Abstract
We previously identified Celsr3, an atypical cadherin, as essential for normal inhibitory circuit formation in the inner retina. Its absence during retinal development leads to increases in GABA receptor numbers on ON-bipolar cell terminals and consequent changes in retinal physiology, but does not cause obvious cell spacing or synaptic lamination defects. This study focuses on defining the subset of amacrine cells that express celsr3 during development of the wild type zebrafish retina. We have developed a BAC transgene expressing EGFP under the control of celsr3 promoter, Tg(celsr3:EGFP). Similar to the retinal expression of the endogenous gene, the transgene is expressed in amacrine, ganglion and bipolar, but not horizontal or photoreceptor cells. We transiently expressed the BAC in zebrafish larvae and categorized 104 celsr3 expressing amacrine cells based on their shape, arborization and lamination. Ten different amacrine cell types express Tg(celsr3:EGFP). These include narrow, medium and wide-field types of varicose cells. There are many multistratified cells, including one not previously identified and a few specific types of monostratified amacrine cells. Non-varicose amacrine cells do not express the transgene. We propose that celsr3 expression in varicose amacrine cells is key to this molecule’s function in circuitry formation during retinal development. The BAC transgene we have developed provides a useful tool to study Celsr3 function.
Introduction
The development of neural networks is a complex and delicate process much of which is still not well understood. In particular, the molecular basis for proper circuit development is largely unknown (Zipursky and Sanes 2010, Garrett and Burgess 2011). Proper formation of a neuronal field like the eye requires accurate cell migration, dendritic and axonal targeting, synapse formation and refining, and maintenance of the system. Various cadherin family members are thought to play roles in all of these processes.
In the inner retina, amacrine cells, the main class of inhibitory interneurons, form complex connections with bipolar and ganglion cells (Masland 2012). Anatomical studies have identified many amacrine subtypes and physiological studies have revealed some of the mechanisms of inhibitory modulation. Each of the subtypes is thought to be responsible for a particular aspect of normal vision and to have a non-random arrangement within the retina. Little is known about the molecular identity of the different amacrine subtypes.
Celsr3 is an atypical 7-pass cadherin receptor. The ectodomain is comprised of multiple cadherin domains, EGF repeats and also laminin A G-type repeats. A seven transmembrane domain connects this with a G-protein binding intracellular signaling domain. Celsr3 is present in both the developing and adult mouse brain (Ying et al. 2009). It has been functionally implicated in proper cell migration and axon targeting in several areas of the nervous system (Tissir et al. 2005, Zhou et al. 2008, Ying et al. 2009, Fenstermaker et al. 2010, Chai et al. 2014). In vitro work suggests that Celsr3 is also involved in dendritic targeting (Shima et al. 2007).
We have previously described a zebrafish mutant (celsr3w65) with a defect in signal processing within the inner retina due to a premature stop codon within the first exon of the celsr3 gene (Lewis et al. 2011). In situ experiments showed that celsr3 is broadly expressed in many amacrine and ganglion cells as well as some bipolar cells in the retina. celsr3 mutants develop a super-normal b-wave in the electroretinogram due to an increase in GABA receptor number on the ON-bipolar cells This specific alteration of GABAergic signaling suggests changes to subsets of the amacrine network in the celsr3 mutant animals. In order to understand the role of celsr3 in circuitry formation, it is necessary to understand which of the many amacrine cell subtypes express celsr3 during the relevant developmental period, 3 – 5 days post fertilization (dpf).
To define the specific subtypes of amacrine cells that express celsr3, we developed a Bacterial Artificial Chromosome (BAC) transgene, Tg(celsr3:EGFP). Using a transient injection strategy, we identified isolated cells that express celsr3 at 3 through 5 dpf and characterized them based on shape, arborization and lamination. celsr3 is expressed in narrow, medium and large amacrine cells, but only in the varicose varieties of these cells. We identified many of the broadly stratified cell classes, but only two monostratified narrow cell types. These data show that celsr3 expression molecularly defines a wide-ranging but distinct subset of varicose amacrine cells, and suggest that changes in the connectivity of these cells account for the circuitry defects in the celsr3 mutant retinas.
Material and Methods
Zebrafish maintenance
Adult fish and larvae were maintained at 28.5°C in reverse-osmosis distilled water reconstituted for fish compatibility by addition of salts and vitamins (Westerfield 1995) on a 10/14 h dark/light cycle. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the IACUC of the University of Washington. Wild type animals used here were all roy−/−.
