest developments in diagnostic testing, therapeutics and alternatives to hospitalization. Equally important are partnerships between emergency providers and heart failure specialists along with the entire interdisciplinary team caring for HF patients to streamline care from the ED to the inpatient and outpatient settings.
1. Current Approaches to Diagnosis
Although there is no universally accepted terminology to describe “acute” heart failure, for the purpose of clarity we have chosen to use AHF, defined as chronic or de novo HF with new or worsening symptoms requiring acute therapy. Patients present to the ED with signs and symptoms, not diagnoses. While dyspnea is the most common symptom in AHF, it has a large differential diagnosis. Efficient diagnosis is critical as delays in the delivery of care for AHF are associated with increases in mortality, hospital length of stay, and treatment costs.10–14 Thus, an understanding of the strengths and limitations of the history, physical examination, and laboratory and radiographic tests used to assist in the diagnosis of AHF is essential.
History and Physical Examination
Multiple studies suggest there is no historical or physical examination finding that achieves a sensitivity and specificity > 70% for the diagnosis of AHF. Further, only one clinical finding, the S3 gallop, achieves a likelihood ratio positive (LR+) greater than 10 and none carries a LR- less than 0.1.14 In a meta-analysis of 18 studies,13 prior HF was the most useful historical parameter, with a LR+ of 5.8 and LR- of 0.45, respectively. Dyspnea on exertion is the symptom with the lowest LR- at 0.48, but has a LR+ of only 1.313,14 while paroxysmal nocturnal dyspnea, orthopnea, and peripheral edema have the best LR+ (2/1–2.6%), but a notably poor LR- (0.64–0.70).13,14 Notably, emergency physician clinical judgment is only modestly useful with a LR+ of 4.4 and LR- of 0.45.13 Although the S3 has the highest LR+ (11), it has far less utility as a negative predictor (LR-, 0.88)13 and suffers from poor inter-rater reliability.15–18 Hepatojugular reflux (LR+, 6.4) and jugular venous distension (LR+, 5.1), are the only other examination findings with a LR+ above 5.
Chest radiography
Chest radiographs demonstrating pulmonary venous congestion, cardiomegaly, and interstitial edema are the most specific test findings for AHF (Table 1).12,13 However, their absence cannot rule out AHF, as up to 20% of patients with AHF will have no congestion on their ED chest radiograph.19 Particularly in late-stage HF, patients may have few radiographic signs, despite AHF symptoms and elevated pulmonary capillary wedge pressure (PCWP). 12,20,21
Table 1.
Summary of Diagnostic Accuracy of Findings on Chest Radiograph and Electrocardiogram for AHF in ED Patients presenting with Dyspnea
| Finding | Pooled | Summary LR (95% CI) | |||
|---|---|---|---|---|---|
| Sensitivity | Specificity | Positive | Negative | ||
| Chest Radiograph | Pulm Venous Congestion | 0.54 | 0.96 | 12.0 (6.8–21.0) | 0.48 (0.28–0.83) |
| Interstitial Edema | 0.34 | 0.97 | 12.0 (5.2–27.0) | 0.68 (0.54–0.85) | |
| Alveolar edema | 0.06 | 0.99 | 6.0 (2.2–16.0) | 0.95 (0.93–0.97) | |
| Cardiomegaly | 0.74 | 0.78 | 3.3 (2.4–4.7) | 0.33 (0.23–0.48) | |
| Pleural Effusion | 0.26 | 0.92 | 3.2 (2.4–4.3) | 0.81 (0.77–0.85) | |
| Any edema | 0.70 | 0.77 | 3.1 (0.60–16.0) | 0.38 (0.11–1.3) | |
| Pneumonia | 0.04 | 0.92 | 0.50 (0.29–0.87) | 1.0 (1.0–1.1) | |
| Hyperinflation | 0.03 | 0.92 | 0.38 (0.20–0.69) | 1.1 (1.0–1.1) | |
| Electrocardiogram | Atrial fibrillation | 0.26 | 0.93 | 3.8 (1.7–8.8) | 0.79 (0.65–0.96) |
| New T-wave changes | 0.24 | 0.92 | 3.0 (1.7–5.3) | 0.83 (0.74–0.92) | |
| Any abnormal finding | 0.50 | 0.78 | 2.2 (1.6–3.1) | 0.64 (0.47–0.88) | |
| ST elevation | 0.05 | 0.97 | 1.8 (0.80–4.0) | 0.96 (0.95–1.0) | |
| ST depression | 0.11 | 0.94 | 1.7 (0.97–2.9) | 0.95 (0.90–1.0) | |
LR=Likelihood Ratio CI=Confidence Interval Pulm=Pulmonary
Adapted from: Wang CS, Fitzgerald JM, Schulzer M, Mak E, Ayas NT. Does This Dyspneic Patient in the Emergency Department Have Congestive HF? JAMA. 2005;294:1944–1956.
Electrocardiogram
The electrocardiogram is not useful for diagnosis, but may suggest a specific cause or precipitant of AHF such as myocardial ischemia, acute myocardial infarction or arrhythmia. The presence of atrial fibrillation has a high LR+ for AHF; however, new t-wave changes are also associated with AHF (Table 1).13 The electrocardiogram may also offer clues as to the underlying cause of chronic HF (e.g., Q waves in ischemic cardiomyopathy, low voltage in cardiac amyloid).
Biomarkers
The natriuretic peptides (NP), B-type NP (BNP), and its precursor N-terminal Pro-BNP (NTBNP), are the most established AHF diagnostic biomarkers. They add value in the setting of undifferentiated dyspnea by improving diagnostic discrimination22,23 and correlate with cardiac filling pressures and ventricular stretch.24 NP testing is a Class 1 (best evidence) guideline recommendation by both Heart Failure Society of America (HFSA) and American College of Cardiology Foundation / American Heart Association (ACCF/AHA)25,26 and may be particularly useful when the etiology of dyspnea is unclear, and have been shown to have greater utility than CXR for diagnosing AHF. 13,22 Newer markers, such as ST2 and galectin 3, have been explored for prognostic assessment and diagnosis of preclinical HF,27,28 but their role in the ED is less clear.
NP levels can be affected by age, gender, weight and renal function (Table 2).29 Dyspnea not due to AHF can still be associated with NP elevation in a variety of conditions associated with myocardial stretch, (e.g. right ventricular stretch from pulmonary hypertension or pulmonary embolism, acute coronary syndromes) or decreased renal clearance. 30–32 Patients with HF with preserved left ventricular ejection fraction (HFpEF) have a smaller left ventricular radius and thicker walls compared to patients with reduced LVEF (HFrEF) resulting in proportionally lower NP levels for similar degrees of AHF, suggesting different diagnostic thresholds are needed depending on whether left ventricular ejection fraction (LVEF) is preserved or reduced.33 Beyond clinical factors, additional variation in NP levels can arise from heritable34 and specific genetic variants that have been shown to alter assay performance.35 In general, changes over 50% from baseline represent worsening HF; however, significant variation in NP levels can occur within the same patient and intra-individual differences between measurements do not necessarily represent an acute clinical event. 36 Despite these limitations and a substantial ‘grey zone’ when interpreting results, NP testing remains useful and is additive to physician assessment.37
Table 2.
Factors affecting Natriuretic Peptide Levels
Point of care ultrasonography
While not a substitute for comprehensive echocardiography, point of care cardiac ultrasonography can be valuable in determining the etiology of dyspnea, providing an assessment of left ventricular function and volume status and looking for signs of pericardial effusion. Point of care ultrasonography can accurately estimate LVEF, has good inter-rater reliability, and is most likely to be of use immediately or shortly after ED arrival in those patients without a prior HF diagnosis.20 Volume status may be assessed by inferior vena cava (IVC) diameter and its degree of change with respiratory variation. Inspiration causes the IVC to decrease in size as more blood leaves the IVC than collecting within it.38,39 The IVC collapse index, defined as IVC diameter during expiration minus the IVC diameter during inspiration divided by IVC diameter during expiration, can help determine volume status in the setting of AHF. In AHF patients with volume overload the IVC is dilated without the normal amount of change in IVC diameter during the respiratory cycle. Thus the IVC collapse index is closer to 1, compared to patients without HF where it is typically between 0.25 and 0.75.40
Lung water can also be assessed with ultrasound by looking for sonographic B-lines (also called lung comets or comet tail artifacts) each of which implies thickened interstitial or fluid-filled alveoli. B-lines occur most commonly in patients with AHF and correlate with elevated PCWP and extravascular pulmonary water.41 In the ED setting, the presence of B lines on pleural ultrasonography is associated with fluid overload, enhancing diagnostic accuracy when coupled with examination and NP measurement.42 Several authors have shown bedside pulmonary ultrasound is accurate in detecting AHF with reported sensitivities of 86–100% and specificities of 95–98% (LR- = 0.01–0.14 and LR+ = 17.2–49.5).43,44 Ultrasonography has been shown to be more accurate than auscultation or chest radiography for the detection of pleural effusion, consolidation, and alveolar interstitial syndrome in the critical care setting.45
Other Diagnostic Technologies
Obtained using stand-alone machines or from an implanted cardiac device, impedance cardiography (ICG) may provide an additional hemodynamic measure.46–48 However, studies evaluating the utility of externally applied ICG devices to guide acute therapy have had mixed results49,50 and their role in the ED remains unclear. Further, while implanted devices offer extensive monitoring capabilities (heart rate, heart rate variability, activity level, thoracic impedance) for chronic HF disease management,51 their utility in the ED is not established. Because data from indwelling devices provide important diagnostic information, they may ultimately prove useful, particularly for subtyping HF based on hemodynamic, developing tailored treatment strategies, and quantifying fluid status to aid in transition care decisions. Routine interrogation of implantable pacemakers or cardioverter-defibrillators, which provide ongoing rhythm recordings, can be used to assess for atrial and/or ventricular arrhythmias as precipitants to AHF.52 Additional technologies to assist with non-invasive hemodynamic assessment such as finger cuff pulse-wave analysis may also be of value but their utility in the ED has yet to be determined.53
2. Current Guideline Recommendations for AHF Therapy
The ACCF/AHA Guidelines for the Management of HF is a comprehensive document using standardized methodology to evaluate the available evidence for HF management,26 with one section providing guidance on the hospitalized patient. While the guidelines do not provide specific recommendations on the early evaluation and management of AHF, they do suggest continuation and initiation of Guideline-Directed Medical Therapy (GDMT) for the hospitalized patient, which can be somewhat extrapolated to the ED phase of care. GDMT may include angiotensin converting enzyme inhibitors (ACE-I) or angiotensin receptor blockers (ARB), beta blockers (BB), mineralocorticoid antagonists (MRA ) depending on variables such as race, LVEF, and contraindications. For instance, in patients with HFrEF, it is recommended (Class I, LOE B) that, in the absence of hemodynamic instability or contraindications, GDMT be maintained and furthermore it is reasonable to consider early administration of such oral therapy in the ED. Other recommendations relevant to early care (Table 3) include initiation of intravenous loop diuretics at a dose greater than or equal to their chronic oral daily dose (Class I, LOE B), consideration of parenteral nitrates (nitroglycerin, nitroprusside) or nesiritide as adjuvant therapy for non-hypotensive patients with continued symptoms, (Class IIb, LOE A) and broad use of thromboembolism prophylaxis (Class I, LOE B).
Table 3.
