Abstract
Background & Aims
Adult-to-adult living donors and recipients were studied to characterize patterns of liver growth and identify associated factors in a multicenter study.
Methods
350 donors and 353 recipients in A2ALL (Adult to Adult Living Donor Liver Transplantation Cohort Study) transplanted between March 2003 and February 2010 were included. Potential predictors of 3-month liver volume included total and standard liver volumes (TLV, SLV), the model for end-stage liver disease (MELD) score (in recipients), remnant and graft size, remnant to donor and graft to recipient weight ratio (RDWR, GRWR), remnant/TLV, and graft/SLV.
Results
Among donors, 3-month absolute growth was 676±251g (mean± SD) and percent reconstitution was 80%±13%. Among recipients, GRWR was 1.3%±0.4% (8<0.8%). Graft weight was 60%±13% of SLV. Three-month absolute growth was 549±267g and percent reconstitution was 93%±18%.
Predictors of greater 3-month liver volume included larger patient size (donors, recipients), larger graft volume (recipients), and larger TLV (donors). Donors with the smallest remnant/TLV ratios had larger than expected growth, but also had higher postoperative bilirubin and international normalized ratio at 7 and 30 days. In a combined donor-recipient analysis, donors had smaller 3-month liver volumes than recipients adjusted for patient size, remnant or graft volume, and TLV or SLV (p=0.004). Recipient graft failure in the first 90 days was predicted by poor graft function at day 7 (HR=4.50, p=0.001), but not by GRWR or graft fraction (p>0.90 for each).
Conclusions
Both donors and recipients had rapid yet incomplete restoration of tissue mass in the first 3 months, confirming previous reports. Recipients achieved a greater percentage of expected total volume. Patient size and recipient graft volume significantly influenced 3 month volumes. Importantly, donor liver volume is a critical predictor of the rate of regeneration, and donor remnant fraction impacts post-resection function.
Keywords: graft volume, graft loss, right vs. left lobe, liver growth, weight ratios
Liver regeneration is critical in adult living donor liver transplantation (LDLT), and size considerations affect the selection of appropriate donor and recipient pairs [1, 2]. Single center studies have shown that recipients have rapid liver regeneration but many donors do not regain total liver volume, even after 1 year [3–6]. Portal hemodynamics, vascular outflow, graft to recipient weight ratios (GRWR), humoral factors, and graft quality have all been implicated in affecting liver regeneration. Left lobe donors provide even smaller grafts, making the procedure potentially safer for the donor, but increasing risk for the recipient [7, 8].
A principal aim of the Adult to Adult Living Donor Liver Transplantation Cohort Study (A2ALL) is to characterize liver regeneration and function and their impact on outcomes in both donors and recipients. This is the first multi-center study examining the clinical manifestations of liver regeneration in LDLT and characterizing growth patterns common to donors and recipients using a prospectively defined clinical cohort.
Methods
Data sources
Study population
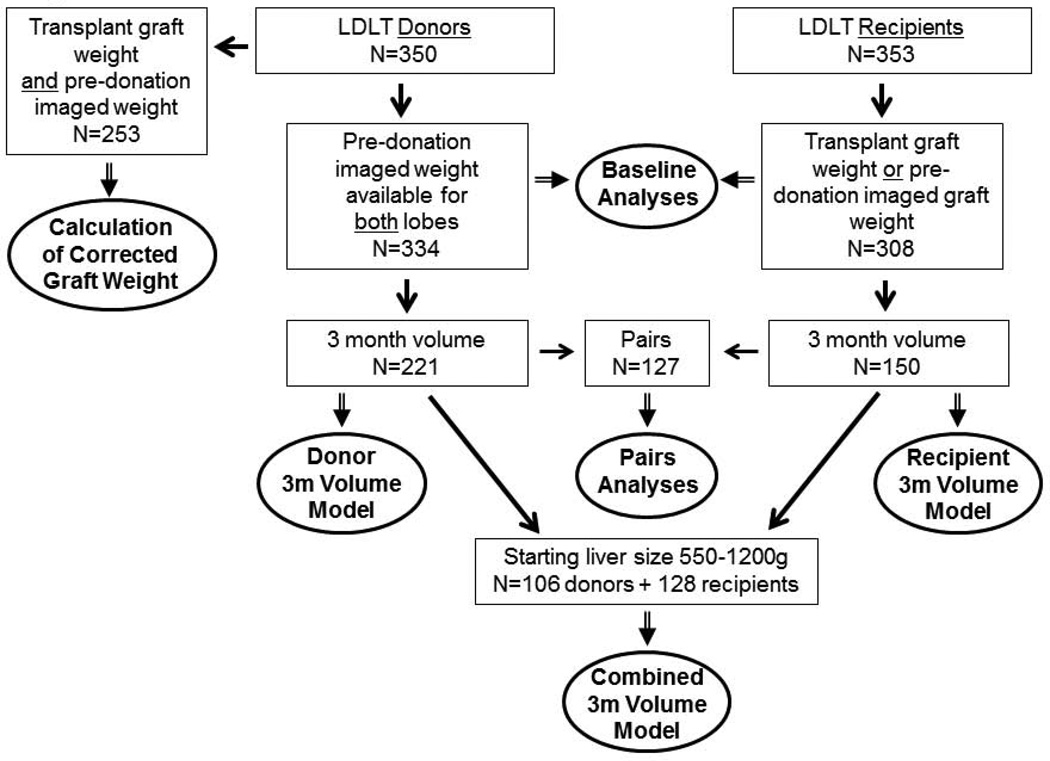
A2ALL enrolled potential living donors and their recipients at nine participating transplant centers. Transplants occurred between March 2003 and February 2010, with follow-up through August 2010. Donors had preoperative volumetric imaging by magnetic resonance imaging (MRI) or computed tomography (CT) to determine total liver volume (TLV) and right and left lobe volumes. Grafts were weighed (or volumes measured by displacement) in the operating room (OR) after removal. Donors and recipients had imaging data at 3 months post- donation/transplant [9]. Volume (cc), as measured on imaging, and weight (g), as measured in the OR, are used interchangeably and adjusted for blood that is drained from the removed lobe. Demographic information, clinical variables, and laboratory values were collected preoperatively and at 3 months following transplantation. Preoperative imaging was available in 334 donors (310 right, 24 left lobes); investigation of remnant regeneration was limited to 221 (211 right, 10 left lobes) who also had graft weight (from intraoperative weight or volume or preoperative imaging), and 3-month imaged volumes. Graft weights were available for 308 recipients (291 right, 17 left lobes). Investigation of graft regeneration included 150 (145 right, 5 left lobes) with 3-month imaged volumes. Of the 158 recipients without 3-month imaging, 24 died or lost their graft within 3 months (6 died [5 right, 1 left lobe], 16 lost their graft [all right lobes], and 2 lost their grafts and died [both right lobes]). There were 127 (122 right, 5 left lobe) donor-recipient pairs where both had complete volumetric data.
Corrected Graft Volumes
In donors, graft volumes estimated by preoperative imaging were higher than intraoperative graft weights, thought to be due primarily to the weight of blood in vivo [6, 8, 10]. Imaged volume exceeded measured weight by a mean of 146g±10.6g (18.6%, p<0.0001). To combine data from intraoperative and imaging measurements, an equation for in vivo graft volume based on intraoperative graft weight was developed for 253 donors who had data from both sources. The corrected graft volume was estimated as 198 + 0.939*graft weight (R2=0.55; Supplementary Figure S1). When the graft weight was not measured in the OR (n=82), the preoperative imaged graft volume was used.