BAC development and preparation
We used standard BAC recombineering strategies to place EGFP downstream of the celsr3 promoter (Dhaka et al. 2007) (Chatterjee and Lufkin 2011). We used BAC CH73-42M24 obtained from Children’s Hospital Oakland Research Institute (http://bacpac.chori.org/zebrafish71.htm), which is labeled as zH42M24T7 on the Sanger Welcome Trust web site. Briefly, PCR was performed to create a fragment containing EGFP and the kanamycin resistance gene flanked by LoxP sites. Engineered on either side of this construct were domains containing homology to celsr3 exon1 in the BAC. This was done using primers forward (GGATTTCACTAGACTAATGGTGAGCAAGGGCGAGGAG) and reverse (CTTGCTCACCATTAGTCTAGTGAAATCCTTTCTCTCTC). Bacteria containing the BAC were electroporated with the fragment and then recombination was induced and selected for by kanamycin resistance. Colonies that were kanamycin resistant were grown and prepped using an Invitrogen Midi prep kit according to manufacturer instructions, except that DNA was pipetted a minimum number of times to avoid shearing. Colonies were checked by PCR and sequencing. After EGFP insertion was confirmed, the DNA was treated with Cre recombinase to remove the kanamycin resistance gene. The final DNA product was then re-electroporated into Bacteria.
BAC injections
Injections of the BAC into zebrafish eggs were done using a fresh DNA solution (no more than 3 weeks old) at 30μM/ml. We injected approximately 1000 eggs to obtain approximately 30 – 40 fish with an average of 3 completely isolated amacrine cells/fish. Only isolated cells were analyzed by microscopy.
Cryosections and Confocal microscopy
For live imaging, larvae were treated with 0.003% 1-phenyl-2-thiourea (PTU) in EM at ~24 hours post fertilization to prevent melanization (Westerfield 1995). At the desired age, larvae were anaesthetized in Tricaine (Sigma) and mounted in warm 0.5–1% low mount agarose. Embedded larvae were covered in EM containing PTU and Tricaine and imaged on an Olympus FV1000 using a 40X or 60X water immersion objective. To obtain cryosections, fish were grown to the indicated age in days, euthanized by immersion in ice, and then fixed in 4% paraformaldehyde (1xPBS, 3% sucrose) for 2hrs at room temperature (rt) or overnight (o/n) at 4 °C. Fixed fish were washed 1x in PBS and then immersed in 30% sucrose (1xPBS) o/n at 4 °C. Fish were incubated in 50% OCT, 15% sucrose for 30min at rt then frozen in 100% OCT on dry ice. 40μM slices were cut using a Leica cm1850 cryostat. Slides were mounted in Vectashield (Vector Laboratories), and imaged using a 60 X objective (N.A. 1.35) and an Olympus FV1000 scanning confocal microscope. Z-stacks were taken with a step size of 0.5 μM. 3D images were prepared using the Image J software. Isolated individual cells were located in each image, and then rendered in 3 dimensions. Length measurements were done using the image J line tool at two different angles across the arbor of the cell.
Determining IPL Boundaries
In live images, other nearby cells and the position of cell somas were used to visualize the boundries of the IPL. Monostratified cells that laminated in the outer layers of the IPL generally had adjacently positioned somas. In slides, the edges of the IPL were also seen by auto-fluorescence. Sublaminae were assigned by visually dividing the IPL into sections.
Results
Developmental expression of Tg(celsr3:EGFP)
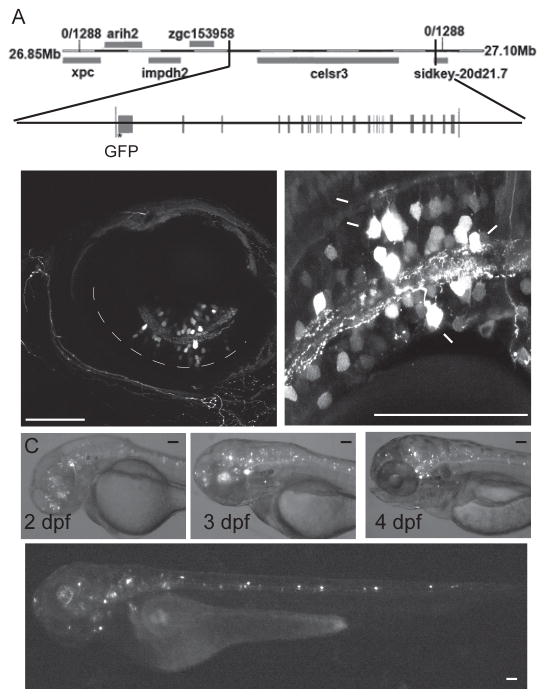
We constructed a BAC expression system to result in EGFP expression under the celsr3 promoter. Our initial attempts to isolate celsr3 promoter fragments suggested that proper expression required regulatory sequences located outside of the immediate region upstream of the gene. In order to drive fluorescent gene expression in cells that normally express celsr3 we used a BAC that contains 15.5kb of sequence upstream of the start of celsr3 as well as the entire gene, all 39 exons and introns (fig 1A). We reasoned that this BAC likely contained the promoter as well as other important regulatory sequences possibly within introns that are required for proper celsr3 expression.