Synopsis of recommended AHF therapies based on ESC, HFSA and ACCF/AHA, and CCS guidelines.
| RECOMMENDATION | ESC | HFSA | ACCF/AHA | CCS |
|---|---|---|---|---|
| Diuretic Use | ||||
| • AHF patients with fluid overload should receive diuretics |
I/B | B | I/B | SR/MQ |
| • Dosing | IV, to symptom improvement, Consider high dose regimen. No specific recommendation (NSR) |
IV, Titrate to symptom relief, minimize AE (C) |
Initial IV dose should equal or exceed oral daily dose, then adjust based on response (I/B) |
We recommend a loop diuretic, such as furosemide, for most patients with HF and congestive symptoms. When acute congestion is cleared, the lowest dose should be used that is compatible with stable signs and symptoms (SR/LQ). |
| • Inadequate Diuresis | Consider doubling loop diuretic (NSR) Consider add thiazide Consider dopamine @ 2.5 mcg/kg/min (NSR) |
Increase dose (C) Continuous infusion (C) Add thiazide (C) |
Add thiazide (IIa/B) Increase loop dose (IIa/B) Consider renal dose dopamine (IIb/B) |
Increases in loop diuretics, cautious addition of a second diuretic (a thiazide or low dose metolazone) (WR/MQ). |
| Ultrafiltration | Consider in refractory cases (NSR) |
Refractory cases (C) In lieu of diuretics- selected pts (C) |
In refractory cases of overload (IIb/B) Pulmonary congestion (IIb/C) |
Patients with persistent congestion despite diuretic therapy, with or without impaired renal function, may, under experienced supervision, receive continuous venovenous ultrafiltration. (Practical tip) |
|
Vasodilators, Other Pharmacologic Therapy |
Titrated to systolic BP (SBP) > 100 mm Hg, for relief of dyspnea in hemodynamically stable patients (SBP > 100 mm Hg) |
|||
| • Nitrates | In pulmonary congestion and SBP>110 mmHg (IIa/B) caution in Aortic/Mitral Stenosis |
+ diuretic and no hypotension (B) |
+ diuretic therapy (IIb/A) |
SR/MQ |
| • Nesiritide | NSR | After first line therapy(C) |
+ diuretic therapy (IIb/A) |
WR/HQ |
| • Vasopressin Antagonist | NSR | NSR | Severe volume overload and Na <135 (IIb/B) |
symptomatic or severe hyponatremia (< 130 mmol/L) and persistent congestion despite standard therapy, to correct hyponatremia and the related symptoms (WR/MQ). |
| • Opiates +/− antiemetic | IIa/C | NSR | NSR | NSR |
| Fluid restriction | NSR | <2L/d if Na<130 mEq/L; stricter if <125 mEq/L (C) |
1.5–2 L/d esp in Na<135 and congestion (IIa/C) |
NSR |
|
Thromboembolism Prophylaxis |
I/A | If no contraindication (B) |
UFH, LMWH (I/B) | NSR |
| Respiratory Support | ||||
| • Oxygen | If SaO2<90% (I/C) | If hypoxia (C) | NSR | If hypoxia; titrated to an SaO2>90% (SR/MQ) |
| • NIV | For RR>20, caution in SBP<85 mmHg (IIa/B) |
In severe dyspnea (A) | NSR | We recommend CPAP or BIPAP not be used routinely (SR/MQ) |
| Inotropic Support | SBP<85 mmHg or hypoperfusion (IIa/C) |
In selected hypotensive pt (C) |
In short term support of selected pts (IIb/B) |
We recommend hemodynamically stable patients do not routinely receive inotropes like dobutamine, dopamine, or milrinone (SR/HQ). |
NSR= no specific recommendation; SR/LQ= Strong Recommendation, Low-Quality Evidence; WR/MQ= Weak Recommendation, Moderate-Quality Evidence; SR/MQ= Strong Recommendation, Moderate- Quality Evidence; SR/HQ= Strong Recommendation, High-Quality Evidence; WR/HQ= Weak Recommendation, High-Quality Evidence
Using methodology similar to ACCF/AHA, the HFSA published Comprehensive Practice Guidelines in 2010.25 Section 12 of this guideline focuses on AHF and suggests that treatment start with clinical classification based on history, examination and secondary risk stratification using diagnostic adjuncts. In general, the recommendations are consistent with the ACCF/AHA (Table 3) but the HFSA guidelines specifically mention non-invasive ventilation (NIV) in patients with severe pulmonary congestion (strength of evidence [SOE] A) and suggest that inotropic therapy (milrinone, dobutamine) be considered in patients with signs of diminished end organ perfusion, LV dilatation, reduced EF and marginal systolic blood pressure (<90mmHg), or in those who are poorly responsive to diuretics or are intolerant to vasodilators (SOE C). An update to the HFSA guideline on AHF was published in 2013,54 and provides information on recently published trials 55–57 that have yielded important data to inform both current clinical practice and future research directions.
The European Society of Cardiology (ESC) guidelines section 12 specifically addresses the treatment of AHF.58 Overall, the ESC Guidelines represent an approachable, clinically relevant synopsis of the available evidence in the treatment of patients with AHF (Table 3). Formatted to address clinical scenarios these guidelines provide treatment recommendations for AHF patients with pulmonary congestion, hypotension, acute coronary syndrome, atrial fibrillation with rapid ventricular response, heart block, isolated right ventricular failure, cardiorenal syndrome and adult congenital heart disease. While the recommendations are generally consistent with the ACCF/AHA and HFSA, the ESC makes a few additional pertinent recommendations. The use of intravenous opiates with antiemetics was given IIa (LOE C) recommendation for patients who are anxious, restless or distressed. When using opiates, the authors suggest close monitoring and caution regarding respiratory depression, which may be important to prevent adverse events, as suggested in a recent analysis.58 With respect to invasive monitoring, an arterial line or a pulmonary artery catheter is recommended only in patients with refractory hypotension, in whom LV filling pressures are uncertain, or who are surgical candidates.
The American College of Emergency Physicians (ACEP) does not publish comprehensive guidelines, focusing instead on clinical policy statements targeted at critical aspects of ED care for common conditions. In 2007, ACEP produced their only clinical policy dedicated to AHF. In it, some of the more controversial and important issues confronting ED management of AHF were addressed; however, significantly more data has become available since its publication.55,59,60
3. Current ED therapy: applicability of recent evidence and ongoing trials
Current Approach to ED Treatment of AHF
It is noteworthy that neither the ACCF/AHA nor the HFSA guidelines include any Class I, Level A recommendations for the pharmacologic treatment of AHF.25,26,58 ED treatment is not emphasized in the expert recommendations from the ACCF/AHA, and little has changed regarding AHF treatment in the ED since the 1970s.26,61 This is not a reflection upon guidelines; rather it highlights the absence of robust ED clinical trial data and an ill-defined standard practice.(Table 4)
Table 4.
Randomized Diagnostic and Phase III Therapeutic AHF Studies over the past 10 years.
| Study/Author | Year/ Sample Size |
Primary Endpoint | Findings | Limitations |
|---|---|---|---|---|
| Diagnostic Studies | ||||
| BASEL Mueller |
2004 (n=452) |
Time to discharge and cost of treatment |
Time to discharge and costs of treatment were reduced in patients with undifferentiated dyspnea who were randomized to rapid, bedside BNP testing |
Conducted in Europe- median LOS and healthcare systems much different than US |
| IMPROVE-CHF Moe |
2007 (n=500) |
ED LOS and total direct medical costs of treatment |
ED LOS and cost of treatment were reduced with addition of NT-proBNP to clinical gestalt for patients with undifferentiated dyspnea |
Conducted in Canada which has different healthcare cost structure than US |
| REDHOT II Singer |
2009 (n=447) |
Hospital LOS | No statistical difference in length of stay with serial BNP testing |
Convenience sample; potentially underpowered |
| Therapeutic Studies | ||||
| SURVIVE Mebazaa |
2007 (n=1327) |
All cause mortality at 180 days |
No difference in mortality in patients requiring inotrope therapy with randomization to levosidemendan or dobutamine |
Conducted in Europe with a drug (levosimendan) that was never FDA approved in the US. Bolus hypotension may have been a significant contributor to adverse events |
| EVEREST Gheorghiade |
2007 (n=4133) |
Composite of global clinical status and body weight at day 7 |
Compared to placebo tolvaptan had significantly greater improvement in the composite |
The composite endpoint was largely driven by changes in body weight |
| VERITAS McMurray |
2007 (n=1435) |
Change in dyspnea over 24 hours and incidence of death or WHF at day 7 |
No significant difference in dyspnea or death/WHF between tezosentan and standard therapy |
|
| 3CPO Gray |
2008 (n=1069) |
Death or intubation within 7 days |
No difference in mortality with NIPPV versus standard oxygen therapy or either end-point with use of CPAP versus BiPAP |
Open label study with extensive crossover to NIPPV in patients randomized to standard oxygen therapy |
| PROTECT Massie |
2010 (n=2033) |
Overall treatment success defined as early dyspnea improvement and no death, HF readmission or WRF |
No significant difference between Rolofylline and placebo |
|
| ASCEND O'Connor |
2011 (n=7141) |
Dyspnea and rehospitalization/death within 30 days |
Prespecified dyspnea endpoint not met; no differences in death between nesiritide and standard care |
Patients enrolled long after ED stay; significantly greater proportion with hypotension in nesiritide group |
| DOSE-AHF Felker |
2011 (n=308) |
Dyspnea and WRF at 72 hours |
No significant difference between bolus/drip or high/low dose furosemide |
Patients randomized up to 24 hours after ED presentation; population largely white males with low EF, Not powered for longer term outcomes |
| RELAX-AHF-1 Teerlink |
2013 (n=1161) |
Improvement in dyspnea measured by both Likert and VAS at day 5 |
Significant improvement in VAS by serelaxin compared to placebo |
No difference in Likert between serelaxin and placebo; clinical meaning of VAS difference unclear |
| ROSE-AHF Chen |
2013 (n=360) |
72-hour urine volume and change in Cystatin-C |
No difference between low-dose dopamine or low-dose nesiritide compared to placebo in either endpoint |
Not powered for longer term outcomes |
| REVIVE II Packer |
2013 (n=600) |
Clinical composite of ‘improved’, ‘unchanged’ or ‘worse’ at 6hrs, 24 hrs, and 5 days |
More improvement in levosimendan treated patients with less worsening. However, more hypotension and arrhythmias were observed with a numerically higher number of deaths |
Bolus hypotension may have been a significant contributor to adverse events |
| PRONTO Peacock |
2014 (n=104) |
Targeted BP control in first 30 minutes of intravenous vasodilator |
Clevidipine provided more rapid BP control compared to standard therapy |
Open label study, more BP overshoot in clevidipine arm, efficacy was monitored only to 12 hours, not powered for longer term outcomes |
WRF=worsening renal function; WHF= worsening heart failure; LOS= length of stay; BP = blood pressure; EF=ejection fraction; ED = emergency department
AHF Treatment: Consideration of Presenting Blood Pressure
Expert opinion, supported by smaller cohort and randomized studies, suggests our current approach should reconsider the “diuretics only” treatment paradigm, and incorporate other clinical parameters into the initial management of AHF.12,62 While several classification schemes are proposed; none have been prospectively validated. There have been no randomized trials evaluating ED treatment strategies based on initial blood pressure. Based on prior and recent evidence we suggest an initial treatment approach based on presenting systolic blood pressure (SBP),63–67 dividing patients into three groups (Table 5): 1) hypertensive (SBP > 140mmHg); 2) normotensive (SBP 100–140mmHg); and 3) hypotensive (SBP < 100mmHg).12 This approach is based on the initial BP (ambulance or ED) and not BP measurements taken several hours later, after initial ED management.