Volume measurements
For donors, remnant volume was calculated by subtracting corrected graft volume from TLV. For recipients, “normal” liver volume was estimated by standard liver volume (SLV=1072.8 × Body Surface Area (BSA) – 345.7, where BSA = (weight [kg])0.425 × (height [cm])0.725 × 0.007184) [9], and liver size at transplant was defined as corrected graft volume. The liver fraction was defined as the percentage of the “normal” whole liver volume that the remnant or graft represented (remnant volume/TLV for donors; corrected graft weight/SLV for recipients). The GRWR was calculated from corrected graft weight in the OR and preoperative recipient weight. The remnant to donor weight ratio (RDWR) was calculated similarly for donors.
Outcome measures and regeneration parameters
Imaged 3-month liver volume was the primary outcome measure, and three additional measures of regeneration were calculated: 1) Absolute volume increase in cc was defined as the difference between the 3-month imaged volume and the graft or remnant volume; 2) Percent volume increase was the percentage increase of liver volume from time of transplant or resection to 3 months post-LT; and 3) Percent reconstitution in cc was defined as the percentage of the normal whole liver volume (TLV for donors; SLV for recipients) achieved by 3 months.
We chose the 3-month liver volume as the main outcome of interest because its measurement does not directly depend on remnant/graft volume. Two of the other outcomes (absolute and percent volume increase) use remnant/graft volume in their calculation, thus preventing the latter from being a proper independent variable in statistical models of these outcomes.
Early allograft dysfunction and small for size syndrome (SFSS) were defined by the presence of jaundice (bilirubin>10 on day 7) or coagulopathy (international normalized ratio [INR]>1.6 on day 7), without technical complications as modified from previous definitions (10–12).
Human subjects protection
The study was approved by the Institutional Review Boards and Privacy Boards of the University of Michigan Data Coordinating Center and each of the nine participating transplant centers. All subjects provided written informed consent. No donor organs were obtained from executed prisoners or other institutionalized persons.
Statistical analyses
Correlation coefficients were used to assess relationships among graft and remnant fractions, measures of regeneration, and laboratory values. T-tests were used to compare GRWR and liver fraction for recipients with and without poor function at day 7. Linear regression was used to identify predictors of 3-month liver volume separately in donors and recipients, as well as in a combined model. Potential explanatory factors were tested based on significant findings in prior studies [11–17]. For associations in donors, donor sex, age, weight, height, body mass index (BMI), BSA, TLV, remnant lobe type (left or right), remnant volume, RDWR, and remnant liver fraction were tested. For associations in recipients, donor and recipient sex, age, weight, height, BMI, and BSA; graft lobe type (left or right), graft weight, GRWR, liver fraction, and cold ischemia time; and recipient SLV, hepatitis C virus (HCV) diagnosis, and model for end-stage liver disease (MELD) score at transplant were tested.
Analysis of donor and recipient 3-month liver volumes together was restricted to subjects with a range of liver volumes at transplant common to both groups, which was 550–1200g (n=106 donors, n=128 recipients). Variables considered for inclusion were patient type (donor or recipient), lobe type, and variables significant in the separate models (weight, TLV, graft or remnant volume, liver fraction). Statistical interactions between patient type and each of the latter factors were tested.
Logistic regression was used to test for associations between incomplete regeneration (defined as <75% reconstitution of TLV or SLV by 3 months) and 7- and 30-day postoperative albumin, bilirubin, INR, and creatinine. Logistic regression was used to examine the association between poor function at 7 days and 3-month liver volume in recipients adjusted for graft size and patient weight.
We used three sets of Cox regression models to investigate predictors of graft failure (including death). The first set followed patients from transplant, and tested separately whether graft weight, GRWR, or liver fraction predicted graft failure overall or in the first 90 days. The second set followed patients from day 7 after transplant, and tested whether poor function at day 7 predicted subsequent graft failure overall or in the first 90 days. The third set followed patients from day 90 after transplant, and tested separately whether absolute growth, volume reconstitution, or percent volume increase at day 90 predicted subsequent graft failure.
Among the donor/recipient pairs, correlation coefficients were used to assess relationships between paired graft/remnant absolute growth, percentage reconstitution, percent volume increase, and 3-month volume. All analyses were performed using SAS version 9.2 (SAS Institute; Cary, North Carolina, USA).
Results
Figure 1 shows the available study sample for each set of results described below.
Figure 1.
Study population and analysis subsets.
Baseline analyses: Donor and recipient characteristics
A total of 334 donors had TLV measurements (Table 1 and Supplementary Table S1, by lobe). Mean age was 38 years, approximately half were men, and the majority were non-Hispanic white and biologically related to the recipient. Right (n=310) lobe donors differed from left lobe (n=24) in mean graft weight (right lobes, mean 1021±187g; left lobes, 672±146g; p<0.0001) and the donor remnant size in both weight (mean 548±213g after right lobe donation, 982±192g after left lobe donation, p<0.0001) and as a fraction of TLV (34% vs. 59%, p<0.0001). Remnant fraction was less than 35% of TLV for 168 donors (50%); it was less than 25% of TLV for 41 (12%), all right lobe donors.
Table 1.
Characteristics of Donors (N=334) and Recipients (N=308)
| Donors | Recipients | |||||
|---|---|---|---|---|---|---|
| Characteristic | N | Mean (sd) or % |
Range (or IQR†) |
N | Mean (sd) or % |
Range (or IQR†) |
| Age at Donation or Transplant | 334 | 38 (10) | 20 – 63 | 308 | 52 (11) | 18 – 72 |
| Sex | ||||||
| Male | 168 | 50 | 165 | 54 | ||
| Female | 166 | 50 | 143 | 46 | ||
| Ethnicity | ||||||
| Hispanic/Latino | 45 | 13 | 41 | 13 | ||
| Non-Hispanic/Non-Latino | 289 | 87 | 265 | 86 | ||
| Missing | 0 | 0 | 2 | 1 | ||
| Race | ||||||
| White | 308 | 92 | 278 | 91 | ||
| African-American | 10 | 3 | 11 | 4 | ||
| Asian | 5 | 1 | 6 | 2 | ||
| Other | 11 | 4 | 11 | 4 | ||
| Right Lobe Donor or Recipient | 310 | 93 | 289 | 94 | ||
| Height at Evaluation (cm) | 332 | 172 (10) | 135 – 196 | 302 | 171 (11) | 140 – 203 |
| Weight at Evaluation (kg) | 327 | 78 (15) | 47 – 135 | 299 | 78 (17) | 40 – 143 |
| Body Mass Index at Evaluation (kg/m2) | 326 | 26 (4) | 16 – 41 | 297 | 27 (5) | 16 – 48 |
| Total Liver Volume by Imaging at Evaluation (cc) or Calculated Standard Liver Volume at Transplant (cc) | 334 | 1566 (298) | 1353 – 1763† | 288 | 1684 (263) | 1501 – 1855† |
| Graft Weight at Donation (g)‡ | 334 | 987 (204) | 860 – 1108† | 308 | 989 (196) | 860 – 1108† |
| Calculated Remnant Volume at Donation (g) | 334 | 579 (240) | 413 – 722† | -- | -- | -- |
| Remnant or Graft Liver Fraction* | 334 | 36% (11%) | 30% – 42%† | 288 | 60% (13%) | 51% – 67%† |
| Liver Volume at Month 3 (cc) | 221 | 1241 (271) | 1022 – 1401† | 150 | 1542 (304) | 1338 – 1743† |
| Lab Values at Day 7 | ||||||
| Bilirubin (IU/L) | 334 | 1.6 (1.4) | 0.4 – 10.6 | 306 | 5.0 (5.1) | 0.3 – 36.7 |
| Albumin (g/dl) | 280 | 3.2 (0.4) | 1.8 – 4.5 | 268 | 2.8 (0.7) | 1.5 – 5.8 |
| INR | 289 | 1.2 (0.2) | 0.9 – 1.8 | 232 | 1.3 (0.2) | 0.8 – 2.4 |
| Lab Values at Day 30 | ||||||
| Bilirubin (IU/L) | 311 | 0.7 (0.5) | 0.1 – 5.2 | 304 | 2.1 (4.4) | 0.1 – 42.5 |
| Albumin (g/dl) | 295 | 3.8 (0.5) | 2.1 – 5.2 | 277 | 3.2 (1.1) | 1.4 – 18.0 |
| INR | 262 | 1.1 (0.1) | 0.7 – 2.6 | 223 | 1.2 (1.1) | 0.8 – 17.0 |
Interquartile range
Measured graft weight in operating room corrected by blood
Calculated Residual Lobe Volume/Total Liver Volume by Imaging for donors, Graft Weight/Standard Liver Volume for recipients
Mean age of recipients was 52 years, 54% were men, and 86% were non-Hispanic white. Left lobe recipients (n=17) were more often female than right lobe recipients (n=291) (94% vs. 44%, p<0.0001), and therefore also shorter (p<0.0001) and lighter (p=0.0071). Mean graft volume was 989cc, with right lobes averaging 1007cc and left lobes averaging 685cc (Table 1 and Supplementary Table S2, by lobe). Left lobe recipient grafts as a fraction of SLV (48% vs. 61%, p=0.0001) and GRWR (1.1% vs. 1.3%, p=0.005) were smaller than right lobe recipient grafts. Eight (6 right, 2 left) grafts had a GRWR<0.8%, and eleven (9 right, 2 left) had a liver fraction less than 40% (7 grafts met both criteria).