Fig 1.
The BAC - Tg (celsr3:EGFP) is expressed in the eye and brain. A) Diagram of the genetic region of celsr3, and the portion of the genome that is included in the BAC and the exon/intron structure of celsr3. EGFP was inserted at the start codon of the exon 1 of celsr3. B) Tg (celsr3:EGFP) is expressed in the amacrine (AC), bipolar (BC) and ganglion cells (GC), but not in the horizontal or photoreceptor cells. Each image is a different transiently injected animal. C) The BAC is expressed in the eye, brain and spine starting at 2 dpf. These images show the same transiently injected fish over time. D) The BAC is expressed in neurons throughout the brain and spinal cord. Scale bars are 50 μm.
Using recombination in bacteria mediated by homologous flanking sequences (Dhaka et al. 2007, Chatterjee and Lufkin 2011), we inserted a PCR product containing the EGFP gene with its stop codon and a kanamycin resistance gene flanked by loxP sites at the start site of the celsr3 gene. After successful insertion, the kanamycin resistance gene was removed through Cre recombination. The final BAC construct is diagrammed in fig 1A.
We injected this construct into wild type zebrafish embryos at the single cell stage. For optimal results, we found that the transgenic BAC construct needed to be freshly prepared and then used within a very narrow concentration range. Using these optimized injection conditions, we obtained up to 30% of the expected number of EGFP positive cells, as judged from our previous in situ experiments (Lewis et al. 2011). By labeling cells in a mosaic fashion, we were able to identify individual cells that expressed the transgene. The injected fish showed no morphological defects even at the highest levels of BAC expression and their OKR visual behavioral responses were normal (data not shown).
Tg(celsr3:EGFP) expression correlates with native Celsr3 expression
Our previous detailed analysis of celsr3 mRNA expression within the retina showed that expression started at 2 dpf and was restricted to bipolar, amacrine and ganglion cells with no apparent expression in the photoreceptor or horizontal cells (Lewis et al. 2011). Expression of Tg(celsr3:EGFP) correlated with these previous results (fig 1B). Transgene expression within the retina began at 2dpf, and increased steadily from 2 – 5dpf. In the present study, we observed EGFP expression in a variety of types of bipolar cells, amacrine cells and ganglion cells throughout the retina, but never detected transgene expression in any horizontal or photoreceptor cells (fig 1B). Thus within the retina, transgene expression appeared to accurately recapitulate the expression of celsr3 mRNA.
We also detected Tg(celsr3:EGFP) expression in the brain and along the spine starting at 1dpf (fig 1C&D). This expression continued through the final time point at 5 dpf. Previous in situ results using whole zebrafish at 5 dpf also indicated that celsr3 mRNA is expressed in the brain, however in those experiments we did not detect celsr3 mRNA in the spine (Lewis et al. 2011). The levels of native celsr3 expression are quite low, and it is possible that our whole animal in-situ was not sensitive enough to visualize transcript expression within the spinal neurons. Another possibility is that the negative regulatory elements controlling spine expression are missing in our BAC construct.
Tg(celsr3:EGFP) directs EGFP expression in a specific subset of amacrine cells
In order to be able to visualize individual Tg(celsr3:EGFP) positive amacrine cells, we injected wild type fish at the one cell stage to generate mosaic animals in which a small number of cells were labeled. Mosaic animals had variable numbers of labeled cells (fig 1B, C&D), and these cells were at varying depths within the retina. Animals were initially visualized live by confocal microscopy. After that they were euthanized, embedded and retinas were cryosectioned at 40 microns to obtain images of cells deeper in the retina. Using this transient injection and imaging strategy, we identified ten distinct amacrine subtypes that express Tg(celsr3:EGFP). We categorized these amacrine subtypes based on arbor size, lamination within the inner plexiform layer (IPL) and dendrite characteristics.
