Abstract
Clinical evidence indicates brain serotonin (5-HT) stores and neurotransmission may be inadequate in subpopulations of individuals with autism, and this may contribute to characteristically impaired social behaviors. Findings that depletion of the 5-HT precursor tryptophan (TRP) worsens autism symptoms support this hypothesis. Yet dietetic studies show and parents report that many children with autism consume less TRP than peers. To measure the impact of dietary TRP content on social behavior, we administered either diets devoid of TRP, with standard TRP (0.2 gm%), or with 1% added TRP (1.2 gm%) overnight to three mouse strains. Of these, BTBRT+Itpr3tf/J and 129S1/SvImJ consistently exhibit low preference for social interaction relative to C57BL/6. We found that TRP depletion reduced C57BL/6 and 129S social interaction preference, while TRP enhancement improved BTBR sociability (p < 0.05; N= 8–10). Subsequent marble burying was similar regardless of grouping. After behavior tests, brain TRP levels and plasma corticosterone were higher in TRP enhanced C57BL/6 and BTBR, while 5-HT levels were reduced in all strains by TRP depletion (p <0.05; N= 4 −10). Relative hyperactivity of BTBR and hypoactivity of 129S, evident in self-grooming and chamber entries during sociability tests, were uninfluenced by dietary TRP. Our findings demonstrate mouse sociability and brain 5-HT turnover are reduced by acute TRP depletion, and can be enhanced by TRP supplementation. This outcome warrants further basic and/or clinical studies employing biomarker combinations such as TRP metabolism and 5-HT regulated hormones to characterize the conditions wherein TRP supplementation can best ameliorate sociability deficits.
Keywords: 129S1/SvImJ, autism, BTBR, C57BL/6, grooming, marble burying, serotonin, social behavior, tryptophan
1. INTRODUCTION
Sociability deficits, specifically interpersonal interaction impairments such as social anxiety, withdrawal, inattentiveness, or lack of social motivation are characteristic of autism spectrum disorders. Serotonin (5-HT) system dysfunctions are implicated in some forms of autism, and may contribute to characteristic social interaction impairments (Lam et al., 2006; Rubin et al., 2013; Yang et al., 2014). During fetal and juvenile brain development, 5-HT plays many critical roles (Daws and Gould, 2011). Clinical and basic research findings indicate that 5-HT-regulated brain developmental trajectories are disrupted in autism, either via deficient or excessive central 5-HT availability (Chandana et al., 2005; Azmitia et al., 2011; Madden and Zup, 2014; Yang et al., 2014).
Among individuals with autism, brain 5-HT availability and neurotransmission are variable, since a diverse range of genetic and environmental risk factors can manifest in common core behavioral deficits (Unwin et al., 2013; Whitehouse and Stanley, 2013). Yet subpopulations with distinct autism phenotypes can be identified, including a group with physiological markers and behavioral symptoms consistent with central hyposerotonemia (Brune et al., 2006; McNamara et al., 2008; Veenstra-VanderWeele et al., 2012). Such markers comprise reduced 5-HT transporter binding in frontal cortex (Makkonen et al., 2008; Nakamura et al., 2010), low oxytocin and low melatonin levels (Alabdali et al., 2014, Ruggeri et al., 2014). Selective 5-HT reuptake inhibitors (SSRIs) improve autism symptoms in some patients (West et al., 2009; Kumar et al., 2012; Hollander et al., 2012; Politte et al., 2014)1, suggesting 5-HT neurotransmission may be reduced and/or brain 5-HT depleted.
Tryptophan (TRP) is the essential amino acid 5-HT precursor. Acute TRP depletion can be used to assess the prognosis of patients with depression to benefit from 5-HT-based treatments (Delgado, 2006; Toker et al., 2010). With TRP depletion, depression symptoms worsen and cognitive functions decline in patients that responded favorably to SSRI treatments, or in individuals with high 5-HT turnover rates (Bell, 2001; Delgado, 2006; Feder et al., 2011). TRP depletion in individuals with autism likewise worsens core behavior symptoms, indicating heightened sensitivity to fluctuations in TRP and 5-HT availability (McDougle et al., 1993; 1996). On the other hand, increasing dietary TRP intake ameliorated autism symptoms in a case study (Beretich, 2009). This presents a paradox, in light of reports that many individuals with autism prefer foods with relatively low TRP content or have aversions to high dietary protein content (Kidd, 2002; Arnold et al., 2003; Herndon et al., 2009; Hyman et al., 2012; Johnson et al., 2014).
Given this, we tested the hypothesis that acute dietary TRP depletion should impair social behavior, while TRP enhancement might improve it. Inbred mice expressing the high-functioning TRP hydroxylase 2 (Tph2) enzyme isoform to convert TRP to 5-HT, with well-characterized sociability phenotypes (Carneiro et al., 2009; Moy et al., 2007) were used. These included socially deficient BTBR T+ Itpr3tf/J (BTBR) and 129S1/SvImJ (129S), and relatively gregarious C57BL/6J (C57) mice. Preferences for social interaction and novelty, chamber entries, self-grooming during sociability tests and subsequent marble burying were compared among strains and overnight TRP diet treatments. After behavior tests, brain TRP, 5-HT turnover and plasma corticosterone (CORT) -- since it can be suppressed by central 5-HT transmission (Gould et al., 2014) -- were measured in tissues collected from all strain × diet treatment groups to assess their central 5-HT status.
2. METHODS
2.1. Mice and Acute Dietary Tryptophan Manipulation
All procedures involving live mice were approved by the UTHSCSA Institutional Animal Care and Use Committee, and were in accordance with current NIH guidelines. Mice tested were fifth and sixth generation male offspring bred in the laboratory animal facilities at The University of Texas Health Science Center at San Antonio (UTHSCSA), San Antonio, TX. BTBR, C57 and 129S, founders came from Jackson Laboratory (Bar Harbor, ME, USA).
Mice were maintained at 22–25°C on 14:10 light dark cycles, with lights on at 0700 h, and ad-libitum access to Teklad LM-485 mouse/rat irradiated food pellets (#7912, Harlan, Madison, WI) and water in cages lined with wood-chip bedding that were changed biweekly. Mice were weaned at postnatal days 20–22 and were housed in same-sex groups of 3–5 per cage. Dietary TRP manipulations and behavior tests were conducted in 3–4 month-old males. For 24–30 hours prior to behavior testing (beginning 0900 or 1000 h), mice had ad libitum access to purified ingredient or “open source” standard diets with either a) control levels of TRP (2.1 g/kg or 0.2% = green pellets), b) diet devoid of TRP (-TRP, 0% = red), or c) diet with 1% added TRP (+TRP 12.6g/kg = yellow) from Research Diets Inc. (New Brunswick, NJ). Nutritional information for open-standard purified diets and the Teklad chow the mice were reared and maintained on are provided in Table 1.
Table 1.
Comparison of nutrient content of sustaining mouse chow that was given prior to study to the experimental open standard diet that was acutely administered before testing.
| Dietary Component | Teklad LM-485 (Irradiated 7012, Harlan) |
Exp. Open Standard (A11022501, Research Diets) |
|---|---|---|
| Macronutrients (% kcal) | ||
| Protein | 25 | 18 |
| Carbohydrates | 58 | 66 |
| Fat | 17 | 16 |
| Fiber (gm%) | 14% | 10% |
| Essential2 L-Amino Acids (gm%) | ||
| Arginine | 1.2 | 0.6 |
| Histidine | 0.5 | 0.4 |
| Isoleucine | 0.8 | 0.7 |
| Leucine | 1.7 | 1.5 |
| Lysine | 1.0 | 1.3 |
| Methionine | 0.4 | 0.5 |
| Phenylalanine | 0.9 | 0.8 |
| Threonine | 0.8 | 0.7 |
| Tryptophan | 0.3 | 0.2 (-TRP 0, +TRP 1.2) |
| Valine | 0.9 | 0.9 |
| Vitamins (IU/g) | ||
| A | 30 | 4000 |
| D | 2.4 | 1000 |
| E | 0.2 | 50 |
| Vitamins (mg/kg) | ||
| Menadione (K3) | 80 | 0.5 |
| B-complex | ||
| Thiamine (B1) | 95 | 6 |
| Riboflavin (B2) | 14 | 6 |
| Niacin (B3) | 100 | 30 |
| Pantothenate (B5) | 87 | 16 |
| Pyroxidine (B6) | 17 | 7 |
| Biotin (B7) | 0.8 | 0.2 |
| Folate (B9) | 7 | 2 |
| Cobaltamin (B12) | 0.09 | 0.01 |
| Minerals (gm%) | ||
| Calcium | 1.0 | 0.9 |
| Phosphorus | 0.7 | 0.3 |
| Sodium | 0.3 | 1.7 |
| Potassium | 0.8 | 0.6 |
| Chloride | 0.5 | 1.0 |
| Magnesium | 0.2 | 0.5 |
| Minerals (mg/kg) | ||
| Zinc | 63.0 | 29.0 |
| Manganese | 93.0 | 59.0 |
| Copper | 23.0 | 6.0 |
| Iodine | 3.0 | 0.2 |
| Iron | 240.0 | 37.0 |
For juvenile mice, per John and Bell, 1976.