Table 5.
Associated clinical characteristics and treatment approaches based on ED presentation phenotype.
| ED Presentation Phenotype |
Clinical Characteristics | Treatment |
|---|---|---|
| Low BP (SBP < 100) |
|
|
| Normal BP (SBP 100 – 140) |
|
|
| High BP (SBP > 140) |
|
|
+ = Relative intensity of use; NIV = non-invasive ventilation; HTN = hypertension; CAD = coronary artery disease; LVEF = left ventricular ejection fraction; CRI = chronic renal insufficiency; SL=sublingual; BP=blood pressure
Elevated SBP may be an especially important target, as a sizable proportion of patients with hypertensive AHF present with volume redistribution rather than volume overload as their predominant phenotype. In such patients, congestion is due to increased afterload with rapid onset of dyspnea and flash pulmonary edema representing a classic presentation.64 Rarely do these patients complain of symptoms days to weeks prior to presentation; rather, symptoms are abrupt and with less evidence of peripheral edema or weight gain.68,69 While elevated systemic vascular resistance is thought to be the primary mechanism, volume mobilization from the large venous reservoir contained within the splanchnic circulation has also been proposed as a contributory mechanism.70 For volume overload patients, IV loop diuretics remain the primary ED pharmacologic therapy, but for those with volume redistribution vasodilation may be the preferred approach to treatment. Small studies suggest safety and significant clinical benefit with nitrates or calcium channel blockers in the hypertensive AHF phenotype63,66,67 and results from several on-going, large trials of targeted vasodilator therapy are eagerly anticipated.
This simple approach prompts greater usage of vasodilators, as approximately 50% of patients have a SBP > 140mmHg.68 However, independent of BP, an assessment of volume status is critical as patients in any group may still require diuresis. This is particularly true for hypotensive patients as many will have a low SBP at baseline and fluid overload may still be present. Importantly, for such patients inotropes should only be used when other treatments have failed or there is clear evidence of shock or organ hypoperfusion.25
Clinical Trials Evidence in Acute HF: Past and Current
Hospitalizations, once viewed as episodes of fluid overload requiring intravenous diuretics in an otherwise stable chronic HF course, are now recognized as a fundamental change in the clinical trajectory of patients with HF. Whether AHF simply heralds a sicker chronic HF cohort or reflects a distinct pathophysiologic entity is unclear. Compared to outpatients with a comparable degree of cardiac dysfunction, hospitalized patients have significantly worse outcomes.71,72 Despite an improved understanding of the pathophysiology and prognosis of patients with AHF, treatment remains primarily targeted at short-term decongestion using diuretics, and to a much lesser degree, vasodilators. Multiple Phase III clinical trials failed to show clear improvement in symptoms, survival or rehospitalization rates.60,73–79
Although intravenous nitroglycerin is commonly used in AHF, there are no large randomized trials evaluating its impact on long-term outcomes.80 A recent study of low-dose dopamine and low-dose nesiritide in patients with AHF and renal dysfunction showed no impact on renal function or clinical outcomes for either agent compared to placebo.81 In the DOSE trial, there were no significant differences in dyspnea improvement or worsening renal function between intermittent bolus or infusion of furosemide. Higher dose diuretic regimens demonstrated a nonsignificant improvement in dyspnea, and slightly higher risk of worsening renal function, when compared to the low-dose strategy.81 None of these studies assessed the cost-effectiveness of care and very few enrolled AHF patients in the ED.
4. Disposition Decision making – can a subset of ED patients be sent home?
The ED is increasingly being relied on to evaluate more complex patients, some of whom previously were directly admitted from an outpatient setting.82 As a result, emergency physicians serve as major decision makers for approximately half of all US inpatient admissions for AHF.83 However, this may present challenges as emergency physicians have a low tolerance of ‘risk’ in relation to ED discharge decisions. For AHF, a less than 0.5% risk of 30-day death or serious nonfatal complication (acute myocardial infarction, reperfusion therapy, respiratory failure or cardiopulmonary resuscitation) has been suggested as an emergency physician’s typical threshold for ED discharge, a proportion most likely lower than for cardiologists or primary care providers.84 Collaborative decision making coupled with capacity for close follow-up may safely change the dominant flow of patients from the ED to the inpatient setting.
Compounded by a lack of disposition recommendations from national guidelines, the absence of validated decision tools identifying patients with limited susceptibility to post-discharge events12,85, and high rates of relapse and mortality after an ED discharge,86,87 such risk intolerance leads to admission for more than 80% of patients treated in the ED for AHF. Consensus guidelines have addressed AHF risk stratification, but provide little objective instruction for ED disposition decision-making26 or base recommendations on disparate studies of isolated predictors.25,88
Prediction of Low Risk
There have been many investigations of high-risk physiologic markers in ED patients with AHF (Table 6), but most were retrospective, involved hospitalized patients, lacked a measurement of additive value to clinical assessment, and did not use a rigorous AHF diagnostic standard.89,90 These limitations notwithstanding, renal dysfunction, low blood pressure, low serum sodium, and elevated cardiac biomarkers (troponin or NP) have been consistently associated with an increased risk of morbidity and mortality.91 As the majority of patients are already admitted to the hospital, characterizing AHF clinical profiles safe for either ED discharge, or discharge after a brief period of treatment and observation, would be of even greater utility. Unfortunately, risk-prediction instruments in AHF have been largely unsuccessful when attempting to define a cohort of patients safe for early discharge and at low-risk of 30-day mortality and readmission.92,93 More importantly, risk tools have not been implemented in the ED to determine how they augment, or detract from, clinician judgment. As a result, published tools have had little impact on ED disposition decision making. Those patients with high-risk features or significant comorbidities need, and often benefit from, inpatient admission. However, up to 50% of hospital admissions may not be necessary and should be the focus of future research.94,95
Table 6.
Selected and recent AHF risk-stratification studies determining events within 30-days or less of index ED presentation.
| First Author Year |
Study Type* |
N | Predicted Outcome | Variables In Final Model |
|---|---|---|---|---|
|
Lassus155 2013 |
I,P | 441–4,450 (pooled analysis, total n varied by biomarker evaluated) |
30-day and 1-year mortality |
ST2, MR-proADM, CRP, NT-proBNP, BNP, MR- proANP in addition to clinical model (age, gender, blood pressure on admission, estimated glomerular filtration rate <60 mL/min/1.73 m2, sodium and hemoglobin levels, and heart rate) |
|
Stiell156 2013 |
E, P | 559 | 30-day death and 14-day serious non-fatal events |
h/o TIA/CVA, Vital signs, ECG and lab findings |
|
Lee88 2012 |
E, R | 12,591 | 7-day mortality | HR, creatinine, BP, O2 sat, Tn, cancer, home metolazone, EMS transport |
|
Peterson157 2010 |
I, R | 39,783 | In-hospital mortality | Age, SBP, BUN, HR, Na, COPD, nonblack race |
|
Fonarow158 2008 |
I, R | 5,791 | In-hospital mortality, 60 –90 day mortality or rehospitalization |
Pneumonia, cardiac ischemia, worsening renal function |
|
Hsieh10 2008 |
I, R | 8,384 | Inpatient mortality or serious medical complications, 30-day mortality |
pH, pulse, renal function, WBC, glucose, sodium |
|
Abraham159 2008 |
I, R | 48,612 | In-hospital mortality | Age, HR, SBP, Na, creatinine, HF as primary cause for admission, LVSD |
|
Rohde160 2006 |
I, P | 779 | In-hospital mortality | Cancer, SBP, creatinine, BUN, Na, age |
|
Diercks97 2006 |
E, P | 499 | Stay <24 hours in observation and no 30- day adverse cardiac events |
Tn, Systolic BP |
|
Auble99 2005 |
R | 33,533 | Inpatient mortality or serious medical complications, 30-day mortality and AHF readmission |
pH, pulse, renal function, WBC, glucose, sodium |
|
Fonarow161 2005 |
I, R | 65,275 | In-hospital mortality | BUN, Systolic BP, Creatinine |
I = inpatient, E = emergency department patients, R = retrospective, P = prospective BUN= blood urea nitrogen, WBC= white blood cell count, BP= blood pressure, TIA= transient ischemic attack, HR= heart rate, COPD= chronic obstructive pulmonary disease, LVSD= center ventricular systolic dysfunction, CVA= cerebrovascular accident, Tn= troponin, BNP= b-type natriuretic peptide, CRP= C-reactive protein, ANP= atrial natriuretic peptide
Previous studies have defined 30-day AHF readmission rates of 15–20% and mortality rates of less than 1% as “low-risk” characteristics in ED patients.84,95–98 A rule derived and validated in two separate AHF datasets identified a subset of 19.2% of patients who would be considered low-risk for 30-day morbidity and mortality.10,99 However, its additive value on top of clinical judgment has yet to be prospectively tested. An evaluation of four AHF prediction rules suggests they would have limited utility to help emergency providers identify patients safe for discharge.100
In a Canadian study of over 12,000 patients, a prediction rule for 7-day mortality was derived and validated. Their model (Table 7) suggests several ED variables can be used to categorize patients at low-risk of 7-day mortality. However, this study had several limitations including retrospective patient identification, excluding early readmission for AHF as an outcome in the model, a lack of prospective testing compared to clinician judgment, and a practice environment not reflective of the United States.88
Table 7.