Only cells that could be completely visualized were characterized and counted. Thus, cells had to be visually separable from all of the neighboring cells. Additionally, in the cryosections cells had to be contained completely within a single section. However, both of these requirements created a bias in our sampling against cells with very large arbors, which were more likely to overlap neighboring cells, and to be cut in the sectioning process. Thus, it is possible that the numbers presented here underrepresent the number of wide-field amacrine cells that express celsr3.
Cells that could be completely visualized and isolated from their neighbors were rendered in 3D, and the approximate arbor size was determined by measuring the width of the arbor on two axes. These numbers were averaged to give the approximate radius of the arbor. Arbor sizes were divided into one of three categories, narrow, medium and wide. These three arbor sizes were non-overlapping, and each cell fell into one of the three categories. The average size for a narrow-field arbor in our studies was 13.91 ± 2.6 microns. The medium-field cells averaged 26.6 ± 4.6 microns, and the wide-field cells had a variable range of sizes between 40 and 100 microns. Table 1 and Figure 4 summarize our results.
Table I.
Detailed description of the ten amacrine cell types identified in this study.
| Cell type | N | size | Stratification type | Stratification depth |
|---|---|---|---|---|
| Narrow displaced varicose | 15 | 15.6 ± 2.7 | monostratified | S5/6 |
| Narrow varicose 1 | 26 | 13.9 ± 2.6 | monostratified | S1 |
| Narrow varicose OFF | 8 | 14.4 ± 3.7 | Broadly stratified | S1 to 3 |
| Narrow varicose ON | 6 | 10.2 ± 2.7 | Broadly stratified | S4 to 6 |
| Narrow varicose ON/OFF | 23 | 13.3 ± 5.3 | Broadly stratified | S1/2 to S5/6 |
| Medium varicose 1 | 11 | 23.9 ± 3.0 | monostratified | S1 |
| Medium varicose OFF | 4 | 27.0 ± 6.5 | Broadly stratified | S1 to 3 |
| Medium varicose ON | 6 | 30.4 ± 3.1 | Broadly stratified | S3 to 6 |
| Wide varicose 2 | 3 | 97 ± 24 | monostratified | S2 |
| Wide varicose 3 | 2 | 51.2 ± 10.6 | monostratified | S3/4 |
| Total | 104 |
N= number of cells.
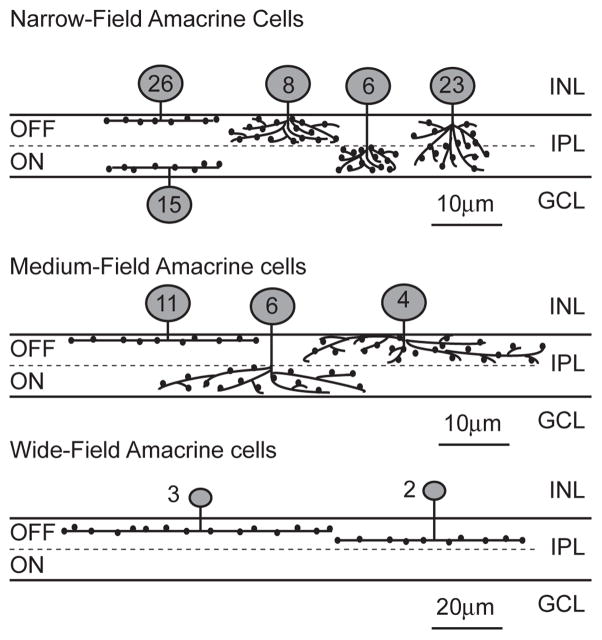
Fig 4.
Diagram of all ten amacrine cell types that were identified in this study. The numbers on the cell body indicate the number of each cell type that was identified in this study.