2.2. Sociability Tests, Self Grooming During Tests and Subsequent Marble Burying
Preference tests for social interaction and social novelty were performed in three chambered testing arenas between 9:00 and 16:00 h CST, as described in prior studies (Gould et al., 2011; 2014; Silverman et al., 2010; Yang et al., 2011). Conditioning and sociability tests were conducted under low red lighting (16 lux). A daily experiment schedule is provided, and sociability-testing arena illustrated in the on-line supplement (Appx A.1.a and b). Subject mice (4–6 tested in different arenas at the same time, 1 per treatment group) were brought from housing to the testing room 30 min prior to testing 24, 26 or 28 hours (typically 0900, 1100 or 1400h CST) after diets were administered to acclimate. Next, subjects acclimated to and explored the sociability arenas for 20 min. Then, subjects were confined to the center chamber for ≥1 min while pre-conditioned ‘strangers’ (novel male 129S mice, 8–10 weeks old) and novel objects (empty wire cages) were placed on either end chamber. Stimulus placement for testing \was randomized and counter-balanced among groups. Ten min tests were videorecorded for subsequent data collection. Preference for social interaction was tested in the first 10 min with a stranger mouse in a cup cage at one end and an empty cage at the other end chamber. Then subjects were re-confined in the center while new strangers (stranger #2) were placed under empty cages and old strangers (stranger #1) were re-positioned in the arena (Appendix A.1.b). Preference for social novelty was measured in the second 10 min phase. Between subjects strangers were returned to home cages, and arenas cleaned with 70% ethanol and dried with paper towels.
Data collected by treatment-blind observers from 10–11 min videorecordings of social interaction and novelty preference tests included time spent in chambers, sniffing and grooming. Chamber dwelling was tracked as subjects entered new chambers by recording the time and each chamber entered and into a spreadsheet, subtracting the exit times to determine each dwelling duration, and adding durations separately for each chamber and each test phase. Sniffing was recorded by timer when a subject directed its nose toward strangers or novel objects (cup cages) from a distance of < 1 cm, and ended when they turned their head or stepped away. Self-grooming was recorded by timer when a stationary subject was observed to lick or use forepaws to smooth its head, body or tail and ended when they stopped moving its head and paws or stepped away from the site where grooming occurred.
2.3. Marble Burying
Immediately following each bought of social novelty testing, each subject was transferred to a 50 × 28 × 23 cm cage filled to a depth of 8 cm with bedding, on top of which was placed 15 blue flattened marbles spaced evenly apart in a 3 × 5 grid. Cages were covered with filter tops and mice had 30 min to bury marbles. For each mouse marbles ≥ 2/3 buried were tallied.
2.4 Whole Brain TRP Levels and 5-HT Turnover
After marble burying (at 1100, 1300 or 1600h CST) subject mice were sacrificed by decapitation, brains were harvested and frozen at −80°C, and trunk blood collected into tubes containing 25 μl of 20 mM ethylenediaminetetraacetic acid (Sigma, St Louis, MO).
Levels of TRP, 5-HT and its metabolite 5-hyroxyindoleacetic acid (5-HIAA) were measured in whole brains by high performance liquid chromatography (HPLC) with electrochemical detection. HPLC was performed as in prior studies (Callaghan et al., 2007), with minor modifications (e.g. software and system updates). In summary a gradient mobile phase was used, and samples were analyzed using an ESA coulometric detector (Chelmsford, MA) and a C-18 column, on a Waters system (Milford, MA). Peak heights were converted to compound concentrations using BSA EZ Start software.
2.5 Plasma Corticosterone (CORT) Levels
Plasma isolated by centrifugation (≈3000 rpm) for 10 min at 4°C was frozen at −80°C until use. CORT levels were measured following the ‘small sample protocol’ for ELISA (#ADI-900-097, Enzo Life Sciences, Farmingdale, NY) on a plate reader (Molecular Devices, Sunnyvale, CA). A non-linear standard curve was generated and concentrations determined using Prism (GraphPad, San Diego, CA).
2.6. Statistical Analyses
Three-way (strain × test-phase × diet) repeated-measures multivariate analysis of variance (RM-MANOVA) comparisons of chamber-dwelling and sniffing data were performed to reveal any differences in preference between strains and among diets across social-interaction and social-novelty test-phases. Repeated measures results across strains are in Appendix (A.2 on-line supplement). Next, by strain, effects of test-phase (1. social interaction or 2. social novelty) × diet on chamber-dwelling and sniffing preferences were compared by two-way RM-ANOVA. Within each strain, diet and test-phase, significant chamber preference differences were resolved via two-tailed t-tests. Then, within each strain and test-phase, to compare effects of diet on each preference-related time variable, ANOVA was performed and significant differences were resolved by Fisher’s least significant difference (LSD) test. Other variables such as chamber entry, self-grooming, marble burying, brain TRP, 5-HT, 5-HIAA and plasma corticosterone was compared by two-way MANOVA or ANOVA, correlation and Fisher’s LSD post-hoc tests. Analyses were performed using Statistica (Statsoft, Tulsa, OK).
3. RESULTS
3.1.1. Effects of Dietary Tryptophan Manipulation on C57 Sociability
During social-interaction preference tests (phase 1), diet and chamber-dwelling interacted (F2,24 = 5.93, p < 0.01) for C57 mice since only controls dwelled more with strangers than objects (F2,24 = 3.68, p < 0.05, LSD p <0.05, t8 = 5.45, p < 0.001, Fig. 1a). By contrast, +TRP and -TRP C57 dwelled in novel-object chambers moreso than controls (F2,24 = 11.2, p < 0.001, LSD p < 0.001, Fig. 1a). For C57 sniffing, diet × chamber-preference interacted (F2,24 = 5.9, p < 0.01) since controls sniffed strangers more than objects (F2,24 = 9.5, p < 0.01, t8 = 4.94 p < 0.01, Fig. 1b), while + TRP or -TRP C57 mice sniffed novel objects more than controls (F2,24 = 6.33, p < 0.01, LSD p < 0.001, Fig. 1b).
Fig. 1.
C57 mouse preference for social interaction or novelty is differentially altered by acute dietary tryptophan (TRP) manipulation. Bars represent means and lines show standard error. The symbol * indicates significant preference for interaction or novelty (p < 0.05), τ indicates trend (p < 0.1) toward novelty preference, and ** indicates a change (an increase) in attention toward novel objects (p <0.05). TRP enhancement (+) or depletion (-) produced a loss of interaction preference in (a) chamber dwelling and (b) sniff time. However, preference for novelty in (c) chamber dwelling or (d) sniffing was enhanced by –TRP. N = 9 mice/group.
In social-novelty preference tests (phase 2), C57 chamber preference differed among diets (F1,24 = 10.71, p < 0.005), as only -TRP C57 mice dwelled near stranger 2 moreso than stranger 1 (t8 = 2.47, p < 0.05, Fig. 1c). However both -TRP and +TRP C57 mice sniffed stranger 2 more than stranger 1 (F1,24 = 20.96, p < 0.001, t8 = 3.63 or 4.66, p < 0.05, Fig. 1d).