Components of the Emergency HF Mortality Risk Grade (calculation available online at www.annals.org).88
|
|
Current Challenges in ED Disposition Decisions
What remains elusive from the ED standpoint is defining who, despite a reassuring clinical profile, is at risk for early readmission, cardiovascular events, or death after discharge home. Compared to a group with worsening renal and cardiac biomarker profiles, as well as an increased risk of 180-day mortality, those with more reassuring biomarker profiles and a decreased risk of mortality unfortunately have a similar risk for readmission.56,101
Multidimensional criteria, such as McKesson Interqual102 or Milliman Care Guidelines,103 are widely used by hospital utilization management to guide and justify level of care (e.g discharge, observation, inpatient floor, or ICU). Their purpose is to help healthcare organizations assess the clinical appropriateness and safety of patient services across the continuum of care, whether prospectively, concurrently, or retrospectively. While they may serve as a useful tool for real-time risk stratification of AHF patients, the small amount of evidence evaluating the accuracy of such criteria in determining appropriate ED disposition for AHF suggests they are limited.104
Among patients stable for discharge, early follow-up is critical and has been associated 105 with a decreased risk of readmission. Collaborative post discharge follow-up involving cardiology and primary care has also been associated with better guideline adherence and lower mortality.106 Further, disease management programs providing close monitoring and follow-up have lowered readmission rates.107 Connecting low-risk patients to these programs represents an opportunity that should be systematically evaluated. Unfortunately, assurance of follow-up post-ED care is challenging and even the best risk prediction tool may not be able to influence physician or patient behavior and facilitate discharge after initial management in the ED.8,84
Beyond Physiologic Risk Predictors
Social, behavioral, and environmental factors strongly influence one’s ability to implement the lifestyle changes required to optimally manage a chronic illness.108,109 Furthermore, patient self-care, and implementing strategies to overcome barriers to successful self-care, are associated with optimal outpatient management and reduced readmissions.110–114 Unfortunately, emergency physicians have largely avoided the complex social, environmental, and behavioral aspects of chronic HF care. Attempts to address these issues in the ED are often thwarted by the lack of the necessary resources or time. Factors unrelated to acute treatment (e.g. health literacy and numeracy, disease knowledge, symptom monitoring, transportation) result in patients being admitted for further management of AHF, despite objective evidence of stability. Combining future risk prediction instruments with clinicians’ judgment as well as obtaining and addressing patients’ self-care barriers may provide the tools necessary to change physicians’ practice patterns.85
Recommendations
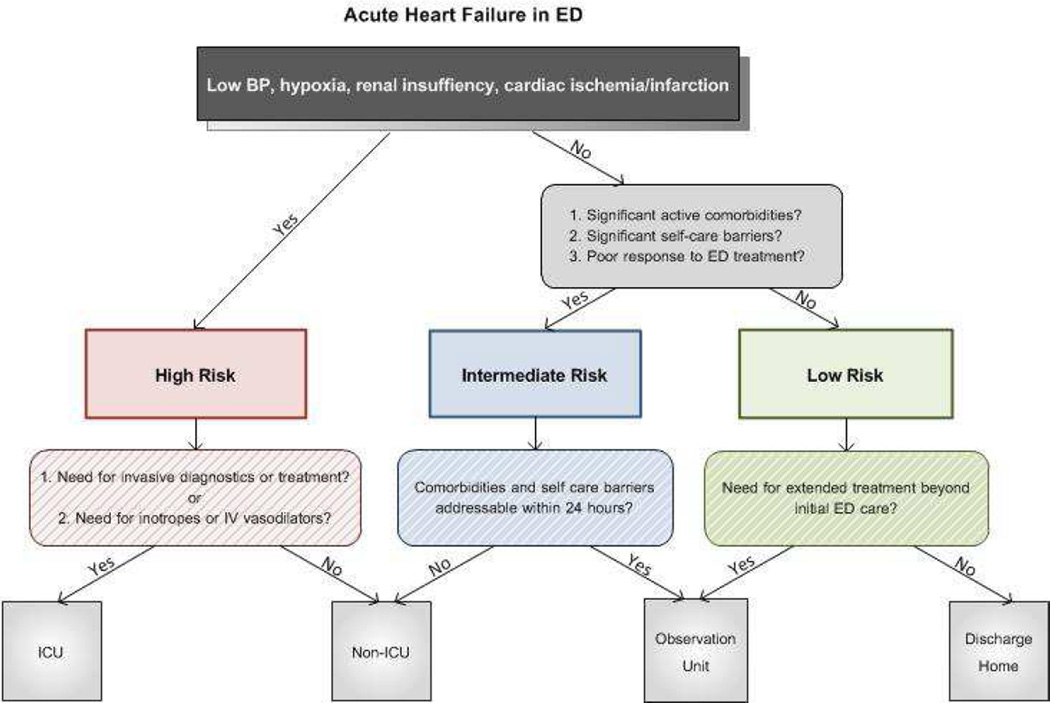
To augment clinical judgment when making disposition decisions, we suggest ED patients with AHF be divided into 3 broad categories (Figure 1). Those at high-risk for mortality or serious adverse events should be admitted to the hospital, with an ICU admission needed in those who require invasive monitoring, ventilator support or other ICU level treatment. Those without high-risk features should be further risk-stratified based on active comorbidities, response to initial therapy, and their ability to manage their illness as an outpatient. Those with active comorbidities or significant self-care barriers may be better suited for inpatient management. Those without active comorbidities who have an incomplete response to initial therapy may be ideal candidates for an observation unit (OU). Candidates for ED discharge are those with adequate response to ED therapy and no high-risk markers, significant comorbidities, or self-care barriers.
Figure 1.
A conceptual model of AHF risk stratification in the ED based upon known predictors of risk for mortality or serious adverse events, presence of absence of comorbidities, and self-care issues. Such an algorithm may augment clinical judgment in disposition decisions.
5. Observation Units and Observation Services- their role in ED Patients with AHF
The pathway from ED to inpatient for AHF patients may not be the best use of resources, as relatively few require intensive acute care, mechanical ventilation, mechanical or pharmacologic circulatory support, or undergo invasive diagnostic or therapeutic interventions.115,116 The majority of AHF patients require intravenous diuresis in a monitored setting until symptoms subjectively improve. These patients, especially if they do not require a prolonged hospital stay, may best be ideal candidates for observation services.
Observation Services
Observation is a billing status indicating a patient is still an outpatient, although they may be receiving care in a hospital or ED-based setting. The rules governing observation status, as generated by the Center for Medicare and Medicaid Services (CMS) and generally followed by private payers, have undergone many changes since their inception in the 1990s. However, CMS has been consistent in that observation services should not span more than 48 hours, and generally should necessitate 24 hours or less. Despite this, the mean length of stay for adult general medical patients in observation status is 41.1 hours, with 26% of stays lasting greater than 48 hours.117
Observation status is independent of the actual location of care delivery. The most common, and likely least efficient approach involves intermixing observation patients amongst the general medical population. 118 While this approach requires the least in terms of infrastructure and resources, it frequently results in lengths of stay beyond the typical 24 to 48 hours. Generally, patients appropriate for observation care will be less ill; they will require less intensive monitoring than higher acuity inpatients on the same service or hospital unit. A more efficient model is to geographically cluster observation patients in a unit that is designed explicitly to deliver care to the short stay population by inpatient specialists,119 hospitalists,120,121 or emergency providers (representing 56% of all dedicated OUs).118 This placement may facilitated the goals of providing frequent reassessment, adjustment of treatment needs based on response to therapy, and ready access to adjunctive diagnostic studies so the decision to discharge or admit the patient can be made within 24 hours of presentation.
AHF and Observation Care
Many patients with AHF may be ideal for management in an observation setting. Frequent reassessment, diuresis, afterload reduction, and targeted patient education can be accomplished in a relatively short time. In consultation with cardiology or primary care providers, outpatient medication regimens can be adjusted, and where available, patients can be linked with specialty HF programs upon discharge. While prospective, randomized, controlled trials evaluating this strategy are lacking, preliminary evidence suggests AHF patients managed in an OU setting have similar outcomes and improved resource utilization compared to a risk-matched group of admitted patients.122,123
The Society for Cardiovascular Patient Care (formerly the Society of Chest Pain Centers) published evidence- and consensus-based guidelines for patient selection and management in the observation setting,124 which were later externally validated in an independent data set (Table 8).95 However, the evidence base behind such recommendations is still not robust. As suggested previously, the absence of high risk does not equate to being low risk. Therefore, it may be easier to define who is not appropriate for short stay / OU care. In addition to those measures in Table 7, patients requiring acute critical care measures such as aggressive titration of parenteral vasoactive medications or ongoing non-invasive positive pressure ventilation (NIPPV) after initial stabilization are candidates for a critical care unit, not an OU. Patients with minimally elevated troponin levels may still be candidates for observation management, especially if serial troponin measurements are followed to exclude ACS. However, patients with a mildly elevated troponin are at increased risk for “failing” observation care and requiring inpatient management.97 Likewise, patients with complex psychosocial needs, such as end stage HF or lack of social support necessitating consideration of skilled nursing facility (SNF) placement, frequently need inpatient resources. Conversely, simpler social barriers, such as medication availability, lack of follow up care, or patient education may be addressed in an observation setting.
Table 8.
Recommendations for appropriate candidates for an OU Stay.98
| Clinical Features of Patients Considered for Observation Management | ||
|---|---|---|
| Recommended | Suggested | |
| Blood pressure | SBP > 100 mmHg | SBP > 120 mmHG |
| Respiratory rate | < 32 breaths/min | NR |
| Renal function | BUN < 40 mg/dl | NR |
| Creatinine < 3.0 mg/dl | NR | |
| ACS | No ischemic changes or elevated troponin | NR |
| Natriuretic peptides | NR | BNP<1000; NT-proBNP<5000 |
NR = no recommendation
6. Unanswered Questions and Future Research Agenda
While the need to alter the current course of research related to AHF is widely recognized, there is a lack of clarity on the specifics of a new agenda. 54 For new therapies, does lack of clinical trial success reflect an absence of drug efficacy, suboptimal study design, or both? Indeed many trials may appear unsuccessful due to the heterogeneous nature of the AHF population. Targeting therapy to more specific patient characteristics and avoidance of a one-size-fits all approach is an important alternative to explore. However, it is unclear what agents are best for a given phenotypic variant and precisely when they should be administered during the course of treatment. Evolving data suggest that early interruption of acute pathophysiology may be a critical consideration, but it can take time for certain perturbations to manifest, and intervention before adequate information has been obtained may increase the potential for therapeutic misalignment. The hypothesis that early treatment with effective novel agents may limit organ damage and improve outcomes has gained momentum in recent years but study of this concept is just beginning.56,63,101,125
In addition to therapeutic considerations, there is a need to better define clinically meaningful end-points of acute intervention. Dyspnea resolution remains the most common goal of treatment and is undoubtedly an important patient-centric outcome, but its subjective nature has made standardization challenging. Moreover, dyspnea correlates poorly with in-hospital worsening HF and post-discharge events (e.g., readmission and mortality) making it difficult to rely on as the sole measure of improvement.126–129 Maneuvers that illicit cardiac stress through provocation with position change (i.e., seated to supine) or physical exertion (i.e., walk-tests) may help delineate more subtle effects of AHF on dyspnea and thus serve as more definitive measures of clinical improvement, but have yet to be tested in prospective trials.130 Further, other HF symptoms, such as fatigue and body swelling, may be as meaningful as dyspnea to patients and important patient-centered endpoints to explore.131 The assessment of frailty, especially in older patients, has also recently evolved as an important marker of risk and prognosis and may become a routine part of the pre-discharge “bundle”.132
Testing Novel Approaches to Therapeutic Decision-Making
To address such unanswered questions and help shape the future research agenda for AHF, new approaches to therapeutic decision-making must be developed. To better understand potential confounders and identify the ideal target population, therapeutic trials are increasingly looking towards timing of intervention and targeted phenotypic groups. Understanding the ED has an active role in the initial management and disposition of AHF patients, many of these trials have focused on inclusion of emergency physicians, both as part of trial design and as principal investigators. Highly successful for acute myocardial infarction trials, such a collaborative approach offers promise for early patient identification and more comprehensive recruitment.
While commonly available parameters such as blood pressure and measures of renal function and myocardial injury are currently used to define patient subgroups, there is growing interest in the utility of biomarkers to enhance phenotypic granularity. Although inclusion and exclusion criteria often consider biomarker profiles, targeting a specific therapy to one biomarker has yet to be implemented in a large-scale study. Trials aimed at administration of a specific drug to a biomarker which identifies patients most likely to benefit are under consideration. Quite unique, this approach would represent a further departure from the one-size-fits-all methodology and facilitate truly tailored therapy. Lastly, the use of implantable hemodynamic monitors to assess intracardiac filling pressures and guide outpatient therapy may reduce the burden of AHF on the department, but require more study.133
Testing Novel Approaches to Disposition Decision-Making
With increasing pressure to avoid unnecessary hospitalizations, there is tremendous interest in improving upon the process of patient disposition. While much research has focused on risk stratification for AHF patients with development of pathways for disposition based on presence or absence of clearly defined variables, little attention has been directed towards the determination of when sufficient cardiac unloading has occurred. Improvement in dyspnea is typically used to demonstrate treatment effectiveness, but there is often no uniform means to determine this beyond patient self-report, making it difficult to quantify differences from one patient to another. As noted above, novel implantable hemodynamic monitors or non-invasive hemodynamic tools may allow clinicians to assess change in filling pressures and optimize timing of discharge and early follow-up.134
Basing disposition on the absence of dyspnea post-provocation would be a novel approach to help ensure symptoms have fully resolved. Studies to test this endpoint are clearly needed. However, even after several days of in-hospital treatment, residual dyspnea is common129 and additional measures to evaluate the effect of treatment on myocardial stress would be beneficial. Although NP levels are strongly associated with cardiac dysfunction, studies of NP guided management have yielded mixed results.9,135 Other biomarkers may be useful for this purpose but to date, none have been clearly identified. Finally, alternative endpoints should be explored. Total days alive out of the hospital, or total hospital days, over a 30-day period, may be more useful than 30-day readmission rates when evaluating the impact of a novel diagnostic or therapeutic investigation on AHF care.