Narrow-field amacrine cells
Previous characterization of the amacrine population in the 5 dpf zebrafish retina found that 135 out of 163 cells examined were narrow-field amacrine cells (Jusuf and Harris 2009). Approximately 55% of these had varicose (knobby) dendrites with the remainder having smooth dendrites. We found that Tg(celsr3:EGFP) was abundantly expressed in many narrow-field cells (78/104), but only in distinct types and none of these had smooth dendritic arbors (fig 2). All of the cells that expressed Tg(celsr3:EGFP) had varicosities on their dendrites. Two of the previously identified six different types of narrow varicose monostratified cells expressed Tg(celsr3:EGFP). Within the total amacrine cell population, the six types of narrow varicose monostratified cells are approximately equally abundant, and each laminate in one of the six stratifications of the IPL (Jusuf and Harris 2009). We identified only two of these types as expressing Tg (celsr3:EGFP); those laminating in layer 1 (26 cells) and displaced amacrines laminating in layers 5&6 (15 cells) (fig 2A &B).
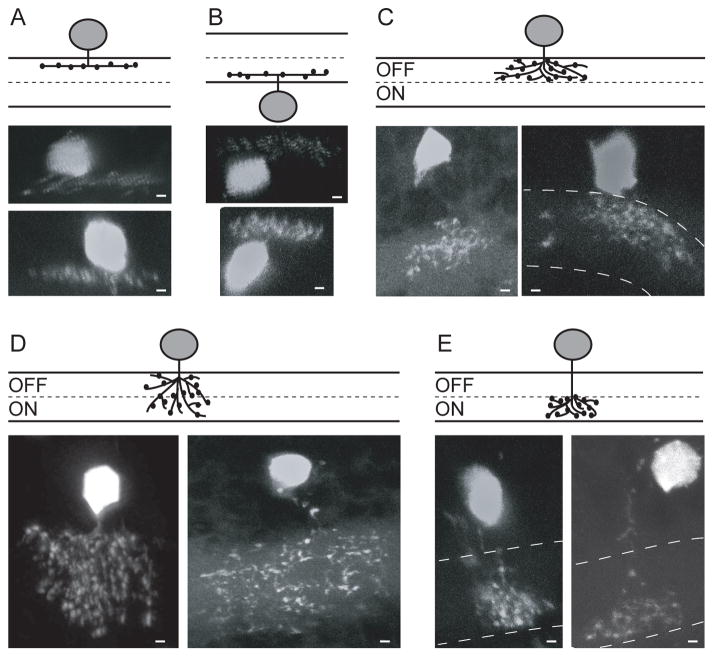
Fig 2.
Five types of narrow-field varicose amacrine cells that expressed Tg (celsr3:EGFP). Two different cells of each type are shown. A) Varicose 1: Mono-stratified cells which stratify in S1. B) Displaced varicose: Displaced mono-stratified cells which stratify in S5 or S6. C) Varicose OFF: Broadly stratified cells which stratify in the OFF layer (S1–3). D) Varicose ON/OFF: Broadly stratified cells which stratify across the IPL (S1–6). E) Varicose ON: Broadly stratified cells which stratify only in the ON layer (S4–6). Scale bars are 1 μm.
We found 8 cells with a broad stratification in the OFF layer (fig 2C), and 23 cells that stratified broadly throughout the IPL (fig 2D). We were not able to easily distinguish broadly stratified spidery from broadly stratified non-spidery cells so these numbers represent both the spidery and non-spidery varieties of these cell types.
We also identified Tg(celsr3:EGFP) expression in 6 narrow-field amacrine cells that were broadly stratified in the ON layer (fig 2E). We found that these 6 cells had slightly smaller arbors than the small varicose OFF cells (10.2 ± 2.7 vs 14.4 ± 3.7 microns).
Medium-field amacrine cells
Tg(celsr3:EGFP) was expressed in all three of the previously identified varicose medium-field amacrine cells (Jusuf and Harris 2009). We identified 11 cells that were monostratified in layer 1 (fig 3A), 6 cells broadly stratified in the ON layer and 4 cells broadly stratified in the OFF layer of the IPL (fig 3B). Similar to previous sampling (Jusuf and Harris 2009), we found about twice as many of the monostratified cells compared to the broadly stratified cells.