Donor and recipient 3-month liver volumes and growth parameters
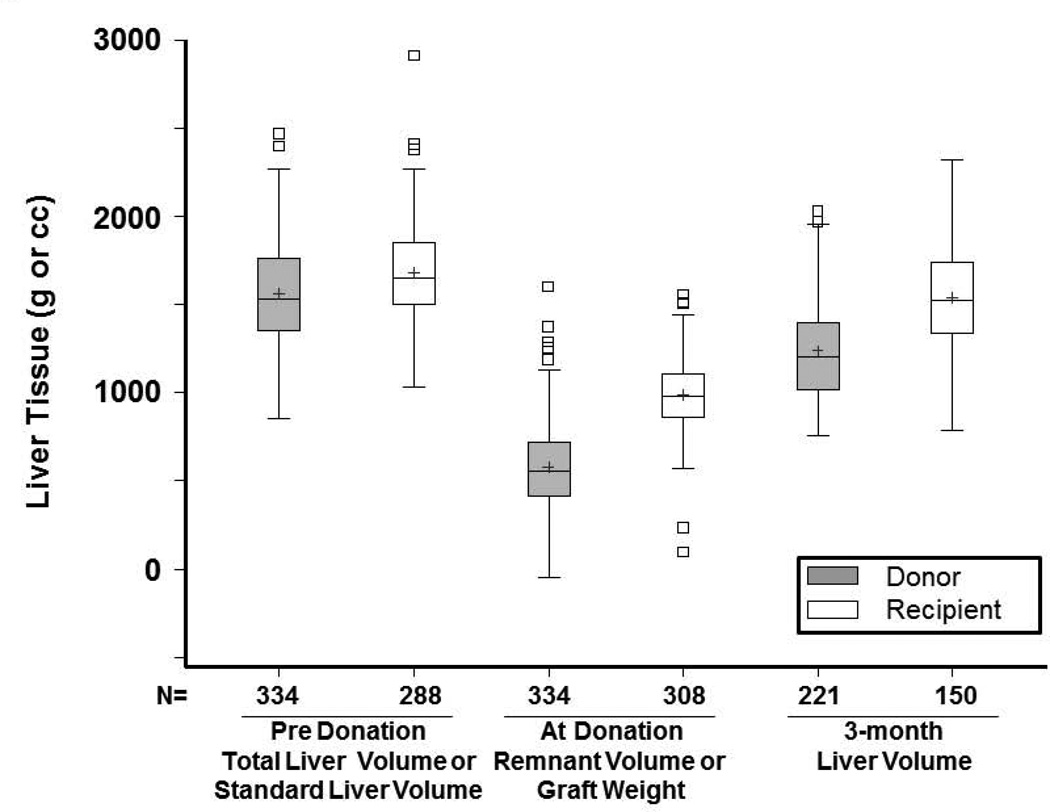
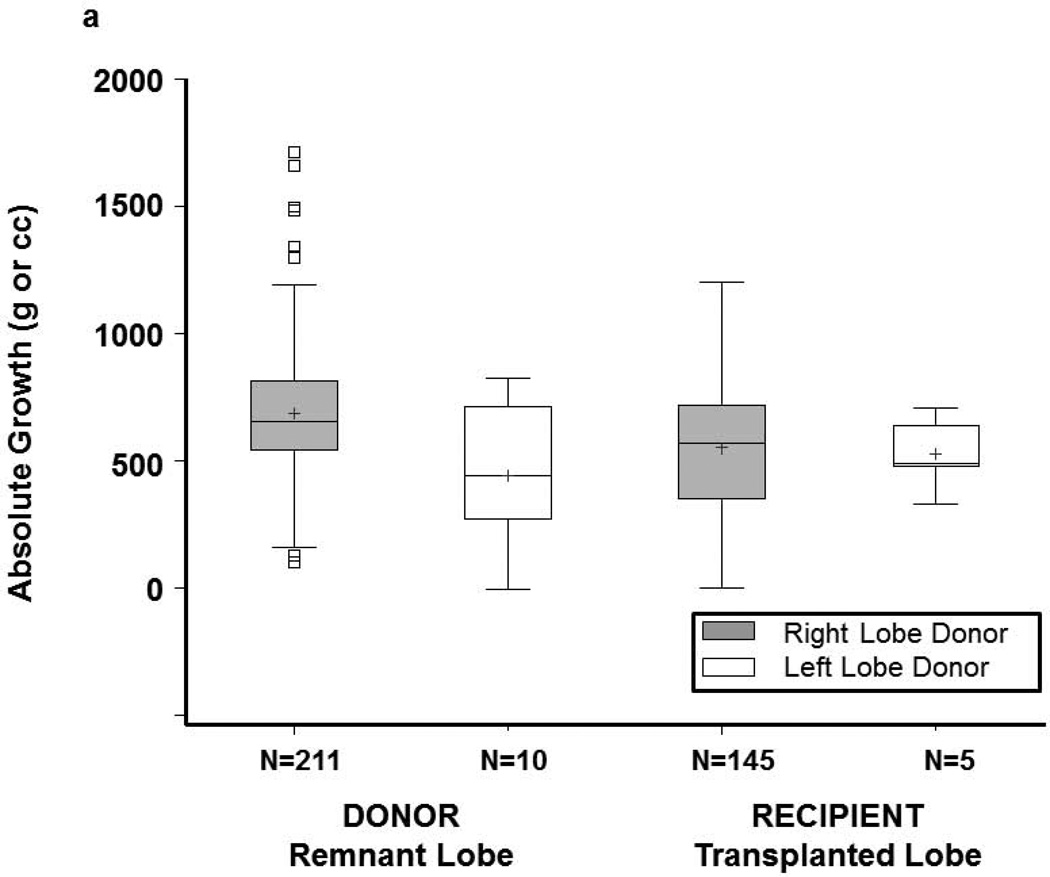
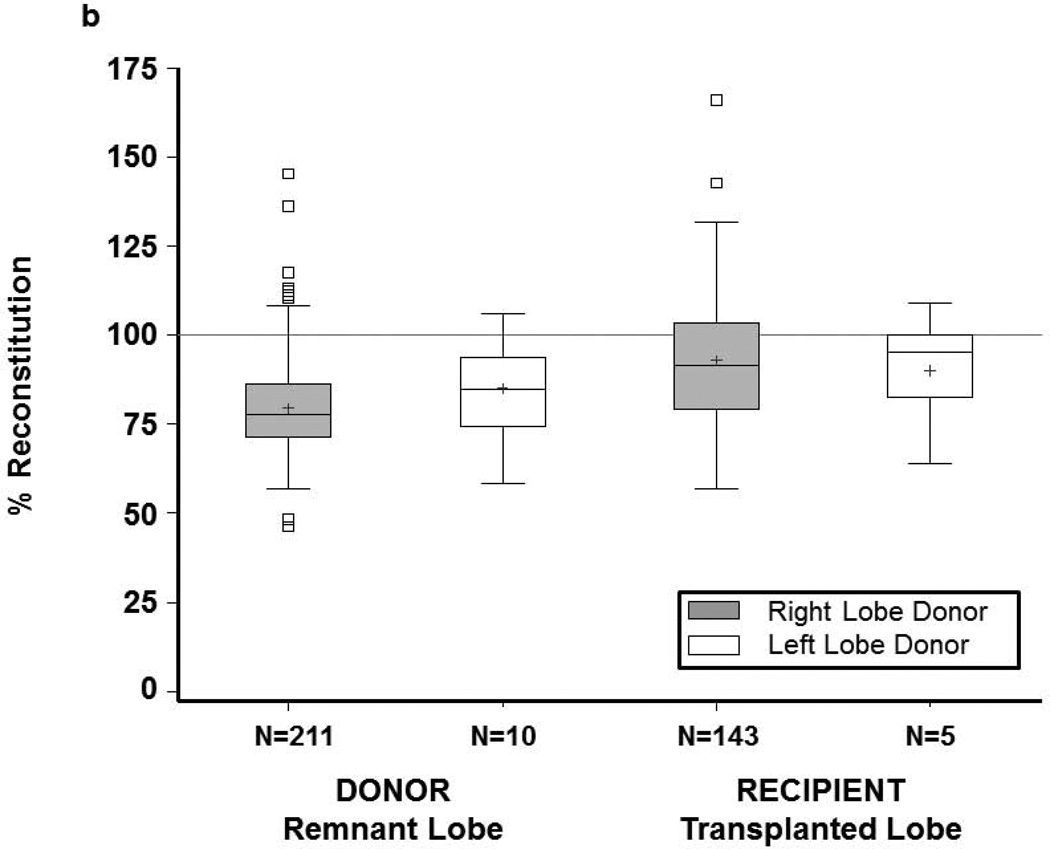
Donor remnants had larger absolute growth at 3 months (676 cc) than recipient grafts (553 cc) (p<0.0001), but lower percent reconstitution at 3 months (80% of starting TLV vs. 93% of expected SLV, p<0.0001) (Figures 2, 3a and 3b). Only 14 donors (6.3%) achieved 100% of starting TLV by 3 months, whereas 52 (35%) recipients achieved their calculated SLV. Percent volume increase of the remnant liver showed a wide range in both donors (median 119%, Q1=86% Q3=176%) and recipients (median 55%, Q1=36% Q3=79%) (p<0.0001). At 3 months, liver volume was smaller for 211 right lobe than 10 left lobe donors (1233±265cc vs. 1413±361cc, p=0.04). Nevertheless, donors of right lobes (who had smaller remnants) had more absolute volume increase than donors of left lobes; recipient growth was comparable, regardless of lobe (Figure S2a). Left lobe donors had a higher percentage reconstitution than right lobe donors. Among recipients, right lobe grafts were significantly larger than left lobe grafts at 3 months (1553±302cc vs. 1225±166cc, p=0.0174). Recipient liver reconstitution was comparable for the two lobes (Figure S2b).
Figure 2.
Box and whisker plot of liver volume before and after transplant. The bottom and top of boxes indicate the 25th and 75th percentile, middle line indicates the median, and + the mean. Whiskers extend up to 1.5 times the interquartile range (IQR) from the bottom and top edges of the box, ending at the last actual data point within the range.