Conclusions
With the rising prevalence of HF and the ED serving as the primary entry point for the majority of AHF admissions, emergency providers will continue to play a critical role in AHF management. Early AHF treatment has begun to evolve from largely a diuretic-only strategy to one that also considers blood pressure management as part of the initial approach. Clinical trials must be conducted in the ED in order to improve the evidence base and drive optimal initial therapy for AHF. Should ongoing and future studies suggest early phenotype-driven therapy improves in-hospital and post-discharge outcomes, ED treatment decisions will need to evolve accordingly. The potential impact of future studies which incorporate risk-stratification into ED disposition decisions cannot be underestimated. Predictive instruments that identify a cohort of patients safe for ED discharge, while simultaneously addressing barriers to successful outpatient management, has the potential to significantly impact quality of life and resource expenditures.
Highlights.
ED treatment of AHF has evolved from a diuretic only strategy to one that considers early use of vasodilators
ED AHF management lacks a strong evidence base
Ongoing and future clinical trial collaborations between ED and heart failure physicians should be conducted in the ED to improve this evidence base
Acknowledgments
Sean Collins: Research Support: NIH/NHLBI, Medtronic, Cardiorentis, Abbott Point-of-Care, Novartis, The Medicines Company, Astellas, Intersection Medical, Radiometer Consulting: Trevena, Novartis, Otsuka, Radiometer, The Medicines Company, Medtronic, BRAHMS, Insys, Intersection Medical, Cardioxyl
Alan B. Storrow: Current or Recent Grant Support Abbott Diagnostics, Centers for Medicaid and Medicare Services (CMS), NIH / NHLBI (K12HL1090), UL1TR000445 National Center for Advancing Translational Sciences, Roche Diagnostics; Current Consultant Roche Diagnostics, Novartis Pharmaceuticals Corp, USA, Alere Diagnostics, Trevena, Beckman Coulter
Javed Butler, MD Dr Butler reports research support from the National Institutes of Health, European Union, and Health Resources Service Administration; and is a consultant to Amgen, Bayer, BG Medicine, Cardiocell, Celladon, Gambro, GE Healthcare, Medtronic, Novartis, Ono Pharma, Takeda, Trevena, and Zensun
G. Michael Felker: Consultant for Novartis, Amgen, Trevena, Roche Diagnostics, Singulex, Sorbent Therapeutics, BMS. Research Funding from NIH, Novartis, Amgen, Roche Diagnostics.
Gregory J. Fermann: Research Support:Novartis, Cardiorentis, Trevena, Portola, Radiometer, Medtronic, Siemens, Insys, The Mayday Foundation, Pfizer; Advisory Board: Intersection Medical, Janssen, Insys, The Medicines Company
Gregg C. Fonarow: Consultant for Medtronic, Novartis, Johnson and Johnson
Michael M. Givertz: Scientific Advisory Board: Merck, Janssen, Cardioxyl; Grant support: NHLBI
Brian Hiestand: has a consulting relationship with Motive Medical Intelligence, Insight PD, and Intersection Medical Inc. He has served on advisory boards for Janssen Pharmaceuticals, and has received research funding from The Medicines Company, Sanofi Aventis, Dyax, Radiometer, Cardiorentis, and Novartis.
Judd Hollander: Consultant for Novartis
David Lanfear: consultant for Bayer, Otsuka, Covis, Biocontrol, Janssen
Phillip D. Levy: Consultant: Novartis Pharmaceuticals; Trevena, Inc; Intersection Medical; The Medicines Co; Ostuka; Janssen; Astellas; AstraZeneca; Beckman Coulter; Research Grant/Support: Novartis Pharmaceuticals; Cardiorentis, Inc; Trevena, Inc; Intersection Medical; Mespere; BG Medicine; NIH/NIMHD 5 R01 MD005849-04
Peter S. Pang: consultant for Janssen, Medtronic, Novartis, Trevena, SpringLeafTx, BG Medicine, Cornerstone Therapeutics; Research support Abbott, Alere, NIH
W. Frank Peacock: Research: Abbott, Alere, Banyan, Cardiorentis, Portola, Roche, The Medicine’s Company; Consultant: BG Medicine, Beckman, Boehringer-Ingelheim, Instrument Labs, The Medicine’s Company; Consultant: Alere, Cardiorentis, Janssen; Ownershit Interests: Comprehensive Research Associates LLC, Emergencies in Medicine LLC.
Douglas B. Sawyer: grant support and consultant for Acorda Therapeutics, Inc
John Teerlink” Consulting fees/ research grants: Amgen, Cardio3 Bioscience, Cytokinetics, Novartis, Sorbent Therapeutics, St. Jude, Trevena.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest
Nancy Albert: none
Justin A. Ezekowitz: conflicts available online at www.vigour.ualberta.ca.
Daniel J. Lenihan: none
Contributor Information
Sean Collins, Vanderbilt University, Nashville Veterans Affairs Medical Center, Nashville, TN.
Alan B. Storrow, Vanderbilt University, Nashville, TN.
Nancy Albert, Cleveland Clinic, Cleveland, OH.
Javed Butler, Emory University, Atlanta, GA.
Justin A. Ezekowitz, University of Alberta, Edmonton, Alberta.
G. Michael Felker, Duke University, Durham, NC.
Gregory J. Fermann, University of Cincinnati, Cincinnati, OH.
Gregg C. Fonarow, Ronald Reagan-UCLA Medical Center, Los Angeles, CA.
Michael M. Givertz, Harvard Medical School, Boston, MA.
Brian Hiestand, Wake Forest University, Winston-Salem, NC.
Judd Hollander, Thomas Jefferson University, Philadelphia, PA.
David Lanfear, Wayne State University, Detroit, MI.
Phillip D. Levy, Wayne State University, Detroit, MI.
Peter S. Pang, Northwestern University, Chicago, IL.
W. Frank Peacock, Baylor University, Houston, TX.
Douglas B. Sawyer, Vanderbilt University, Nashville, TN.
John Teerlink, San Francisco Veterans Affairs Medical Center, University of California at San Francisco, San Francisco, CA.
Daniel J. Lenihan, Vanderbilt University, Nashville, TN.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Storrow AB, Jenkins CA, Self WH, et al. The Burden of Acute Heart Failure on US Emergency Departments. JACC Heart failure. 2014 Apr 30; doi: 10.1016/j.jchf.2014.01.006. online before print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 5.Peacock WF, Emerman C, Costanzo MR, Diercks DB, Lopatin M, Fonarow GC. Early vasoactive drugs improve heart failure outcomes. Congest Heart Fail. 2009;15:256–264. doi: 10.1111/j.1751-7133.2009.00112.x. [DOI] [PubMed] [Google Scholar]
- 6.Maisel AS, Peacock WF, McMullin N, et al. Timing of immunoreactive B-type natriuretic peptide levels and treatment delay in acute decompensated heart failure: an ADHERE (Acute Decompensated Heart Failure National Registry) analysis. Journal of the American College of Cardiology. 2008;52:534–540. doi: 10.1016/j.jacc.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Brar S, McAlister FA, Youngson E, Rowe BH. Do outcomes for patients with heart failure vary by emergency department volume? Circ Heart Fail. 2013;6:1147–1154. doi: 10.1161/CIRCHEARTFAILURE.113.000415. [DOI] [PubMed] [Google Scholar]
- 8.Rame JE, Sheffield MA, Dries DL, et al. Outcomes after emergency department discharge with a primary diagnosis of heart failure. American heart journal. 2001;142:714–719. doi: 10.1067/mhj.2001.118473. [DOI] [PubMed] [Google Scholar]
- 9.Singer AJ, Birkhahn RH, Guss D, et al. Rapid Emergency Department Heart Failure Outpatients Trial (REDHOT II): a randomized controlled trial of the effect of serial B-type natriuretic peptide testing on patient management. Circ Heart Fail. 2009;2:287–293. doi: 10.1161/CIRCHEARTFAILURE.108.826685. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh M, Auble TE, Yealy DM. Validation of the Acute Heart Failure Index. Annals of emergency medicine. 2008;51:37–44. doi: 10.1016/j.annemergmed.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 11.Mueller C, Laule-Kilian K, Schindler C, et al. Cost-effectiveness of B-type natriuretic peptide testing in patients with acute dyspnea. Archives of internal medicine. 2006;166:1081–1087. doi: 10.1001/archinte.166.10.1081. [DOI] [PubMed] [Google Scholar]
- 12.Collins S, Storrow AB, Kirk JD, Pang PS, Diercks DB, Gheorghiade M. Beyond pulmonary edema: diagnostic, risk stratification, and treatment challenges of acute heart failure management in the emergency department. Annals of emergency medicine. 2008;51:45–57. doi: 10.1016/j.annemergmed.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Wang CS, FitzGerald JM, Schulzer M, Mak E, Ayas NT. Does this dyspneic patient in the emergency department have congestive heart failure? JAMA. 2005;294:1944–1956. doi: 10.1001/jama.294.15.1944. [DOI] [PubMed] [Google Scholar]
- 14.Wong GC, Ayas NT. Clinical approaches to the diagnosis of acute heart failure. Curr Opin Cardiol. 2007;22:207–213. doi: 10.1097/HCO.0b013e3280d357e1. [DOI] [PubMed] [Google Scholar]
- 15.Drazner MH, Rame JE, Stevenson LW, Dries DL. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. The New England journal of medicine. 2001;345:574–581. doi: 10.1056/NEJMoa010641. [DOI] [PubMed] [Google Scholar]
- 16.Drazner MH, Hamilton MA, Fonarow G, Creaser J, Flavell C, Stevenson LW. Relationship between right and left-sided filling pressures in 1000 patients with advanced heart failure. J Heart Lung Transplant. 1999;18:1126–1132. doi: 10.1016/s1053-2498(99)00070-4. [DOI] [PubMed] [Google Scholar]
- 17.Collins SP, Lindsell CJ, Peacock WF, et al. The combined utility of an S3 heart sound and B-type natriuretic peptide levels in emergency department patients with dyspnea. Journal of cardiac failure. 2006;12:286–292. doi: 10.1016/j.cardfail.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 18.Collins SP, Lindsell CJ, Peacock WF, Hedger VD, Storrow AB. The Effect of Treatment on the Presence of Abnormal Heart Sounds in Emergency Department Patients with Heart Failure. American Journal of Emergency Medicine. 2005;24:25–32. doi: 10.1016/j.ajem.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Collins SP, Lindsell CJ, Storrow AB, Abraham WT. Prevalence of negative chest radiography results in the emergency department patient with decompensated heart failure. Annals of emergency medicine. 2006;47:13–18. doi: 10.1016/j.annemergmed.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Volpicelli G, Caramello V, Cardinale L, Mussa A, Bar F, Frascisco MF. Bedside ultrasound of the lung for the monitoring of acute decompensated heart failure. Am J Emerg Med. 2008;26:585–591. doi: 10.1016/j.ajem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Chakko S, Woska D, Martinez H, et al. Clinical, radiographic, and hemodynamic correlations in chronic congestive heart failure: conflicting results may lead to inappropriate care. Am J Med. 1991;90:353–359. doi: 10.1016/0002-9343(91)80016-f. [DOI] [PubMed] [Google Scholar]
- 22.Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. The New England journal of medicine. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 23.McCullough PA, Hollander JE, Nowak RM, et al. Uncovering heart failure in patients with a history of pulmonary disease: rationale for the early use of B-type natriuretic peptide in the emergency department. Acad Emerg Med. 2003;10:198–204. doi: 10.1111/j.1553-2712.2003.tb01990.x. [DOI] [PubMed] [Google Scholar]