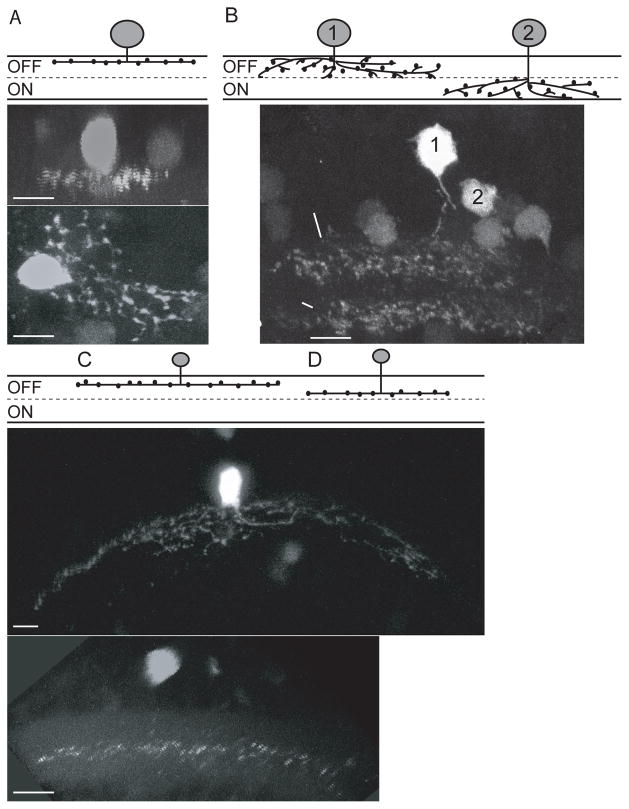
Fig 3.
Types of medium and wide-field varicose amacrine cells that expressed Tg(celsr3:EGFP). A) Medium Varicose 1: Mono-stratified cells which stratify in S1. Two images of the same cell from different angles highlighting the stratification and the distribution of the arbor. B) Medium Varicose OFF and Medium varicose ON: Cell 1 shows a broadly stratified cell with stratification in the OFF layer (S1–3), Cell 2 shows a broadly stratified cell with stratification in the ON layer (S4–6). C) Wide Varicose 2: Mono-stratified cells that stratify in S2. The arbor of this cell is over 100 microns. D) Wide Varicose 3: Mono-stratified in S3/S4 (this class of cells stratifies in either S3 or S4). Scale bars are 5 μm.
Wide-field amacrine cells
There have been 8 types of wide-field cells identified in the 5dpf wild type zebrafish retina and 5 of these types are varicose (Jusuf and Harris 2009). We identified five wide-field cells. Three of these fit the previous classification for wide varicose 2 (fig 3C) and the remaining 2 of our cells appeared similar to wide varicose 3 (fig 3D). Similar to previous work, we found small numbers of wide-field cells of each type, likely due to their relative scarcity within the retina. Interestingly, the wide varicose 2 type cells that we identified had much larger arbors then were previously reported for this subtype; 55.4 ±13.7 microns previously, while our arbors averaged 97±24 microns. One possible reason for this difference in arbor size could be the robustness of EGFP expression. More robust expression under the celsr3 promoter could facilitate identification and imaging of extended dendritic arbors.
Overall, we were able to identify ten different amacrine types (fig 4 and Table 1) representing a distinct subset of the known amacrine cell types. The relative abundance and specificity of these cells indicates a select expression pattern for celsr3 within the young zebrafish retina.
Discussion
The purpose of this study was to identify amacrine subtypes that express the atypical cadherin molecule Celsr3. To this end we created a BAC containing the entire celsr3 locus with an EGFP transgene inserted at the start codon of this gene. We injected this transgenic BAC into single cell embryos, and obtained mosaic animals that contain the transgene in a random subset of their cells. We found that EGFP was only expressed in neurons; in the retina, throughout the brain and along the spinal cord. Within the retina, EGFP expression from the BAC transgene correlated with the pattern of expression that we saw for endogenous celsr3 (Lewis et al. 2011). Similar to previous in situ analysis for endogenous celsr3, Tg(celsr3:EGFP) was expressed in the bipolar, amacrine and ganglion cell layers, but was never found in the horizontal or photoreceptor layers. In addition, celsr3 is broadly expressed in both the mammalian and zebrafish brain (Tissir et al. 2002, Lewis et al. 2011). Thus, the BAC transgene reported here appears to faithfully recapitulate the endogenous expression of celsr3 and represents a useful tool for studying the role of this molecule in retinal development and function.