3.1.2. Effects of Dietary Tryptophan Manipulation on 129S Sociability
During social-interaction tests, diet × chambers interacted (F2,23 = 5.6, p < 0.01) for 129S mice. 129S controls were sociable (F2,23 = 3.9, p <0.05, t9 = 2.42, p < 0.05), while 129S on -TRP and +TRP diets had no preference for social-interaction by chamber-dwelling (F2,23= 11.95, p < 0.001, LSD p < 0.05, Fig. 2a). –TRP 129S spent more time in the arena center than controls (F2,23 = 3.9, p <0.05). For 129S sniffing, diet × chambers interacted (F2,23 = 4.7, p < 0.05), since controls sniffed strangers more than novel-objects (t9 = 2.63 p< 0.05), and -TRP sniffed strangers less than controls (F2,23= 6.9, p < 0.01; LSD p < 0.01 Fig. 2b).
Fig. 2.
129S mouse preference for social interaction or novelty is differentially altered by dietary TRP manipulation. Graph legend is as for Fig. 1, plus # indicates significantly more time spent in the center arena (p <0.05) and *** indicates significantly less time with stranger (p <0.05). TRP depletion resulted in loss of social interaction preference for (a) chamber dwelling and (b) sniff time. TRP enhancement promoted preference for social novelty in (c) chamber dwelling and (d) sniff time. N = 8–10 mice/group.
In 129S social-novelty preference tests +TRP mice exhibited greater preference for the second novel stranger mouse (stranger 2) than stranger 1, both in chamber-dwelling (F1,23 = 6.49, p < 0.01, t7 = 2.75, p < 0.05, Fig. 2c) and sniffing (F1,23 = 7.83, p < 0.01, t7 = 3.98, p < 0.01, Fig. 2d), while 129S controls displayed no such preference for novelty.
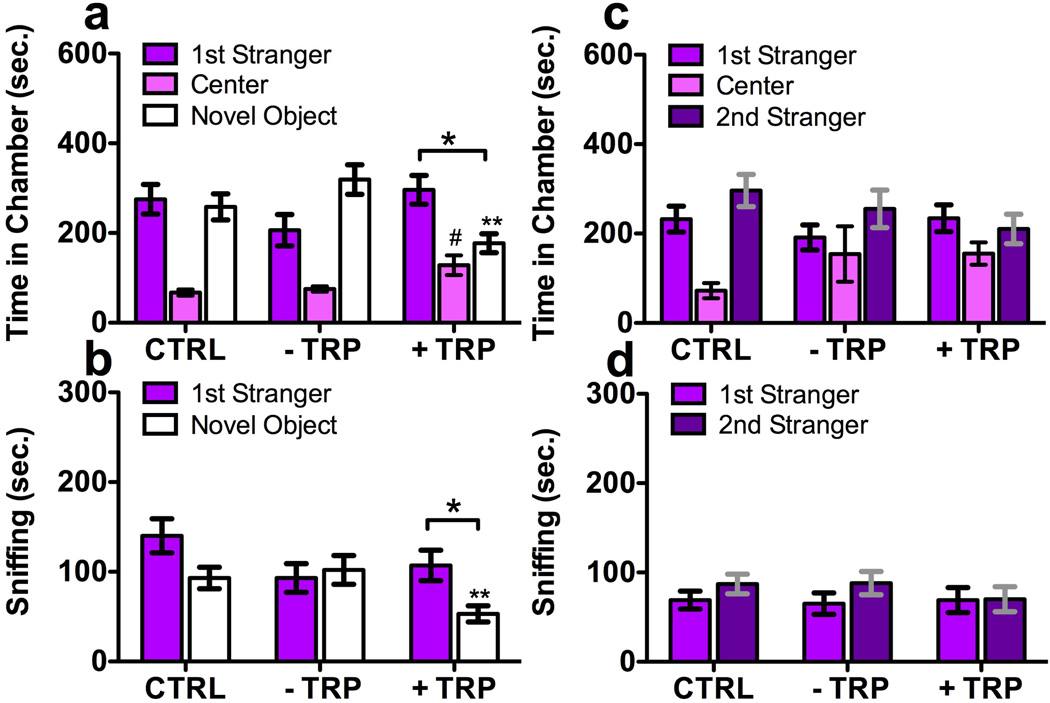
3.1.3. Effects of Dietary Tryptophan Manipulation on BTBR Sociability
In social-interaction tests, BTBR mice given +TRP diet exhibited enhanced sociability in chamber-dwelling (F2,26 = 6.2, p < 0.01, t8 = 2.37, p < 0.05 Fig. 3a), and sniffing (F2,26 = 5.4, p < 0.01, t8 = 2.31, p < 0.05 Fig. 3b) while other groups did not. BTBR given +TRP spent less time in chambers with (F2,26 = 5.44, p < 0.05; LSD p < 0.05) or sniffing (F2,26 = 4.03, p < 0.05; LSD p < 0.05) novel objects.
Fig. 3.
BTBR mouse preference for social interaction or novelty is differentially altered by dietary TRP manipulation. Graph legend is as for Fig. 2. TRP enhancement increased preference for social interaction in (a) chamber dwelling time and (b) social sniffing, through a reduction in the attention paid to novel objects (**). There were no significant differences in the lack of preference for social novelty displayed by all treatment groups for (c) chamber dwelling or (d) sniffing time. N = 8–12 mice/group.
BTBR mice failed to exhibit any preference for the second new stranger introduced in the social-novelty phase, as measured by chamber dwelling (F2,26 = 1.0, p = 0.3, Fig. 3c) or social sniffing (F2,26 = 3.2, p = 0.08, Fig. 3d). Also there was no difference among BTBR diets in lack of social-novelty preference (chambers F2,26 = 1.4, p = 0.26; sniff F2,26 = 0.6, p = 0.54).
3.2. Chamber Entries and Self Grooming During Sociability Tests
The number of chamber entries differed among strains in both social-interaction (F2,73 = 26.38, p < 0.0001) and social-novelty (F2,73 = 13.82, p < 0.0001) preference tests. 129S mice made fewer entries than C57 or BTBR mice during social interaction tests (LSD p < 0.01), while BTBR made more chamber entries than C57 or 129S mice (LSD p < 0.0001, Fig. 4a) during both test phases. BTBR on +TRP diet made more entries (66 ± 5) than BTBR controls (53 ± 8, F2,73 = 3.49, p < 0.05 LSD p < 0.05). There were no other effects or interactions of diet with strain for chamber entries during sociability testing.
Fig. 4.
Chamber entry and grooming behaviors during sociability tests were enhanced in BTBR and reduced in 129S mice. Bars represent means and lines show standard error. The symbol * indicates greater than and ** indicates less than other strains (p < 0.05). (a) BTBR mice made more chamber entries and 129S made fewer during sociability tests. (b) 129S did less self-grooming during preference for interaction tests than the other strains, while BTBR did more self-grooming during preference for novelty tests. N = 8–12 mice/group.
Diet had no effect on self-grooming during sociability tests (F2,73 = 1.61, p = 0.2), but strain (F2,73 = 5.20, p < 0.01), test phase (F2,73 = 17.96, p < 0.0001) and their interaction (F4,73 = 2.63, p < 0.05) were significant. During social-interaction preference tests, 129S did less self grooming than BTBR or C57 mice (F2,73 = 5.8, p<0.01, LSD p <0.005, Fig. 4b). In social-novelty preference tests, BTBR self-groomed more than the other strains (F 2,73 = 3.9, p < 0.05, Fig. 4b).
3.3. Marble Burying after Sociability Testing
Diet treatment (F2,73 = 1.49 p = 0.23) and strain (F2,73 = 1.43 p = 0.25) had no significant effects on marble burying, and they did not interact (F4,73 = 0.1, p <0.98). Mice in all groups buried to a similar extent on these diets, and their pooled mean was 9 ± 0.3 marbles buried.