- 24.Maeda K, Tsutamoto T, Wada A, Hisanaga T, Kinoshita M. Plasma brain natriuretic peptide as a biochemical marker of high left ventricular end-diastolic pressure in patients with symptomatic left ventricular dysfunction. American heart journal. 1998;135:825–832. doi: 10.1016/s0002-8703(98)70041-9. [DOI] [PubMed] [Google Scholar]
- 25.Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. Journal of cardiac failure. 2010;16:e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Karayannis G, Triposkiadis F, Skoularigis J, Georgoulias P, Butler J, Giamouzis G. The emerging role of Galectin-3 and ST2 in heart failure: practical considerations and pitfalls using novel biomarkers. Current heart failure reports. 2013;10:441–449. doi: 10.1007/s11897-013-0169-1. [DOI] [PubMed] [Google Scholar]
- 28.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. Galectin-3, cardiac structure and function, and long-term mortality in patients with acutely decompensated heart failure. Eur J Heart Fail. 2010;12:826–832. doi: 10.1093/eurjhf/hfq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maisel AS, Clopton P, Krishnaswamy P, et al. Impact of age, race, and sex on the ability of B-type natriuretic peptide to aid in the emergency diagnosis of heart failure: results from the Breathing Not Properly (BNP) multinational study. American heart journal. 2004;147:1078–1084. doi: 10.1016/j.ahj.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Pasha SM, Klok FA, van der Bijl N, de Roos A, Kroft LJ, Huisman MV. NT-pro-BNP levels in patients with acute pulmonary embolism are correlated to right but not left ventricular volume and function. Thromb Haemost. 2012;108:367–372. doi: 10.1160/TH11-12-0901. [DOI] [PubMed] [Google Scholar]
- 31.Luchner A, Hengstenberg C, Lowel H, Riegger GA, Schunkert H, Holmer S. Effect of compensated renal dysfunction on approved heart failure markers: direct comparison of brain natriuretic peptide (BNP) and N-terminal pro-BNP. Hypertension. 2005;46:118–123. doi: 10.1161/01.HYP.0000170140.36633.8f. [DOI] [PubMed] [Google Scholar]
- 32.Cavagna L, Caporali R, Klersy C, et al. Comparison of brain natriuretic peptide (BNP) and NT-proBNP in screening for pulmonary arterial hypertension in patients with systemic sclerosis. The Journal of rheumatology. 2010;37:2064–2070. doi: 10.3899/jrheum.090997. [DOI] [PubMed] [Google Scholar]
- 33.Jorge AJ, Freire MD, Ribeiro ML, et al. [Utility of B-type natriuretic peptide measurement in outpatients with heart failure with preserved ejection fraction] Revista portuguesa de cardiologia : orgao oficial da Sociedade Portuguesa de Cardiologia = Portuguese journal of cardiology : an official journal of the Portuguese Society of Cardiology. 2013;32:647–652. doi: 10.1016/j.repc.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 34.Wang TJ, Larson MG, Levy D, et al. Heritability and genetic linkage of plasma natriuretic peptide levels. Circulation. 2003;108:13–16. doi: 10.1161/01.CIR.0000081657.83724.A7. [DOI] [PubMed] [Google Scholar]
- 35.Costello-Boerrigter LC, Boerrigter G, Ameenuddin S, et al. The effect of the brain-type natriuretic peptide single-nucleotide polymorphism rs198389 on test characteristics of common assays. Mayo Clinic proceedings. 2011;86:210–218. doi: 10.4065/mcp.2010.0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maisel A, Mueller C, Adams K, Jr, et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10:824–839. doi: 10.1016/j.ejheart.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 37.McCullough PA, Nowak RM, McCord J, et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002;106:416–422. doi: 10.1161/01.cir.0000025242.79963.4c. [DOI] [PubMed] [Google Scholar]
- 38.Carr BG, Dean AJ, Everett WW, et al. Intensivist bedside ultrasound (INBU) for volume assessment in the intensive care unit: a pilot study. J Trauma. 2007;63:495–500. doi: 10.1097/TA.0b013e31812e51e5. discussion −2. [DOI] [PubMed] [Google Scholar]
- 39.Kimura BJ, Dalugdugan R, Gilcrease GW, 3rd, Phan JN, Showalter BK, Wolfson T. The effect of breathing manner on inferior vena caval diameter. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2011;12:120–123. doi: 10.1093/ejechocard/jeq157. [DOI] [PubMed] [Google Scholar]
- 40.Dean A, Stahmer S. Field JM, editor. Ultrasonography and emergency cardiac care. The textbook of emergency cardiovascular care and CPR: Wolters Kluwer. 2009;129=:48.
- 41.Agricola E, Bove T, Oppizzi M, et al. "Ultrasound comet-tail images": a marker of pulmonary edema: a comparative study with wedge pressure and extravascular lung water. Chest. 2005;127:1690–1695. doi: 10.1378/chest.127.5.1690. [DOI] [PubMed] [Google Scholar]
- 42.Liteplo AS, Marill KA, Villen T, et al. Emergency thoracic ultrasound in the differentiation of the etiology of shortness of breath (ETUDES): sonographic B-lines and N-terminal pro-brain-type natriuretic peptide in diagnosing congestive heart failure. Acad Emerg Med. 2009;16:201–210. doi: 10.1111/j.1553-2712.2008.00347.x. [DOI] [PubMed] [Google Scholar]
- 43.Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest. 2008;134:117–125. doi: 10.1378/chest.07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Volpicelli G, Mussa A, Garofalo G, et al. Bedside lung ultrasound in the assessment of alveolar-interstitial syndrome. Am J Emerg Med. 2006;24:689–696. doi: 10.1016/j.ajem.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 45.Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100:9–15. doi: 10.1097/00000542-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Maines M, Catanzariti D, Angheben C, Valsecchi S, Comisso J, Vergara G. Intrathoracic impedance and ultrasound lung comets in heart failure deterioration monitoring. Pacing and clinical electrophysiology : PACE. 2011;34:968–974. doi: 10.1111/j.1540-8159.2011.03072.x. [DOI] [PubMed] [Google Scholar]
- 47.Mullens W, Oliveira LP, Verga T, Wilkoff BL, Wilson Tang WH. Insights from internet-based remote intrathoracic impedance monitoring as part of a heart failure disease management program. Congest Heart Fail. 2010;16:159–163. doi: 10.1111/j.1751-7133.2010.00149.x. [DOI] [PubMed] [Google Scholar]
- 48.Shochat M, Charach G, Meyler S, et al. Internal thoracic impedance monitoring: a novel method for the preclinical detection of acute heart failure. Cardiovascular revascularization medicine : including molecular interventions. 2006;7:41–45. doi: 10.1016/j.carrev.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Hirschl MM, Kittler H, Woisetschlager C, et al. Simultaneous comparison of thoracic bioimpedance and arterial pulse waveform-derived cardiac output with thermodilution measurement. Critical care medicine. 2000;28:1798–1802. doi: 10.1097/00003246-200006000-00017. [DOI] [PubMed] [Google Scholar]
- 50.Piccoli A, Codognotto M, Cianci V, et al. Differentiation of cardiac and noncardiac dyspnea using bioelectrical impedance vector analysis (BIVA) Journal of cardiac failure. 2012;18:226–232. doi: 10.1016/j.cardfail.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Schlendorf KH, Russell SD. New diagnostic devices in heart failure. Curr Opin Cardiol. 2010;25:262–267. doi: 10.1097/HCO.0b013e3283387962. [DOI] [PubMed] [Google Scholar]
- 52.Neuenschwander JF, Hiestand BC, Peacock WF, et al. A pilot study of implantable cardiac device interrogation by emergency department personnel. Critical pathways in cardiology. 2014;13:6–8. doi: 10.1097/HPC.0000000000000000. [DOI] [PubMed] [Google Scholar]
- 53.Nowak R, DiSomma S, Nanayakkara P, et al. Soc for Acad Emerg Med. Dallas, TX: 2014. The Noninvasive Hemodynamic Phenotyping of Patients Presenting to the Emergency Department with Acute Heart Failure: Prognostic and Therapeutic Implications. 2014. [Google Scholar]
- 54.Givertz MM, Teerlink JR, Albert NM, et al. Acute decompensated heart failure: update on new and emerging evidence and directions for future research. Journal of cardiac failure. 2013;19:371–389. doi: 10.1016/j.cardfail.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 55.Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. The New England journal of medicine. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teerlink JR, Cotter G, Davison BA, et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 57.Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. The New England journal of medicine. 2012;367:2296–2304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 59.Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J. Noninvasive ventilation in acute cardiogenic pulmonary edema. The New England journal of medicine. 2008;359:142–151. doi: 10.1056/NEJMoa0707992. [DOI] [PubMed] [Google Scholar]
- 60.O'Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. The New England journal of medicine. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 61.Ramirez A, Abelmann WH. Cardiac decompensation. The New England journal of medicine. 1974;290:499–501. doi: 10.1056/NEJM197402282900906. [DOI] [PubMed] [Google Scholar]
- 62.Gheorghiade M, Braunwald E. A proposed model for initial assessment and management of acute heart failure syndromes. JAMA. 2011;305:1702–1703. doi: 10.1001/jama.2011.515. [DOI] [PubMed] [Google Scholar]
- 63.Peacock WF, Chandra A, Char D, et al. Clevidipine in acute heart failure: Results of the A Study of Blood Pressure Control in Acute Heart Failure-A Pilot Study (PRONTO) American heart journal. 2014;167:529–536. doi: 10.1016/j.ahj.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 64.Cotter G, Felker GM, Adams KF, Milo-Cotter O, O'Connor CM. The pathophysiology of acute heart failure--is it all about fluid accumulation? American heart journal. 2008;155:9–18. doi: 10.1016/j.ahj.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 65.Cotter G, Metra M, Milo-Cotter O, Dittrich HC, Gheorghiade M. Fluid overload in acute heart failure--re-distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail. 2008;10:165–169. doi: 10.1016/j.ejheart.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 66.Levy P, Compton S, Welch R, et al. Treatment of severe decompensated heart failure with high-dose intravenous nitroglycerin: a feasibility and outcome analysis. Annals of emergency medicine. 2007;50:144–152. doi: 10.1016/j.annemergmed.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 67.Cotter G, Metzkor E, Kaluski E, et al. Randomised trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary oedema. Lancet. 1998;351:389–393. doi: 10.1016/S0140-6736(97)08417-1. [DOI] [PubMed] [Google Scholar]
- 68.Gheorghiade M, Abraham WT, Albert NM, et al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296:2217–2226. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 69.Adams KF, Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) American heart journal. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 70.Fallick C, Sobotka PA, Dunlap ME. Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circ Heart Fail. 2011;4:669–675. doi: 10.1161/CIRCHEARTFAILURE.111.961789. [DOI] [PubMed] [Google Scholar]