Amacrine cells in the 5dpf zebrafish have been characterized using a transient expression strategy and the Ptf1A promoter, which labels all amacrine and horizontal cells (Jusuf and Harris 2009). A total of 28 amacrine cell types grouped into three classes of amacrine cells (narrow, medium and wide) were defined. Cells with smooth dendritic arbors represented a significant portion (~40%) of the total. The most distinct property of all the Tg(celsr3:EGFP) positive cells we classified in this report was the identification of only varicose cells. None of the Tg(celsr3:EGFP) positive cells had smooth dendritic arbors. Varicosities in several amacrine subtypes have been shown to contain reciprocal input/output circuits, allowing for the isolation of local circuitry and localized signaling while minimizing “wiring cost.” For example, the wide-field, varicose, A17 amacrine cell of the rat contains hundreds of isolated “microcircuits” that act as gain control on local rod bipolar cells (Grimes et al. 2010). Although, it is not yet known that the varicosities on our cells are playing any similar role, it is striking that all of the amacrine subtypes that we identified using Tg(celsr3:EGFP) had varicosities, indicating that Celsr3 plays a role specifically in a wide variety of varicose amacrine cells.
We characterized a total of 104 amacrine cells (Table 1 and figure 4) expressing Tg(celsr3:EGFP). We identified all three of varieties of medium-field varicose cells, as well as all of the broadly stratified narrow-field varicose cells previously described (Jusuf and Harris 2009). However, for monstratified narrow-field cells, we identified only two of six described types, those that stratify in layer 1 and displaced amacrines that stratify in either layers 5 or 6. Given the abundance of Tg (celsr3:EGFP) expressing monostratified cells identified in our experiments (greater than 15 for each), we postulate that this selective expression of celsr3 represents a molecular differentiation between these two monostratified varieties and the other monostratified narrow-field amacrine cells. Interestingly, the two monostratified cells types that express celsr3 are located at either edge of the IPL, whereas the other monostratified types stratify more centrally within the IPL.
We identified 6 cells narrow-field cells with a broad stratification in the ON-layer (fig 2). It is unclear whether this cell is a newly identified amacrine subtype in zebrafish. Whereas the Ptf1A-driven GFP transgene did not identify this cell type (Jusuf and Harris 2009), the unique transgenic strain Tg(7.2mab21l2:EGFP)ucd2 did express GFP in a cell classified as a Type I ON cell that could be the same (Cederlund et al. 2011). A direct comparison will be necessary to confirm this. In other animals, such as the rabbit, narrow diffuse cells have been found to stratify in either the OFF or ON layers (MacNeil et al. 1999).
In addition to morphological characterization of amacrine cells, antibodies are often used to classify amacrine subtypes that express transgenic reporters. These studies, done in both zebrafish and mammalian systems, use stable transgenic lines and evaluate overlap between antibody staining and transgenic reporter expression (Sarthy et al. 2007, Jusuf and Harris 2009, Cederlund et al. 2011). There is not a stable transgenic zebrafish line expressing Tg(celsr3:EGFP) and in mosaic animals these studies are of little utility. With the recent optimization of homologous recombination strategies in zebrafish (Shin et al. 2014), another strategy to determine gene expression patterns is to knock-in fluorescent proteins into the genomic locus for a gene of interest. These newly developed strains, together with the other strategies such as cell transplantation and live imaging, are powerful approaches to dissect circuit formation in the vertebrate nervous system.
In summary, we have characterized a subset of amacrine cells that express the atypical cadherin celsr3. Our morphological classification of the amacrine cell types expressing celsr3 represents a further step to understanding the role of this molecule in the formation of inhibitory circuits within the inner retina.
Normal inhibitory circuit development in retina requires celsr3.
We made a BAC transgene and categorized amacrine subtypes that express celsr3.
Ten amacrine cell types with varicose (knobby) dendrites express celsr3.
Amacrine cells with smooth dendrites do not express the celsr3 transgene.
Multistratified and a few types of monostratified amacrines express celsr3.
The select expression pattern is likely key to celsr3 function in circuitry formation.