Figure 3.
(a) Donor and recipient absolute growth at 3 months by lobe. (b) Donor and recipient percent liver reconstitution at 3 months by lobe. Each regeneration measure is based on remnant lobe for donors and on transplanted lobe for recipients. The bottom and top of boxes indicate the 25th and 75th percentile respectively, middle line indicates the median, and + indicates the mean. Whiskers extend up to 1.5 times the interquartile range (IQR) from the bottom and top edges of the box, ending at the last actual data point within the range.
Predictors of 3-month volume: Donors and recipients
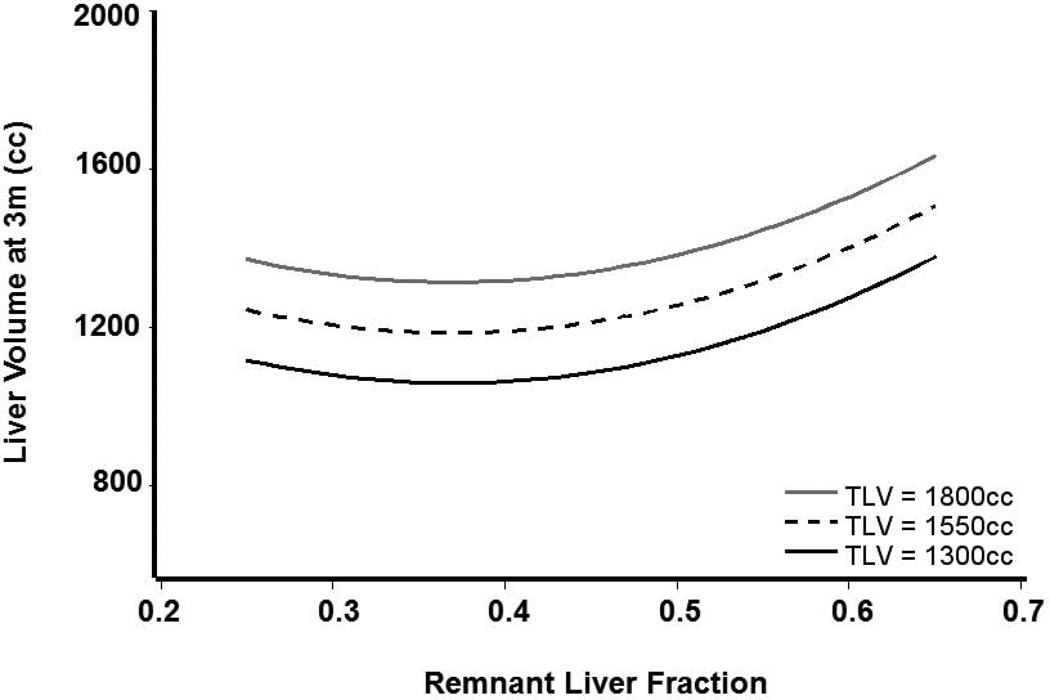
Among donors, greater body weight, TLV, and liver fraction were significantly and positively associated with greater 3-month liver volume (Table 2). When adjusted for these three measures, neither remnant volume nor lobe donated had a statistically significant effect on 3-month volume. On average, liver volume at 3 months was 43.8cc higher for every 10kg of donor body weight (p<0.0001), and 51cc higher for every 100cc of original TLV (p<0.0001), the latter demonstrated in Figure 4. This figure demonstrates the predicted 3-month liver volume for donors with three different TLVs for remnant liver fractions between 0.2 and 0.7, accounting for 87.3% of donors. The relationship with liver fraction was not linear; 3-month liver volumes were similar for remnant liver fractions between 0.2 and 0.5, with increasing 3-month volumes for liver fractions >0.5.
Table 2.