- 71.Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. American heart journal. 2007;154:260–266. doi: 10.1016/j.ahj.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 72.Solomon SD, Dobson J, Pocock S, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–1487. doi: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 73.Cuffe MS, Califf RM, Adams KF, Jr, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. Jama. 2002;287:1541–1547. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 74.Konstam MA, Gheorghiade M, Burnett JC, Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297:1319–1331. doi: 10.1001/jama.297.12.1319. [DOI] [PubMed] [Google Scholar]
- 75.Gheorghiade M, Gattis WA, O'Connor CM, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA. 2004;291:1963–1971. doi: 10.1001/jama.291.16.1963. [DOI] [PubMed] [Google Scholar]
- 76.Mebazaa A, Nieminen MS, Packer M, et al. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA. 2007;297:1883–1891. doi: 10.1001/jama.297.17.1883. [DOI] [PubMed] [Google Scholar]
- 77.Gheorghiade M, Bohm M, Greene SJ, et al. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA. 2013;309:1125–1135. doi: 10.1001/jama.2013.1954. [DOI] [PubMed] [Google Scholar]
- 78.Massie BM, O'Connor CM, Metra M, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. The New England journal of medicine. 2010;363:1419–1428. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- 79.McMurray JJ, Teerlink JR, Cotter G, et al. Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials. JAMA. 2007;298:2009–2019. doi: 10.1001/jama.298.17.2009. [DOI] [PubMed] [Google Scholar]
- 80.Wakai A, McCabe A, Kidney R, et al. Nitrates for acute heart failure syndromes. Cochrane Database Syst Rev. 2013;8:CD005151. doi: 10.1002/14651858.CD005151.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen HH, Anstrom KJ, Givertz MM, et al. Low-Dose Dopamine or Low-Dose Nesiritide in Acute Heart Failure With Renal Dysfunction: The ROSE Acute Heart Failure Randomized Trial. JAMA. 2013 doi: 10.1001/jama.2013.282190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schuur JD, Venkatesh AK. The growing role of emergency departments in hospital admissions. The New England journal of medicine. 2012;367:391–393. doi: 10.1056/NEJMp1204431. [DOI] [PubMed] [Google Scholar]
- 83.NCHS. Health, United States, 2012: With a special feature on emergency care. Hyattsville, MD: 2013. [PubMed] [Google Scholar]
- 84.McCausland JB, Machi MS, Yealy DM. Emergency physicians' risk attitudes in acute decompensated heart failure patients. Acad Emerg Med. 2010;17:108–110. doi: 10.1111/j.1553-2712.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 85.Collins SP, Storrow AB. Moving Toward Comprehensive Acute Heart Failure Risk Assessment in the Emergency Department: The Importance of Self-Care and Shared Decision Making. JACC Heart failure. 2013;1:273–280. doi: 10.1016/j.jchf.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ezekowitz JA, Bakal JA, Kaul P, Westerhout CM, Armstrong PW. Acute heart failure in the emergency department: short and long-term outcomes of elderly patients with heart failure. Eur J Heart Fail. 2008;10:308–314. doi: 10.1016/j.ejheart.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 87.Lee DS, Schull MJ, Alter DA, et al. Early deaths in patients with heart failure discharged from the emergency department: a population-based analysis. Circ Heart Fail. 2010;3:228–235. doi: 10.1161/CIRCHEARTFAILURE.109.885285. [DOI] [PubMed] [Google Scholar]
- 88.Lee DS, Stitt A, Austin PC, et al. Prediction of heart failure mortality in emergent care: a cohort study. Ann Intern Med. 2012;156:767–775. doi: 10.7326/0003-4819-156-11-201206050-00003. [DOI] [PubMed] [Google Scholar]
- 89.Pang PS, Jesse R, Collins SP, Maisel A. Patients with acute heart failure in the emergency department: do they all need to be admitted? Journal of cardiac failure. 2012;18:900–903. doi: 10.1016/j.cardfail.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 90.Weintraub NL, Collins SP, Pang PS, et al. Acute heart failure syndromes: emergency department presentation, treatment, and disposition: current approaches and future aims: a scientific statement from the American Heart Association. Circulation. 2010;122:1975–1996. doi: 10.1161/CIR.0b013e3181f9a223. [DOI] [PubMed] [Google Scholar]
- 91.Peacock WFt, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. The New England journal of medicine. 2008;358:2117–2126. doi: 10.1056/NEJMoa0706824. [DOI] [PubMed] [Google Scholar]
- 92.Collins SP, Lindsell CJ, Jenkins CA, et al. Risk stratification in acute heart failure: rationale and design of the STRATIFY and DECIDE studies. American heart journal. 2012;164:825–834. doi: 10.1016/j.ahj.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Collins SP, Storrow AB. Acute heart failure risk stratification: can we define low risk? Heart Fail Clin. 2009;5:75–83. vii. doi: 10.1016/j.hfc.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 94.Butler J, Hanumanthu S, Chomsky D, Wilson JR. Frequency of low-risk hospital admissions for heart failure. Am J Cardiol. 1998;81:41–44. doi: 10.1016/s0002-9149(97)00851-5. [DOI] [PubMed] [Google Scholar]
- 95.Collins SP, Lindsell CJ, Naftilan AJ, et al. Low-risk acute heart failure patients: external validation of the Society of Chest Pain Center's recommendations. Critical pathways in cardiology. 2009;8:99–103. doi: 10.1097/HPC.0b013e3181b5a534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Collins SP, Peacock WF, Lindsell CJ, et al. S3 detection as a diagnostic and prognostic aid in emergency department patients with acute dyspnea. Annals of emergency medicine. 2009;53:748–757. doi: 10.1016/j.annemergmed.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 97.Diercks DB, Peacock WF, Kirk JD, Weber JE. ED patients with heart failure: identification of an observational unit-appropriate cohort. Am J Emerg Med. 2006;24:319–324. doi: 10.1016/j.ajem.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 98.Peacock WF, Fonarow GC, Ander DS, et al. Society of Chest Pain Centers Recommendations for the evaluation and management of the observation stay acute heart failure patient: a report from the Society of Chest Pain Centers Acute Heart Failure Committee. Critical pathways in cardiology. 2008;7:83–86. doi: 10.1097/01.hpc.0000317706.54479.a4. [DOI] [PubMed] [Google Scholar]
- 99.Auble TE, Hsieh M, Gardner W, et al. A prediction rule to identify low-risk patients with heart failure. Acad Emerg Med. 2005;12:514–521. doi: 10.1197/j.aem.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 100.Auble TE, Hsieh M, McCausland JB, Yealy DM. Comparison of four clinical prediction rules for estimating risk in heart failure. Annals of emergency medicine. 2007;50:127–135. 35, e1–e2. doi: 10.1016/j.annemergmed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 101.Metra M, Cotter G, Davison BA, et al. Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. Journal of the American College of Cardiology. 2013;61:196–206. doi: 10.1016/j.jacc.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 102. [Accessed April 3, 2014];InterQual Actionable Evidence-Based Criteria Portfolio. (at www.mckeeson.com.)
- 103. [Accessed April 3, 2014];Indicia for Utilization Review. (at http://www.careguidelines.com/.)
- 104.Wang H, Robinson RD, Coppola M, et al. The accuracy of interqual criteria in determining the need for observation versus hospitalization in emergency department patients with chronic heart failure. Critical pathways in cardiology. 2013;12:192–196. doi: 10.1097/HPC.0b013e3182a313e1. [DOI] [PubMed] [Google Scholar]
- 105.Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–1722. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 106.Lee DS, Stukel TA, Austin PC, et al. Improved outcomes with early collaborative care of ambulatory heart failure patients discharged from the emergency department. Circulation. 2010;122:1806–1814. doi: 10.1161/CIRCULATIONAHA.110.940262. [DOI] [PubMed] [Google Scholar]
- 107.Takeda A, Taylor SJ, Taylor RS, Khan F, Krum H, Underwood M. Clinical service organisation for heart failure. Cochrane Database Syst Rev. 2012;9:CD002752. doi: 10.1002/14651858.CD002752.pub3. [DOI] [PubMed] [Google Scholar]
- 108.Wu JR, Moser DK, Lennie TA, Burkhart PV. Medication adherence in patients who have heart failure: a review of the literature. The Nursing clinics of North America. 2008;43:133–153. vii–viii. doi: 10.1016/j.cnur.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 109.van der Wal MH, Jaarsma T. Adherence in heart failure in the elderly: problem and possible solutions. International journal of cardiology. 2008;125:203–208. doi: 10.1016/j.ijcard.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 110.Jovicic A, Holroyd-Leduc JM, Straus SE. Effects of self-management intervention on health outcomes of patients with heart failure: a systematic review of randomized controlled trials. BMC cardiovascular disorders. 2006;6:43. doi: 10.1186/1471-2261-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Barnason S, Zimmerman L, Young L. An integrative review of interventions promoting self-care of patients with heart failure. J Clin Nurs. 2012;21:448–475. doi: 10.1111/j.1365-2702.2011.03907.x. [DOI] [PubMed] [Google Scholar]
- 112.McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. Journal of the American College of Cardiology. 2004;44:810–819. doi: 10.1016/j.jacc.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 113.Riegel B, Carlson B. Facilitators and barriers to heart failure self-care. Patient Educ Couns. 2002;46:287–295. doi: 10.1016/s0738-3991(01)00165-3. [DOI] [PubMed] [Google Scholar]
- 114.Riegel B, Moser DK, Anker SD, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141–1163. doi: 10.1161/CIRCULATIONAHA.109.192628. [DOI] [PubMed] [Google Scholar]
- 115.Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. Journal of the American College of Cardiology. 2007;50:768–777. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 116.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. Journal of the American College of Cardiology. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 117.Sheehy AM, Graf B, Gangireddy S, et al. Hospitalized but not admitted: characteristics of patients with "observation status" at an academic medical center. JAMA internal medicine. 2013;173:1991–1998. doi: 10.1001/jamainternmed.2013.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wiler JL, Ross MA, Ginde AA. National study of emergency department observation services. Acad Emerg Med. 2011;18:959–965. doi: 10.1111/j.1553-2712.2011.01151.x. [DOI] [PubMed] [Google Scholar]
- 119.Somekh NN, Rachko M, Husk G, Friedmann P, Bergmann SR. Differences in diagnostic evaluation and clinical outcomes in the care of patients with chest pain based on admitting service: the benefits of a dedicated chest pain unit. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2008;15:186–192. doi: 10.1016/j.nuclcard.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 120.Leykum LK, Huerta V, Mortensen E. Implementation of a hospitalist-run observation unit and impact on length of stay (LOS): a brief report. J Hosp Med. 2010;5:E2–E5. doi: 10.1002/jhm.642. [DOI] [PubMed] [Google Scholar]
- 121.Lucas BP, Kumapley R, Mba B, et al. A hospitalist-run short-stay unit: features that predict length-of-stay and eventual admission to traditional inpatient services. J Hosp Med. 2009;4:276–284. doi: 10.1002/jhm.386. [DOI] [PubMed] [Google Scholar]