3.4 Whole Brain TRP Levels and 5-HT Turnover Following Behavior Tests
Two-way MANOVA revealed significant interactions between strain and diet (Wilks’ λ16,70 = 0.18, p < 0.001), with respect to whole brain wet tissue content of TRP (F4,26 = 3.6, p < 0.05), 5-HT (F 4,26 = 4.2, p < 0.01), 5-HIAA (F4,26 = 4.3, p < 0.01) and 5-HT turnover (% 5-HIAA/5-HT F4,26 = 3.2, p < 0.05) in HPLC measurements of whole brains collected after behavior tests. C57 and BTBR mice had increased brain TRP content with either TRP enhancement or depletion relative to controls (Fig. 5 a, LSD p < 0.05). As shown in Fig. 5b and 5c, acute TRP depletion significantly reduced (LSD p<0.05) brain 5-HT and 5-HIAA content in all strains relative to controls. However TRP depletion in only C57 reduced 5-HT turnover, while TRP enhancement only in BTBR mice enhanced 5-HT turnover (Fig. 5d).
Fig. 5.
Whole brain TRP and 5-HT turnover after social interaction and marble burying test battery. (a) Dietary TRP enhancement (+TRP) or depletion (-TRP) significantly increased brain TRP levels in C57 and BTBR mice (**p < 0.05). Depletion of TRP reduced brain (b) 5-HT levels and (c) 5-HIAA levels after behavior tests (* p < 0.05) in all strains, but it reduced (d) 5-HT turnover in C57 mice only. On the other hand TRP enhancement only increased 5-HT turnover in BTBR mice (d, **p <0.05). N = 4 mice/group (randomly selected).
3.5 Plasma Corticosterone Levels Following Behavior Tests
There was a significant interaction between strain and diet treatment (F4,49 = 3.3, p < 0.05), in plasma CORT levels measured in representative samples following our behavior test battery, as shown in Fig. 6. Specifically both TRP depleted and TRP enhanced C57 mice had higher CORT than those on standard control diet (LSD p <0.05). Only TRP-enhanced BTBR mice had higher CORT levels than controls. By contrast, TRP-enhanced 129S had lower CORT than controls (LSD p < 0.05). For reference, baseline plasma CORT levels from aged-matched naïve mice were 20 ± 4 in C57, 32 ± 8 in 129S, and 28 ± 4 in BTBR (N = 3–5).
Fig. 6.
Plasma corticosterone after behavior tests varied with strain and dietary TRP. The symbol * indicates greater than and ** indicates less than other strains (p < 0.05). In C57 mice either supplementing (+TRP) or depleting (-TRP) resulted in higher CORT relative to controls. +TRP reduced CORT significantly in 129S while it increased CORT in BTBR mice. Serum was collected after 70 min of behavior tests. N = 4–9 mice/group (randomly selected).
4. DISCUSSION
4.1. Summary of Key Experimental Findings
This study utilized 24–30 h of ad libitum feeding on purified diet to assess the impact of acute TRP manipulation on sociability of three inbred mouse strains. Indeed, dietary TRP depletion altered murine sociability in three-chamber tests, but in different ways dependent upon mouse strain. For example in typically sociable C57 mice, preference for social- interaction was lost in -TRP mice, as they investigated novel objects more than controls (Fig. 1). Likewise, 129S on –TRP diet dwelled less with strangers, spending more time in central and novel-object chambers (Fig. 2). However in BTBR mice that usually exhibit sociability deficits, –TRP diet had little effect on social-interaction preference, while +TRP improved BTBR sociability (Fig. 3). Specifically +TRP BTBR paid less attention to novel objects, both in chamber-dwelling and sniff, and spent more time in the center (closer to strangers) relative to control or -TRP mice (Fig. 3). These findings support our hypothesis that deficits in social interaction preference can stem from reduced 5-HT availability, and parallel observations in patients with autism (McDougle et al., 1993; 1996, Beretich, 2009; Daly et al., 2012).
An unanticipated finding was that 129S mice on control diet were sociable. Previously, naïve 129S fed Teklad chow exhibited impaired sociability, in agreement with Moy et al. (2007), even as collinear (129S) strangers were used (Gould et al., 2014). However, distinct oxytocin-mediated responses to same vs. different mouse strains were found in other studies (Macbeth et al., 2009; Hattori et al., 2014). To assess whether collinear (129S) strangers influenced 129S sociability herein, we replicated overnight (24h) control-diet treatment and tested 129S sociability using C57 strangers instead (Appendix A.3, Fig. 7). Even with C57 strangers, 129S controls displayed preference for social-interaction. This enhanced 129S sociability may instead stem from some aspect of the control diet, such as its composition or novelty. Likewise, purified diets may have precipitated the loss of BTBR’s characteristic social-novelty preference (Fig. 3), with little effect on C57 controls (Fig. 1).
The texture of the open standard diet was similar to Teklad chow (Table 1); it has similar fat content and was not oily, and like cereal chow it was compressed to prevent crumbling. However, open standard diets are made from purified ingredients, so due to their refinement they may have a different aroma than Teklad chow (Ricci and Ulman, 2005). Hence introducing purified diets might have presented odor and flavor novelty. Yet mice in all groups consumed their diets, and feces color matched the group’s diet color. While not empirically quantified, feeding did not appear to be suppressed, as comparable volume reductions to overnight feeding on Teklad diets were evident across groups.
However aside from variable TRP, other differences in macronutrient content between Teklad chow and open-standard diets were notable and may have enhanced social interaction preference in 129S controls or reduced social novelty preference in BTBR. Specifically, vitamin A, D, and E contents were greater in purified diets. Vitamin A deficiency has anxiogenic effects (Bonhomme et al., 2014), and E deficiency alters CORT release in rats (Terada et al., 2011). Vitamin D influences sniffing and following (Kalueff et al., 2006), and can modulate 5-HT synthesis (Patrick and Ames, 2014). Thne level of menadione, an oxidative stressor (Giustarini et al., 2006) was reduced in purified diets. Finally, consistent with National Research Council (1995) recommended 35 mg/kg, open standard diets had 37 mg/kg of iron, while Teklad chow contained 240 mg/kg iron.
Also 129S mice on +TRP diet exhibited social-novelty preference (Fig. 2c). We postulate this change may stem from confluence of the following factors: 1) novelty of diet, 2) +TRP in diet, 3) sociability test duration and phase (social novelty test after 30 min in arena), and 4) previously characterized contextual fear-extinction deficiencies in 129S that resemble post-traumatic stress disorder (Camp et al., 2012; Temme et al., 2014). Previously we found evidence for involvement of hippocampal 5-HT1A receptors in the abnormal CORT responses of 129S mice, highlighting an important role for 5-HT in this process (Gould et al., 2014). In accord, the CORT response in +TRP 129S after behavior tests was relatively blunted (** in Fig. 6). Otherwise, plasma CORT following behavior tests differed among strain such that in C57 higher CORT in +TRP and –TRP corresponded with loss of preference for interaction, whereas higher CORT in +TRP BTBR corresponded with reduced interest in novel objects. Overall correlations between % 5-HT turnover (Fig. 5d) and plasma CORT were r = −0.36 (p = 0.27) for C57, r = − 0.59 (p < 0.05) for 129S, and r = 0.66 (p < 0.1) for BTBR, more detailed correlations are presented in Appendix A4.
As with +TRP, BTBR sociability is also enhanced by treatment with the SSRI fluoxetine, indicating that deficient 5-HT neurotransmission may underlie its impaired sociability (Gould et al., 2011). Given this, it is interesting that CORT blocks ancillary transporters of 5-HT (i.e. “uptake 2”) such as organic cation transporters (OCTs) from clearing extracellular 5-HT in the brain (Baganz et al., 2010; Hill et al., 2011). This lends support to the hypothesis that their blockade may be a useful strategy for treating sociability deficits, warranting further studies in this area.
4.2. Prior Findings with Acute or Chronic Dietary Tryptophan Depletion in Mice
In C57 mice Van Donkelaar et al. (2010) found that TRP depletion achieved by oral gavage of solutions with a high ratio of large neutral amino acids relative to TRP failed to alter serum TRP, central 5-HT levels, 5-HT metabolism or behavior in forced swim and zero mazes. Blood samples were taken 30 min prior to start, at gavage (t0) and 30, 60 and 120 min after, and brain samples were collected at 20, 40, 60 and 240 min after. The authors attributed this difference to slower TRP metabolism and higher baseline TRP in C57 mice. Rat responses to TRP manipulation also vary by strain, both when TRP-to-5-HT metabolism in the brain and behavior are considered (Jans et al., 2010). Biskup et al. (2012) compared C57 and BALB/c responses to a TRP-free nutrient solution with low methionine (Moja-De) on a schedule including post-gavage tissue measures from 90–330 min, and saw depression- like behaviors were augmented by TRP depletion (Biskup et al. 2012). They also discovered both strains had less TRP and reduced 5-HT and 5-HIAA levels in brain (similar to Fig. 5 herein). However, when given a balanced amino acid mixture including more TRP (0.7g/10 kg) 5-HT synthesis was not enhanced (Biskup et al., 2012). Acute TRP depletions in mice by oral gavage are expeditious, but associated procedures such as food deprivation, restraint, forced-feeding, and repeated blood sampling, are stressful (van Donkelaar et al., 2010; 2011). Since stress-sensitive social behavior tests were our main interest, we opted instead to deplete TRP via ad libitum administration of purified diet overnight to achieve this end.