Predictors of 3-Month Liver Volume by Multiple Linear Regression Analysis among Donors, among Recipients, and among both Donors and Recipients
| Predictor | Effect on Liver Volume (g) |
95% CI | p-value |
|---|---|---|---|
| (a) Donor Model1 | |||
| (N=221, R-squared=0.57) | |||
| Weight* (per 10 kg) | 43.8 | (22.0, 65.5) | <0.0001 |
| Total Liver Volume (per 100 g) | 50.9 | (38.8, 63.0) | <0.0001 |
| Remnant Liver Fraction† (per 1%) | 10.5 | (5.8, 15.3) | <0.0001 |
| Remnant Liver Fraction† squared (per 1%) | 0.40 | (0.25, 0.55) | <0.0001 |
| (b) Recipient Model2 | |||
| (N=149††, R-squared=0.38) | |||
| Weight* (per 10 kg) | 71.5 | (46.5, 96.4) | <0.0001 |
| Graft Volume (per 100 g) | 60.5 | (39.5, 81.4) | <0.0001 |
| (c) Combined Model for Donors and Recipients3 | |||
| (N=232††, R-squared=0.42)** | |||
| Donor (vs. Recipient) | −110.4 | (−185.0, −35.9) | 0.004 |
| Weight* (per 10 kg) | 44.1 | (14.3, 73.8) | 0.004 |
| Remnant or Graft Volume (per 100 g) | 53.9 | (32.6, 75.3) | <0.0001 |
| Total Liver Volume or SLV (per 100 g) | 24.4 | (3.9, 44.8) | 0.020 |
If weight is replaced by BSA the R-squared values are very similar for all three models
Restricted to 106 donors and 128 recipients with remnant or graft liver volume (of 550–1200g), which included 48% of remnant lobes and 85% of donated lobes.
Liver fraction is remnant/total size, centered on 50%
One recipient missing weight was excluded from recipient and combined models. One recipient missing height (needed to calculate SLV) was also excluded from the combined model.
In donor model, donor gender, age, height, BMI, BSA (see ** above), remnant lobe type (left or right), remnant volume, and RDWR were tested but not significant.
In recipient model, donor and recipient gender, age, height, and BMI, and BSA; donor weight; graft lobe type (left or right), GRWR, liver fraction, and cold ischemia time; and recipient standard liver volume (SLV), hepatitis C virus (HCV) diagnosis, and model for end-stage liver disease (MELD) at transplant were tested but not significant.
In combined model, lobe type (left or right; remnant for donor, graft for recipient) and liver fraction were tested but not significant as were interactions between donor and all factors.
Figure 4.
Predicted donor liver volume at 3 months (Y axis) and remnant liver fraction (X axis) at transplantation based on donor model. Predicted values are shown for three hypothetical 78kg donors with TLV of 1300cc, 1550cc, and 1800cc.
Recipient body weight and graft size were positively and significantly associated with 3-month liver volume (Table 2). Liver volume at 3 months was on average 71cc higher for every 10kg recipient weight (p<0.0001) and 60cc higher for every 100cc transplanted graft weight (p<0.0001). Unlike the donors, the relationship between starting liver fraction and 3-month liver volume was linear, without the parabolic tail seen for the smaller remnants (p=0.42).
We tested weight, continuous BMI, and BMI categories (normal, overweight, and obese) in donors and recipients for all measures of regeneration. In both donor and recipient models, we found weight to be the stronger predictor of regeneration as measured by 3-month volume in donors and recipients, and absolute volume increase in the donors. After adjusting for weight, BMI provided no additional predictive information. We assessed the influence of HCV, HCC, and diabetes on liver growth. None of these variables were significantly associated with parameters of liver regeneration.
Previous reports have used the additional three measures of liver growth to assess liver regeneration: absolute growth, percent volume increase, and percent reconstitution. The significant predictors for these outcomes were: liver fraction (all outcomes), remnant/graft size (percent reconstitution), and donor/recipient weight (percent reconstitution and absolute volume increase). In addition, the following donor/recipient variables were tested and found to not be significant. For donors: sex, age, BMI, and graft type (left/right lobe). For recipients: graft type (left/right lobe), SLV, sex, age, BMI, HCV, HCC, diabetes, MELD, cold ischemia time, and donor age, sex, and BMI. Thus, the main predictors of liver regeneration, no matter how they are measured, are remnant/graft size, liver fraction, and weight.
Predictors of 3-month volume: Combined donors and recipients
Predictors of 3-month liver volume in 106 donors and 128 recipients were examined over the range of common lobe weights (550–1200g) (Table 2). Positive associations with 3-month donor and recipient volumes were seen for donor or recipient weight, remnant or graft volume, and donor TLV. The effects of these three features were similar for donors and recipients, i.e., with no significant interaction between patient type and any of these factors. Most importantly, we found that after adjusting for these factors, 3-month volumes were greater for recipient than for donor livers by 110g on average (p=0.004).
Paired comparisons between lobes
Donor and recipient data were complete in 127 pairs. There was no correlation between the liver lobes for absolute growth (r=−0.1, p=0.28), percent reconstitution (r=−0.14, p=0.11), or percent volume increase (r=−0.07, p= 0.46). There was a significant correlation between 3-month volumes for these pairs (r=0.26, p=0.003) that was lost when adjusted for donor TLV (r=−0.03, p=0.73).
Clinical correlates
Correlation of recipient regeneration with graft function and failure
Twenty-four recipients died or lost their graft in the first 90 days. Forty-nine recipients (44 right, 5 left lobe) had early allograft dysfunction and symptoms of SFSS (16% overall; 15% of right lobe, 29% of left lobe recipients). Of these, 38 (34 right, 4 left lobe) survived at least 90 days with a functioning graft. Poor graft function at day 7 predicted progression to graft failure both overall (HR=2.5, p=0.004) and in the first 90 days following transplant (HR=4.5, p=0.001).
Ten of 12 recipients with GRWR<0.8% or graft fraction <40% survived at least 90 days with a functioning graft. Neither GRWR nor graft fraction was associated with graft failure overall or in the first 90 days, or with poor function at day 7. Adjusted for graft size and patient weight, grafts with dysfunction at 1 week (with 3-month imaging) were a mean of 140cc larger at 3 months (p=0.02) than those without early dysfunction.
There were only 15 graft failures beyond 3 months, and none of the measurements of liver regeneration (3-month volume, absolute or percent volume increase, or percent reconstitution) were significantly associated with subsequent graft failure. However, this result must be considered in light of the low statistical power.
Correlation with laboratory values in donors and recipients
In donors (Table 3), remnant fractions correlated with 7-day and 30-day bilirubin and INR and 30-day albumin and creatinine. Bilirubin at 7 days post-donation correlated with liver volume at 3 months (r=0.19; p-value=0.0036). Platelet counts decreased from evaluation to year 1 post-donation more for donors with smaller remnants (Table 3). Compared to left lobe donors, right lobe donors had significantly higher bilirubin at days 7 and 30, and lower albumin at 30 days (3.8 vs. 4.1, p=0.0059) (Supplementary Table S1).
Table 3.
Clinical Correlates of Donor Remnant Fraction and Recipient Graft Fraction
| Donor Remnant Fraction | Recipient Graft Fraction | |||
|---|---|---|---|---|
| Lab | r | p-value | r | p-value |
| Day 7 Post-donation | ||||
| Bilirubin | −0.13 | 0.02 | 0.03 | 0.61 |
| INR | −0.13 | 0.03 | −0.27 | <0.0001 |
| Albumin | 0.09 | 0.13 | 0.004 | 0.95 |
| Creatinine | 0.05 | 0.37 | 0.03 | 0.63 |
| Day 30 Post-donation | ||||
| Bilirubin | −0.12 | 0.03 | 0.005 | 0.93 |
| INR | −0.13 | 0.03 | 0.01 | 0.88 |
| Albumin | 0.18 | 0.002 | 0.13 | 0.04 |
| Creatinine | 0.22 | 0.0001 | 0.007 | 0.91 |
| 1 Year Post-donation | ||||
| Platelet drop since evaluation | −0.13 | 0.08 | -- | -- |
Among recipients, significant correlations with graft liver fractions were seen for 7-day INR and 30-day albumin (Table 3). Liver volume at 3 months was also correlated (p<0.05) with bilirubin and creatinine at 7 days post-transplant, and albumin and creatinine at 30 days post-transplant.