- 122.Schrager J, Wheatley M, Georgiopoulou V, et al. Favorable bed utilization and readmission rates for emergency department observation unit heart failure patients. Acad Emerg Med. 2013;20:554–561. doi: 10.1111/acem.12147. [DOI] [PubMed] [Google Scholar]
- 123.Storrow AB, Collins SP, Lyons MS, Wagoner LE, Gibler WB, Lindsell CJ. Emergency department observation of heart failure: preliminary analysis of safety and cost. Congest Heart Fail. 2005;11:68–72. doi: 10.1111/j.1527-5299.2005.03844.x. [DOI] [PubMed] [Google Scholar]
- 124.Peacock WF, Fonarow GC, Ander DS, et al. Society of Chest Pain Centers recommendations for the evaluation and management of the observation stay acute heart failure patient-parts 1–6. Acute Card Care. 2009;11:3–42. doi: 10.1080/02652040802688690. [DOI] [PubMed] [Google Scholar]
- 125.Teerlink JR, Metra M, Felker GM, et al. Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): a multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet. 2009;373:1429–1439. doi: 10.1016/S0140-6736(09)60622-X. [DOI] [PubMed] [Google Scholar]
- 126.Ezekowitz JA, Hernandez AF, O'Connor CM, et al. Assessment of dyspnea in acute decompensated heart failure: insights from ASCEND-HF (Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure) on the contributions of peak expiratory flow. Journal of the American College of Cardiology. 2012;59:1441–1448. doi: 10.1016/j.jacc.2011.11.061. [DOI] [PubMed] [Google Scholar]
- 127.Pang PS, Konstam MA, Krasa HB, et al. Effects of tolvaptan on dyspnoea relief from the EVEREST trials. European heart journal. 2009;30:2233–2240. doi: 10.1093/eurheartj/ehp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Metra M, O'Connor CM, Davison BA, et al. Early dyspnoea relief in acute heart failure: prevalence, association with mortality, and effect of rolofylline in the PROTECT Study. European heart journal. 2011;32:1519–1534. doi: 10.1093/eurheartj/ehr042. [DOI] [PubMed] [Google Scholar]
- 129.Metra M, Teerlink JR, Felker GM, et al. Dyspnoea and worsening heart failure in patients with acute heart failure: results from the Pre-RELAX-AHF study. Eur J Heart Fail. 2010;12:1130–1139. doi: 10.1093/eurjhf/hfq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pang PS, Cleland JG, Teerlink JR, et al. A proposal to standardize dyspnoea measurement in clinical trials of acute heart failure syndromes: the need for a uniform approach. European heart journal. 2008;29:816–824. doi: 10.1093/eurheartj/ehn048. [DOI] [PubMed] [Google Scholar]
- 131.Redfield M, Butler J, O'Connor CM, et al. ACC. Washington, D.C.: 2014. Reliable Evaluation of Dyspnea in the ROSE AHF trial (RED ROSE) 2014. [Google Scholar]
- 132.Afilalo J, Alexander KP, Mack MJ, et al. Frailty Assessment in the Cardiovascular Care of Older Adults. Journal of the American College of Cardiology. 2014;63:747–762. doi: 10.1016/j.jacc.2013.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Abraham WT, Adamson PB, Bourge RC, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658–666. doi: 10.1016/S0140-6736(11)60101-3. [DOI] [PubMed] [Google Scholar]
- 134.Sharma GV, Woods PA, Lindsey N, et al. Noninvasive monitoring of left ventricular end-diastolic pressure reduces rehospitalization rates in patients hospitalized for heart failure: a randomized controlled trial. Journal of cardiac failure. 2011;17:718–725. doi: 10.1016/j.cardfail.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 135.Maisel A, Hollander JE, Guss D, et al. Primary results of the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT). A multicenter study of B-type natriuretic peptide levels, emergency department decision making, and outcomes in patients presenting with shortness of breath. Journal of the American College of Cardiology. 2004;44:1328–1333. doi: 10.1016/j.jacc.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 136.McCullough PA, Duc P, Omland T, et al. B-type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study. Am J Kidney Dis. 2003;41:571–579. doi: 10.1053/ajkd.2003.50118. [DOI] [PubMed] [Google Scholar]
- 137.Sanford SD, Lichstein KL, Durrence HH, Riedel BW, Taylor DJ, Bush AJ. The influence of age, gender, ethnicity, and insomnia on Epworth sleepiness scores: A normative US population. Sleep Med. 2006 doi: 10.1016/j.sleep.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 138.McCord J, Mundy BJ, Hudson MP, et al. Relationship between obesity and B-type natriuretic peptide levels. Archives of internal medicine. 2004;164:2247–2252. doi: 10.1001/archinte.164.20.2247. [DOI] [PubMed] [Google Scholar]
- 139.Goto K, Arai M, Watanabe A, Hasegawa A, Nakano A, Kurabayashi M. Utility of echocardiography versus BNP level for the prediction of pulmonary arterial pressure in patients with pulmonary arterial hypertension. International heart journal. 2010;51:343–347. doi: 10.1536/ihj.51.343. [DOI] [PubMed] [Google Scholar]
- 140.Logeart D, Lecuyer L, Thabut G, et al. Biomarker-based strategy for screening right ventricular dysfunction in patients with non-massive pulmonary embolism. Intensive Care Med. 2007;33:286–292. doi: 10.1007/s00134-006-0482-1. [DOI] [PubMed] [Google Scholar]
- 141.Hsich EM, Grau-Sepulveda MV, Hernandez AF, et al. Relationship between sex, ejection fraction, and B-type natriuretic peptide levels in patients hospitalized with heart failure and associations with inhospital outcomes: Findings from the Get With The Guideline-Heart Failure Registry. American heart journal. 2013;166:1063–1071. e3. doi: 10.1016/j.ahj.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 142.Krim SR, Vivo RP, Krim NR, et al. Racial/Ethnic differences in B-type natriuretic Peptide levels and their association with care and outcomes among patients hospitalized with heart failure: findings from get with the guidelines-heart failure. JACC Heart failure. 2013;1:345–352. doi: 10.1016/j.jchf.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 143.Anwaruddin S, Lloyd-Jones DM, Baggish A, et al. Renal function, congestive heart failure, and amino-terminal pro-brain natriuretic peptide measurement: results from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) Study. Journal of the American College of Cardiology. 2006;47:91–97. doi: 10.1016/j.jacc.2005.08.051. [DOI] [PubMed] [Google Scholar]
- 144.Hess G, Runkel S, Zdunek D, Hitzler WE. N-terminal pro-brain natriuretic peptide (NT-proBNP) in healthy blood donors and in patients from general practitioners with and without a diagnosis of cardiac disease. Clin Lab. 2005;51:167–172. [PubMed] [Google Scholar]
- 145.Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: The International Collaborative of NT-proBNP Study. European heart journal. 2006;27:330–337. doi: 10.1093/eurheartj/ehi631. [DOI] [PubMed] [Google Scholar]
- 146.Bayes-Genis A, Lloyd-Jones DM, van Kimmenade RR, et al. Effect of body mass index on diagnostic and prognostic usefulness of amino-terminal pro-brain natriuretic peptide in patients with acute dyspnea. Archives of internal medicine. 2007;167:400–407. doi: 10.1001/archinte.167.4.400. [DOI] [PubMed] [Google Scholar]
- 147.Das SR, Drazner MH, Dries DL, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation. 2005;112:2163–2168. doi: 10.1161/CIRCULATIONAHA.105.555573. [DOI] [PubMed] [Google Scholar]
- 148.Henzler T, Roeger S, Meyer M, et al. Pulmonary embolism: CT signs and cardiac biomarkers for predicting right ventricular dysfunction. Eur Respir J. 2012;39:919–926. doi: 10.1183/09031936.00088711. [DOI] [PubMed] [Google Scholar]
- 149.Li ZF, Zhou DX, Wang QB, Pan WZ, Zhang L, Ge JB. Plasma N-terminal pro-brain natriuretic peptide levels are positively correlated with pulmonary arterial pressure in atrial septal defect patients. Regul Pept. 2013;183C:13–16. doi: 10.1016/j.regpep.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 150.Chi SY, Kim EY, Ban HJ, et al. Plasma N-terminal pro-brain natriuretic peptide: a prognostic marker in patients with chronic obstructive pulmonary disease. Lung. 2012;190:271–276. doi: 10.1007/s00408-011-9363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mauritz GJ, Rizopoulos D, Groepenhoff H, et al. Usefulness of serial N-terminal pro-B-type natriuretic peptide measurements for determining prognosis in patients with pulmonary arterial hypertension. Am J Cardiol. 2011;108:1645–1650. doi: 10.1016/j.amjcard.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 152.Curiati MN, Silvestre OM, Pires LJ, et al. Agreement of BNP and NT-proBNP and the influence of clinical and laboratory variables. Einstein (Sao Paulo) 2013;11:273–277. doi: 10.1590/S1679-45082013000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Choi EY, Bahrami H, Wu CO, et al. N-terminal pro-B-type natriuretic peptide, left ventricular mass, and incident heart failure: Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2012;5:727–734. doi: 10.1161/CIRCHEARTFAILURE.112.968701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Krauser DG, Chen AA, Tung R, Anwaruddin S, Baggish AL, Januzzi JL., Jr Neither race nor gender influences the usefulness of amino-terminal pro-brain natriuretic peptide testing in dyspneic subjects: a ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) substudy. Journal of cardiac failure. 2006;12:452–457. doi: 10.1016/j.cardfail.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 155.Lassus J, Gayat E, Mueller C, et al. Incremental value of biomarkers to clinical variables for mortality prediction in acutely decompensated heart failure: the Multinational Observational Cohort on Acute Heart Failure (MOCA) study. International journal of cardiology. 2013;168:2186–2194. doi: 10.1016/j.ijcard.2013.01.228. [DOI] [PubMed] [Google Scholar]
- 156.Stiell IG, Clement CM, Brison RJ, et al. A risk scoring system to identify emergency department patients with heart failure at high risk for serious adverse events. Acad Emerg Med. 2013;20:17–26. doi: 10.1111/acem.12056. [DOI] [PubMed] [Google Scholar]
- 157.Peterson PN, Rumsfeld JS, Liang L, et al. A validated risk score for in-hospital mortality in patients with heart failure from the American Heart Association get with the guidelines program. Circulation Cardiovascular quality and outcomes. 2010;3:25–32. doi: 10.1161/CIRCOUTCOMES.109.854877. [DOI] [PubMed] [Google Scholar]
- 158.Fonarow GC, Abraham WT, Albert NM, et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Archives of internal medicine. 2008;168:847–854. doi: 10.1001/archinte.168.8.847. [DOI] [PubMed] [Google Scholar]
- 159.Abraham WT, Fonarow GC, Albert NM, et al. Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) Journal of the American College of Cardiology. 2008;52:347–356. doi: 10.1016/j.jacc.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 160.Rohde LE, Goldraich L, Polanczyk CA, et al. A simple clinically based predictive rule for heart failure in-hospital mortality. Journal of cardiac failure. 2006;12:587–593. doi: 10.1016/j.cardfail.2006.06.475. [DOI] [PubMed] [Google Scholar]
- 161.Fonarow GC, Adams KF, Jr, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]