Modified pellet diets were employed in prior studies to examine the effects of chronic TRP depletion on rodent behaviors. In one study, dietary TRP depletion for 7 and 14 day increased immobility in forced swim, decreased TRP and 5-HT in brain, and increased serum CORT at both times in rats (Franklin et al., 2012). One-month dietary TRP depletion in C57 mice enhanced aggression, dominance and hyperactivity (Uchida et al. 2005). In C57 and BALB/c mice, Browne et al. (2012) examined chronic effects of dietary TRP manipulation (depleted, deficient, control and enhanced) in emotionality tests, and on TRP and 5-HT turnover. Pertinent to our finding of enhanced sociability in BTBR by +TRP diet (Fig. 3) was their discovery that TRP enhancement promoted nesting behavior (Browne et al., 2012).
With TRP depletion, plasma and brain TRP and 5-HT turnover was reduced and nesting and marble-burying were suppressed (Browne et al., 2012). Marble burying is typically sensitive to serotonergic manipulations (Deacon et al., 2006), and a limitation inherent in the present study is that marble-burying was measured after sociability tests, and associated CORT increases may have obscured effects of TRP manipulation. Yet we measured marble burying after sociability tests before and found drug (SSRI)-induced changes (Gould et al., 2011). This indicates that dietary TRP effects may be less robust than SSRI effects.
4.3. Other Considerations: Tph Metabolic Capacity and Supplementation
Our observations that 5-HT availability can impact social BTBR social behavior are paralleled in other inbred (BALB/c) and transgenic (Tph2 knock-out) mice with impaired sociability (Flood et al., 2012; Kane et al., 2012). Indeed, metabolic TRP abnormalities due to enzymatic deficiencies may contribute to brain hyposerotonemia in some patients with autism (Boccuto et al., 2013; Schwartz, 2014). In case-control studies, children with autism had elevated levels of plasma-free TRP and blood 5-HT levels, suggestive of compromised Tph2 function in brain (Hoshino et al., 1984; 1986; Coon et al., 2005). Thus, low dietary TRP intake or compromised TRP to 5-HT conversion in brain can impair social behavior. In the latter, TRP supplementation may be more problematic than helpful. However if capacity to produce 5-HT is compromised, drug interventions prolonging presence of 5-HT in the synapse, as might be achieved by OCT3 blockade (Baganz et al., 2008; Horton et al., 2013), may be of great therapeutic benefit for the treatment of sociability impairments.
Developmental depletion of cortical 5-HT by 5,7-dihydroxytryptamine in mice had transient effects on 5-HT transporter and receptor expression, yet it produced persistent increases in anxiety in response to change (Hohmann et al., 2007). On the other hand monoamine oxygenase-A knock-out mice have reduced capacity to degrade 5-HT and are aggressive, antisocial and bury many marbles, effects that were blocked by the Tph blocker p-chloro-phenylalanine ((PCPA) Bortolato et al., 2013). PCPA treatment was found to impair object recognition in mice (Alkam et al., 2011). Given this it will be of great interest learn of the effects of PCPA treatment on sociability in these strains in future studies.
4.4. Clinical Relevance: Dietary TRP Concerns in Autism and Social Behavior
‘Picky eating’ is commonly reported among children with autism, and manifests in a limited range of acceptable foods, high frequency intake of a single food type, or food refusal (Marí-Bauset et al., 2013). Selective avoidance of proteins rich in amino acids such as TRP may relate to the fact that about one-third of patients with autism exhibit “platelet hyperserotonemia” or higher blood 5-HT levels than normal (Anderson et al., 1990; Croonenberghs et al., 2005). This biomarker may result from excess intestinal 5-HT synthesis and release, or reduced hepatic 5-HT removal (Janušonis, 2008; Gabriele et al., 2014). So TRP avoidance by some children with autism might be a response to discomfort associated with elevated gut 5-HT (Aitken, 2008; Nakamura and Hasegawa, 2009).
To the limited extent that plasma amino acids and dietary intake in children with autism have been studied, evidence of protein and nutrient malnutrition has emerged, including relatively low levels of TRP, particularly in 4 and 8 year olds; and this is even more profound with casein/gluten restricted diets (Arnold et al., 2003; Herndon et al., 2009; Kałuzna-Czaplinska et al., 2010; Hyman et al., 2012; Tanoue et al., 2012; Naushad et al., 2013). While popular among alternative-approaches to manage autism symptoms, in general the effects of restricted diets have not been well-characterized (Marcason, 2009). However there is evidence that restrictions can worsen symptoms or hinder social development in children with central hyposerotonemia and autism (Christison and Ivany 2006; Hijej et al., 2008; Johnson et al., 2014). On the other hand, while TRP supplementation may ameliorate symptoms in some patients (Lakhan and Vieira, 2008), evidence for its effects in a broader population of autism patients is lacking. Hence studies on developmental and long-term effects of manipulating TRP intake are also warranted.
4.5. Conclusions: Significance of Experimental Findings
In conclusion, we have shown that overnight ad libitum dietary TRP manipulation alters social behaviors in BTBR, C57 and 129S mice in different ways: C57 and 129S mice behaviors were sensitive to TRP depletion (reduced preference for social interaction) that corresponded with clinical responses in autistic patients (McDougle et al., 1993; 1996). Also enhanced social interaction preference in BTBR after TRP supplementation resembled the response of a similarly-treated autism patient (Beretich, 2009). More clinical studies examining effects of dietary TRP supplementation are needed to determine how prevalent such beneficial responses might be in the greater population of patients with autism. However, it appears that screening 5-HT regulated hormones such as cortisol, oxytocin or prolactin, could provide some insight into the functional status of 5-HT system in patients. Such screens may help to determine if TRP supplements or 5-HT-based therapeutic interventions would be beneficial on an individualized basis.
Supplementary Material
Highlights.
Acute dietary tryptophan (TRP) effects on social behavior were studied.
Mouse strains used were BTBR T+Itpr3tf/J, 129S1/SvImJ and C57BL/6J.
TRP depletion impaired sociability in two strains and reduced brain 5-HT.
TRP enhancement improved sociability in BTBR mice.
Serum corticosterone was increased by sociability testing.
ACKNOWLEDGEMENTS
This study was supported by grants from the Department of Defense Congressionally-Directed Medical Research Program (#AR110109), the Morrison Trust, and Lindow, Stephens & Treat, LLP (to GGG), and the National Institutes of Health (MH093320 to LCD). This project was also supported by student involvement through sub-awards from the UTHSCSA Undergraduate Research START-UP program (GM097632), and Voelcker Biomedical Research program. We thank Catherine Gonzalez of Health Careers High School, San Antonio, TX making her students’ participation possible. We also thank Angela Gajda from Research Diets Inc., New Brunswick, NJ for assistance with diets, and Clover Moten for assistance with behavior tests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
However, parallel benefits of SSRI treatment are frequently muted or absent in children with autism (Henry et al., 2009; Williams et al., 2013; Politte et al., 2014).