We also correlated lab values (bilirubin, albumin, and INR) with the three measures of liver regeneration (absolute and percent volume increase, and percent reconstitution) for donors and recipients. In donors, the only significant correlation was between day 7 bilirubin and absolute volume increase. In recipients, day 7 bilirubin was consistently correlated with liver regeneration, and INR was correlated with two of the regeneration measures (Supplemental Table S4).
Discussion
In living donor transplantation, donor and recipient livers need to regenerate while maintaining adequate metabolic function. This process is central to donor safety and avoiding liver dysfunction in the recipient [7, 18, 19]. In the current study, we have confirmed previous observations that regeneration was brisk in donors and recipients with substantial, though not always complete, mass restoration by 3 months (14, 20). A unique aspect of this study is that this is the first multi-center study of the clinical manifestations of liver regeneration in LDLT in the west. Using a prospectively defined clinical cohort, we were able to characterize growth patterns common to donors and recipients, despite the vagaries of local surgical practice.
An important finding is the apparent relationship between donor regeneration and both TLV and remnant liver fraction. Uncertainty still remains within the living donor community regarding the minimum remnant liver size in the donor, with proposed lower limits between 25% and 35% of total volume. Though right lobes and left lobes differed markedly with respect to both graft size and remnant liver volume, they appear to regenerate in a similar pattern. Importantly, donor remnant size is a critical predictor of the rate of regeneration. Regeneration in donors was related to body weight, pre-donation total liver volume, and the fraction of total liver remaining after donation, regardless of lobe used, which aligns with recent findings by others. Klink et al recently reported on regeneration in 47 donors followed up to 84 months (21). Regeneration at 1 year was 87.3% for right lobes and 80% for left lobes. No serious complications were observed in long term follow-up. Early regeneration was assessed by Gruttadauria et al in a series of 70 right lobe donors. Their modeling identified greater BMI, a smaller FLV, and a higher ratio of SLV/FLV as positive predictors of regeneration (5). In a series of 101 cases of LDLT, Tanemura et al identified donor age as a significant predictor of regeneration, an observation not made in our study (22).
Interestingly, analyses of regeneration in donor-recipient pairs did not show any correlation in the regeneration parameters between the two parts of the same liver, indicating that the host plays a significant role in driving the process. In A2ALL, a significant number of the donors had less than 35% calculated residual volume, all after right lobe resection. We demonstrated a parabolic relationship in donors between 3-month volume and remnant fraction, with the smallest remnants regenerating faster. Despite very rapid regeneration, early postoperative hepatic function, as measured by bilirubin, INR, and albumin, was compromised with very small remnant liver size, demonstrating an association between liver mass and function (23–25). Avoidance of very small remnants in donors is one element supporting the trend toward greater use of the left lobe in LDLT to minimize the extent of hepatectomy.
Recipients also demonstrated rapid regeneration, achieving 93% of calculated SLV by 3 months. Larger recipients and those who received a larger graft had greater volume at 3 months. Unlike the donors, a smaller fraction of SLV was not associated with 3-month liver volume, possibly due to SLV being only a rough approximation to original liver size. With regard to function, smaller grafts had higher INR at 1 week and 1 month.
A unique contribution of our study is the comparative analysis of liver growth between the donor and recipient. In a combined model of donors and recipients, patient weight, larger starting liver volume (remnant or graft), larger TLV or SLV, and patient type (donor vs. recipient) each significantly predicted 3-month liver volume. We demonstrated that recipient liver grafts grew more rapidly in the first 3 months than the donor remnants. This recipient/donor divergence did not appear to be due to the variations in the starting lobe size, as we demonstrated that in recipients and donors with similar graft or remnant sizes, the recipient liver growth was greater. Fewer donors achieved their baseline liver volume than recipients who achieved their predicted liver volumes by 3 months. Only 6.3% of donors achieved 100% of starting TLV, whereas 35% of recipients achieved 100% of SLV. Others have noted related observations (4), which likely reflects the very distinct physiology between donors and recipients. We speculate that the ischemic stress and metabolic demands in recipients provide a growth stimulus to activate priming cytokines for initiation of liver regeneration, whereas the donor has less metabolic demand and no immune pressure or other obvious outward regenerative enhancing stimuli. The relative illness of the recipient may be an impetus for more rapid growth due to higher metabolic demand (26). Both mechanisms are clinically plausible, shifting the energy balance of the recipient toward faster and greater growth than the donor. Finally, it is possible that the larger size of the recipient livers may not reflect “normal” hepatocyte mass but perhaps increased water content or other metabolic alterations of the recipient parenchyma as compared to the regenerating donor tissue. Our ongoing mechanistic studies in liver regeneration may provide supporting molecular evidence for these findings. Recognizing these differences in magnitude of regeneration, we also demonstrated some commonalities between donor and recipient in that both donor and/or recipient weight, TLV, and/or SLV, as well as remnant liver and/or graft volume, were all predictors of 3-month volume.
Several large studies, including A2ALL, have shown that older recipients, older donor age, cold ischemia time, MELD score, and graft size affect outcome (2, 13, 27). There is evidence that small liver grafts transplanted into a metabolically stressed recipient, e.g., those in the ICU, those with fulminant failure, renal failure, and high MELD scores, have less favorable outcomes than those transplanted into the more advantageous environment of a healthier recipient (26, 28). However, several recent studies have shown that smaller grafts can be utilized effectively if other parameters are carefully managed or limited, such as donor and recipient age or the presence of significant portal hypertension (16, 17). Others have shown that it is possible to successfully transplant patients with higher MELD scores with living donors if carefully selected (29, 30). In this current study, we did not observe an effect of MELD on regeneration but there were very few subjects with high MELD scores, and this warrants further investigation.
We were also unable to demonstrate a relationship between any of the regeneration parameters and graft loss in this cohort. Early graft dysfunction, as evidenced by persistent jaundice or coagulopathy at day 7, was seen in 16% of recipients, with more left lobe recipients displaying these features (29% vs. 15%). In addition, poorly functioning grafts were 2.5 times more likely to have subsequent graft failure. However, we did not observe a correlation between postoperative graft failure and graft size, suggesting that early graft dysfunction is dependent on a combination of factors. Identifying these other factors will be important for extending the limits of donation in the future, and is a continued focus of A2ALL.
We recognize the limitations of this study primarily due to missing volumetric data in a significant percentage of patients. In addition, clinical variables that we did not collect, such as variables that better define poor graft function or intraoperative physiologic measurements such as portal pressures and flows, may affect regeneration. The study of regeneration in vivo depends on the reliability of image-based volumetry of the liver and presents numerous challenges, including prediction of the projected graft based on an imaged transection line, the normal variation of the ratio of liver size to body weight or surface area, and the shape and relative lobar volumes. We also noted significant center variation in measurement discrepancies. Though others have used differing approaches (10, 31–34), we estimated the volume of blood in the liver as a linear relationship between the in vivo volume and the weight of the resected lobe, not forced to pass through the origin.
In conclusion, current practice in adult to adult LDLT is well within the limits of safe regeneration for both donors and recipients. Thus, it should be possible to move further by understanding how the quality of the parenchyma, the size of the remnant lobe and the graft, and the status of the recipient affects early regeneration and function. Certainly, the error and variability in measuring liver volume in vivo must be considered when evaluating a potential donor and recipient pair to determine residual remnant fraction and graft volume. Better definitions of these parameters and limits will lead to expanded use of adult LDLT. To this end, mechanistic studies of biomarkers associated with regeneration are ongoing, using specimens collected in these A2ALL subjects. Because LDLT provides a laboratory to observe liver growth in the human setting, it is important for the research community to utilize this unique opportunity to study biological interventions that may enhance liver growth and improve liver function.