Acknowledgments
This work was funded by NIH grants EY01018814 and EY015165 (to SEB). We thank Dr. Sara Hayden for comments on the manuscript and members of the Dhaka lab (UW, Dept. of Biological Structure) for assistance with BAC recombineering.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cederlund ML, Vendrell V, Morrissey ME, Yin J, Gaora PO, Smyth VA, Higgins DG, Kennedy BN. mab21l2 transgenics reveal novel expression patterns of mab21l1 and mab21l2, and conserved promoter regulation without sequence conservation. Dev Dyn. 2011;240(4):745–754. doi: 10.1002/dvdy.22573. [DOI] [PubMed] [Google Scholar]
- Chai G, Zhou L, Manto M, Helmbacher F, Clotman F, Goffinet AM, Tissir F. Celsr3 is required in motor neurons to steer their axons in the hindlimb. Nat Neurosci. 2014;17(9):1171–1179. doi: 10.1038/nn.3784. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Lufkin T. Fishing for function: zebrafish BAC transgenics for functional genomics. Mol Biosyst. 2011;7(8):2345–2351. doi: 10.1039/c1mb05116d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54(3):371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Fenstermaker AG, Prasad AA, Bechara A, Adolfs Y, Tissir F, Goffinet A, Zou Y, Pasterkamp RJ. Wnt/planar cell polarity signaling controls the anterior-posterior organization of monoaminergic axons in the brainstem. J Neurosci. 2010;30(47):16053–16064. doi: 10.1523/JNEUROSCI.4508-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett AM, Burgess RW. Candidate molecular mechanisms for establishing cell identity in the developing retina. Dev Neurobiol. 2011;71(12):1258–1272. doi: 10.1002/dneu.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes WN, Zhang J, Graydon CW, Kachar B, Diamond JS. Retinal parallel processors: more than 100 independent microcircuits operate within a single interneuron. Neuron. 2010;65(6):873–885. doi: 10.1016/j.neuron.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusuf PR, Harris WA. Ptf1a is expressed transiently in all types of amacrine cells in the embryonic zebrafish retina. Neural Dev. 2009;4:34. doi: 10.1186/1749-8104-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis A, Wilson N, Stearns G, Johnson N, Nelson R, Brockerhoff SE. Celsr3 is required for normal development of GABA circuits in the inner retina. PLoS Genet. 2011;7(8):e1002239. doi: 10.1371/journal.pgen.1002239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH. The shapes and numbers of amacrine cells: matching of photofilled with Golgi-stained cells in the rabbit retina and comparison with other mammalian species. J Comp Neurol. 1999;413(2):305–326. [PubMed] [Google Scholar]
- Masland RH. The tasks of amacrine cells. Vis Neurosci. 2012;29(1):3–9. doi: 10.1017/s0952523811000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy V, Hoshi H, Mills S, Dudley VJ. Characterization of green fluorescent protein-expressing retinal cells in CD 44-transgenic mice. Neuroscience. 2007;144(3):1087–1093. doi: 10.1016/j.neuroscience.2006.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima Y, Kawaguchi SY, Kosaka K, Nakayama M, Hoshino M, Nabeshima Y, Hirano T, Uemura T. Opposing roles in neurite growth control by two seven-pass transmembrane cadherins. Nat Neurosci. 2007;10(8):963–969. doi: 10.1038/nn1933. [DOI] [PubMed] [Google Scholar]
- Shin J, Chen J, Solnica-Krezel L. Efficient homologous recombination-mediated genome engineering in zebrafish using TALE nucleases. Development. 2014;141(19):3807–3818. doi: 10.1242/dev.108019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissir F, Bar I, Jossin Y, De Backer O, Goffinet AM. Protocadherin Celsr3 is crucial in axonal tract development. Nat Neurosci. 2005;8(4):451–457. doi: 10.1038/nn1428. [DOI] [PubMed] [Google Scholar]
- Tissir F, De-Backer O, Goffinet AM, Lambert de Rouvroit C. Developmental expression profiles of Celsr (Flamingo) genes in the mouse. Mech Dev. 2002;112(1–2):157–160. doi: 10.1016/s0925-4773(01)00623-2. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio) Eugene: University of Oregon Press; 1995. [Google Scholar]
- Ying G, Wu S, Hou R, Huang W, Capecchi MR, Wu Q. The protocadherin gene Celsr3 is required for interneuron migration in the mouse forebrain. Mol Cell Biol. 2009;29(11):3045–3061. doi: 10.1128/MCB.00011-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Bar I, Achouri Y, Campbell K, De Backer O, Hebert JM, Jones K, Kessaris N, de Rouvroit CL, O’Leary D, Richardson WD, Goffinet AM, Tissir F. Early forebrain wiring: genetic dissection using conditional Celsr3 mutant mice. Science. 2008;320(5878):946–949. doi: 10.1126/science.1155244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky SL, Sanes JR. Chemoaffinity revisited: dscams, protocadherins, and neural circuit assembly. Cell. 2010;143(3):343–353. doi: 10.1016/j.cell.2010.10.009. [DOI] [PubMed] [Google Scholar]