REFERENCES
- Aitken KJ. Intersubjectivity, affective neuroscience, and the neurobiology of autistic spectrum disorders: a systematic review. Keio J Med. 2008;57:15–36. doi: 10.2302/kjm.57.15. [DOI] [PubMed] [Google Scholar]
- Alabdali A, Al-Ayadhi L, El-Ansary A. Association of social and cognitive impairment and biomarkers in autism spectrum disorders. J Neuroinflammation. 2014;11:4. doi: 10.1186/1742-2094-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkam T, Hiramatsu M, Mamiya T, Aoyama Y, Nitta A, Yamada K, Kim HC, Nabeshima T. Evaluation of object-based attention in mice. Behav Brain Res. 2011;220:185–193. doi: 10.1016/j.bbr.2011.01.039. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Horne WC, Chatterjee D, Cohen DJ. The hyperserotonemia of autism. Ann. N Y Acad. Sci. 1990;600:331–340. doi: 10.1111/j.1749-6632.1990.tb16893.x. [DOI] [PubMed] [Google Scholar]
- Arnold GL, Hyman SL, Mooney RA, Kirby RS. Plasma amino acids profiles in children with autism: potential risk of nutritional deficiencies. J Autism Dev Disord. 2003;33:449–454. doi: 10.1023/a:1025071014191. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Singh JS, Whitaker-Azmitia PM. Increased serotonin axons (immunoreactive to 5-HT transporter) in postmortem brains from young autism donors. Neuropharmacology. 2011;60:1347–154. doi: 10.1016/j.neuropharm.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Baganz NL, Horton RE, Calderon AS, Owens WA, Munn JL, Watts LT, Koldzic-Zivanovic N, Jeske NA, Koek W, Toney GM, Daws LC. Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc Natl Acad Sci U S A. 2008;105:18976–18981. doi: 10.1073/pnas.0800466105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baganz N, Horton R, Martin K, Holmes A, Daws LC. Repeated swim impairs serotonin clearance via a corticosterone-sensitive mechanism: organic cation transporter 3, the smoking gun. J Neurosci. 2010;30:15185–15195. doi: 10.1523/JNEUROSCI.2740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C, Abrams J, Nutt D. Tryptophan depletion and its implications for psychiatry. Br J Psychiatry. 2001;178:399–405. doi: 10.1192/bjp.178.5.399. [DOI] [PubMed] [Google Scholar]
- Beretich GR. Reversal of autistic symptoms by removal of low-relative tryptophan foods: case report. Med Hypotheses. 2009;73:856–857. doi: 10.1016/j.mehy.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Biskup CS, Sánchez CL, Arrant A, Van Swearingen AE, Kuhn C, Zepf FD. Effects of acute tryptophan depletion on brain serotonin function and concentrations of dopamine and norepinephrine in C57BL/6J and BALB/cJ mice. PLoS One. 2012;7:e35916. doi: 10.1371/journal.pone.0035916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccuto L, Chen CF, Pittman AR, Skinner CD, McCartney HJ, Jones K, Bochner BR, Stevenson RE, Schwartz CE. Decreased tryptophan metabolism in patients with autism spectrum disorders. Mol Autism. 2013;4:16. doi: 10.1186/2040-2392-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhomme D, Minni AM, Alfos S, Roux P, Richard E, Higueret P, Moisan MP, Pallet V, Touyarot K. Vitamin A status regulates glucocorticoid availability in Wistar rats: consequences on cognitive functions and hippocampal neurogenesis? Front Behav Neurosci. 2014;8:20. doi: 10.3389/fnbeh.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Godar SC, Tambaro S, Li FG, Devoto P, Coba MP, Chen K, Shih JC. Early postnatal inhibition of serotonin synthesis results in long-term reductions of perseverative behaviors, but not aggression, in MAO A-deficient mice. Neuropharmacology. 2013;75:223–232. doi: 10.1016/j.neuropharm.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne CA, Clarke G, Dinan TG, Cryan JF. An effective dietary method for chronic tryptophan depletion in two mouse strains illuminates a role for 5-HT in nesting behaviour. Neuropharmacology. 2012;62:1903–1915. doi: 10.1016/j.neuropharm.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Brune CW, Kim SJ, Salt J, Leventhal BL, Lord C, Cook EH., Jr 5-HTTLPR Genotype-specific phenotype in children and adolescents with autism. Am J Psychiatry. 2006;163:2148–2156. doi: 10.1176/ajp.2006.163.12.2148. [DOI] [PubMed] [Google Scholar]
- Callaghan PD, Owens WA, Javors MA, Sanchez TA, Jones DJ, Irvine RJ, Daws LC. In vivo analysis of serotonin clearance in rat hippocampus reveals that repeated administration of p-methoxyamphetamine (PMA), but not 3,4- methylenedioxymethamphetamine (MDMA), leads to long-lasting deficits in serotonin transporter function. J Neurochem. 2007;100:617–627. doi: 10.1111/j.1471-4159.2006.04247.x. [DOI] [PubMed] [Google Scholar]
- Camp MC, Macpherson KP, Lederle L, Graybeal C, Gaburro S, Debrouse LM, Ihne JL, Bravo JA, O’Connor RM, Ciocchi S, Wellman CL, Lüthi A, Cryan JF, Singewald N, Holmes A. Genetic strain differences in learned fear inhibition associated with variation in neuroendocrine, autonomic, and amygdala dendritic phenotypes. Neuropsychopharmacology. 2012;37:1534–1547. doi: 10.1038/npp.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro AM, Airey DC, Thompson B, Zhu CB, Lu L, Chesler EJ, Erikson KM, Blakely RD. Functional coding variation in recombinant inbred mouse lines reveals multiple serotonin transporter-associated phenotypes. Proc Natl Acad Sci. 2009;106:2047–2052. doi: 10.1073/pnas.0809449106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadman KK. Fluoxetine but not risperidone increases sociability in the BTBR mouse model of autism. Pharmacol Biochem Behav. 2011;97:586–594. doi: 10.1016/j.pbb.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Chandana SR, Behen ME, Juhász C, Muzik O, Rothermel RD, Mangner TJ, Chakraborty PK, Chugani HT, Chugani DC. Significance of abnormalities in developmental trajectory and asymmetry of cortical serotonin synthesis in autism. Int J Dev Neurosci. 2005;23:171–182. doi: 10.1016/j.ijdevneu.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Christison GW, Ivany K. Elimination diets in autism spectrum disorders: any wheat amidst the chaff? J Dev Behav. Pediatr. 2006;27:S162–S171. doi: 10.1097/00004703-200604002-00015. [DOI] [PubMed] [Google Scholar]
- Coon H, Dunn D, Lainhart J, Miller J, Hamil C, Battaglia A, Tancredi R, Leppert MF, Weiss R, McMahon W. Possible association between autism and variants in the brain-expressed tryptophan hydroxylase gene (TPH2) Am J Med Genet B Neuropsychiatr Genet. 2005;135B:42–46. doi: 10.1002/ajmg.b.30168. [DOI] [PubMed] [Google Scholar]
- Croonenberghs J, Verkerk R, Scharpe S, Deboutte D, Maes M. Serotonergic disturbances in autistic disorder: L-5-hydroxytryptophan administration to autistic youngsters increases the blood concentrations of serotonin in patients but not in controls. Life Sci. 2005;76:2171–2183. doi: 10.1016/j.lfs.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Daly EM, Deeley Q, Ecker C, Craig M, Hallahan B, Murphy C, Johnston P, Spain D, Gillan N, Brammer M, Giampietro V, Lamar M, Page L, Toal F, Cleare A, Surguladze S, Murphy DG. Serotonin and the neural processing of facial emotions in adults with autism: an fMRI study using acute tryptophan depletion. Arch Gen Psychiatry. 2012;69:1003–1013. doi: 10.1001/archgenpsychiatry.2012.513. [DOI] [PubMed] [Google Scholar]
- Daws LC, Gould GG. Ontogeny and regulation of the serotonin transporter: providing insights into human disorders. Pharmacol Ther. 2011;131:61–79. doi: 10.1016/j.pharmthera.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon RM. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nat Protoc. 2006;1:122–124. doi: 10.1038/nprot.2006.20. [DOI] [PubMed] [Google Scholar]