Supplementary Material
Acknowledgments
Financial Support:
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases through cooperative agreements (grants U01-DK62444, U01-DK62467, U01-DK62483, U01-DK62484, U01-DK62494, U01-DK62496, U01-DK62498, U01-DK62505, U01-DK62531, and U01-DK62536). Additional support was provided by Health Resources and Services Administration (HRSA), and the American Society of Transplant Surgeons (ASTS).
The following individuals were instrumental in the planning and conduct of this study at each of the participating institutions as follows:
Columbia University Medical Center, New York, NY (DK62483): PI: Jean C. Emond, MD; Co-Is: Robert S. Brown, Jr., MD, MPH, James Guarrera, MD, FACS, Martin R. Prince, MD, PhD, Benjamin Samstein, MD, Elizabeth Verna, MD, MS; Study Coordinators: Taruna Chawla, MD, Scott Heese, MPH, Theresa Lukose, PharmD, Rudina Odeh-Ramadan, PharmD, Jonah Zaretsky, BS.
Northwestern University, Chicago, IL (DK62467): PI: Michael M.I. Abecassis, MD, MBA; Co-Is: Talia Baker, MD, Laura M. Kulik, MD, Daniela P. Ladner, MD; Study Coordinator: Patrice Al-Saden, RN, CCRC.
University of California Los Angeles, Los Angeles, CA (DK62496): PI: Johnny C. Hong, MD; Co-I: Ronald W. Busuttil, MD, PhD; Study Coordinator: Janet Mooney, RN, BSN.
University of California San Francisco, San Francisco, CA (DK62444): PI: Chris E. Freise, MD, FACS; Co-I: Norah A. Terrault, MD, MPH; Study Coordinator: Dulce MacLeod, RN.
University of Colorado Denver, Aurora, CO (DK62536): PI: James R. Burton, Jr., MD; Co-Is: Gregory T. Everson, MD, FACP, Igal Kam, MD, James Trotter, MD; Study Coordinators: Carlos Garcia, RN, BS, Anastasia Krajec, RN.
University of Michigan Health System, Ann Arbor, MI (DK62498): PI: Robert M. Merion, MD, FACS; DCC Staff: Mary Akagi, MS, CCRP, Douglas R. Armstrong, BSN, MS, Abby Brithinee, BA, Margaret Hill-Callahan, BS, LSW, Lisa Holloway, BS, CCRC, Terese A. Howell, BS, CCRC, Brenda W. Gillespie, PhD, Beth Golden, BScN, Anna S.F. Lok, MD, Monique Lowe, MSI, Akinlolu O. Ojo, MD, PhD, Samia Shaw, AAIT, Abigail Smith, MS, Robert A. Wolfe, PhD.
University of North Carolina, Chapel Hill, NC (DK62505): PI: Paul H. Hayashi, MD, MPH; Study Coordinator: Tracy Russell, MA.
University of Pennsylvania, Philadelphia, PA (DK62494): PI: Abraham Shaked, MD, PhD; Co-Is: Kim M. Olthoff, MD, FACS, K. Rajender Reddy, MD, Mark A. Rosen, MD, PhD; Study Coordinators: Brian Conboy, PA, MBA, Mary Kaminski, PA-C, Debra McCorriston, RN, Mary Shaw, RN, BBA.
University of Virginia (DK62484): PI: Carl L. Berg, MD; Co-I: Timothy L. Pruett, MD; Study Coordinator: Jaye Davis, RN.
Virginia Commonwealth University - Medical College of Virginia, Richmond, VA (DK62531): PI: Robert A. Fisher, MD, FACS; Co-Is: Adrian Cotterell, MD, FACS, R. Todd Stravitz, MD, FACP; Study Coordinators: April Ashworth, RN, BSN, Joanne Davis, RN, Ede Fenick, RN, Andrea Lassiter, BS, Cheryl Rodgers, RN, Jose Rodriguez, MPH, Luke Wolfe, MS.
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: Edward Doo, MD, James E. Everhart, MD, MPH, Jay H. Hoofnagle, MD, Stephen James, MD, Patricia R. Robuck, PhD, Leonard B. Seeff, MD, Rebecca J. Torrance, RN, MS.
Abbreviation list
- A2ALL
Adult to Adult Living Donor Liver Transplantation Cohort Study
- BSA
Body surface area
- CT
Computed tomography
- GRWR
Graft weight to recipient weight ratio (100%*(g/g))
- LDLT
Living donor liver transplant
- MELD
Model for End-stage Liver Disease (score)
- MRI
Magnetic resonance imaging
- OR
Operating room
- RDWR
Remnant weight to donor weight ratio (100%*(g/g))
- SFSS
Small for size syndrome
- SLV
Standard liver volume, calculated from the Heinemann equation (ref 8)
- TLV
Total liver volume (estimated in donors by imaging)
Footnotes
This study was presented in part at the 9th annual meeting of the American Transplant Congress, Boston, MA, May 30 – June 3, 2009.
Conflicts of Interest:
The authors have no conflicts of interest to disclose.
Contributor Information
Kim M. Olthoff, Email: kim.olthoff@uphs.upenn.edu.
Jean C. Emond, Email: je111@columbia.edu.
Tempie H. Shearon, Email: tshearon@umich.edu.
Greg Everson, Email: greg.everson@ucdenver.edu.
Talia B. Baker, Email: tabaker@nmh.org.
Robert A. Fisher, Email: rafisher@vcu.edu.
Chris E. Freise, Email: Chris.Freise@ucsfmedctr.org.
Brenda W. Gillespie, Email: bgillesp@umich.edu.
James E. Everhart, Email: EverhartJ@extra.niddk.nih.gov.
References
- 1.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 2.Olthoff K, Abecassis M, Emond J, Kam I, Merion RM, Gillespie BW, Tong L. Outcomes for Adult Living Donor Liver Transplantation: Comparison of A2ALL and National Experience. Liver Transpl. 2011;17(7):789–797. doi: 10.1002/lt.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pomfret EA, Pomposelli JJ, Gordon FD, Erbay N, Lyn Price L, Lewis WD, et al. Liver regeneration and surgical outcome in donors of right-lobe liver grafts. Transplantation. 2003;76:5–10. doi: 10.1097/01.TP.0000079064.08263.8E. [DOI] [PubMed] [Google Scholar]
- 4.Humar A, Kosari K, Sielaff TD, Glessing B, Gomes M, Dietz C, et al. Liver regeneration after adult living donor and deceased donor split-liver transplants. Liver Transpl. 2004;10:374–378. doi: 10.1002/lt.20096. [DOI] [PubMed] [Google Scholar]
- 5.Gruttadauria S, Parikh V, Pagano D, Tuzzolino F, Cintorino D, Miraglia R, et al. Early regeneration of the remnant liver volume after right hepatectomy for living donation: a multiple regression analysis. Liver Transpl. 2012;18:907–913. doi: 10.1002/lt.23450. [DOI] [PubMed] [Google Scholar]
- 6.Marcos A, Fisher RA, Ham JM, Shiffman ML, Sanyal AJ, Luketic VA, et al. Liver regeneration and function in donor and recipient after right lobe adult to adult living donor liver transplantation. Transplantation. 2000;69:1375–1379. doi: 10.1097/00007890-200004150-00028. [DOI] [PubMed] [Google Scholar]
- 7.Botha JF, Langnas AN, Campos BD, Grant WJ, Freise CE, Ascher NL, et al. Left lobe adult-to-adult living donor liver transplantation: small grafts and hemiportocaval shunts in the prevention of small-for-size syndrome. Liver Transpl. 2011;16:649–657. doi: 10.1002/lt.22043. [DOI] [PubMed] [Google Scholar]
- 8.Chen HL, Chen CL, Huang TL, Chen TY, Tsang LL, Ou HY, et al. Regeneration rate of left liver grafts in adult living donor liver transplant. Transplant Proc. 2010;42:699–700. doi: 10.1016/j.transproceed.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Heinemann A, Wischhusen F, Püschel K, Rogiers X. Standard liver volume in the caucasian population. Liver Transpl Surg. 1999;5:366–368. doi: 10.1002/lt.500050516. [DOI] [PubMed] [Google Scholar]
- 10.Gruttadauria S, Marsh JW, Vizzini GB, di Francesco F, Luca A, Volpes R, et al. Analysis of surgical and perioperative complications in seventy-five right hepatectomies for living donor liver transplantation. World J Gastroenterol. 2008;14:3159–3164. doi: 10.3748/wjg.14.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahm F, Georgiev P, Clavien PA. Small-for-size syndrome after partial liver transplantation: definition, mechanisms of disease and clinical implications. Am J Transplant. 2005;5:2605–2610. doi: 10.1111/j.1600-6143.2005.01081.x. [DOI] [PubMed] [Google Scholar]
- 12.Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943–949. doi: 10.1002/lt.22091. [DOI] [PubMed] [Google Scholar]
- 13.Abt PL, Mange KC, Olthoff KM, Markmann JF, Reddy KR, Shaked A. Allograft survival following adult-to-adult living donor liver transplantation. Am J Transplant. 2004;4:1302–1307. doi: 10.1111/j.1600-6143.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 14.Cheng YF, Huang TL, Chen TY, Tsang LL, Ou HY, Yu CY, et al. Liver graft regeneration in right lobe adult living donor liver transplantation. Am J Transplant. 2009;9:1382–1388. doi: 10.1111/j.1600-6143.2009.02626.x. [DOI] [PubMed] [Google Scholar]
- 15.Haga J, Shimazu M, Wakabayashi G, Tanabe M, Kawachi S, Fuchimoto Y, et al. Liver regeneration in donors and adult recipients after living donor liver transplantation. Liver Transpl. 2008;14:1718–1724. doi: 10.1002/lt.21622. [DOI] [PubMed] [Google Scholar]
- 16.Shah SA, Cattral MS, McGilvray ID, Adcock LD, Gallagher G, Smith R, et al. Selective use of older adults in right lobe living donor liver transplantation. Am J Transplant. 2007;7:142–150. doi: 10.1111/j.1600-6143.2006.01596.x. [DOI] [PubMed] [Google Scholar]
- 17.Hill MJ, Hughes M, Jie T, Cohen M, Lake J, Payne WD, et al. Graft weight/recipient weight ratio: how well does it predict outcome after partial liver transplants? Liver Transpl. 2009;15:1056–1062. doi: 10.1002/lt.21846. [DOI] [PubMed] [Google Scholar]
- 18.Patel S, Orloff M, Tsoulfas G, Kashyap R, Jain A, Bozorgzadeh A, et al. Living-donor liver transplantation in the United States: identifying donors at risk for perioperative complications. Am J Transplant. 2007;7:2344–2349. doi: 10.1111/j.1600-6143.2007.01938.x. [DOI] [PubMed] [Google Scholar]
- 19.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 20.Aoki T, Imamura H, Matsuyama Y, Kishi Y, Kobayashi T, Sugawara Y, et al. Convergence process of volumetric liver regeneration after living-donor hepatectomy. J Gastrointest Surg. 2011;15:1594–1601. doi: 10.1007/s11605-011-1590-y. [DOI] [PubMed] [Google Scholar]
- 21.Klink T, Simon P, Knopp C, Ittrich H, Fischer L, Adam G, et al. Liver remnant regeneration in donors after living donor liver transplantation: long-term follow-up using CT and MR imaging. RoFo. 2014;186:598–605. doi: 10.1055/s-0033-1355894. [DOI] [PubMed] [Google Scholar]
- 22.Tanemura A, Mizuno S, Wada H, Yamada T, Nobori T, Isaji S. Donor age affects liver regeneration during early period in the graft liver and late period in the remnant liver after living donor liver transplantation. World J Surg. 2012;36:1102–1111. doi: 10.1007/s00268-012-1496-1. [DOI] [PubMed] [Google Scholar]
- 23.Cho JY, Suh KS, Kwon CH, Yi NJ, Lee HH, Park JW, et al. Outcome of donors with a remnant liver volume of less than 35% after right hepatectomy. Liver Transpl. 2006;12:201–206. doi: 10.1002/lt.20592. [DOI] [PubMed] [Google Scholar]
- 24.Nadalin S, Testa G, Malago M, Beste M, Frilling A, Schroeder T, et al. Volumetric and functional recovery of the liver after right hepatectomy for living donation. Liver Transpl. 2004;10:1024–1029. doi: 10.1002/lt.20182. [DOI] [PubMed] [Google Scholar]
- 25.Trotter JF, Gillespie BW, Terrault NA, Abecassis MM, Merion RM, Brown RS, et al. Laboratory test results after living liver donation in the Adult to Adult Living Donor Liver Transplantation Cohort Study (A2ALL) Liver Transpl. 2011;17(4):409–417. doi: 10.1002/lt.22246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olthoff KM. Small size and disease severity in living donation: a difficult match. Liver Transpl. 2009;15:457–459. doi: 10.1002/lt.21737. [DOI] [PubMed] [Google Scholar]
- 27.Dayangac M, Taner CB, Yaprak O, Demirbas T, Balci D, Duran C, et al. Utilization of elderly donors in living donor liver transplantation: when more is less? Liver Transpl. 2011;17:548–555. doi: 10.1002/lt.22276. [DOI] [PubMed] [Google Scholar]
- 28.Campsen J, Blei AT, Emond JC, Everhart JE, Freise CE, Lok AS, et al. Outcomes of living donor liver transplantation for acute liver failure: the adult-to-adult living donor liver transplantation cohort study. Liver Transpl. 2008;14:1273–1280. doi: 10.1002/lt.21500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selzner M, Kashfi A, Cattral MS, Selzner N, McGilvray ID, Greig PD, et al. Live donor liver transplantation in high MELD score recipients. Ann Surg. 2010;251:153–157. doi: 10.1097/SLA.0b013e3181bc9c6a. [DOI] [PubMed] [Google Scholar]
- 30.Yi NJ, Suh KS, Lee HW, Shin WY, Kim J, Kim W, et al. Improved outcome of adult recipients with a high model for end-stage liver disease score and a small-for-size graft. Liver Transpl. 2009;15:496–503. doi: 10.1002/lt.21606. [DOI] [PubMed] [Google Scholar]
- 31.Kim KW, Lee J, Lee H, Jeong WK, Won HJ, Shin YM, et al. Right lobe estimated blood-free weight for living donor liver transplantation: accuracy of automated blood-free CT volumetry--preliminary results. Radiology. 2010;256:433–440. doi: 10.1148/radiol.10091897. [DOI] [PubMed] [Google Scholar]
- 32.Pomposelli JJ, Tongyoo A, Wald C, Pomfret EA. Variability of standard liver volume (SLV) estimation compared to software assisted total liver volume measurement. Liver Transpl. 2012;18(9):1083–1092. doi: 10.1002/lt.23461. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki K, Epstein ML, Kohlbrenner R, Garg S, Hori M, Oto A, et al. Quantitative radiology: automated CT liver volumetry compared with interactive volumetry and manual volumetry. Am J Roentgenol. 2011;197:W706–W712. doi: 10.2214/AJR.10.5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satou S, Sugawara Y, Tamura S, Yamashiki N, Kaneko J, Aoki T, et al. Discrepancy between estimated and actual weight of partial liver graft from living donors. J Hepatobiliary Pancreat Sci. 2011;18:586–591. doi: 10.1007/s00534-011-0374-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.