- Delgado PL. Monoamine depletion studies: implications for antidepressant discontinuation syndrome. J Clin Psychiatry. 2006;67:22–26. [PubMed] [Google Scholar]
- Feder A, Skipper J, Blair JR, Buchholz K, Mathew SJ, Schwarz M, Doucette JT, Alonso A, Collins KA, Neumeister A, Charney DS. Tryptophan depletion and emotional processing in healthy volunteers at high risk for depression. Biol Psychiatry. 2011;69:804–807. doi: 10.1016/j.biopsych.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood ZC, Engel DL, Simon CC, Negherbon KR, Murphy LJ, Tamavimok W, Anderson GM, Janušonis S. Brain growth trajectories in mouse strains with central and peripheral serotonin differences: relevance to autism models. Neuroscience. 2012;210:286–295. doi: 10.1016/j.neuroscience.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Franklin M, Bermudez I, Murck H, Singewald N, Gaburro S. Sub-chronic dietary tryptophan depletion--an animal model of depression with improved face and good construct validity. J Psychiatr Res. 2012;46:239–247. doi: 10.1016/j.jpsychires.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Gabriele S, Sacco R, Persico AM. Blood serotonin levels in autism spectrum disorder: A systematic review and meta-analysis. Eur Neuropsychopharmacol. 2014 doi: 10.1016/j.euroneuro.2014.02.004. In press. [DOI] [PubMed] [Google Scholar]
- Giustarini D, Dalle-Donne I, Cavarra E, Fineschi S, Lungarella G, Milzani A, Rossi R. Metabolism of oxidants by blood from different mouse strains. Biochem Pharmacol. 2006;71:1753–1764. doi: 10.1016/j.bcp.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior. J Neurochem. 2011;116:291–303. doi: 10.1111/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GG, Burke TF, Osorio MD, Smolik CM, Zhang WQ, Onaivi ES, Gu TT, DeSilva MN, Hensler JG. Enhanced novelty-induced corticosterone spike and upregulated serotonin 5-HT1A and cannabinoid CB1 receptors in adolescent BTBR mice. Psychoneuroendocrinology. 2014;39:158–169. doi: 10.1016/j.psyneuen.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T, Kanno K, Nagasawa M, Nishimori K, Mogi K, Kikusui T. Impairment of interstrain social recognition during territorial aggressive behavior in oxytocin receptor-null mice. Neurosci Res. 2014 doi: 10.1016/j.neures.2014.05.003. In press. [DOI] [PubMed] [Google Scholar]
- Henry CA, Shervin D, Neumeyer A, Steingard R, Spybrook J, Choueiri R, Bauman M. Retrial of selective serotonin reuptake inhibitors in children with pervasive developmental disorders: a retrospective chart review. J Child Adolesc Psychopharmacol. 2009;19:111–117. doi: 10.1089/cap.2008.037. [DOI] [PubMed] [Google Scholar]
- Herndon AC, DiGuiseppi C, Johnson SL, Leiferman J, Reynolds A. Does nutritional intake differ between children with autism spectrum disorders and children with typical development? J Autism Dev Disord. 2009;39:212–222. doi: 10.1007/s10803-008-0606-2. [DOI] [PubMed] [Google Scholar]
- Hjiej H, Doyen C, Couprie C, Kaye K, Contejean Y. Substitutive and dietetic approaches in childhood autistic disorder: interests and limits. Encephale. 2008;34:496–503. doi: 10.1016/j.encep.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Hill JE, Makky K, Shrestha L, Hillard CJ, Gasser PJ. Natural & synthetic corticosteroids inhibit uptake 2-mediated transport in CNS neurons. Physiol Behav. 2011;104:306–311. doi: 10.1016/j.physbeh.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Hohmann CF, Walker EM, Boylan CB, Blue ME. Neonatal serotonin depletion alters behavioral responses to spatial change and novelty. Brain Res. 2007;1139:163–177. doi: 10.1016/j.brainres.2006.12.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Soorya L, Chaplin W, Anagnostou E, Taylor BP, Ferretti CJ, Wasserman S, Swanson E, Settipani C. A double-blind placebo-controlled trial of fluoxetine for repetitive behaviors and global severity in adult autism spectrum disorders. Am J Psychiatry. 2012;169:292–299. doi: 10.1176/appi.ajp.2011.10050764. [DOI] [PubMed] [Google Scholar]
- Horton RE, Apple DM, Owens WA, Baganz NL, Cano S, Mitchell NC, Vitela M, Gould GG, Koek W, Daws LC. Decynium-22 enhances SSRI-induced antidepressant-like effects in mice: uncovering novel targets to treat depression. J Neurosci. 2013;33:10534–10543. doi: 10.1523/JNEUROSCI.5687-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y, Yamamoto T, Kaneko M, Tachibana R, Watanabe M, Ono Y, Kumashiro H. Blood serotonin and free tryptophan concentration in autistic children. Neuropsychobiology. 1984;11:22–27. doi: 10.1159/000118045. [DOI] [PubMed] [Google Scholar]
- Hoshino Y, Yamamoto T, Kaneko M, Kumashiro H. Plasma free tryptophan concentration in autistic children. Brain Dev. 1986;8:424–427. doi: 10.1016/s0387-7604(86)80064-x. [DOI] [PubMed] [Google Scholar]
- Hyman SL, Stewart PA, Schmidt B, Cain U, Lemcke N, Foley JT, Peck R, Clemons T, Reynolds A, Johnson C, Handen B, James SJ, Courtney PM, Molloy C, Ng PK. Nutrient intake from food in children with autism. Pediatrics. 2012;130:S145–S153. doi: 10.1542/peds.2012-0900L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans LA, Korte-Bouws GA, Korte SM, Blokland A. The effects of acute tryptophan depletion on affective behaviour and cognition in Brown Norway and Sprague Dawley rats. J Psychopharmacol. 2010;24:605–614. doi: 10.1177/0269881108099424. [DOI] [PubMed] [Google Scholar]
- Janušonis S. Origin of the blood hyperserotonemia of autism. Theor Biol Med Model. 2008;5:10. doi: 10.1186/1742-4682-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John AM, Bell JM. Amino acid requirements of the growing mouse. J Nutr. 1976;106:1361–1367. doi: 10.1093/jn/106.9.1361. [DOI] [PubMed] [Google Scholar]
- Johnson CR, Turner K, Stewart PA, Schmidt B, Shui A, Macklin E, Reynolds A, James J, Johnson SL, Manning CP, Hyman SL. Relationships between feeding problems, behavioral characteristics and nutritional quality in children with ASD. J Autism Dev Disord. 2014;44:2175–2184. doi: 10.1007/s10803-014-2095-9. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Keisala T, Minasyan A, Kuuslahti M, Miettinen S, Tuohimaa P. Behavioural anomalies in mice evoked by “Tokyo” disruption of the Vitamin D receptor gene. Neurosci Res. 2006;54:254–260. doi: 10.1016/j.neures.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Angoa-Peréz M, Briggs DI, Sykes CE, Francescutti DM, Rosenberg DR, Kuhn DM. Mice genetically depleted of brain serotonin display social impairments, communication deficits and repetitive behaviors: possible relevance to autism. PLoS One. 2012;7:e48975. doi: 10.1371/journal.pone.0048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd PM. Autism, an extreme challenge to integrative medicine: Part 2. Medical management. Altern Med Rev. 2002;7:472–499. [PubMed] [Google Scholar]
- Kałuzna-Czaplinska J, Michalska M, Rynkowski J. Determination of tryptophan in urine of autistic and healthy children by gas chromatography/mass spectrometry. Med Sci Monit. 2010;16:CR488–CR492. [PubMed] [Google Scholar]
- Kumar B, Prakash A, Sewal RK, Medhi B, Modi M. Drug therapy in autism: a present and future perspective. Pharmacol Rep. 2012;64:1291–1304. doi: 10.1016/s1734-1140(12)70927-1. [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Vieira KF. Nutritional therapies for mental disorders. Nutr J. 2008;7:2. doi: 10.1186/1475-2891-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KS, Aman MG, Arnold LE. Neurochemical correlates of autistic disorder: a review of the literature. Res Dev Disabil. 2006;27:254–289. doi: 10.1016/j.ridd.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Macbeth AH, Lee HJ, Edds J, Young WS. Oxytocin and the oxytocin receptor underlie intrastrain, but not interstrain, social recognition. Genes Brain Behav. 2009;8:558–567. doi: 10.1111/j.1601-183X.2009.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden AM, Zup SL. Effects of developmental hyperserotonemia on juvenile play behavior, oxytocin and serotonin receptor expression in the hypothalamus are age and sex dependent. Physiol Behav. 2014;128:260–269. doi: 10.1016/j.physbeh.2014.01.036. [DOI] [PubMed] [Google Scholar]
- Makkonen I, Riikonen R, Kokki H, Airaksinen MM, Kuikka JT. Serotonin and dopamine transporter binding in children with autism determined by SPECT. Dev Med Child Neurol. 2008;50:593–597. doi: 10.1111/j.1469-8749.2008.03027.x. [DOI] [PubMed] [Google Scholar]
- Marcason W. What is the current status of research concerning use of a gluten-free, casein-free diet for children diagnosed with autism? J Am Diet Assoc. 2009;109(3):572. doi: 10.1016/j.jada.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Marí-Bauset S, Zazpe I, Mari-Sanchis A, Llopis-González A, Morales-Suárez-Varela M. Food Selectivity in Autism Spectrum Disorders: Systematic Review. J Child Neurol. 2013 doi: 10.1177/0883073813498821. In press. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Naylor ST, Goodman WK, Volkmar FR, Cohen DJ, Price LH. Acute tryptophan depletion in autistic disorder: a controlled case study. Biol Psychiatry. 1993;33:547–550. doi: 10.1016/0006-3223(93)90011-2. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Naylor ST, Cohen DJ, Aghajanian GK, Heninger GR, Price LH. Effects of tryptophan depletion in drug-free adults with autistic disorder. Arch Gen Psychiatry. 1996;53:993–1000. doi: 10.1001/archpsyc.1996.01830110029004. [DOI] [PubMed] [Google Scholar]
- McNamara IM, Borella AW, Bialowas LA, Whitaker-Azmitia PM. Further studies in the developmental hyperserotonemia model (DHS) of autism: social, behavioral and peptide changes. Brain Res. 2008;1189:203–214. doi: 10.1016/j.brainres.2007.10.063. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavior tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Hasegawa H. Production and Peripheral Roles of 5-HTP, a Precursor of Serotonin. Int J Tryptophan Res. 2009;2:37–43. doi: 10.4137/ijtr.s1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Sekine Y, Ouchi Y, Tsujii M, Yoshikawa E, Futatsubashi M, Tsuchiya KJ, Sugihara G, Iwata Y, Suzuki K, Matsuzaki H, Suda S, Sugiyama T, Takei N, Mori N. Brain serotonin and dopamine transporter bindings in adults with high-functioning autism. Arch Gen Psychiatry. 2010;67:59–68. doi: 10.1001/archgenpsychiatry.2009.137. [DOI] [PubMed] [Google Scholar]
- Naushad SM, Jain JM, Prasad CK, Naik U, Akella RR. Autistic children exhibit distinct plasma amino acid profile. Indian J Biochem Biophys. 2013;50:474–478. [PubMed] [Google Scholar]
- National Research Council. Nutrient Requirements of Laboratory Animals. 4th Edition. Washington, DC: National Academies Press; 1995. Nutrient requirements of the mouse; pp. 80–102. [Google Scholar]
- Patrick RP, Ames BN. Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism. FASEB J. 2014;28:2398–2413. doi: 10.1096/fj.13-246546. [DOI] [PubMed] [Google Scholar]
- Politte LC, Henry CA, McDougle CJ. Psychopharmacological interventions in autism spectrum disorder. Harv Rev Psychiatry. 2014;22:76–92. doi: 10.1097/HRP.0000000000000030. [DOI] [PubMed] [Google Scholar]
- Ricci MR, Ulman EA. Laboratory animal diets: a critical part of your in vivo research. Animal Lab News. 2005;4:1–6. [Google Scholar]
- Rubin DH, Althoff RR, Ehli EA, Davies GE, Rettew DC, Crehan ET, Walkup JT, Hudziak JJ. Candidate gene associations with withdrawn behavior. J Child Psychol Psychiatry. 2013;54:1337–1345. doi: 10.1111/jcpp.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri B, Sarkans U, Schumann G, Persico AM. Biomarkers in autism spectrum disorder: the old and the new. Psychopharmacology (Berl) 2014;231:1201–1216. doi: 10.1007/s00213-013-3290-7. [DOI] [PubMed] [Google Scholar]
- Schwartz CE. Aberrant tryptophan metabolism: the unifying biochemical basis for autism spectrum disorders? Biomark Med. 2014;8:313–315. doi: 10.2217/bmm.14.11. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme SJ, Bell RZ, Pahumi R, Murphy GG. Comparison of inbred mouse substrains reveals segregation of maladaptive fear phenotypes. Front Behav Neurosci. 2014;8:282. doi: 10.3389/fnbeh.2014.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue K, Matsui K, Takamasu T. Fried-potato diet causes vitamin A deficiency in an autistic child. JPEN J Parenter Enteral Nutr. 2012;36:753–755. doi: 10.1177/0148607111436280. [DOI] [PubMed] [Google Scholar]
- Terada Y, Okura Y, Kikusui T, Takenaka A. Dietary vitamin E deficiency increases anxiety-like behavior in juvenile and adult rats. Biosci Biotechnol Biochem. 2011;75:1894–1899. doi: 10.1271/bbb.110190. [DOI] [PubMed] [Google Scholar]
- Toker L, Amar S, Bersudsky Y, Benjamin J, Klein E. The biology of tryptophan depletion and mood disorders. Isr J Psychiatry Relat Sci. 2010;47:46–55. [PubMed] [Google Scholar]
- Uchida S, Kitamoto A, Umeeda H, Nakagawa N, Masushige S, Kida S. Chronic reduction in dietary tryptophan leads to changes in the emotional response to stress in mice. J Nutr Sci Vitaminol. 2005;51:175–181. doi: 10.3177/jnsv.51.175. [DOI] [PubMed] [Google Scholar]
- Unwin LM, Maybery MT, Wray JA, Whitehouse AJ. A “bottom-up” approach to aetiological research in autism spectrum disorders. Front Hum Neurosci. 2013;7:606. doi: 10.3389/fnhum.2013.00606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Donkelaar EL, Blokland A, Lieben CK, Kenis G, Ferrington L, Kelly PA, Steinbusch HW, Prickaerts J. Acute tryptophan depletion in C57BL/6 mice does not induce central serotonin reduction or affective behavioural changes. Neurochem Int. 2010;56:21–34. doi: 10.1016/j.neuint.2009.08.010. [DOI] [PubMed] [Google Scholar]
- van Donkelaar EL, Blokland A, Ferrington L, Kelly PA, Steinbusch HW, Prickaerts J. Mechanism of acute tryptophan depletion: is it only serotonin? Mol. Psychiatry. 2011;16:695–713. doi: 10.1038/mp.2011.9. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, Ye R, Kerr TM, Carneiro AM, Crawley JN, Sanders-Bush E, McMahon DG, Ramamoorthy S, Daws LC, Sutcliffe JS, Blakely RD. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci. 2012;109:5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West L, Waldrop J, Brunssen S. Pharmacologic treatment for the core deficits and associated symptoms of autism in children. J Pediatr Health Care. 2009;23:75–89. doi: 10.1016/j.pedhc.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Whitehouse AJO, Stanley FJ. Is autism one or multiple disorders? Med J. Aust. 2013;28:302–303. doi: 10.5694/mja12.11667. [DOI] [PubMed] [Google Scholar]
- Williams K, Brignell A, Randall M, Silove N, Hazell P. Selective serotonin reuptake inhibitors (SSRIs) for autism spectrum disorders. Cochrane DB Syst Rev. 2013;8:CD004677. doi: 10.1002/14651858.CD004677.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CJ, Tan HP, Du YJ. The developmental disruptions of serotonin signaling may be involved in autism during early brain development. Neuroscience. 2014;267C:1–10. doi: 10.1016/j.neuroscience.2014.02.021. [DOI] [PubMed] [Google Scholar]
- Yang M, Silverman JL, Crawley JN. Automated three-chambered social approach task for mice. Chapter 8: Unit 8.26. Curr Protoc Neurosci. 2011 doi: 10.1002/0471142301.ns0826s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Beaulieu JM, Sotnikova TD, Gainetdinov RR, Caron MG. Tryptophan hydroxylase-2 controls brain serotonin synthesis. Science. 2004;305:217. doi: 10.1126/science.1097540. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.